Abstract
The extraction of optic flow cues is fundamental for successful locomotion. During forward motion, the focus of expansion (FoE), in conjunction with knowledge of eye position, indicates the direction in which the individual is heading. Therefore, it is expected that cortical brain regions that are involved in the estimation of heading will be sensitive to this feature. To characterize cortical sensitivity to the location of the FoE or, more generally, the center of flow (CoF) during visually simulated self-motion, we carried out a functional MRI (fMRI) adaptation experiment in several human visual cortical areas that are thought to be sensitive to optic flow parameters, namely, V3A, V6, MT/V5, and MST. In each trial, two optic flow patterns were sequentially presented, with the CoF located in either the same or different positions. With an adaptation design, an area sensitive to heading direction should respond more strongly to a pair of stimuli with different CoFs than to stimuli with the same CoF. Our results show such release from adaptation in areas MT/V5 and MST, and to a lesser extent V3A, suggesting the involvement of these areas in the processing of heading direction. The effect could not be explained either by differences in local motion or by attention capture. It was not observed to a significant extent in area V6 or in control area V1. The different patterns of responses observed in MST and V6, areas that are both involved in the processing of egomotion in macaques and humans, suggest distinct roles in the processing of visual cues for self-motion.
Keywords: functional MRI, visual cortex, self-motion, global motion
when an individual moves around the environment, a coherent pattern of motion known as optic flow reaches the retina. Movement is typically forward, generating expanding optic flow, and the focus of expansion (FoE) indicates, in retinotopic coordinates, the direction toward which the individual is heading (Gibson 1950; Warren and Hannon 1988). Because the FoE is highly informative for determining heading parameters, it is expected that cortical areas encoding visual self-motion will be sensitive to this feature. In this study we simulate self-motion that contains forward, backward, and rotational components in equal measure, and so we use the more general term center of flow (CoF) to describe it.
Studies of visual motion sensitivity in macaque point toward a role of area MST in the computation of egomotion (Duffy and Wurtz 1995; Gu et al. 2006, 2007; Saito et al. 1989). Cells in the dorsal portion of MST (MSTd) are well suited for the analysis of optic flow: they have large receptive fields, and they show sensitivity to specific optic flow components (Duffy and Wurtz 1991b) and to heading direction (Gu et al. 2007; Page and Duffy 2003). Furthermore, electrical stimulation of MST neurons shifts the heading judgments of monkeys viewing optic flow fields (Britten and van Wezel 1998). Area VIP has similar properties (Schaafsma and Duysens 1996; Zhang and Britten 2011), but it is thought to integrate multisensory self-motion parameters to a greater extent than MSTd (Bremmer et al. 2002; Duhamel et al. 1998).
In humans, sensitivity to optic flow structure has been demonstrated in area MST and to a lesser extent in areas MT/V5, V3A, and V3B (Greenlee 2000; Morrone et al. 2000; Smith et al. 2006; Wall et al. 2008). Further studies have identified flow-sensitive areas that have not been studied in macaques in this context, such as human V6, the cingulate sulcus visual area (CSv), and the precuneus (Cardin and Smith 2010; Wall and Smith 2008). Moreover, responses in the POS (putative V6) and the dorsal IPS (Brandt et al. 1998; Kovács et al. 2008) have been found in studies of vection—the illusion of egomotion induced by optic flow.
Sensitivity to heading direction has seldom been studied in the human brain. A previous study by Peuskens et al. (2001) showed that MT+ and the dorsal IPS were active when participants judged the displacement of a CoF. Koyama et al. (2005) showed that the strongest activations in V3A occur in the region of the visual field that represents the location of the CoF, suggesting that the extraction of this feature could start in intermediate stages of the visual hierarchy. In this study, we used functional MRI (fMRI) adaptation to determine sensitivity to heading direction in optic flow-sensitive visual regions of the human brain. The rationale was that if a region is involved in extracting visual cues for the computation of heading direction, it should contain populations of neurons that are sensitive to CoFs located in specific parts of the visual field. If so, then when a stimulus with a given CoF is presented twice in rapid succession, the response to the second presentation will be attenuated by the first, because of adaptation or “repetition suppression” (Grill-Spector et al. 1999; Grill-Spector and Malach 2001). In contrast, when the two stimuli have different CoFs the response to the second will be less attenuated.
The aim of the study was to determine sensitivity to heading direction in various predefined, motion-sensitive human brain areas. Following the above rationale, if there are separate neural populations encoding different directions of heading in a given visual area, trials with pairs of stimuli with different CoFs will result in larger compound responses, because the second stimulus will activate relatively unadapted neurons, than trials in which the two stimuli have the same CoF. It should be noted that “heading” normally relates to an upright person moving, and looking, forward. In our case, the subjects were supine in the scanner, looking upward. Expanding optic flow would therefore indicate upward motion in world-centered coordinates. However, optic flow is equivalent in the two cases, and we assume here that the BOLD responses we record in visual areas are comparable to those that would be obtained in an upright person.
MATERIALS AND METHODS
Participants.
Seven individuals (including two of the authors, L. Hemsworth and A. T. Smith) with normal or corrected-to-normal vision gave written consent to participate in the study reported here, which was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee at Royal Holloway, University of London. Standard MRI screening procedures were followed for all participants, and naive volunteers were paid for their participation.
Stimuli and experimental design.
An fMRI adaptation experiment was conducted. Stimuli were presented in pairs, one trial consisting of an adapting stimulus (S1, adapter) and a test stimulus (S2, probe). S1 was presented for 3 s, followed by a 1-s interstimulus interval and finally by S2 for 1 s (Fig. 1A). This timing was based on previous studies of fMRI adaptation in the visual cortex (Ashida et al. 2007; Larsson et al. 2006; Smith and Wall 2008) together with pilot studies. A fixed intertrial interval (ITI) of 8 s followed before the start of the next trial. To provide a demanding task that diverted attention from the motion stimuli, participants were instructed to fixate a small (1.2° × 1.6°) central letter that changed identity throughout the run at a rate of 2 Hz. On each update, the letter was drawn from a set of seven (E, F, H, I, L, P, T) with the constraint that the same letter could not be drawn twice in succession and “E” and “F” together had the same probability of being presented as each of the other individual letters. fMRI adaptation responses have been shown to be influenced by perceptual expectation and capture of attention (Larsson and Smith 2011; Summerfield et al. 2008). However, a highly demanding task that diverts attention from the relevant properties of the stimuli is effective in eliminating these confounding effects so as to isolate genuine adaptation effects (Larsson and Smith 2011). For this purpose, and to ensure central fixation, participants counted the instances of “E” and “F,” adding 1 to their mental count each time they were presented with the former and subtracting 1 when presented with the latter. The count started at 10 to reduce the need to mentally increment and decrement negative numbers. Participants reported their final count verbally at the end of each run. The mean correlation between the reported and the actual count was 0.92 (P = 0.2 × 10−16), indicating that participants were concentrating on the task. As well as diverting attention from the motion stimuli, this task ensured good fixation. Eye movements were not monitored since good performance provided an indicator of good fixation, the letter being small. As a check that the task successfully diverted attention from the stimuli, an “Attention Control” condition (see below) was included.
