Abstract
Voltage-dependent calcium and sodium channels mediating persistent inward currents (PICs) amplify the effects of synaptic inputs on the membrane potential and firing rate of motoneurons. CaPIC channels are thought to be relatively slow, whereas the NaPIC channels have fast kinetics. These different characteristics influence how synaptic inputs with different frequency content are amplified; the slow kinetics of Ca channels suggest that they can only contribute to amplification of low frequency inputs (<5 Hz). To characterize frequency-dependent amplification of excitatory postsynaptic potentials (EPSPs), we measured the averaged stretch-evoked EPSPs in cat medial gastrocnemius motoneurons in decerebrate cats at different subthreshold levels of membrane potential. EPSPs were produced by muscle spindle afferents activated by stretching the homonymous and synergist muscles at frequencies of 5–50 Hz. We adjusted the stretch amplitudes at different frequencies to produce approximately the same peak-to-peak EPSP amplitude and quantified the amount of amplification by expressing the EPSP integral at different levels of depolarization as a percentage of that measured with the membrane hyperpolarized. Amplification was observed at all stretch frequencies but generally decreased with increasing stretch frequency. However, in many cells the amount of amplification was greater at 10 Hz than at 5 Hz. Fast amplification was generally reduced or absent when the lidocaine derivative QX-314 was included in the electrode solution, supporting a strong contribution from Na channels. These results suggest that NaPICs can combine with CaPICs to enhance motoneuron responses to modulations of synaptic drive over a physiologically significant range of frequencies.
Keywords: persistent inward currents, resonance
an individual motoneuron must convert synaptic current delivered by thousands of excitatory and inhibitory inputs on its dendritic tree into a train of action potentials whose frequency controls the force developed by its muscle unit. The contractile properties of muscle act to low-pass filter the motoneuron command delivered to them (Baldissera et al. 1998). The response of motoneurons to somatic current injection suggests that conductances on and around the soma of motoneurons can high-pass filter their inputs (Baldissera et al. 1982, 1984), and that this can act to “precompensate” for the muscle's low-pass filtering, allowing muscles to produce rapid changes in force when needed (Baldissera et al. 1998). However, both experimental and simulation work suggests that without some form of amplification, currents generated by dendritic synapses are low-pass filtered and attenuated as they travel toward the soma and thus cannot provide enough current to the spike-generating conductances to produce either maximum steady discharge rates (Cushing et al. 2005; Powers and Binder 2001; Rall et al. 1967) or rapid changes in discharge rate.
Recent evidence (cf. Heckmann et al. 2005; Powers and Binder 2001) suggests that under conditions of tonic neuromodulatory drive, persistent inward currents (PICs) generated predominantly by voltage-dependent calcium (Cav1.3) channels on the dendrites provide robust amplification of synaptic currents. However, the kinetics of these channels appear to be slow (e.g., Lee and Heckman 1998; Li and Bennett 2003), and their activation is thought to give rise to all-or-none plateau potentials in the dendrites, thus exaggerating the low-pass filtering properties of dendrites. There is also evidence that persistent current carried by dendritic Na channels can contribute to amplification (Jones and Lee 2006). The persistent component of current carried by Na channels has fast kinetics, and so could mediate amplification of rapidly varying synaptic inputs. Furthermore, Na currents together with the prominent hyperpolarization-activated cation (IH) currents seen in motoneurons could act to mediate resonant behavior (Manuel et al. 2007). It is not clear, however, whether the distribution and voltage dependence of the channels carrying IH and fast inward currents are appropriate to facilitate resonant transfer of synaptic current.
Experimental studies of amplification in motoneurons largely have been confined to the analysis of steady-state or slowly varying inputs (e.g., Lee and Heckman 2000; Lee and Heckman 1996; Lee et al. 2003; Prather et al. 2001). There is only one study of amplification of the high-frequency (180 Hz) component of excitatory synaptic input (Jones and Lee 2006). A recent study of subthreshold resonance in motoneurons examined the interaction of IH and simulated PICs, but the PIC was simulated by a dynamic conductance clamp applied through the somatic recording electrode, and frequency-dependent amplification was measured by the response to somatically injected current (Manuel et al. 2007). The experiments described below examine frequency-dependent amplification of an excitatory synaptic input that is predominantly distributed to motoneuron dendrites, under conditions of tonic, neuromodulatory drive, over a physiological important range of input frequencies (2–50 Hz).
We measured excitatory synaptic inputs with different frequency content by recording intracellularly from triceps surae motoneurons (predominantly medial gastrocnemius) in decerebrate cats during sinusoidal stretching of the triceps surae muscle at frequencies of 2–50 Hz. The stretch amplitude at each frequency was adjusted so that the peak-to-peak amplitude of the stretch-evoked excitatory postsynaptic potentials (EPSPs) measured at the resting potential was similar across different frequencies of stretch. We combined muscle stretch with de- and hyperpolarizing-injected current pulses to measure voltage-dependent synaptic amplification at each stretch frequency. We found voltage-dependent amplification at all stretch frequencies. The amount of amplification generally increased with increasing depolarization and decreased with increasing stretch frequency, although in many cells the amount of amplification was greater at 10 Hz than at 5 Hz. In a subset of cells, we measured synaptic amplification with the lidocaine derivative QX-314 included in the electrode solution. In most of these cases, synaptic amplification was reduced or eliminated, suggesting a prominent contribution of Na channels to amplification over the entire frequency range studied. A preliminary account of these results has been presented in abstract form (Powers et al. 2010).
