Abstract
Despite the diverse methods vertebrates use for locomotion, there is evidence that components of the locomotor central pattern generator (CPG) are conserved across species. When zebrafish begin swimming early in development, they perform short episodes of activity separated by periods of inactivity. Within these episodes, the trunk flexes with side-to-side alternation and the traveling body wave progresses rostrocaudally. To characterize the distribution of the swimming CPG along the rostrocaudal axis, we performed transections of the larval zebrafish spinal cord and induced fictive swimming using N-methyl-d-aspartate (NMDA). In both intact and spinalized larvae, bursting is found throughout the rostrocaudal extent of the spinal cord, and the properties of fictive swimming observed were dependent on the concentration of NMDA. We isolated series of contiguous spinal segments by performing multiple spinal transections on the same larvae. Although series from all regions of the spinal cord have the capacity to produce bursts, the capacity to produce organized episodes of fictive swimming has a rostral bias: in the rostral spinal cord, only 12 contiguous body segments are necessary, whereas 23 contiguous body segments are necessary in the caudal spinal cord. Shorter series of segments were often active but produced either continuous rhythmic bursting or sporadic, nonrhythmic bursting. Both episodic and continuous bursting alternated between the left and right sides of the body and showed rostrocaudal progression, demonstrating the functional dissociation of the circuits responsible for episodic structure and fine burst timing. These findings parallel results in mammalian locomotion, and we propose a hierarchical model of the larval zebrafish swimming CPG.
Keywords: central pattern generator, transection, N-methyl-d-aspartate, locomotion
central pattern generators (CPGs) are neural circuits capable of producing a patterned motor output even in the absence of a patterned input (Marder and Calabrese 1996). The spinal cord contains a CPG or CPGs that are important to the production of locomotion (Grillner 2006). Despite the diverse methods vertebrates use for locomotion, there is evidence that components of the CPG are conserved across species (Grillner and Jessell 2009; Kiehn 2011). Larval zebrafish are an attractive species for studying motor systems (Fetcho 2007; Fetcho and Liu 1998; McLean and Fetcho 2011). Although the neuronal circuits for some motor behaviors such as escape (Koyama et al. 2011; O'Malley et al. 1996; Satou et al. 2009) and early touch response (Downes and Granato 2006; Pietri et al. 2009) have been characterized, the CPG circuits that control swimming in larval zebrafish are less well characterized (Eklöf-Ljunggren et al. 2012; Kimura et al. 2006; McLean et al. 2008).
Zebrafish begin swimming at 3 days postfertilization (dpf) (Buss and Drapeau 2001; Fuiman and Webb 1988; Muller and Von Leeuwen 2004) and perform infrequent episodes of locomotion that last several seconds (Buss and Drapeau 2001). Later, at 4 dpf, larvae transition to perform more frequent but shorter episodes of locomotor activity, sometimes described as a “beat and glide” pattern (Buss and Drapeau 2001). The muscle contractions within these swimming episodes, in both swimming behaviors, are timed to alternate side to side and have rostrocaudal progression (Batty et al. 1984; Borla et al. 2002), which we collectively refer to as “coordination.” Similarly, paralyzed preparations of larval zebrafish produce episodes of peripheral nerve activity indicative of motor neuron firing (Masino and Fetcho 2005). Within episodes, peripheral nerve activity is organized into discrete bursts that have the same coordinated pattern as trunk flexions in free-swimming zebrafish larvae (Masino and Fetcho 2005), similar to the correspondence between fictive and real behavior in several other swimming vertebrates (Cohen and Wallen 1980; Fetcho and Svoboda 1993; Grillner 1974; Kahn and Roberts 1982). Normal swimming in larval zebrafish at 4 dpf and later, both real and fictive, is defined by episodic organization and coordination.
When one is mapping a CPG, it is helpful to know the distribution of its neuronal components. One approach to characterizing the distribution of these CPG components is through the use of lesions (Ho and O'Donovan 1993; Kjaerulff and Kiehn 1996). There are two benefits to this approach. First, locomotor functions produced by spatially distinct neuronal circuits can be dissociated. Sagittal sections of the spinal cord have been used in several species to demonstrate that the locomotor rhythm does not depend on connections that cross the midline (Cangiano and Grillner 2003; Kahn and Roberts 1982; Kjaerulff and Kiehn 1996). Second, lesions can be used to simplify the system being studied. Horizontal transections of the spinal cord have localized the CPG to the ventral spinal cord (Kjaerulff and Kiehn 1996). Similarly, in a CPG composed of segmentally reiterated oscillators (Matsushima and Grillner 1990), transections may allow investigation of simpler isolated oscillators.
Transections of the spinal cord produce “series” of contiguous spinal segments. Short series of spinal segments from dogfish and lamprey are sufficient to produce fictive locomotion regardless of rostrocaudal position in the spinal cord (Cohen and Wallen 1980; Grillner 1974). Among limbed vertebrates, the bulk of the locomotor circuits are located in the spinal limb enlargements. In some limbed vertebrate model systems, such as embryonic chick, there is not a strong effect of rostrocaudal position within the limb enlargement on the capacity of segments to produce the locomotor rhythm (Ho and O'Donovan 1993). Other model systems show a strong effect of rostrocaudal position on rhythm-generating capacity (Kjaerulff and Kiehn 1996), including extreme examples where a CPG is located in one or two spinal segments (Deliagina et al. 1983; Wheatley et al. 1994). A previous report in larval zebrafish showed episodic motor neuron spiking in an isolated series of two body segments (McDearmid and Drapeau 2006); however, the effect the of rostrocaudal position of the segments and the coordination of the pattern produced was not described. Given the differences in rostrocaudal distribution of CPG elements between species, it was our objective to characterize the rostrocaudal distribution of the swimming CPG in larval zebrafish.