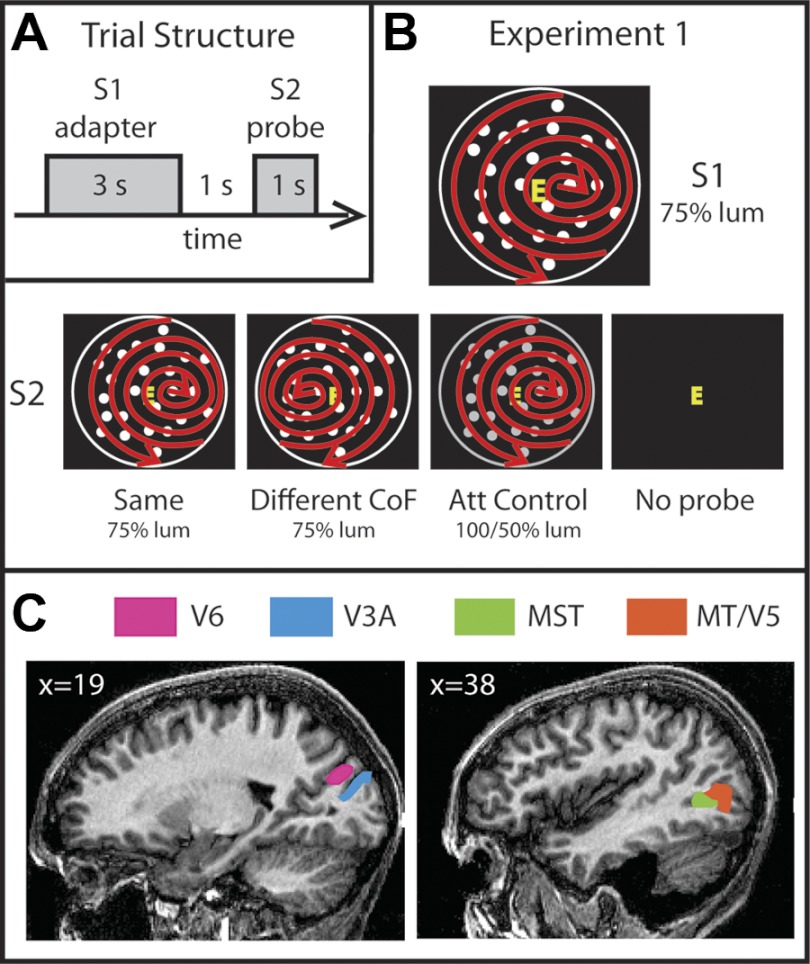
Fig. 1.
Stimuli, experimental design, and anatomical location of regions of interest (ROIs). A: schematic representation of a single trial sequence. The experiment was designed to test for adaptation effects in several motion areas. For that purpose, in every trial a pair of stimuli were presented sequentially. The first stimulus (S1), referred to as the adapter, was shown for 3 s, followed by a 1-s presentation of a black background displaying only a fixation square. After this, the second stimulus (S2), the probe, was presented for 1 s. Trials were separated by 8-s periods of background presentation. B: stimuli. There were 4 conditions: Same (S1 = S2), Different CoF [center of flow (CoF) of S2 located in the opposite left-right location to S1], Attention Control (S2 had the same CoF but a different luminance), and No Probe (no S2). In all trials, the adapter consisted of dots expanding, contracting, and rotating from a CoF located toward the right or left of fixation, as indicated by the red arrows. The arrows and the white circle surrounding the dots were not present in the stimuli and are shown only for diagrammatic purposes. The red arrows indicate only that spiral global motion occurred and do not represent dot trajectories. C: cortical ROIs. The locations of areas V6, V3A, MST, and MT/V5 on sagittal slices of the brain of 1 participant are shown. Slice coordinates are in Talairach space.
The stimuli were generated with Psychophysics Toolbox version 3 (http://psychtoolbox.org) and MATLAB 7.9 (MathWorks) and projected onto a rear-projection screen with a LCD projector. To obtain wide-field visual stimulation, emulating natural optic flow, the screen was viewed via a custom optical device that magnified the image to give a 60° field of view. The device was monocular and was positioned over the participant's preferred eye; the unstimulated eye was occluded.
The experiment was designed to test for sensitivity to different directions of heading, in several visual areas. Since it is impossible to change the CoF without changing local motion, it is essential to establish whether any observed adaptation effects reflect local differences or global differences. The stimuli consisted of a 56°-diameter circular field of 300 light dots on a dark background moving in a coherent pattern. Global flow cycled repeatedly through spiral space to give, at different times, expansion, contraction, and rotation components. This simulates optic flow elicited by self-motion on a varying spiral trajectory (Morrone et al. 2000; see Cardin and Smith 2010 and Wall and Smith 2008 for more details). It was created by updating the trajectory of each dot over time, such that each dot had a curved trajectory consistent with the changing simulated self-motion. This stimulus was selected because it provides a means of balancing local dot motion. During one cycle of the stimulus (which lasted 1 s), the local motion at any given location occurs once in each possible direction at some point in time. Local direction changes smoothly at each point, with a different temporal phase at each point. Thus, when integrated over the 1-s unit of stimulus presentation, there were no local motion differences between patterns with the same or different CoF and any response differences must be due to global differences. To avoid introducing local differences between stimuli that would compromise this careful construction, the dot size and dot speed were not scaled with eccentricity as would occur in natural flow but were the same at all points in the image.