METHODS
Data were collected from 29 medial gastrocnemius (MG) and 5 lateral gastrocnemius-soleus (LGS) motoneurons in 5 adult cats (2.5–3.5 kg). All surgical and experimental procedures had the approval of the Wright State University Institutional Animal Care and Use Committee. Anesthesia was induced with an intramuscular injection of ketamine (10 mg/kg) and xylazine (1 mg/kg) and maintained throughout the initial dissection with a gaseous mixture of isofluorane (1.5–2.5%) in O2 administered via a tracheal cannula. Artificial respiration was adjusted to hold end-tidal CO2 between 3 and 4%. The right carotid artery and jugular vein were cannulated for monitoring blood pressure and administering fluids, respectively, and the left carotid artery was ligated. The lumbosacral enlargement was exposed by a laminectomy from L4 to S1 to provide access to MG and LGS motoneurons. The left hindlimb was dissected to expose the MG and LGS muscle nerves and the common peroneal nerve, and the triceps surae muscles were separated from their surrounding tissues. The common peroneal nerve was crushed to prevent tonic Ia inhibition from the antagonist muscles, which can reduce persistent inward currents (Kuo et al. 2003). After separating the plantaris tendon, the triceps surae muscles were freed of surrounding tissue, and their common tendon of insertion (Achilles tendon) was detached from the calcaneous. The animal was then mounted in a recording frame, and an intercollicular decerebration was performed. Anesthesia was discontinued after the decerebration. At the end of the recording session, animals were euthanized using a lethal dose of intravenous pentobarbital.
Intracellular recordings from MG and LGS motoneurons were made with glass micropipettes filled with either 2 M K-acetate or 2 M K-acetate with 0.1 M QX-314 (resistances of 5–10 MΩ) connected to an Axoclamp-2A amplifier operated in either bridge or DCC mode. Recordings were taken from motoneurons with resting potentials more negative than −60 mV and action potential amplitudes of at least 70 mV upon impalement. (In most cells impaled with the QX-314-containing electrodes, the action potentials attenuated rapidly after impalement.) We first collected a series of antidromic action potentials, followed by a series of 50-ms current pulses of different amplitude to determine rheobase (Zengel et al. 1985). A series of 1-ms, suprathreshold current pulses were applied to elicit direct action potentials and afterhyperpolarizations (AHPs), followed by a series of ±5 nA, 500-ms current steps to measure input resistance (Zengel et al. 1985) and the sag ratio, which was calculated as the peak change in voltage following a −5-nA current step, divided by the mean change in voltage over the last 100 ms of the current step (Manuel et al. 2007).
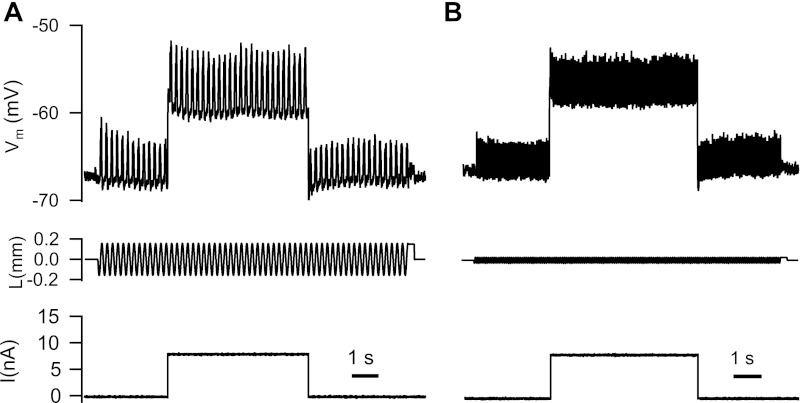
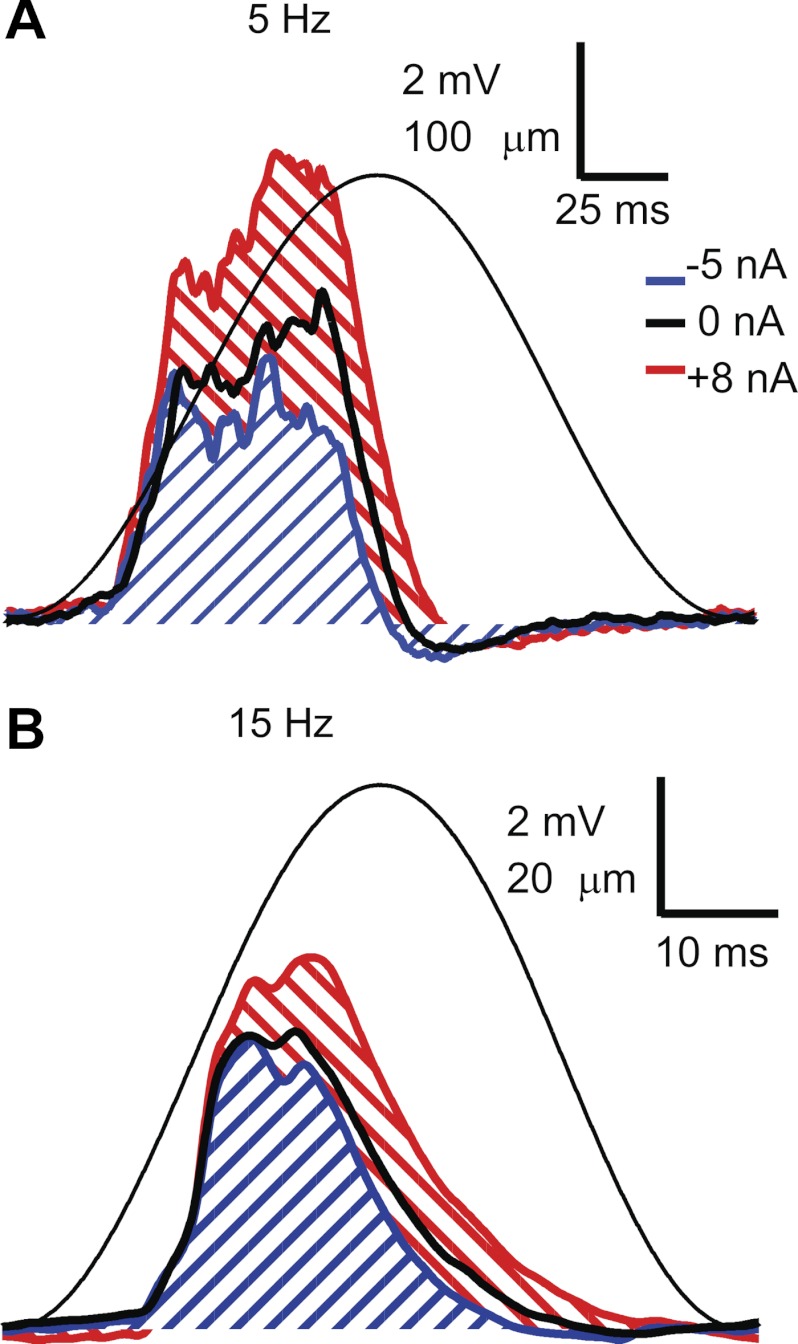
Following the initial characterization of motoneuron properties, we measured EPSPs produced by activation of primary muscle spindle (Ia) afferents in response to sinusoidal stretch of the triceps surae muscles at different frequencies. To stretch the muscles, the Achilles tendon was tied directly to the lever of a motor system (model 310C-LR, Aurora Scientific). The motor was used both to stretch the triceps surae muscles with specific sinusoidal frequencies and to record length and force at the lever. The stretch amplitude was decreased at higher frequencies to compensate for the dynamic sensitivity of Ia afferents (Matthews 1972) to produce EPSPs of approximately the same peak-to-peak amplitude in a given cell (2–6 mV measured at the resting potential) at different stretch frequencies. The mean membrane potential was varied in different trials by injecting de- and hyperpolarizing current steps during stretch. Figure 1 shows example records of membrane potential (Vm), muscle length (L), and injected current (I) in an MG motoneuron during stretching of the triceps surae at 5 Hz (Fig. 1A) and 15 Hz (Fig. 1B). At each level of membrane potential, we averaged the stretch-evoked EPSP across several stretch cycles. We typically recorded one series at the resting potential, one during a 5-nA hyperpolarizing current step, and one at one or more levels of depolarizing current up to a level that was just subthreshold for discharge. Figure 2 shows averaged stretch-evoked EPSPs at 5 Hz (Fig. 2A) and 15 Hz (Fig. 2B) for three different levels of injected current.
Fig. 1.
Basic experimental protocol. Sinusoidal muscle stretch at 5 Hz (A) and 15 Hz (B) was combined with a range of injected current levels (+8 nA in this example). The 3 traces in each panel are membrane voltage (Vm, top), muscle length (L, relative to the resting length, middle), and injected current (I, bottom). Note that the amplitude of sinusoidal stretch was adjusted down at higher frequencies to produce roughly comparable levels of depolarization.
Fig. 2.
Average stretch-evoked excitatory postsynaptic potentials (EPSPs) during 5 Hz (A) and 15 Hz (B) muscle stretch during different levels of injected current (blue: −5 nA, thick black line: 0 nA, red: +8 nA). The thin solid line is the averaged length trace (note different length calibration in A and B). Diagonal lines indicate EPSP areas for the EPSPs recorded at hyperpolarized and depolarized levels.
In cells recorded with K acetate electrodes, increasing depolarization led to an increase in both the EPSP peak-to-peak amplitude and EPSP duration. Voltage-dependent amplification was quantified by expressing the integral of the stretch-evoked EPSP at depolarized levels (Fig. 2) as a percentage of the value measured when the membrane is hyperpolarized. In cells recorded using electrodes containing QX-314, “amplification” was quantified in the same way, although depolarization often led to a decrease in EPSP area. We collected several sets of stretch-evoked EPSPs in cells recorded with QX-314 electrodes interspersed with measurement of a series of antidromic action potentials to assess the degree of sodium channel block.
Statistical comparisons under different conditions are based on either Student's t-test or the non-parametric Kruskal-Wallis test, with the Bonferroni correction for multiple comparisons. The Tukey HSD test was used to compare amplification at different frequencies for QX-314-containing and control electrodes. Variability about the mean is expressed by the standard deviation in the text and standard error bars in the figures.
RESULTS
Basic cell properties.
We recorded stretch-evoked EPSPs in 25 triceps surae motoneurons (20 MG and 5 LGS) using K acetate electrodes (control), and an additional 9 motoneurons (all MG) using electrodes containing K acetate and 0.1 mM QX-314. The average input resistance for the control cells was 0.85 ± 0.56 MΩ (range 0.39–2.0 MΩ), and for the QX-314 cells was 0.79 ± 0.39 MΩ (range 0.31–1.4 MΩ). The sag ratios for the two groups were also similar; control: 1.40 ± 0.2 (range: 1.11–1.93), QX-314: 1.38 ± 0.15 (range: 1.14–1.58). The average rheobase of the control cells was 14.1 ± 8.5 nA (range 3–32 nA). Although we attempted to determine rheobase as quickly as possible after impalement with QX-314-containing electrodes, in two cells the spike was blocked so quickly that spikes could not be elicited by 50-ms current pulses. In the remaining seven cells, rheobase values were similar to those of control cells: 15.6 ± 8 nA (range 5–29 nA). The afterhyperpolarization durations were also similar in the two samples; control: 52.0 ± 13.9 ms (range: 34.0–92.6 ms), QX-314: 51.8 ± 16.1 ms (range: 34.4–85.2 ms). None of the intrinsic properties differed significantly between the two samples (Kruskal-Wallis, P > 0.1 in all cases).