We hypothesized several possible architectures for the larval zebrafish swimming CPG: 1) the CPG is composed of segmentally reiterated oscillators; 2) there is a single CPG distributed throughout the entire spinal cord; or 3) there is a single CPG located in a small region of the spinal cord. We evaluated the episodic structure and coordination of the fictive swim pattern produced by series of spinal segments of different lengths in response to N-methyl-d-aspartate (NMDA), an activator of the larval zebrafish swimming CPG (McDearmid and Drapeau 2006). We found that only longer series (≥12 of 33 total spinal segments) produced episodic fictive swimming and that shorter series often produced continuous rhythmic bursting or sporadic, nonrhythmic bursting. Fewer rostral spinal segments (≥12) than caudal segments (≥23) were necessary to produce episodic swimming. We also found that the degree of coordination, that is, side-to-side alternation and rostrocaudal progression, was not different between preparations that produced episodic fictive swimming and those that produced tonic bursting. This result prompted us to propose a model of the larval zebrafish swimming CPG that separates the circuits for episode generation and coordination.
MATERIALS AND METHODS
Animals and solutions.
All procedures were approved by the Animal Care and Use Committee of the University of Minnesota Twin Cities. Wild-type adult zebrafish (Danio rerio; Segrest Farms, Gibsonton, FL) were maintained in a zebrafish core facility. Adult zebrafish were set up to produce daily clutches of embryos with timed fertilization between 8:45 and 9:00 AM. Embryos and larval zebrafish were maintained in petri dishes filled with embryo water (60 μg/ml Instant Ocean salt mix; Cincinnati, OH) in a 28.5°C incubator with a 14:10-h light-dark cycle. Larval zebrafish 4 to 6 dpf were used in these studies. Chemicals and drugs were obtained from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise noted. Zebrafish Ringer solution was composed of (in mM) 134 NaCl, 2.9 KCl, 1.2 MgCl2, 2.1 CaCl2, 10 HEPES buffer, and 10 glucose, adjusted to pH 7.8 with NaOH (Drapeau et al. 1999; Legendre and Korn 1994).
Procedure.
The following procedure was adapted from Masino and Fetcho (2005). Zebrafish larvae were anesthetized for 5 min with 0.02% Tricaine-S (Western Chemical, Ferndale, WA) in Ringer solution. For unilateral recordings, larvae were transferred to Sylgard-lined dissecting dishes and pinned on their sides through the notochord using short pieces of fine tungsten wire (0.001-in. diameter). Typically, a rostral pin was placed between body segment (S) 5 and S8 and a caudal pin between S20 and S25 (Fig. 1A). To access the peripheral motor nerves and provide better perfusion access, the skin between the tungsten pins was removed using a sharp tungsten probe and fine forceps (Fine Science Tools, Foster City, CA). For bilateral recordings, skin was removed from both sides of the larvae, and the larvae were repositioned dorsal side up with the use of additional pins to hold them in position. To prevent muscle contractions during recording, larvae were paralyzed by adding 5 μl of 0.1 mM α-bungarotoxin (Tocris, Ellisville, MO) to the small amount (∼15 μl) of Ringer solution in the dissection dish. The larvae were kept in α-bungarotoxin for 10 min before superfusion with Ringer solution began. Larvae were left intact, spinalized (spinal cord transected at S3 to separate brain from spinal cord), or transected at multiple points along the body with the use of a razor blade shard (FA-10 Feather S; Ted Pella, Redding, CA) clamped by a blade holder (Fine Science Tools, Foster City, CA). In the intact preparation, fictive swimming occurred spontaneously or was evoked by shining light on the fish. In the spinalized preparation, swimming was induced with 50–200 μM NMDA in Ringer solution.
Fig. 1.
Quantification of episodic organization (EO). A: a diagram of a typical unilateral fictive swimming preparation. Two tungsten pins (filled circles) were used to stabilize the larvae. The region between these pins was skinned. B–D: peripheral nerve recording from the midbody region of a spinalized zebrafish during bath application of 200 μM N-methyl-d-aspartate (NMDA). C: the episode duration and inter-episodic long interburst period (IBP) are indicated by horizontal bars. D: the intra-episodic short IBP and burst duration are indicated by horizontal bars. The inverse of the IBP is the instantaneous burst frequency. E: histogram of IBP distribution from the representative recording in B–D. The values that were used to calculate EO (critical value, mean short IBP, and mean long IBP) are indicated by dashed vertical lines.
Electrophysiology.
After the larva were transferred to the stage of an Olympus BX51 WI microscope (Center Valley, PA), superfusion with Ringer solution at room temperature (21–25°C) was started. Glass suction electrodes were fashioned from 1.5-mm-o.d. × 1.12-mm-i.d. glass tubes (A-M Systems, Sequim, WA) and pulled on a P-97 electrode puller (Sutter Instruments, Novato, CA). Electrodes were filled with Ringer solution. Tip sizes ranged from 9 to 15 μm. The electrodes were placed in electrode holders that were inserted into headstages (50 MΩ) attached to an Axon Multiclamp 700B amplifier (Molecular Devices, Union City, CA). The electrodes were positioned over the peripheral nerves using micromanipulators (Siskiyou, Grants Pass, OR). Current-clamp signals were digitized by using an Axon Instruments Digidata 1440A data acquisition system (Molecular Devices). Data were recorded using pClamp 10 software (Molecular Devices). Signals were sampled at 10 kHz and bandpass filtered to 100–1,000 Hz.
Data analysis.
Organized fictive swimming in larval zebrafish consists of motor neuron bursts clustered into episodes (Fig. 1, B and C). A program written in Matlab (The MathWorks, Natick, MA) was used to analyze episodically organized extracellular peripheral nerve voltage recordings. The program detected the presence or absence of activity at each voltage sample v(n). For each v(n), the algorithm determined a voltage autocorrelation cn(k) over a small window (3 ms) centered at v(n). These “windowed” autocorrelations were computed as cn(k) = ∑i=−N0i=N0 v(n − i)v(n − i − k) (Eq. 1), where 3-ms windowing was implemented in Eq. 1 by setting N0 = (3 ms·fsam)/2, where fsam is the sampling frequency, and by setting v(j) = 0 for j outside the interval [n − N0, n + N0].
A subset of the autocorrelation values (lags) from Eq. 1 were used to compute a test statistic for each v(n) with the same lags (k⃗0 = [k1, k2, …, km]) used for all voltage samples. Building on Eq. 1, for each v(n), a test statistic cn was computed as cn = ∑k=k1k=km ∑i=−N0i=N0 v(n-i)v(n-i-k) (Eq. 2), where Eq. 2 is the sum of the cn(k) from Eq. 1 specified by k⃗0. k⃗0 was set at k⃗0 = [1, 2] because we found that these values effectively separate the distributions of the test statistics {cn} for samples of noise and samples of activity across a broad range of recording quality.