The CoF of the pattern was located 14° to the right or left of the center of the display, to simulate two very different heading directions. There were four conditions (Fig. 1B): 1) Same—the CoF was located in the same position (either left or right) for S1 and S2; 2) Different CoF—in S2, the CoF was displaced from fixation in the opposite direction to S1; 3) Attention Control—here, the CoF was the same for S1 and S2, as in the “Same” condition; however, dot contrast differed between S1 and S2 (see below); 4) No Probe—in this condition there was no S2, in order to isolate the response to the probe.
The Attention Control condition was included as a check that any effect observed with pairs of different stimuli was not due to attentional modulation caused by the novelty of the second stimulus. This is not expected, because of the demanding attentional task at fixation, but an empirical check is important in view of the reported confounds of attention and expectation in adaptation studies. In the Attention Control condition, S1 and S2 had the same CoF, as in the “Same” condition, but differed on another salient (attention capturing) stimulus dimension that is of little relevance to visual processing in the dorsal cortical areas under examination, namely, contrast. It is well known that in macaques the contrast response functions saturate at a low contrast in MT/V5 and MST (Sclar et al. 1990; Wall and Smith 2008), so a change in contrast has little effect on the response, except at very low contrasts. Whereas in all the other conditions the luminance of the dots in both S1 and S2 was 75% of the maximum available, in this condition the luminance of S1 was also 75% of the maximum but the luminance of S2 increased or decreased, with equal probability, to 100% or 50% of maximum, respectively. This was intended to attract attention at least as strongly as a change in CoF. A result in line with the “Same” condition indicates that attention was successfully diverted from the motion stimuli; results in line with the “Different” condition suggest that the larger response in that condition reflects attention differences rather than adaptation. It cannot be assumed that responses in dorsal regions are completely independent of contrast, and certainly this is not the case in V1, which is used here to provide a comparison with higher areas, but the fact that luminance increased and decreased equally often balances any direct effects of contrast. This approach has been used successfully in previous studies (Lingnau et al. 2009; Wall et al. 2008).
There were six scan runs per scanning session, each lasting ∼7 min. In each scan run, there were 32 trials (8 of each type) that were included in the analysis. The first of these was preceded by an additional trial that was not analyzed, so that the first analyzed trial conformed to the balanced pattern of preceding trials, giving 33 trials in total per run. The trial order was different for each scan run but always conformed to the same counterbalancing regimen. The order of presentation of trials within a given run was determined such that each trial type was preceded equally often by each of the trial types, including itself. This was to ensure that cross-trial contamination affected all conditions equally. The same set of six trial sequences was used for each participant; the order of the runs was counterbalanced across participants to eliminate any possible order effects. Throughout each run, S1 was the same in every trial. This is a precaution in case adaptation carries over, to some extent, from one trial to the next, as it ensures that there is adaptation to only one stimulus. Therefore, for half of the runs the CoF of the adapting stimulus was located to the right of fixation, while for the other half it was located to the left; the responses to the two versions of each condition were averaged. Thus “Same” trials could be either a pair of optic flow stimuli both expanding from the left or a pair both expanding from the right. This balances any differences in the absolute magnitudes of the responses to the two stimuli.
Data acquisition.
Images were acquired with a 3-T MR scanner (Magnetom Trio, Siemens, Erlangen, Germany) equipped with a custom eight-channel posterior-head array headcoil (Stark Contrast, Erlangen, Germany). A standard gradient-echo, echoplanar (EPI) sequence was used [repetition time (TR) = 2,500 ms, echo time (TE) = 31 ms, flip angle = 90°, voxel size = 3 × 3 × 3 mm, 35 axial slices, bandwidth = 1,396 Hz/pixel]. For coregistration purposes, at the beginning of each scanning session we acquired two single-volume EPI images that had the same position parameters as the experimental runs: one (BC-EPI) using the scanner's integral whole-body coil, to give uniform contrast, and another immediately after, acquired with the posterior array head coil (PA-EPI). We also acquired an anatomical T1-weighted image (MPRAGE, 160 axial slices, in-plane resolution 256 × 256, 1-mm isotropic voxels, TR = 1,830 ms, TE = 4.43 ms, flip angle = 11°, bandwidth = 130 Hz/pixel).
For each participant, a high-resolution T1-weighted three-dimensional (3D) anatomical image [modified driven-equilibrium Fourier transform (MDEFT) (Deichmann et al. 2004); 176 axial slices, in-plane resolution 256 × 256, 1-mm isotropic voxels, TR = 7.92 ms, TE = 2.45 ms, flip angle = 16°, bandwidth = 195 Hz/pixel] was acquired in a different session with a standard (whole head) Siemens eight-channel head coil. MDEFT was chosen in place of standard 3D anatomical sequences because of its improved contrast between gray matter and white matter, which is beneficial for segmentation and flattening. This anatomical image was used as a reference to which all the functional images were coregistered.
Analysis.
All data were preprocessed and analyzed with BrainVoyager QX (version 2.0, Brain Innovation). EPIs were corrected for head motion and slice timing. To remove low-frequency drifts, a GLM with 2 pairs of sine/cosine predictors of 1 and 2 cycles per run and a linear regressor was used to estimate the low-frequency components of the time series and subtract them from the original data. No spatial smoothing was applied. All functional images were aligned to the PA-EPI acquired at the beginning of the scan session. Because of the steep posterior-to-anterior gradient of the EPIs acquired with the posterior array head coil, direct coregistration of these images to the anatomy was found to be poor. Therefore, we coregistered the BC-EPI to the MPRAGE structural image corrected for inhomogeneities and assumed no head movements between the acquisition of the BC-EPI and the PA-EPI. The MPRAGE was also coregistered to the MDEFT image. Coregistration accuracy was always checked visually. Parameters from both coregistration steps were then used to build functional 3D time series in the same space as the MDEFT image.
The first three volumes of each experimental run were discarded to allow for T1 equilibration effects. Time series were extracted from all the visually responsive voxels within each region of interest (ROI; see ROI definition). Visually responsive voxels were defined as those that showed a significant response to all the stimuli at P < 0.001. Since we were mainly interested in the response to S2, the baseline (0% signal change) for each trial was calculated as the average signal between −2 s (where 0 s is the onset of S1) and 5 s (just after the presentation of S2). For each hemisphere, the time courses for each condition were averaged across trials and across voxels within the ROI; all conditions were then normalized to the largest peak obtained in any condition in each ROI. Finally, the results were averaged across hemispheres.