Frequency-dependent amplification.
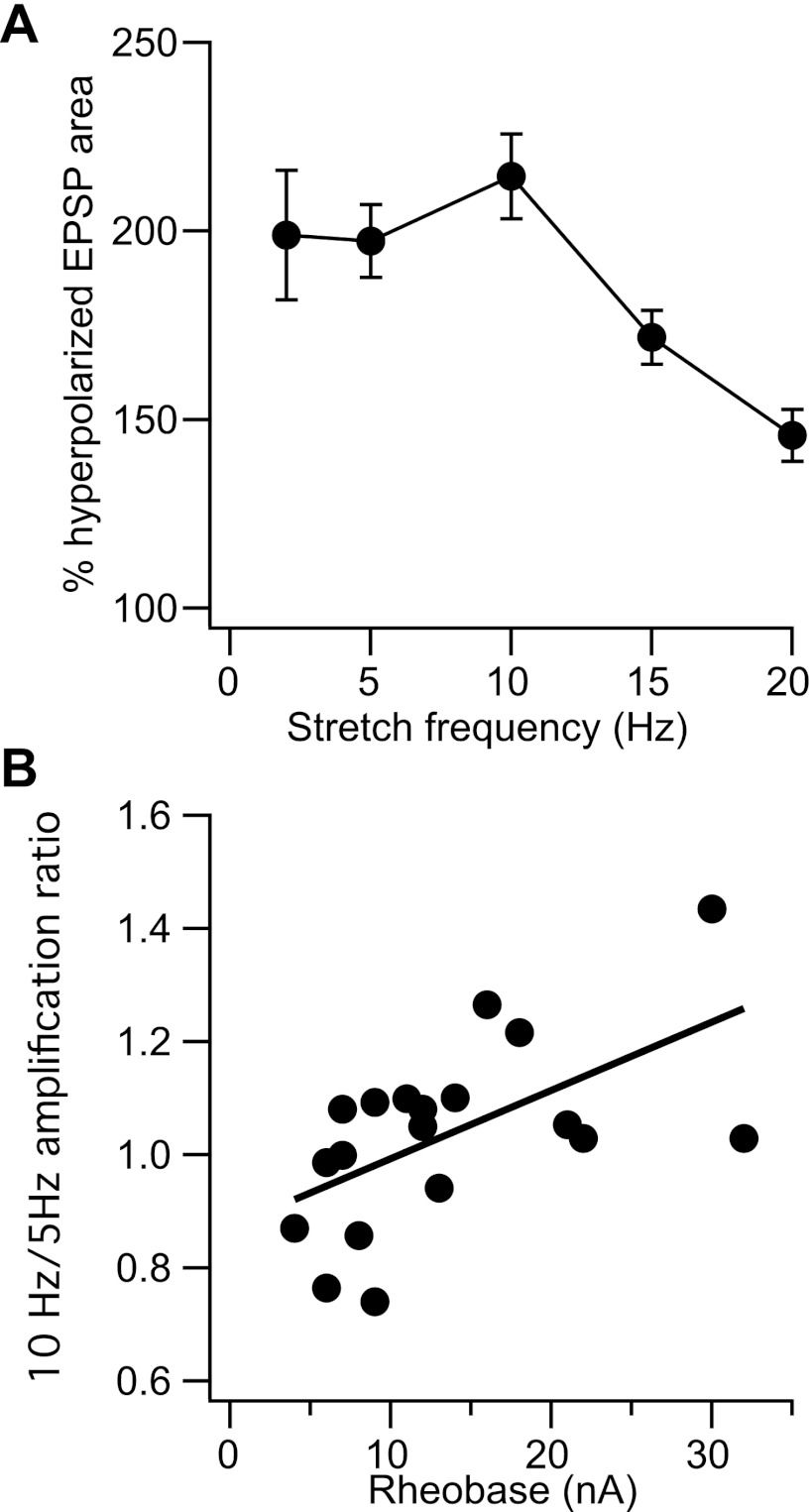
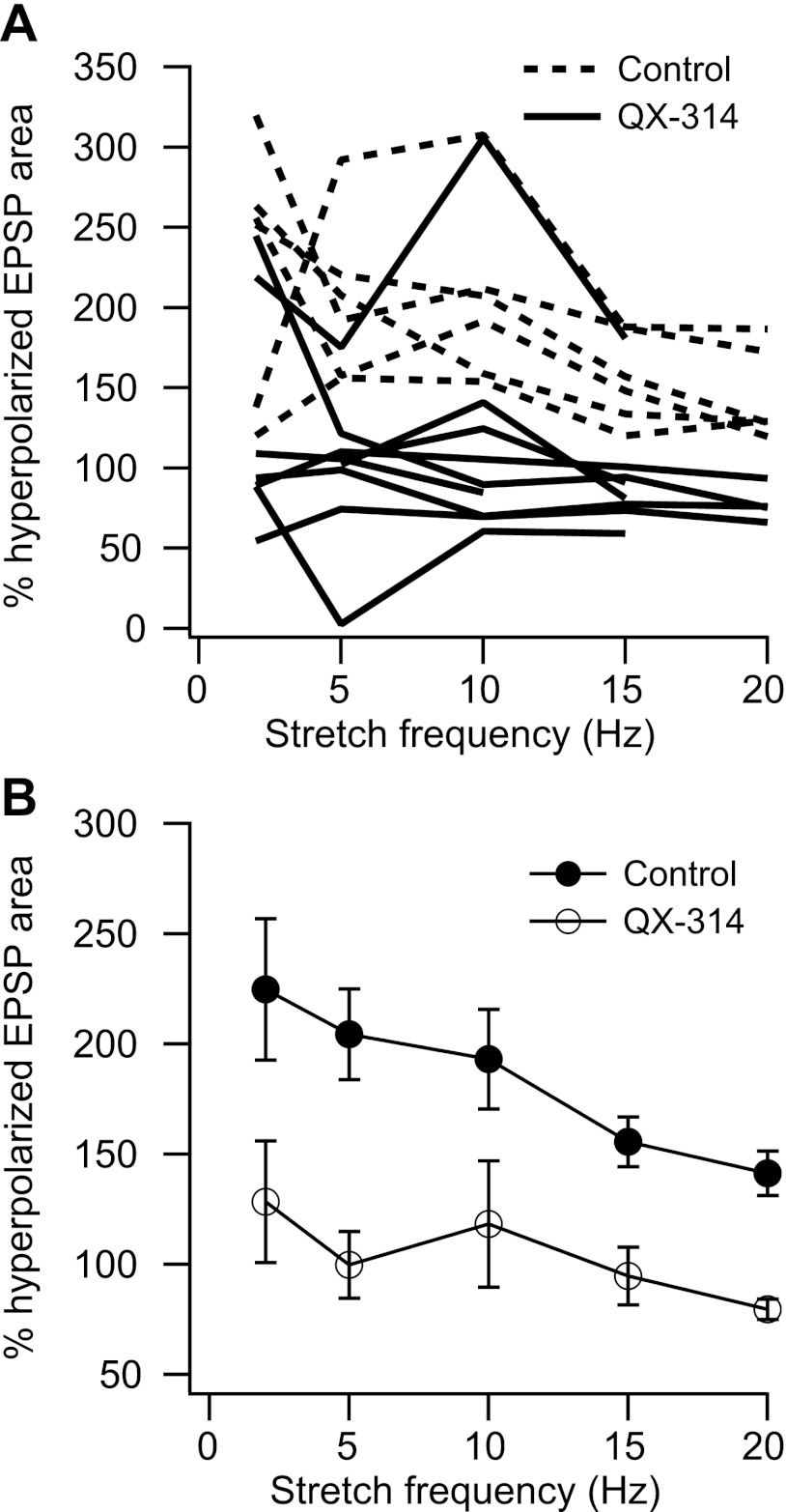
In our control sample, membrane depolarization led to EPSP amplification in all cases for stretching frequencies from 2 to 20 Hz. Due to changes in recording conditions, we were not able to record the effects of membrane polarization on stretch-evoked EPSPs for all frequencies in every cell. We obtained the most data for stretch frequencies from 2 to 20 Hz (2 Hz: 14 cells, 5Hz: 22 cells, 10 Hz: 23 cells, 15 Hz: 13 cells, 20 Hz: 19 cells). Figure 3A shows the mean and standard error of the amount of EPSP amplification observed near threshold (expressed as a percentage of the EPSP area at hyperpolarized potentials). EPSPs evoked by 50 Hz stretching were amplified in 6/7 cells studied (mean: 126 ± 18.9%; data not shown). The frequency dependence of amplification differed across cells. In some cells, the amount of amplification decreased at increasing stretch frequencies, whereas in others there was a clear resonant peak in amplification, generally at 10 Hz. Since we did not examine the entire range of stretch frequencies in every cell, we quantified the tendency of amplification to increase up to 10 Hz by taking the ratio of amplification at 10 Hz to that at 5 Hz. Figure 3B shows this amplification ratio as a function of rheobase. There was a significant positive correlation between the amplification ratio and rheobase (r = 0.58, P < 0.01, n = 19), indicating a tendency for increased resonance in higher threshold cells. Although this could reflect a higher density of the HCN channels mediating membrane sag (Manuel et al. 2007), the amplification ratio was not significantly correlated with the sag ratio in our sample.