Finally, activity was considered present at v(n) only when cn was greater than a detection threshold T. T was set as the maximum of a set of {cn} corresponding to the {v(n)} in one contiguous second of the voltage recording where activity was confirmed to be absent (typically the first second of the recording), and was set this way for each individual voltage recording to account for differences in baseline noise levels. Fictive locomotor bursts were detected and grouped into episodes, and the burst and episode properties were determined as follows. Episode duration is the time from the onset of the first burst of an episode to the offset of the final burst in the same episode (Fig. 1C). Burst duration is the time from the onset to the offset of each burst, as defined by cn and described above (Fig. 1D). Burst frequency is the inverse of the interburst period (IBP), which is defined for each pair of bursts within episodes as the time from the onset of the first burst to the onset of the second burst (Fig. 1D). IBPs between episodes (Fig. 1C) are excluded from burst frequency analysis. Rostrocaudal delay per body segment is the time between the onset of each burst in the more rostral recording and the onset of the corresponding burst in the more caudal recording divided by the number of intervening body segments. Contralateral phase is measured on a cycle-by-cycle basis as the time between the onset of each burst on the left and each corresponding burst on the right divided by the IBP of the left.
Because nonepisodic swimming could not be processed using our Matlab program, the rostrocaudal delay and contralateral phase of nonepisodically organized swimming was quantified using auto- and cross-correlation (see Fig. 7). Signals were processed using the Python programming language and the SciPy signal library (http://www.scipy.org/) to rectify and low-pass filter the signal to 90 Hz. Rostrocaudal delay was defined as the time of peak cross-correlation between the rostral and caudal signals (see Fig. 7E). Rostrocaudal delay was normalized to the number of body segments separating the recording locations. Cross-correlation-derived IBP was defined as the time of peak autocorrelation from 10 to 200 ms and was converted to frequency by taking the reciprocal (see Fig. 7C). Contralateral offset was defined as the average of the time of highest cross-correlation greater than zero and the absolute value of the time of the highest peak less than zero (see Fig. 7G). Contralateral phase was defined as the contralateral offset divided by the IBP and expressed as a percentage.
Fig. 7.
Nonepisodic bursting is coordinated following spinal transection. A: representative traces from ipsilateral (left) and bilateral (right) two-point recordings of fictive swimming in the S3–15 series. Bursting was induced by bath application of 100 μM NMDA. Rostrocaudal delay in the ipsilateral record is indicated by a gray line. The timing of bursts from the left channel of each contralateral record is indicated by a gray-shaded box. B: traces from A following processing. C: autocorrelation of the ipsilateral processed signal in B. The gray line shows the IBP. D: plot of the burst frequency determined by autocorrelation against NMDA concentration (100 and 200 μM). E: cross-correlation of the ipsilateral processed signal in B. The gray line shows the rostrocaudal delay. F: plot of the rostrocaudal delay against NMDA concentration. G: cross-correlation of the bilateral processed signal in B. The gray line shows the contralateral delay reflected across 0-ms lag. H: plot of the contralateral phase against NMDA concentration. *Statistically significant difference.
Episodic organization.
To evaluate the degree to which the episodic structure of normal zebrafish locomotion was perturbed in these experiments, we developed a tool to quantify the degree of episodic organization (EO) of bursting. Because our Matlab program could not reliably identify bursts in nonepisodically organized recordings, we used Clampfit (Molecular Devices) to detect bursts by using a voltage threshold. Because many bursts crossed the threshold multiple times, crossings with an inter-event period of <15 ms were grouped into a single burst. The 15-ms threshold was chosen on the basis of our observation that, in these recordings, most crossings within a burst occurred within 12 ms and most interburst intervals were >30 ms. In normal swimming, the inter-episode interval is much longer than the intra-episode burst period. To separate “long” IBPs (inter-episode-like) from “short” IBPs (intra-episode-like), we defined a critical value for separating the IBPs into short and long as the mean of all IBPs plus two standard deviations (Fig. 1E). The means of the short and long IBPs were then calculated. EO is defined as the log10 ratio of the mean long IBP to the mean short IBP. To enhance readability, the EO scores of spinalized and transected larvae are reported as a percentage of the EO of fictive swimming in spinalized larvae at the same NMDA concentration.
Statistical analysis.
For the comparisons between intact and NMDA-induced fictive swimming along the rostrocaudal axis, data were analyzed using a two (intact, spinalized NMDA) × three-factor (rostral, midbody, caudal) independent groups factorial design. All other tests were single-factor independent group designs. Tests for significance were carried out using one- and two-way ANOVAs and subsequent protected t-tests, or two-tailed t-tests using SYSTAT software (SigmaPlot, San Jose, CA). The Pearson's correlation coefficient was calculated using R software (http://www.r-project.org/). Data with P < 0.05 were accepted as statistically significant. Data are expressed as means with SD.
RESULTS
The larval zebrafish spinal cord produces fictive swimming throughout its rostrocaudal extent.
To determine the baseline bursting activity at points along the rostrocaudal axis of the spinal cord, we measured motor neuron bursts during fictive swimming in intact and spinalized larval zebrafish. Spontaneous fictive swimming episodes were recorded from intact larvae (n = 21 peripheral nerves, 3 larvae), and NMDA (50 μM)-induced fictive swimming was recorded in spinalized larvae, that is, larvae with a spinal transection at S3 (n = 37 peripheral nerves, 17 larvae; Fig. 2A). Series of contiguous spinal segments are referred to by the body segment of the rostral and caudal boundaries; e.g., a spinalized zebrafish is referred to as S3–33.
Fig. 2.
Fictive swimming is produced throughout the rostrocaudal extent of the spinal cord. A: diagrams of larval zebrafish illustrating the design of the experiment. Recordings were performed in intact larvae (left) and spinalized larvae (right). The body of the larvae is divided into 3 regions: rostral (dark gray), midbody (light gray), and caudal (white). Circles above the illustration indicate location and number of recordings. Representative traces of spontaneous (intact) and chemically evoked (spinalized) fictive swimming recorded in the midbody region are shown below the respective diagrams. B–D: plots of episode duration (B), burst duration (C), and burst frequency (D) against recording location and preparation type (rostral, midbody, and caudal) in intact (left) and spinalized larvae (right). *Statistically significant difference.