To quantify the degree of adaptation, we calculated an adaptation index in a time window of 8–10 s (around the peak of the response to S2; see Figs. 2 and 3). The response to the “Same” condition was subtracted from that of the relevant “Different” condition and the result divided by the sum of the absolute values of the two responses [(diff − same)/(diff + same)] (Larsson et al. 2006). Positive scores on this index represent sensitivity to the tested feature (e.g., a positive score in condition “Different CoF” suggests sensitivity to heading direction).
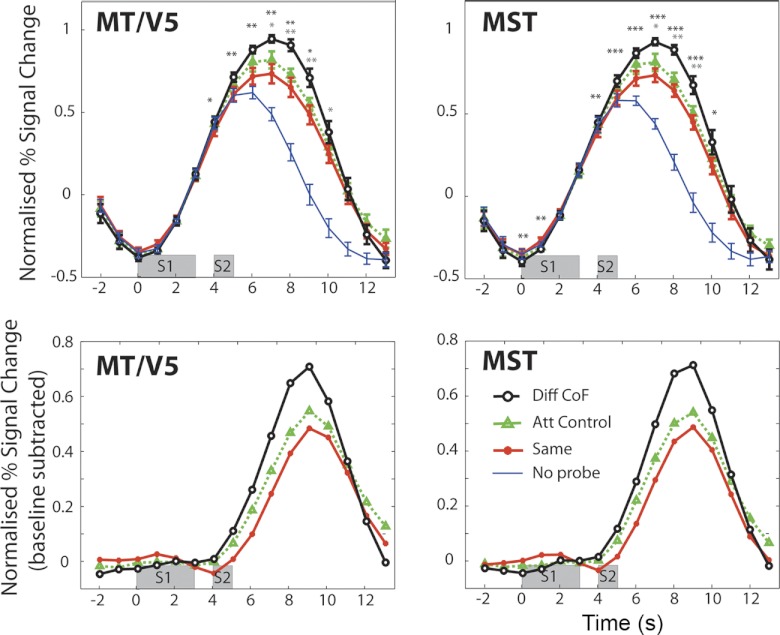
Fig. 2.
Sensitivity to CoF in visual motion areas MT/V5 and MST. Top: time courses of the indicated ROIs in response to the 4 experimental conditions. Time courses represent averages across hemispheres (n = 14), and error bars show ±SE. Bottom: response to S2 isolated (i.e., “No Probe” time course subtracted from that of the relevant condition). Time windows indicated in gray represent the periods of stimulus presentation. Diff CoF, Different CoF; Att Control, Attention Control. Black asterisks indicate significant differences between “Same” and “Different CoF” based on the data from single time points. Gray asterisks indicate significant differences between “Different CoF” and “Attention Control” (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 3.
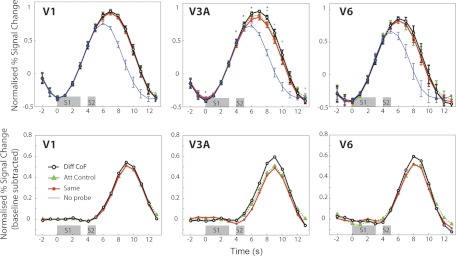
Sensitivity to CoF in areas V1, V3A, and V6. All details as in Fig. 2, apart from n = 12 in V3A and V6. *P < 0.05.
ROI definition.
Scanning runs to define ROIs were performed in a separate session. Images were acquired and preprocessed as in the main experiment, but in this case volumes consisted of 28 slices and TR = 2,000 ms. MT/V5 and MST were defined by the use of an ipsilateral stimulus based on Huk et al. (2002) and previously used in our laboratory (Smith et al. 2006). A circular patch of dots (8° in diameter) was presented with its center placed 10° to the left or right of fixation (note that these dimensions are different from those used by Huk et al.). Blocks of 15 s in which the dots were static were alternated with blocks in which the dots moved alternately inward and outward along the radial axes (thus creating alternating contraction and expansion). Sixteen blocks (8 static and 8 moving) were presented in each scanning run; one scanning run was completed with the stimulus on the left and another with it on the right. With this procedure, MT/V5 and MST can be differentiated in terms of the absence or presence, respectively, of significant ipsilateral drive. On average, 128 ± 14 (SE) voxels were labeled as MT and 78 ± 13 as MST (Table 1).
Table 1.
Talairach coordinates and number of voxels for each ROI
| Right |
Left |
|||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | N | x | y | z | N | |
| V3A | 22 | −86 | 16 | 187 ± 10 | −24 | −88 | 13 | 180 ± 19 |
| V6 | 15 | −77 | 26 | 55 ± 17 | −15 | −79 | 23 | 68 ± 24 |
| MT | 43 | −69 | −1 | 113 ± 11 | −44 | −69 | 1 | 143 ± 26 |
| MST | 45 | −62 | 0 | 84 ± 20 | −43 | −63 | 2 | 72 ± 17 |
Values are mean center coordinate and mean ± SE number of voxels (N) for each region of interest (ROI).
Retinotopic mapping was performed to demarcate retinotopic areas V1 and V3A. V3A is thought to be important for processing motion, and V1 was included as a check that effects of CoF were not seen where they are not expected. Standard retinotopic mapping procedures were used (Engel et al. 1994; Sereno et al. 1995). Two stimulus runs were performed in a separate scanning session conducted with binocular viewing via a mirror in accordance with our routine procedures. In each run, a counterphasing checkerboard “wedge” stimulus (a 24° sector) rotated clockwise at a rate of 64 s/cycle (8 cycles per run). Check size was scaled by eccentricity in approximate accordance with the cortical magnification factor. The diameter of the stimulus was 30°. Images were acquired and preprocessed as in the MT/MST localizer. Data were analyzed by fitting a model to the time course obtained with the rotating wedge stimulus. This consisted of a rectangular wave of duty cycle 24/360, reflecting the duration of stimulation at any portion of the visual field, convolved with a standard hemodynamic response function (HRF). The phase of the fitted response was taken as an index of visual field location, in terms of polar angle. Reversals of the direction of phase change across the cortical surface were taken as boundaries of visual areas. The boundaries of visual areas V1, V2, V3, and V3A were drawn by eye, on the basis of these reversals viewed on a flattened version of each participant's reference anatomy. It was not possible to localize area V3A in one of our participants. The flattened representation of each hemisphere was created by segmenting and reconstructing the border between gray and white matter within each hemisphere of the MDEFT scan with BrainVoyager. The resulting surfaces were smoothed, inflated, and cut along the calcarine sulcus. Finally, the surface was flattened and corrected for linear distortions.