Fig. 3.
Frequency-dependent amplification of stretch-evoked EPSPs. A: mean and standard error of amplification as a function of frequency. B: ratio of amplification at 10 Hz to that at 5 Hz as a function of cell rheobase.
Effects of QX-314 on amplification of stretch-evoked EPSPs.
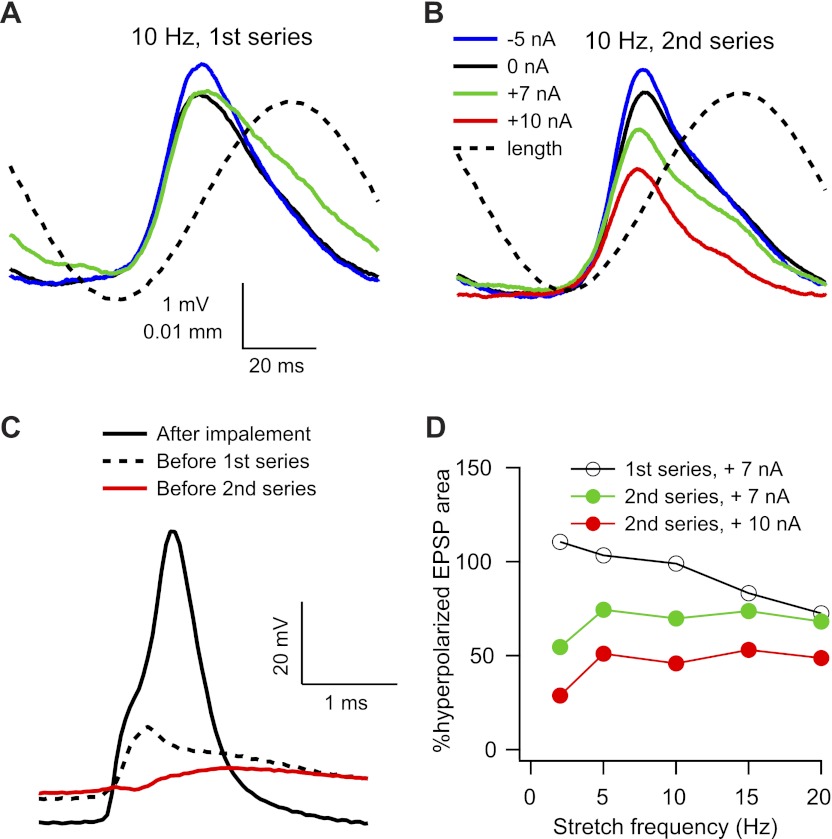
Amplification of stretch-evoked EPSPs was generally reduced or abolished in cells that were impaled with electrodes containing QX-314, although this effect could depend on the amount of time that had elapsed since impalement. Figure 4 shows the effects of membrane depolarization on the amplitude of stretch-evoked EPSPs measured at two different times in the same cell. Shortly after impalement, a nearly full-sized (70 mV) antidromic action potential was recorded, but after measuring AHP properties, input resistance, and rheobase (∼3 min later), only an attenuated initial segment spike remained. We then collected a complete series of stretch responses at resting, depolarized, and hyperpolarized membrane potentials at stretching frequencies from 2 to 50 Hz. Figure 4A shows the averaged EPSPs evoked by 10 Hz stretching at three different levels of injected current. The peak-to-peak amplitudes of the EPSPs at rest (black) and during depolarizing current injection (green) are smaller than that of the EPSP recorded during hyperpolarizing current injection, but the falling phase of the EPSP recorded during depolarization was prolonged so that the area of the depolarized EPSP was approximately equal to that of the hyperpolarized EPSP.