Both spontaneous (intact) and chemically evoked (spinalized) swimming consists of episodes (Fig. 1B) that are composed of high-frequency bursts (Fig. 1, C and D). In both intact and spinalized larvae, peripheral nerves along the rostrocaudal extent of the spinal cord produced episodes of fictive swimming. To compare the motor output produced by different regions of the spinal cord, we grouped the data into three anatomic divisions: rostral (S1–10), midbody (S11–20) and caudal (S21–33). There were statistically significant differences in episode duration, burst duration, and burst frequency between spontaneous (intact) and chemically evoked (spinalized) fictive swimming (all F > 6.8, all P < 0.01; Fig. 2, B–D). In intact larvae, there were no significant differences in the episode duration (Table 1; all t < 0.01, all P > 0.99), burst duration (Table 1; all t < 0.88, all P > 0.38), or burst frequency (Table 1; all t < 0.71, all P > 0.57) along the rostrocaudal axis (Fig. 2, B–D, left). In spinalized larvae, there were no significant differences in the episode duration (Table 1; all t < 0.39, all P > 0.7) or burst frequency (Table 1; all t < 0.07, all P > 0.95) along the rostrocaudal axis (Fig. 2, B and D, right), but the burst duration was significantly longer in the rostral region than in the midbody or caudal regions (Table 1; t > 3.2, P < 0.002; Fig. 2C, right).
Table 1.
Properties of fictive swimming along the rostrocaudal axis
| Preparation | Recording Location | Episode Duration, ms | Burst Duration, ms | Burst Frequency, Hz |
|---|---|---|---|---|
| Intact, spontaneous | Rostral | 257 (34) | 10.9 (0.89) | 30 (1.2) |
| Midbody | 254 (30) | 11.1 (2.0) | 30 (1.8) | |
| Caudal | 250 (28) | 12.7 (1.3) | 29 (2.3) | |
| Spinalized, 50 μM NMDA | Rostral | 3,484 (1,694) | 11.7 (0.58) | 16.5 (1.0) |
| Midbody | 3,707 (1,575) | 8.0 (0.67) | 16.5 (1.1) | |
| Caudal | 3,575 (2,554) | 7.2 (1.3) | 16.5 (0.6) |
Values are means (SD).
Fictive swimming characteristics vary with NMDA concentration.
To determine the effect of NMDA concentration on the properties of fictive swimming, we recorded activity from peripheral nerves in spinalized larvae (n = 6) at three concentrations of NMDA: 50, 100, and 200 μM (Fig. 3). Because the burst duration of NMDA-induced fictive swimming differs along the rostrocaudal axis (Fig. 2C) and to simplify our analysis, all recordings were performed in the midbody region due to its relative accessibility. Episodic fictive swimming was observed at all three NMDA concentrations (Fig. 3A). Increasing NMDA concentration significantly decreased episode duration (Table 2; F = 20.3, P < 0.001; Fig. 3B). Increasing NMDA concentration also significantly increased the number of episodes produced per minute (Table 2; F = 28.8, P < 0.001; Fig. 3C). NMDA concentration did not have a significant effect on burst duration (Table 2; F = 2.3, P = 0.13; Fig. 3D) but increased burst frequency at 200 μM NMDA (Table 2; all t > 4.0, all P < 0.006; Fig. 3E). To determine the independence of burst frequency and episode duration, we tested their correlation. There was a significant negative correlation between episode duration and burst frequency across NMDA concentrations (r = −0.63; t = −3.2, P = 0.006), but there was no correlation between episode duration and burst frequency across preparations within NMDA concentrations (r = 0.05, t = 0.2, P = 0.84).
Fig. 3.
Characteristics of fictive swimming depend on NMDA concentration. A: representative traces of NMDA-induced fictive swimming in a spinalized larval zebrafish at 50, 100, and 200 μM NMDA. B–E: plots of episode duration (B), episodes per minute (C), burst duration (D), and burst frequency (E) against NMDA concentration (50, 100, and 200 μM). *Statistically significant difference.
Table 2.
Properties of chemically evoked fictive swimming in reduced preparations of larval zebrafish
| Preparation | NMDA Concentration, μM | Episode Duration, ms | Episodes per Minute | Burst Duration, ms | Burst Frequency, Hz |
|---|---|---|---|---|---|
| S3–33 | 50 | 3,150 (952) | 12 (4.6) | 11.2 (1.2) | 15 (1.4) |
| S3–33 | 100 | 1,950 (459) | 24 (4.2) | 12.0 (1.9) | 16 (1.2) |
| S3–33 | 200 | 898 (87) | 33 (5.1) | 13.8 (3.0) | 21 (2.7) |
| S10–33 | 200 | 1,130 (372) | n.d. | 10.6 (1.1) | 19.5 (5.8) |
| S3–15 | 200 | 1,425 (386) | n.d. | 8.9 (1.4) | 18.3 (4.0) |
| S8–20 | 200 | 1,087 (412) | n.d. | 9.8 (4.8) | 16.3 (4.0) |
Values are means (SD); n.d., not determined.
Series of contiguous spinal segments retain the ability to produce fictive swimming following spinal transection.