V6 was identified with a separate localizer scan in which participants viewed a 58° visual display of egomotion-consistent (EC) or egomotion-inconsistent (EI) optic flow as previously described (Cardin and Smith 2010). Our work (Cardin et al. 2011) shows good correspondence between the V6 ROI identified with retinotopic mapping, the method published by Pitzalis et al. (2006), and the method used here. The localizer consisted of 6 event-related scans of 32 trials each. The stimuli were presented for 3 s, with ITIs varying between 2 and 10 s. Images were acquired and preprocessed as in the main experiment. The ROIs were identified with the statistical contrast (EC − EI). We localized V6 in both hemispheres of six participants, but accurate localization was not possible in either hemisphere of one of our participants. With this localizer it is also possible to identify areas VIP, CSv, p2v, PIVC, and PcM. However, we do not report results from these ROIs because with our scanning parameters the responses in these ROIs were relatively small and the time courses were too noisy to study adaptation effects. Figure 1C illustrates, in one participant, the locations of the key cortical regions discussed in results (V3A, MT, MST, and V6).
RESULTS
The goal of our study was to determine the sensitivity to heading direction of various cortical visual motion areas. For this purpose, we identified visual areas in the human brain that have been shown previously to be involved in optic flow and egomotion processing (Cardin and Smith 2010; Greenlee 2000; Koyama et al. 2005; Morrone et al. 2000; Peuskens et al. 2001; Pitzalis et al. 2010; Smith et al. 2004; Wall and Smith 2008) and we measured heading-specific adaptation.
The experiment had four conditions: “Same,” “Different CoF,” “Attention Control,” and “No Probe.” In condition “Same,” S1 and S2 comprised an array of 300 moving dots expanding, rotating, and contracting from the same point (14° from fixation). In condition “Different CoF,” S1 and S2 had their CoFs located in different positions (Fig. 1B). In the “Attention Control” condition, the stimuli were different in luminance but not CoF. Only S1 was present in the “No Probe” condition; this was to isolate the response to S2 from the other conditions.
Figures 2 and 3 show the results obtained in all the areas studied. In all cases, the response to “No Probe” trials, which contain only an S1, peaks between 5–6 s after the beginning of the trial (Figs. 2 and 3, top). The response to “Same,” “Different CoF,” and “Attention Control” trials, which contain both S1 and S2, show an extended response that peaks around 6–7 s (Figs. 2 and 3, top). To isolate the response to S2 (the probe), the mean time course for the “No Probe” condition was subtracted from each of the other conditions (Figs. 2 and 3, bottom). This shows that the peak of the response to the S2 occurs at 9 s after the beginning of the trial, as expected. In areas MT/V5 and MST, the response to “Different CoF” is significantly higher than the response to “Same” in the regions of the response where the S2 response is dominant (from 5 to 12 s in MT/V5 and from 5 to 13 s in MST), which suggests sensitivity to heading direction in these areas (Fig. 2). The biggest difference between “Same” and “Different CoF” at a single time point (9 s) is similar in both cases (MT: 0.255, t[13] = 3.41, P = 0.0046; MST: 0.247, t[13] = 4.49, P = 0.0006) There was also a significant difference between these conditions in area V3A (maximal at 9 s), but it was considerably smaller (0.114, t[11] = 2.23, P = 0.047) (Fig. 3). Gray asterisks in Figs. 2 and 3 denote points at which condition “Different CoF” was significantly higher than “Attention Control.” No significant difference between “Same” and “Different CoF” was observed at any time point in V6, an area previously shown to be differentially sensitive to optic flow patterns that are consistent with egomotion. As expected, there was no time point at which “Different CoF” was significantly higher than “Same” in V1 (Fig. 3).
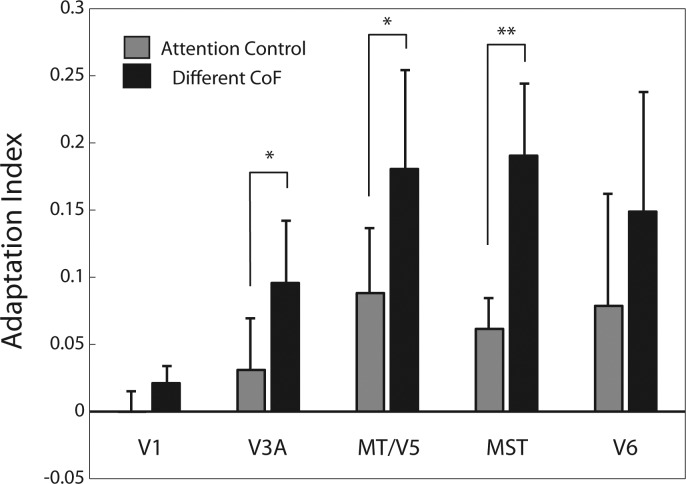
To quantify the adaptation effect, we calculated adaptation indexes (see materials and methods; Fig. 4) based on a time window of 8 to 10 s, where the response to S2 is prominent (Fig. 2 and 3, bottom). We subtracted the “Same” response from the relevant “Different” response and divided the result by the total sum of the absolute values of the “Different” and “Same” responses. A positive score on this index indicates adaptation in the expected direction, while a zero or negative value indicates no effect or a reversed effect, respectively. The results of this analysis are shown in Fig. 4 for each visual area included in Figs. 2 and 3. The adaptation index is significantly higher for “Different CoF” compared with “Attention Control” in areas V3A (t[11] = 2.54, P = 0.027), MT/V5 (t[13] = 2.2, P = 0.046), and MST (t[13] = 2.97, P = 0.01). Reflecting the trends observed in the time courses in Fig. 3, the adaptation indexes are not significantly different in V1 (t[13] = 1.8, P = 0.09), where sensitivity to heading direction is not expected, or in V6 (t[11] = 1.36, P = 0.2). In V6, there is a nonsignificant trend in the same direction as in V3A (see Figs. 3 and 4); however, this trend comes entirely from 3 of 12 hemispheres, and these can be regarded as oddballs. It should also be pointed out that the adaptation index for “Different CoF” is not even significantly different from zero (t[11] = 1.67, P = 0.12).