Fig. 4.
Effects of QX-314 on voltage dependence of stretch-evoked EPSPs. A and B: average EPSPs evoked by 10 Hz stretch starting around 3 (A) and 10 (B) min after impalement. C: antidromic action potentials recorded immediately after impalement (solid black line), after 3 min (dotted black line), and after 10 min (red line). D: effects of depolarization on EPSP area at different frequencies for the first and second series of responses.
After the first series of stretch responses (which took ∼10 min to collect), we recorded another set of antidromic spikes, and at this time the initial segment spike was blocked and only the M-spike remained (Fig. 4C). We then collected another series of stretch responses, and Fig. 4B shows the average responses to 10 Hz stretches at four different levels of injected current. For this series, both the peak-to-peak amplitude and the area of the EPSPs clearly decreased with increasing depolarization, and the area under the EPSP at the most depolarized level was only ∼50% the EPSP recorded during hyperpolarization. This level of attenuation is unlikely to be due solely to a change in EPSP driving force, since the difference in mean membrane potential for the two EPSPs was ∼19 mV (from −75 mV to −56 mV), which would correspond to about a 25% reduction in driving force for somatic EPSPs (assuming a reversal potential of 0 mV) and even less for dendritic EPSPs. This and previous evidence (Clements et al. 1986) suggests a contribution from an outward current active in this voltage range (see discussion).
Figure 4D shows the average EPSP area at stretch frequencies of 2–20 Hz for the two series of responses. EPSP area was reduced by depolarization at all frequencies for the second series of responses, particularly at the higher level of depolarizing current injection. EPSP areas at depolarized levels (expressed as a percentage of the hyperpolarized response) evoked by 50 Hz stretches were similar to those obtained at 20 Hz (data not shown).
We used electrodes containing QX-314 in two experiments. To control for changes in excitability over the course of the experiment, we recorded additional control cells using K acetate alone both before and after using the QX-314-containing electrodes. Figure 5A shows amplification vs. frequency for the control (n = 6) and QX-314 (n = 9) cells recorded in these two experiments. Amplification in the QX-314 cells was lower than control values except for one cell that showed normal amplification at the lowest stretch frequency and another cell that showed amplification across all frequencies even though the somato-dendritic spike was blocked and the initial segment spike was partially blocked. Across the entire QX-314 sample, amplification was not significantly different from zero (i.e., depolarized EPSP area = 100% of hyperpolarized EPSP area) at any stretch frequency (Student's t-test, P > 0.1). Figure 5B shows the mean and standard error of normalized EPSP area vs. frequency for the two samples. Amplification was significantly reduced by QX-314 at all stretch frequencies (Tukey's HSD test, q = 26.85, P < 0.05).
Fig. 5.
A: EPSP area as a percentage of hyperpolarized area for stretch frequencies of 2–20 Hz for control (dotted lines) and QX-314 (solid lines) cells recorded in the same experiments. B: mean and standard error of EPSP area for the 2 samples. The difference between the 2 samples was statistically significant at all frequencies (Tukey's HSD, P < 0.05).
Specificity of QX-314 effects.