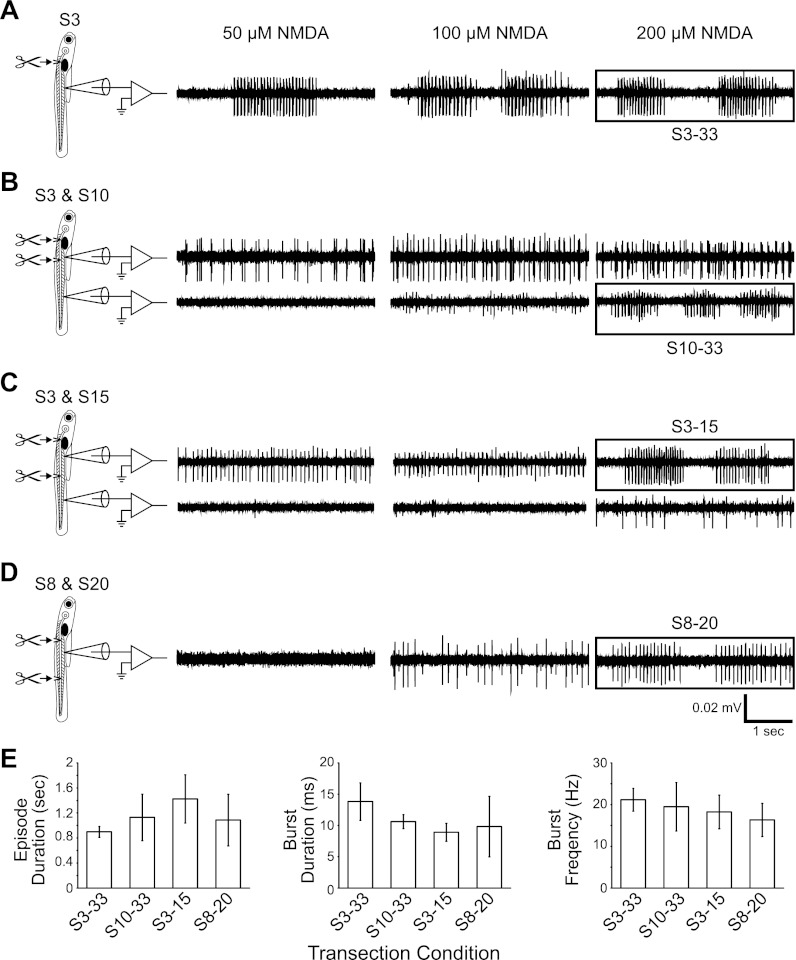
To characterize the rostrocaudal distribution of the spinal locomotor circuit in spinalized (S3 transection) larval zebrafish, we performed a series of spinal transections at S8, S10, S15, or S20, dividing the spinal cord into series of spinal segments of different lengths. In all spinalized larvae with an additional spinal transection, episodically organized bursting was no longer observed at 50 μM NMDA (n = 24; Fig. 4, B–D). When the NMDA concentration was increased to 200 μM, some of the series of contiguous spinal segments produced episodically organized bursting (Fig. 4, B–D). In spinalized larvae transected at S10 (n = 5; Fig. 4B), the caudal portion of the spinal cord (S10–33) produced normal, episodically organized bursting, whereas the rostral portion of the spinal cord (S3–10) produced regular, nonepisodic bursting. In spinalized larvae transected at S15 (n = 5; Fig. 4C), the rostral portion of the spinal cord (S3–15) produced episodically organized bursting, whereas the caudal portion (S15–33) produced low-frequency, sporadic bursting. In larvae transected at S8 and S20 (n = 6; Fig. 4D), the midbody series (S8–20) in most (4 of 6) larvae produced episodic bursting. The fictive swimming produced showed no significant differences in episode duration (Table 2; F = 2.3, P = 0.12; Fig. 4E). There was a nonsignificant trend toward shorter burst duration in shorter series of spinal segments compared with spinalized larvae (Table 2; F = 3.0, P = 0.07; Fig. 4E). There were no significant differences in burst frequency (Table 2; F = 1.0, P = 0.41) among the spinalized larvae and shorter series of spinal segments at 200 μM NMDA (Fig. 4E).
Fig. 4.
Length and location of contiguous segments determine the episodic nature of NMDA-induced fictive swimming. A–D: representative traces illustrating the typical fictive motor output from each contiguous spinal region. Chemically evoked fictive locomotor activity is shown at 50, 100, and 200 μM NMDA. A black box in each panel indicates experimental conditions that produced episodically organized fictive swimming that is quantified in E. A: fictive swimming activity produced by a spinalized larva. B: activity from S3–10 (top trace) and S10–33 (bottom trace). C: activity from S3–15 (top trace) and S15–33 (bottom trace). D: activity from S8–20. E: plots of episode duration (left), burst duration (middle), and burst frequency (right) against the series of contiguous spinal segments that produced fictive swimming.
Rostral spinal segments have greater capacity for producing episodic fictive swimming.
To map the distribution of episode organizing capacity along the rostrocaudal extent of the spinal cord, we compared the degree of episodic organization (EO) produced by series of contiguous spinal segments by their length and location along the spinal cord. EO is a tool for quantifying the degree to which bursts are organized into episodes (see materials and methods; Fig. 5A). To provide more closely matched lengths of spinal segments between rostral and caudal series, we measured bursting produced by spinalized larvae additionally transected at S20 (n = 4) as well as at S10 and S15 (rostral: 7-, 12-, and 17-segment series; caudal: 13-, 18-, and 23-segment series). Because high NMDA concentrations were more likely to produce episodic fictive swimming in all series of spinal segments, 200 μM NMDA was used for this analysis. There was a positive correlation between the number of contiguous spinal segments and EO for both the rostral series (S3–10, S3–15, and S3–20) and the caudal series (S10–33, S15–33, and S20–33) (r > 0.99 for both; Fig. 5B). Rostral series of spinal segments produced greater EO per contiguous spinal segment than caudal series [rostral: 5.3% (SD 0.6), caudal: 2.9% (SD 0.1), t = 7.3, P = 0.018]. When series of contiguous spinal segments were compared that were roughly the same length (12–13 spinal segments) but from different regions of the spinal cord, there was a trend toward the rostral spinal segments producing fictive swimming with a higher EO than the caudal spinal segments, but this trend was not statistically significant [S3–15: 60% (SD 15), S8–20: 56% (SD 14), S20–33: 39% (SD 25), F = 1.7, P = 0.22; Fig. 5C].
Fig. 5.
Production of episodic fictive swimming is rostrally biased. A: representative traces illustrating the patterns of bursting that correspond to episodic, intermediate, and nonepisodic EO scores. Fictive swimming was evoked by 200 μM NMDA. B: plot of %EO against the length of the series of contiguous spinal segments. The length of rostral spinal regions (open circles) was measured from the transection at S3 to the location of a more caudal transection. The length of caudal spinal regions (filled diamonds) was measured from the site of the midbody transection to S33. The rostral series were S3–10, S3–15, and S3–20. The caudal series were S10–33, S15–33, and S20–33. C: plot of %EO against transection type: rostral (S3–15), midbody (S8–20), or caudal (S15–33). Data are normalized to the average EO of spinalized larvae in 200 μM NMDA.