Fig. 4.
Adaptation indexes. Adaptation indexes for conditions “Different CoF” and “Attention Control” are shown separately for each ROI. Bars indicate the mean ± SE index across hemispheres. Asterisks indicate significant differences between conditions “Different CoF” and “Attention Control” (*P < 0.05, **P = 0.01).
DISCUSSION
Visual cues are highly informative in the calculation of self-motion. In particular, the CoF of the optic flow pattern that reaches the retina during locomotion is, when taken together with knowledge of eye position and head position, an accurate indicator of the direction in which the individual is heading. In this study, we have shown differential sensitivity to optic flow patterns that simulate different directions of heading in human cortical areas MT/V5 and MST, suggesting that extraction of visual cues for the processing of heading direction occurs in these areas. We used fMRI adaptation, which allows the isolation of responses from populations of neurons with different specificities within the same functional area. Results from our experiment showed an increase in the response of areas MT/V5 and MST, and to a lesser extent in V3A, when a probe had a different CoF from an adapter, suggesting sensitivity to heading direction in these areas. This effect was not observed in V6, which is also sensitive to optic flow, showing a dissociation between the functions of these flow-responsive visual areas.
MST.
In macaques, the regions traditionally believed to be involved in heading processing are MST and VIP. These regions are interconnected, their neurons respond selectively to different optic flow patterns, and they are selective for heading direction in both the visual and vestibular modalities (Bremmer et al. 2002; Duffy and Wurtz 1991a, 1992b, 1995; Duhamel et al. 1998; Gu et al. 2006, 2007, 2008; Page and Duffy 2003; Saito et al. 1989; Schlack et al. 2002; Takahashi et al. 2007). In humans, homologous regions have also been shown to process egomotion and optic flow (Morrone et al. 2000; Peuskens et al. 2001; Wall and Smith 2008). In this study, we observe strong sensitivity to heading direction in area MST, in accordance with the results obtained in macaque. We were not able to study the response of area VIP: although visual responses were obtained, they were too small and variable to permit measurement of adaptation.
MT and V3A.
MT also shows selectivity for optic flow patterns with different CoFs. This is more surprising if one is expecting results that relate directly to macaque physiology, but it is consistent with results from a previous fMRI study of MT/V5 showing selectivity to optic flow patterns (Wall et al. 2008). MT/V5 has been shown to be a visual motion area in macaques (Allman and Kaas 1971; Dubner and Zeki 1971; Zeki 1974) and humans (Tootell and Taylor 1995; Watson et al. 1993; Zeki et al. 1991). However, macaque MT neurons are not thought to differentiate between different global flow components. Receptive fields are smaller than those in MST (Desimone and Ungerleider 1986; Komatsu and Wurtz 1988), and it has been assumed that large receptive fields are needed to encode optic flow. MT neurons may respond differently to different flow patterns, but this could simply reflect sensitivity to the direction of translation that falls within their relatively small receptive fields. This interpretation is supported by the fact that flow-specific responses in MT do not show position invariance (i.e., their preference changes when the stimulus is moved to a different position relative to the receptive field; Lagae et al. 1989; Orban et al. 1992). However, although position invariance (as seen in some MSTd neurons) is a reliable sign of sensitivity to global flow structure, lack of position invariance is ambiguous. It is consistent not only with sensitivity to local translation but also with sensitivity to a CoF only when located in a specific part of the retina. After all, a position-invariant cell can specify the presence of a particular flow component, but it cannot convey its position in the receptive field. Although MT receptive fields are too small to capture the full flow field, they are large enough to detect expansion or rotation if the CoF falls within the receptive field. We know of no evidence that demonstrates that they do this, but it has not been ruled out. It is also possible that there is a species difference between macaques and humans in terms of the sensitivity of MT to optic flow.
Another possible interpretation of the apparent CoF specificity of MT is that it is not real but reflects bleed-through from neighboring MST. The definition of MT is imperfect—it is defined as what is left of MT+ after voxels with significant ipsilateral responses are removed. If there are voxels in MST where the ipsilateral response does not reach significance for any reason, these will be incorrectly assigned to MT, which could lead to the observed result. However, visual inspection showed that the CoF-specific adaptation effects of the most posterior MT voxels are just as robust as in the more anterior portion. We therefore think that the MT effect is genuine, although it is impossible to completely rule out such an explanation.
In the human brain, V3A is a motion-responsive region (Tootell et al. 1997; Vanduffel et al. 2002). The constraints imposed by receptive field size discussed in relation to MT also apply to V3A. Nonetheless, V3A has been shown to have a certain degree of sensitivity to optic flow patterns (Wall et al. 2008), while expanding and contracting optic flow patterns with different CoFs activate more strongly the region of V3A that represents the location of the CoF (Koyama et al. 2005). Our results also show in this region a weak sensitivity to the location of the CoF of an optic flow pattern, suggesting that the extraction of visual information for the computation of optic flow parameters might start in this area.
V6.