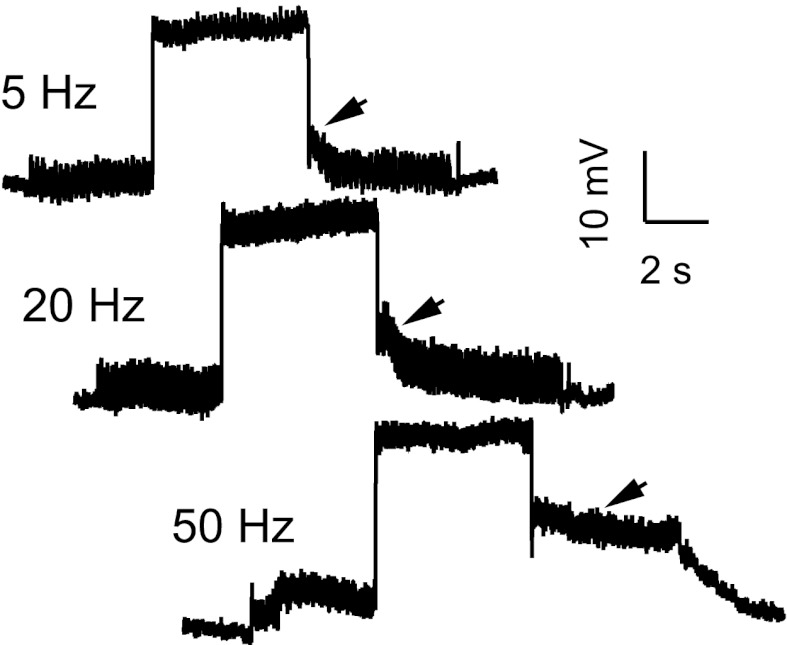
QX-314 not only blocks sodium currents, but has also been shown to reduce calcium currents as well (Talbot and Sayer 1996). However, persistent inward currents and plateau depolarizations can still be recorded in the presence of QX-314 (Bennett et al. 1998; Lee and Heckman 1999; Powers and Binder 2000), suggesting that calcium channels are still available to provide synaptic amplification. To compare EPSPs at similar levels of depolarization in control and QX-314 cells, we generally applied levels of depolarizing current that were just below rheobase, and these currents did not usually evoke plateau potentials in the QX-314 cells. However, in five QX-314 cells we also applied currents that were greater than the rheobase measured shortly after impalement, and these could occasionally evoke plateau potentials. Figure 6 shows an example of this in the same cell whose average stretch-evoked EPSPs are shown in Fig. 4 (second series, depolarizing current of 10 nA). The mean membrane potential showed a delayed return to the resting potential following a depolarizing current step during 5 Hz muscle stretching. This slow decay was more prominent during 20 Hz stretching, and during 50 Hz stretching a sustained plateau followed the injected current step, and membrane potential did not return to the resting potential until the stretching was turned off. These examples show that in the presence of QX-314 the response to the DC component of a depolarizing stimulus (the injected current) could be amplified and prolonged even while the response to the AC component (sinusoidal muscle stretch) was attenuated by membrane depolarization (Fig. 4, B and C).
Fig. 6.
Responses to membrane depolarization and sinusoidal muscle stretch at 3 different frequencies in the presence of QX-314. Depolarization was produced by a +10 nA current step.
DISCUSSION
Our results show that voltage-dependent amplification of excitatory synaptic input is present across a wide range of frequencies and that the frequency dependence of amplification varies among low- and high-threshold motoneurons. The greater relative amplification of high frequencies in high-threshold cells confirms predictions based on somatic current injection (Manuel et al. 2007). Since higher-threshold motoneurons innervate muscle fibers with faster contraction times, this finding suggests that resonant amplification in motoneurons may be matched to muscle contractile properties.
Our results also suggest that Na channels make a prominent contribution to amplification of high-frequency fluctuations in synaptic input. The kinetics of Cav1.3 channels appear to be slow in situ (Lee and Heckman 1998; Li and Bennett 2003), and simulations of Cav1.3 activation in compartmental models typically use activation time constants of 20–60 ms (Bui et al. 2006; Carlin et al. 2000; Elbasiouny et al. 2005). This suggests that Cav1.3 channels can only amplify slowly varying components of synaptic input (<5 Hz), and introducing inward currents with activation time constants of 50 ms via dynamic clamp only amplifies these low input frequencies (Manuel et al. 2007). In contrast, the activation and deactivation time constants of the persistent component of Na current are on the order of 1 ms (Kay et al. 1998), making the NaPIC well suited to amplify high-frequency fluctuations in synaptic input. The reduction or elimination of fast amplification by QX-314 further supports the contribution of Na channels.
The location of the Na channels responsible for fast amplification in motoneurons is not known. In most neurons, the high concentration of Na channels at the axon initial segment makes them the primary source of persistent Na current (e.g., Astman et al. 2006). However, since over 95% of the surface area and synaptic contacts onto motoneurons is on the dendrites, even low levels of dendritic NaPIC can make a significant contribution to amplification. Furthermore, Jones and Lee (2006) found amplification of high-frequency excitatory synaptic currents during somatic voltage clamp, which would have prevented voltage fluctuations from being transmitted to the soma and initial segment. The slow development of the effects of QX-314 on amplification and the failure of QX-314 to block amplification in one cell could reflect the time taken for QX-314 to diffuse to distant dendritic sites.
The resonant amplification in some cells could reflect the interaction of NaPIC and IH, as previously suggested (Manuel et al. 2007). However, the contribution of IH should be relatively small at depolarized potentials, suggesting a potential contribution from another repolarizing current, such as a subthreshold K current. In CA1 pyramidal cells, resonance at voltages positive to the resting potential results from the interaction of NaPIC and an M-current (Hu et al. 2002). The reduction in stretch-evoked EPSPs produced by depolarization in the presence of QX-314 is also consistent with the presence of a subthreshold K current, since the reduction in amplitude is greater than would be expected on the basis of changes in synaptic driving force alone. The presence of a subthreshold K current in motoneurons is also supported by the results of Clements et al. (1986), who found an enhancement of single fiber and compound Ia EPSPs in motoneurons after K channels were blocked with internal TEA.