Coordination of episodically organized bursting in transected spinal cord.
To determine if bursting produced by series of contiguous spinal segments is coordinated (that is, produces side-to-side alternation and rostrocaudal progression), we performed two-point recordings on spinalized and transected larvae. Quantification of delay and phase was performed by using the first burst of each episode as a phase marker. Spinalized larvae produced fictive swimming with side-to-side alternation (n = 3) and rostrocaudal progression (n = 3) in the presence of 200 μM NMDA (Fig. 6A). The S10–33 series, the shortest caudal series of contiguous spinal segments that produced episodically organized fictive swimming [EO = 67% (SD 13)], produced bursting with side-to-side alternation (n = 3) and rostrocaudal delay (n = 3; Fig. 6B). The S3–15 series, the shortest rostral series of contiguous spinal segments that produces episodic fictive swimming [EO = 61% (SD 15)], also produced bursting with side-to-side alternation (n = 3) and rostrocaudal delay (n = 3; Fig. 6C). Neither the rostrocaudal delay per body segment [S3–33: 1.3 ms (SD 0.75), S10–33: 2.5 ms (SD 1.2), S3–15: 1.3 ms (SD 0.38), F = 2.5, P = 0.15] nor the contralateral phase [S3–33: 45% (SD 3.9), S10–33: 49% (SD 5.0), S3–15: 47% (SD 3.6), F = 0.36, P = 0.71] were significantly different between spinalized larvae and shorter series of spinal segments (Fig. 6, D and E).
Fig. 6.
Episodic fictive swimming is coordinated following spinal transection. A–C: representative traces from ipsilateral (left) and bilateral (right) two-point recordings in spinalized larvae. Fictive swimming was induced by bath application of 200 μM NMDA. Rostrocaudal delay in each ipsilateral record is indicated by a gray line. The timing of bursts from the left channel of each contralateral record is indicated by a gray-shaded box. A: spinalized larvae. B: transected larvae recorded in the S10–33 series. C: transected larvae recorded in the S3–15 series. D and E: plots of ipsilateral delay (D) and contralateral phase (E) against transection conditions: spinalized (S3–33), S10–33, and S3–15.
Coordination of nonepisodic bursting in transected spinal cord.
Among the transection conditions that do not produce episodically organized bursting, some (e.g., S3–15, 100 μM NMDA; Fig. 4C) produced highly regular bursting [burst frequency = 14 Hz (SD 0.82), coefficient of variation = 0.48 (SD 0.21)]. Given the regularity of these bursts, we hypothesized that the putative episodic organization circuit was either inactive or functioned in an abnormal fashion that did not lead to episode termination, but that other swimming-related circuits were active. The S3–15 series transitioned from producing tonic bursting [EO = 26% (SD 2.0)] to episodic bursting [EO = 60% (SD 15)] when the NMDA concentration was increased from 100 to 200 μM. Therefore, to determine the necessity of episodic organization for coordination, we compared the coordination of bursts produced by this series of segments in 100 μM NMDA with the coordination of bursts produced in 200 μM NMDA (n = 3 per group). Because the bursting in 100 μM NMDA was nonepisodic, we could not use the first burst of each episode as a phase marker (as in Fig. 6). Instead, we used cross- and autocorrelation to measure the IBP and burst frequency of individual recordings and the relative timing of bursts between recording sites. To facilitate correlation of biphasic bursts (Fig. 7A), recordings were rectified and low-pass filtered at 90 Hz (Fig. 7B), and auto- and cross-correlations of the processed signal were performed (Fig. 7, C and E). The burst frequency of the S3–15 series was significantly slower in 100 μM than in 200 μM NMDA [100 μM: 14 Hz (SD 1.6), 200 μM: 19 Hz (SD 1.7), t = 3.4, P = 0.043; Fig. 7D]. The nonepisodic bursting produced by the S3–15 series in 100 μM NMDA had a rostrocaudal delay per body segment significantly greater than zero [1.48 ms (SD 0.18), t =14.6, P = 0.005]. Rostrocaudal delay per body segment was significantly longer in 100 μM than in 200 μM NMDA [200 μM: 1.00 ms (SD 0.07), t = 4.4, P = 0.02; Fig. 7F]. Contralateral phase was found by dividing the contralateral delay by the IBP (Fig. 7G). There were no significant differences between the contralateral phase of the S3–15 series in 100 and 200 μM NMDA [100 μM: 52% (SD 0.02), 200 μM: 50% (SD 0.03), t = 0.8, P = 0.49; Fig. 7H].
DISCUSSION
We have shown that fictive locomotion is produced along the rostrocaudal extent of the spinal cord in larval zebrafish (Fig. 2) and that this locomotor pattern can be generated from reduced series of spinal segments (Fig. 4). In the transected spinal cord, rostral spinal segments have greater potential for generating episodically organized fictive swimming (Fig. 5). The episodic fictive swimming produced by these series is coordinated normally (Fig. 6). On the basis of these findings, we return to our initial three hypotheses regarding the spatial distribution of the swimming CPG: 1) the CPG is composed of segmentally reiterated oscillators; 2) there is a single CPG distributed throughout the entire spinal cord; or 3) there is a single CPG located in a small region of the spinal cord. Our results are inconsistent with the third hypothesis. Neither the rostral (S3–10) nor caudal (S15–33) regions are necessary for organized locomotion (Fig. 5). Although those transections leave open the possibility that the midbody segments S10–15 contain a critical population of neurons, the S8–20 series contains the largest contiguous midbody region but does not produce the most organized swimming (Fig. 5). On the basis of these results, we conclude that there are no critical segments or series of segments in the larval zebrafish swimming CPG. Our finding that there is a linear trend of EO against number of contiguous segments (Fig. 5) is suggestive of either a robust, distributed CPG or segmentally reiterated oscillators that are too weak to drive fictive swimming independently, but we cannot distinguish between these possibilities on the basis of these data. We also found that normal functioning of the circuit responsible for generation of episodic organization is not necessary for coordination of motor bursts (Fig. 7). Our results support the hypothesis that the zebrafish swimming CPG is composed of functionally separable circuits, one that organizes episodes and another that coordinates bursting side to side and rostrocaudally.