Another region that has been implicated in self-motion is V6, in the parieto-occipital sulcus. It has been suggested that V6 is suitable for the analysis of optic flow and egomotion because it contains a representation of the contralateral visual field that extends up to 80° in which the center is not magnified relative to the periphery (Galletti et al. 1999; Pitzalis et al. 2006). Also, it is reciprocally connected to MST and VIP (Galletti et al. 2001; Shipp et al. 1998) and has a population of neurons that respond to “real motion” (Galletti and Fattori 2003). Responses to egomotion stimulation in area V6 have not been studied in the brain of macaques, but in humans this area has been shown to be sensitive to optic flow (Pitzalis et al. 2010), in particular if it is consistent with self-motion (Cardin and Smith 2010). Furthermore, epileptic seizures due to an ependynoma located near V6 caused linear self-motion perception and occasional body tilts (Wiest et al. 2004). Despite its preference for self-motion over other types of global motion, V6 does not show significant sensitivity in the present study to the location of the CoF—the percent signal change values and the adaptation index for condition “Different CoF” are not significantly different from the “Attention Control” or the “Same” condition. These results suggest that this area does not encode direction of heading. It is possible that V6 simply does not adapt, but this is unlikely since adaptation is ubiquitous in visual cortex. It is possible that larger responses, allowing better assessment, might be obtained in V6 with different stimuli. Specifically, Quinlan and Culham (2007) report an area (dPOS) that may correspond to V6 and shows a preference for objects in near space. It could be that binocular stimulation with converged eyes is necessary for an optimum response. In a previous study, we showed that in V6 sensitivity to optic flow patterns is enhanced when they are combined with binocular disparity cues that are consistent with self-motion (Cardin and Smith 2011). This was not the case in MT or MST. Disparity is most informative for nearby objects that generate relatively large retinal disparities, so together the results suggest that V6 may be concerned with flow for the purpose of avoiding obstacles during self-motion rather than for providing a representation of heading direction. Given this emphasis on objects, a possibility is that V6 is sensitive to optic flow for the purpose of flow parsing—the separation of object motion from self-motion (Warren and Rushton 2009). The segregation of these two types of motion is essential both for the avoidance of obstacles and for planning the handling of nearby objects, and it has been shown to use optic flow as well as local motion signals (Warren and Rushton 2009). Whatever the specific role of V6, our results point toward a functional dissociation between these two visual areas that both show strong sensitivity to optic flow.
Effects of attention.
Previous studies have shown that differences in the capture of attention and the expectation produced by different pairs of stimuli can have a confounding effect on the fMRI adaptation response (Larsson and Smith 2011; Summerfield et al. 2008). To avoid this in the present study, participants had to perform a highly demanding fixation task that directed their attention away from the stimulus properties. As a test of the effectiveness of this strategy, we included a condition in which the stimuli were different in terms of an irrelevant feature (dot luminance). The small or absent effect observed in the “Attention Control” condition in all ROIs suggests that the result obtained with stimuli that have different CoFs cannot be explained by differences in attention capture. Furthermore, our effects cannot be explained by differences in local motion between the stimuli because the flow pattern changed continuously between expansion, rotation, and contraction, which resulted in all dots in the display moving in every possible direction at different points in time. It should be noted that the attention control stimulus is designed for visual areas such as MT and MST where responses are believed to saturate at contrasts well below 50%. There are differing reports of whether this is the case in human V3A (Boynton et al. 1999; Tootell et al. 1997). Arguably, our conclusion regarding V3A could be compromised if it is not the case. However, because contrast increased and decreased equally often in our attention control condition, there should be little net effect of contrast even if the response is sensitive to contrast above 50%. In the case of V6, we know of no contrast response data in V6, either macaque or human. However, since we do not find significant adaptation that is specific to CoF, the question of whether it can be explained by attention does not arise. The same applies to V1.
Conclusion.
In summary, we have shown sensitivity to the location of the CoF within an optic flow pattern in areas MT/V5 and MST and to a lesser extent in V3A, suggesting involvement of these regions in the estimation of heading direction. This sensitivity was not present in V6. These results show a clear dissociation between areas MST and V6, both of which have been implicated in the extraction of visual cues for self-motion. We speculate that area MST could be involved in the extraction of optic flow for the computation of heading direction, whereas V6 may be involved in the extraction of optic flow for obstacle avoidance.
GRANTS
This research was supported by a project grant (ref. 082648) from The Wellcome Trust to A. T. Smith and a bursary from The Nuffield Foundation to L. Hemsworth.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.C. and A.T.S. conception and design of research; V.C. and L.H. performed experiments; V.C. and L.H. analyzed data; V.C. and A.T.S. interpreted results of experiments; V.C. prepared figures; V.C. drafted manuscript; V.C., L.H., and A.T.S. approved final version of manuscript; A.T.S. edited and revised manuscript.
ACKNOWLEDGMENTS
Present address of V. Cardin: Deafness, Cognition and Language Research Centre, University College London, London, WC1H 0PD, UK.
REFERENCES
- Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res 31: 85–105, 1971 [DOI] [PubMed] [Google Scholar]
- Ashida H, Lingnau A, Wall MB, Smith AT. fMRI adaptation reveals separate mechanisms for first-order and second-order motion. J Neurophysiol 97: 1319–1325, 2007 [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Res 39: 257–269, 1999 [DOI] [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction—visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 121: 1749–1758, 1998 [DOI] [PubMed] [Google Scholar]
- Bremmer F, Duhamel JR, Hamed SB, Graf W. Heading encoding in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16: 1554–1568, 2002 [DOI] [PubMed] [Google Scholar]
- Britten KH, van Wezel RJA. Electrical microstimulation of cortical area MST biases heading perception in monkeys. Nat Neurosci 1: 59–63, 1998 [DOI] [PubMed] [Google Scholar]
- Cardin V, Sherrington R, Hemsworth L, Smith AT. Responses of human V6 to random motion, egomotion-incompatible and egomotion-compatible optic flow. J Vis 11: 711, 2011 [Google Scholar]