The combined contribution of Na, K, and Ca channels can potentially provide more flexible amplification of synaptic inputs, particularly if the relative contribution of these channels is under neuromodulatory control. If the level of neuromodulatory drive to motoneurons changes both the amount of synaptic amplification and its frequency dependence, this would represent a trade-off between the ability to sustain high forces (stronger amplification and low-pass filtering) vs. the ability to make rapid changes in muscle force (less amplification of steady synaptic inputs with better preservation of high-frequency components of the synaptic input). The characteristics of frequency-dependent amplification of synaptic input in a given neuromodulatory state have important implications for the control of motoneuron output by ionotropic inputs. If amplification comes at the expense of increased low-pass filtering, rapid changes in motor output can only be achieved by “sculpting” out decreases in output with inhibitory inputs (i.e., “push-pull” inhibition; Johnson and Heckman 2010). Although reciprocal changes in excitation and inhibition have been recently shown to enhance slow, cyclical changes in muscle force (Johnson et al. 2012), it is not known if such a push-pull mechanism would work for faster movements. In any case, if higher-frequency components of excitatory synaptic inputs are amplified as well, then rapid changes in excitatory drive to motoneurons may be sufficient to achieve rapid changes in motor output. Furthermore, since the excitatory input from the stretch-sensitive muscle spindles to motoneurons produces a closed feedback loop, high-pass filtering and phase advance produced by both motoneurons and muscle spindles may be needed to compensate for lags associated with conduction delays and muscle contraction, thus reducing reflex instability and tremor (Matthews 1997).
Our findings demonstrate strong amplification of synaptic excitation in a behaviorally relevant range of muscle length change. Frequencies of 5–10 Hz have been measured for length changes of the MG muscle in normal cats during walking and paw shake (Fowler et al. 1988; Maas et al. 2010). Our results give further indirect support to assertions that amplification of excitatory synaptic drive to motoneurons contributes to performance of motor tasks (e.g., Gorassini et al. 2002; Powers et al. 2008), and here we suggest that this contribution is frequency dependent. The increase in amplification from 5–10 Hz, particularly for the higher-threshold motoneurons (Fig. 3), is likely to support recruitment of fast motor units which are thought to be important for deceleration of limb oscillation during paw shake. Amplification of higher input frequencies (>10 Hz) may enhance phase locking to suprathreshold inputs at these frequencies. The sensitivity of motoneuron spike rate to high frequency components of synaptic input may act to increase the efficiency of synaptic driving of motoneurons (Parkis et al. 2003), and the sensitivity of spike timing to high-frequency inputs may underlie the coherent oscillations between cortical and muscle activity at these frequencies (Farmer et al. 1993). In summary, functional amplification of synaptic inputs in motoneurons is not limited to the support of tonic activation of motoneurons during postural tasks, but is likely to contribute to motoneuron activation during a wide range of motor behaviors.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-06220, NS-071951, and NS-049324.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.K.P., P.N., and T.C.C. conception and design of research; R.K.P., P.N., and T.C.C. performed experiments; R.K.P. analyzed data; R.K.P., P.N., and T.C.C. interpreted results of experiments; R.K.P. prepared figures; R.K.P. drafted manuscript; R.K.P., P.N., and T.C.C. edited and revised manuscript; R.K.P., P.N., and T.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lori Goss for assistance with the initial animal surgery and Beverly Grunden for help with the statistical analyses.
REFERENCES
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci 26: 3465–3473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Campadelli P, Piccinelli L. The dynamic response of cat alpha-motoneurones investigated by intracellular injection of sinusoidal currents. Exp Brain Res 54: 275–282, 1984 [DOI] [PubMed] [Google Scholar]
- Baldissera F, Campadelli P, Piccinelli L. Neural encoding of input transients investigated by intracellular injection of ramp currents in cat α-motoneurones. J Physiol 328: 73–86, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Cerri G. Motoneuronal pre-compensation for the low-pass filter characteristics of muscle. A quantitative appraisal in cat muscle units. J Physiol 511: 611–627, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca(2+) channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol 95: 225–241, 2006 [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci 12: 1635–1646, 2000 [DOI] [PubMed] [Google Scholar]
- Clements JD, Nelson PG, Redman SJ. Intracellular tetraethylammonium ions enhance group Ia excitatory post-synaptic potentials evoked in cat motoneurones. J Physiol 377: 267–282, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol 94: 3465–3478, 2005 [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol 94: 3961–3974, 2005 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg SF, Stephens JA. The frequency content of common synaptic input to motoneurons studied during voluntary contraction in man. J Physiol 463: 83–105, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler EG, Gregor RJ, Roy RR. Differential kinetics of fast and slow ankle extensors during the paw-shake in the cat. Exp Neurol 99: 219–224, 1988 [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol 545: 783–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann NY Acad Sci 1198: 35–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Hyngstrom AS, Manuel M, Heckman CJ. Push-pull control of motor output. J Neurosci 32: 4592–4599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Lee RH. Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol 96: 2200–2206, 2006 [DOI] [PubMed] [Google Scholar]
- Kay AR, Sugimori M, Llinás R. Kinetic and stochastic properties of a persistent sodium current in mature guinea pig cerebellar Purkinje cells. J Neurophysiol 80: 1167–1179, 1998 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol 90: 3617–3624, 2003 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol 76: 2107–2110, 1996 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol 82: 2518–2527, 1999 [DOI] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol 89: 27–39, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 30: 30, 2003 [DOI] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, English AW, Prilutsky BI. Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists. Exp Brain Res 203: 681–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci 27: 12977–12988, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Spindle and motoneuronal contributions to the phase advance of the human stretch reflex and the reduction of tremor. J Physiol 498: 249–275, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. Baltimore: Williams & Winkins, 1972 [Google Scholar]
- Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci 23: 8152–8158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol 83: 483–500, 2000 [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Frequency-dependent amplification of stretch-evoked EPSPs in spinal motoneurons. Soc Neurosci Abstr 685–620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol 85: 43–53, 2001 [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol 30: 1169–1193, 1967 [DOI] [PubMed] [Google Scholar]
- Talbot MJ, Sayer RJ. Intracellular QX-314 inhibits calcium currents in hippocampal CA1 pyramidal neurons. J Neurophysiol 76: 2120–2124, 1996 [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53: 1323–1344, 1985 [DOI] [PubMed] [Google Scholar]