The entire spinal cord produces the same pattern of fictive swimming.
On the basis of our observations of free-swimming larvae, we predicted that there might be different patterns of motor neuron bursting in the midbody and tail of the larvae. For example, one type of free-swimming behavior, slow start swimming, has a relatively tight lateral undulation in the midbody region that rapidly increases in amplitude in the far caudal region (Muller and Von Leeuwen 2004). We proposed that this motion could be due to the whiplike snapping of a passive tail. To determine the spatial distribution of motor activity, we measured peripheral nerve activity at points along the rostrocaudal axis of the larvae. Our prediction of a passive tail was not observed in the range of swim frequencies or spinal segments recorded in our experiments (Fig. 2). Instead, the entire spinal cord produced the same pattern of motor neuron bursting. We conclude that the motor neuron output is distributed throughout the cord, although the interneurons that generate the motor pattern may not be.
Fictive swimming characteristics are dependent on NMDA concentration.
Concentrations of NMDA between 50 and 200 μM reliably evoked fictive swimming in spinalized larval zebrafish (Fig. 3). High concentrations of NMDA produced short-duration episodes of high-frequency bursting, whereas low concentrations produced long-duration episodes of lower frequency bursting. The correlation we observed between burst frequency and episode duration across NMDA concentrations was not observed across preparations following application of the same concentration of NMDA. This suggests that the effect of NMDA concentration accounts for the covariance of these variables and that episode duration and burst frequency are independent of one another.
Series of spinal segments produce fictive swimming following transection.
We performed a series of spinal transections that divided the spinal cord into isolated series of contiguous segments (Fig. 4). Under some conditions, these isolated series of spinal segments produced episodically organized fictive locomotion that was not statistically different from the output produced by spinalized larvae. The effect of these transections was to raise the threshold for production of organized episodes from 50 to 200 μM NMDA and, for some transection conditions, to abolish the capacity for generating episodically organized fictive swimming. The necessity for higher NMDA concentration may be due to removal of ascending and descending intraspinal excitatory projections (e.g., Satou et al. 2012) or to an injury-induced decrease in neuronal excitability. We found that rostral series of segments shorter than 12 segments were not able to produce episodic swimming (Figs. 4 and 5) and that more than 20 body segments were necessary to produce episodic swimming in the caudal region. This finding differs from a previous report that two isolated body segments were sufficient to produce locomotor-like bursting (McDearmid and Drapeau 2006). There are several potential explanations for this discrepancy, including effects of strain, larval stage, NMDA concentration, and transection technique. We believe the most parsimonious explanation arises from the intrinsic properties of motor neurons. McDearmid and Drapeau (2006) performed whole cell recordings from individual motor neurons, whereas we used peripheral nerve recordings. Zebrafish motor neurons have intrinsic bursting properties (Buss et al. 2003) that may be activated by high doses of NMDA, possibly giving the appearance of episodic fictive swimming when one is recording from an individual neuron. However, these oscillations would not likely be correlated between motor neurons, and therefore would not be observed with peripheral nerve recording unless a CPG was driving their activity.
The rostral spinal cord has greater episode organizing potential.
In these experiments, we find that rostral body segments are more capable of producing episodically organized swimming than caudal body segments following transection (Fig. 5). This difference is most dramatically demonstrated by the difference in motor output between the rostral series S3–15 and the caudal series S15–33 (Fig. 4C). Despite the rostral bias for episode generation, rostral spinal segments are not necessary for the production of episodes (Fig. 4). This finding is inconsistent with the hypothesis that the swimming CPG is localized to a small region of the cord and suggests a more distributed model. The strong linear trend we found between the number of body segments and the EO score of the swim pattern (Fig. 5) could be interpreted in two ways. On the one hand, it could be that the episode circuit is segmentally reiterated. On the basis of this structure, we would predict repeating interneuron populations with progressively weaker net synaptic drive onto their targets. On the other hand, it could be that the episode organizing circuit is composed of a non-segmentally organized network of neurons spread throughout the spinal cord. On the basis of this structure, we would predict a gradient of synaptic output from the episode circuit and an interneuron distribution that does not align to segmental boundaries. Modeling studies in tadpole (Wolf et al. 2009) provide a quantitative framework for the distributed hypothesis, but determining which hypothesis is more likely will require additional characterization of neuronal distribution throughout the larval zebrafish spinal cord.
Coordination of bursts is independent of episodic organization.
We found that by manipulating the concentration of NMDA, we activated the putative coordination circuit without observing discretely organized episodes (S3–15, 100 μM NMDA; Figs. 4C and 7). The functional dissociation between the episode organization circuit and the coordination circuit we have observed is similar to models of the leech swimming circuit (Kristan et al. 2005) and multilevel models of the mammalian CPG (McCrea and Rybak 2008). Therefore, we propose the following preliminary model of the functional organization of the zebrafish spinal locomotor CPG.
The hindbrain acts as an activator (Arrenberg et al. 2009; Li et al. 2006; Mori et al. 1978; Noga et al. 1988; Soffe et al. 2009) and makes excitatory connections with the spinal episode circuit (Hägglund et al. 2010; Li et al. 2010). The episode circuit acts as a gating center and makes excitatory connections (Buss and Drapeau 2001; Kyriakatos et al. 2011) with the spinal coordination circuit. The coordination circuit sculpts excitatory input from the episode circuit into a coordinated output and makes excitatory and inhibitory connections with motor neurons (Kyriakatos et al. 2011; McLean et al. 2008). When the hindbrain initiates a locomotor episode, it sends an excitatory signal to the episode circuit, initiating an up-state of high activity. The episode circuit excites the coordination circuit, which then begins driving the motor neurons in a coordinated fashion. The episode generator up-state self-terminates, ending the excitation to the coordination circuit. In the absence of excitation, the coordination circuit is silenced and the motor neurons stop firing.
Conclusions.