- Cardin V, Smith AT. Sensitivity of human visual and vestibular cortical regions to egomotion-compatible visual stimulations. Cereb Cortex 20: 1964–1973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin V, Smith AT. Sensitivity of human visual cortical area V6 to stereoscopic depth gradients associated with self-motion. J Neurophysiol 106: 1240–1249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage 21: 757–767, 2004 [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol 248: 164–189, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Zeki SM. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res 35: 528–532, 1971 [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Responses of monkey MST neurons to optic flow stimuli with shifted centers of motion. J Neurosci 15: 5192–5208, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. II Mechanisms of response selectivity revealed by small-field stimuli. J Neurophysiol 65: 1346–1359, 1991a [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol 65: 1329–1345, 1991b [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol 79: 126–136, 1998 [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature 369: 525, 1994 [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P. Neuronal mechanisms for detection of motion in the field of view. Neuropsychologia 41: 1717–1727, 2003 [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Kutz DF, Gamberini M. Brain location and visual topography of cortical area V6A in the macaque monkey. Eur J Neurosci 11: 575–582, 1999 [DOI] [PubMed] [Google Scholar]
- Galletti C, Gamberini M, Kutz DF, Fattori P, Luppino G, Matelli M. The cortical connections of area V6: an occipito-parietal network processing visual information. Eur J Neurosci 13: 1572–1588, 2001 [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The Perception of the Visual World. Boston, MA: Houghton Mifflin, 1950 [Google Scholar]
- Greenlee MW. Human cortical areas underlying the perception of optic flow: brain imaging studies. Intl Rev Neurobiol 44: 269–292, 2000 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24: 187–203, 1999 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying functional properties of human cortical neurons. Acta Psychol (Amst) 107: 293–321, 2001 [DOI] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, DeAngelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci 11: 1201–1210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci 10: 1038–1047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Watkins PV, Angelaki DE, DeAngelis GC. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci 26: 73–85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci 22: 7195–7205, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol 60: 580–603, 1988 [DOI] [PubMed] [Google Scholar]
- Kovács G, Raabe M, Greenlee MW. Neural correlates of visually induced self-motion illusion in depth. Cereb Cortex 18: 1779–1787, 2008 [DOI] [PubMed] [Google Scholar]
- Koyama S, Sasaki Y, Andersen GJ, Tootell RBH, Matsuura M, Watanabe T. Separate processing of different global-motion structures in visual cortex is revealed by fMRI. Curr Biol 15: 2027–2032, 2005 [DOI] [PubMed] [Google Scholar]
- Lagae L, Gulyas B, Raiguel S, Orban GA. Laminar analysis of motion information processing in macaque V5. Brain Res 496: 361–367, 1989 [DOI] [PubMed] [Google Scholar]
- Larsson J, Landy MS, Heeger DJ. Orientation-selective adaptation to first- and second-order patterns in human visual cortex. J Neurophysiol 95: 862–881, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cereb Cortex 22:567–576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau A, Ashida H, Wall MB, Smith AT. Speed encoding in human visual cortex revealed by fMRI adaptation. J Vis 9: .1–14, 2009 [DOI] [PubMed] [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci 3: 1322–1328, 2000 [DOI] [PubMed] [Google Scholar]
- Orban GA, Lagae L, Verri A, Raiguel S, Xiao D, Maes H, Torre V. First-order analysis of optical flow in monkey brain. Proc Natl Acad Sci USA 89: 2595–2599, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W, Duffy C. Heading representation in MST: sensory interactions and population encoding. J Neurophysiol 89: 1994–2013, 2003 [DOI] [PubMed] [Google Scholar]
- Peuskens H, Sunaert S, Dupont P, Van Hecke P, Orban GA. Human brain regions involved in heading estimation. J Neurosci 21: 2451–2461, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis S, Galletti C, Huang RS, Patria F, Committeri G, Galati G, Fattori P, Sereno MI. Wide-field retinotopy defines human cortical visual area V6. J Neurosci 26: 7962–7963, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis S, Sereno MI, Committeri G, Fattori P, Galati G, Patria F, Galletti C. Human V6: the medial motion area. Cereb Cortex 20: 411–424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan DJ, Culham JC. fMRI reveals a preference for near viewing in the human parieto-occipital cortex. Neuroimage 36: 167–187, 2007 [DOI] [PubMed] [Google Scholar]
- Saito H, Tanaka K, Isono H, Yasuda M, Mikami A. Directionally selective responses of cells in the middle temporal area (MT) of the macaque monkey to the movement of equiluminous opponent color stimuli. Exp Brain Res 75: 1–14, 1989 [DOI] [PubMed] [Google Scholar]
- Schaafsma SJ, Duysens J. Neurons in the ventral intraparietal area of awake macaque monkey closely resemble neurons in the dorsal part of the medial superior temporal area in their responses to optic flow patterns. J Neurophysiol 76: 4056–4068, 1996 [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Interaction of linear vestibular and visual stimulation in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16: 1877–1886, 2002 [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JHR, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res 30: 1–10, 1990 [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893, 1995 [DOI] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur J Neurosci 10: 3171–3193, 1998 [DOI] [PubMed] [Google Scholar]
- Smith A, Williams A, Singh K, Wall M. Sensitivity of human visual areas MT and MST to optic flow stimuli (Abstract). Neuroimage 20: TU307, 2004 [Google Scholar]
- Smith AT, Wall MB. Sensitivity of human visual cortical areas to the stereoscopic depth of a moving stimulus. J Vis 8: 1–12, 2008 [DOI] [PubMed] [Google Scholar]
- Smith AT, Wall MB, Williams AL, Singh KD. Sensitivity to optic flow in human cortical areas MT and MST. Eur J Neurosci 23: 561–569, 2006 [DOI] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egne T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11: 1004–1006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Gu Y, May PJ, Newlands SD, DeAngelis GC, Angelaki DE. Multimodal coding of three-dimensional rotation and translation in area MSTd: comparison of visual and vestibular selectivity. J Neurosci 27: 9742–9768, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci 17: 7060–7078, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Taylor JB. Anatomical evidence for MT and additional cortical visual areas in humans. Cereb Cortex 1: 39–55, 1995 [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science 298: 413–415, 2002 [DOI] [PubMed] [Google Scholar]
- Wall M, Smith A. The representation of egomotion in the human brain. Curr Biol 18: 191–194, 2008 [DOI] [PubMed] [Google Scholar]
- Wall MB, Lingnau A, Ashida H, Smith AT. Selective visual responses to expansion and rotation in the human MT complex revealed by functional magnetic resonance imaging adaptation. Eur J Neurosci 27: 2747–2757, 2008 [DOI] [PubMed] [Google Scholar]
- Warren PA, Rushton SK. Optic flow processing for the assessment of object movement during ego movement. Curr Biol 19: 1555–1560, 2009 [DOI] [PubMed] [Google Scholar]
- Warren WH, Hannon DJ. Direction of self-motion is perceived from optical flow. Nature 336: 162–163, 1988 [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, Hajinal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3: 79–94, 1993 [DOI] [PubMed] [Google Scholar]
- Wiest G, Zimprich F, Prayer D, Czech T, Serles W, Baumgartner C. Vestibular processing in human paramedian precuneus as shown by electrical cortical stimulation. Neurology 62: 473–475, 2004 [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci 11: 641–649, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol 236: 549–573, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Britten KH. Parietal area VIP causally influences heading perception during pursuit eye movements. J Neurosci 31: 2569–2575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]