In summary, using transections of the larval zebrafish spinal cord, we have demonstrated the spatial and functional organization of the episode and coordination circuits in the spinal CPG. We have shown that there is a strong effect of rostrocaudal position on the ability of series of spinal segments to produce episodic swimming. Furthermore, we have shown that normal coordination of bursting is not dependent on episodic organization. Future work is necessary to determine whether the episode organization and coordination circuits are segmentally organized and how the putative episode and coordination circuits interact with one another.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS065054 (to M. A. Masino), Minnesota Medical Foundation Grant 4052-9238-11 (to M. A. Masino), and Grant-in-Aid of Research, Artistry and Scholarship 21934 from the Office of the Dean of the Graduate School of the University of Minnesota (to M. A. Masino).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.D.W., T.M.A., J.H.P., and M.A.M. conception and design of research; T.D.W., T.M.A., J.H.P., and M.A.M. performed experiments; T.D.W., T.M.A., J.E., and J.H.P. analyzed data; T.D.W., T.M.A., J.H.P., and M.A.M. interpreted results of experiments; T.D.W. prepared figures; T.D.W., T.M.A., J.E., and J.H.P. drafted manuscript; T.D.W., T.M.A., J.E., J.H.P., and M.A.M. edited and revised manuscript; T.D.W., T.M.A., J.E., J.H.P., and M.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ronald L. Calabrese, Dr. Eric Newman, Dr. Karen Mesce, and members of the Masino laboratory for helpful comments on this manuscript.
REFERENCES
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA 106: 17968–17973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty RS. Development of swimming movements and musculature of larval herring (Clupea harengus). J Exp Biol 110: 217–229, 1984 [DOI] [PubMed] [Google Scholar]
- Borla MA, Palecek B, Budick S, O'Malley DM. Prey capture by larval zebrafish: evidence for fine axial motor control. Brain Behav Evol 60: 207–229, 2002 [DOI] [PubMed] [Google Scholar]
- Buss RR, Bourque CW, Drapeau P. Membrane properties related to the firing behavior of zebrafish motoneurons. J Neurophysiol 89: 657–664, 2003 [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol 86: 197–210, 2001 [DOI] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Fast and slow locomotor burst generation in the hemispinal cord of the lamprey. J Neurophysiol 89: 2931–2942, 2003 [DOI] [PubMed] [Google Scholar]
- Cohen AH, Wallen P. The neuronal correlate of locomotion in fish. Exp Brain Res 41: 11–18, 1980 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53: 81–90, 1983 [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol 66: 437–451, 2006 [DOI] [PubMed] [Google Scholar]
- Drapeau P, Ali DW, Buss RR, Saint-Amant L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J Neurosci Methods 88: 1–13, 1999 [DOI] [PubMed] [Google Scholar]
- Eklöf-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlén P, Higashijima S, El Manira A. Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci USA 109: 5511–5516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR. The utility of zebrafish for studies of the comparative biology of motor systems. J Exp Zool B Mol Dev Evol 308: 550–562, 2007 [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Liu KS. Zebrafish as a model system for studying neuronal circuits and behavior. Ann NY Acad Sci 860: 333–345, 1998 [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Svoboda KR. Fictive swimming elicited by electrical stimulation of the midbrain in goldfish. J Neurophysiol 70: 765–780, 1993 [DOI] [PubMed] [Google Scholar]
- Fuiman LA, Webb PW. Ontogeny of routine swimming activity and performance in zebra danios (Teleostei: Cyprinidae). Anim Behav 36: 250–261, 1988 [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006 [DOI] [PubMed] [Google Scholar]
- Grillner S. On the generation of locomotion in the spinal dogfish. Exp Brain Res 20: 459–470, 1974 [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19: 572–586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci 13: 246–252, 2010 [DOI] [PubMed] [Google Scholar]
- Ho S, O'Donovan M. Regionalization and intersegmental coordination of rhythm-generating networks in the spinal cord of the chick embryo. J Neurosci 13: 1354–1371, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JA, Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci 296: 229–243, 1982 [DOI] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21: 100–109, 2011 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci 26: 5684–5697, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Kinkhabwala A, Satou C, Higashijima S, Fetcho J. Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain. Proc Natl Acad Sci USA 108: 1170–1175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan WB, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005 [DOI] [PubMed] [Google Scholar]
- Kyriakatos A, Mahmood R, Ausborn J, Porres CP, Büschges A, El Manira A. Initiation of locomotion in adult zebrafish. J Neurosci 31: 8422–8431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Korn H. Glycinergic inhibitory synaptic currents and related receptor channels in the zebrafish brain. Eur J Neurosci 6: 1544–1557, 1994 [DOI] [PubMed] [Google Scholar]
- Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J Neurosci 30: 16609–16620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J Neurosci 26: 4026–4035, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996 [DOI] [PubMed] [Google Scholar]
- Masino MA, Fetcho JR. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J Neurophysiol 93: 3177–3188, 2005 [DOI] [PubMed] [Google Scholar]
- Matsushima T, Grillner S. Intersegmental co-ordination of undulatory movements—a “trailing oscillator” hypothesis. Neuroreport 1: 97–100, 1990 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDearmid JR, Drapeau P. Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J Neurophysiol 95: 401–417, 2006 [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Movement, technology and discovery in the zebrafish. Curr Opin Neurobiol 21: 110–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Masino MA, Koh IYY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11: 1419–1429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M. Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol 41: 1580–1591, 1978 [DOI] [PubMed] [Google Scholar]
- Muller UK, Von Leeuwen J. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J Exp Biol 207: 853–868, 2004 [DOI] [PubMed] [Google Scholar]
- Noga BR, Kettler J, Jordan LM. Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J Neurosci 8: 2074–2086, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley DM, Kao YH, Fetcho JR. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron 17: 1145–1155, 1996 [DOI] [PubMed] [Google Scholar]
- Pietri T, Manalo E, Ryan J, Saint-Amant L, Washbourne P. Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Dev Neurobiol 69: 780–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Higashijima S. Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J Neurosci 32: 1771–1783, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci 29: 6780–6793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffe SR, Roberts A, Li WC. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. J Physiol 587: 4829–4844, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley M, Jovanovic K, Stein RB, Lawson V. The activity of interneurons during locomotion in the in vitro necturus spinal cord. J Neurophysiol 71: 2025–2032, 1994 [DOI] [PubMed] [Google Scholar]
- Wolf E, Soffe SR, Roberts A. Longitudinal neuronal organization and coordination in a simple vertebrate: a continuous, semi-quantitative computer model of the central pattern generator for swimming in young frog tadpoles. J Comput Neurosci 27: 291–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]