Abstract
Disordered reflex activity and alterations in the neural control of walking have been observed after stroke. In addition to impairments in leg movement that affect locomotor ability after stroke, significant impairments are also seen in the arms. Altered neural control in the upper limb can often lead to altered tone and spasticity resulting in impaired coordination and flexion contractures. We sought to address the extent to which the neural control of movement is disordered after stroke by examining the modulation pattern of cutaneous reflexes in arm muscles during arm cycling. Twenty-five stroke participants who were at least 6 mo postinfarction and clinically stable, performed rhythmic arm cycling while cutaneous reflexes were evoked with trains (5 × 1.0-ms pulses at 300 Hz) of constant-current electrical stimulation to the superficial radial (SR) nerve at the wrist. Both the more (MA) and less affected (LA) arms were stimulated in separate trials. Bilateral electromyography (EMG) activity was recorded from muscles acting at the shoulder, elbow, and wrist. Analysis was conducted on averaged reflexes in 12 equidistant phases of the movement cycle. Phase-modulated cutaneous reflexes were present, but altered, in both MA and LA arms after stroke. Notably, the pattern was “blunted” in the MA arm in stroke compared with control participants. Differences between stroke and control were progressively more evident moving from shoulder to wrist. The results suggest that a reduced pattern of cutaneous reflex modulation persists during rhythmic arm movement after stroke. The overall implication of this result is that the putative spinal contributions to rhythmic human arm movement remain accessible after stroke, which has translational implications for rehabilitation.
Keywords: stroke, cerebrovascular accident, locomotion, reflexes, interlimb, rehabilitation, plasticity
a cerebrovascular accident (CVA), or stroke, leads to alterations in the neural control of walking (Kim and Eng 2004; Olney and Richards 2001; Richards and Olney 2001; Zehr et al. 1998) and leg cycling (Brown and Kautz 1998; Brown et al. 1997; Schindler-Ivens et al. 2004). In addition to the many locomotor impairments involving movement ability of the legs, significant impairments are also seen in the activity of arm muscles. Clinical presentation of impaired muscle activity can include exaggerated reflex responses such as spasticity and clonus (Whitlock 1990) as well as reduced muscle responses such as flaccidity and paresis, all of which can alter normal coordination of the arms and legs (Beer et al. 2000; Canning et al. 2000; Cirstea and Levin 2000; Rymer et al. 1998). Hemiparesis and spasticity in the upper limb can often lead to flexion contractures and can also reduce coordinated movement between the arms (Beer et al. 2000; Canning et al. 2000; Cirstea and Levin 2000; Rymer et al. 1998). These alterations in coordination of muscle activity within and between the limbs can significantly affect movement performance. Alterations in descending supraspinal inputs induced by the lesion can allow spinal cord circuits that are exquisitely regulated by interneuronal connections (Jankowska and Hammar 2002) to operate in an unregulated fashion. As a result, activity in spinal pathways may be changed including cutaneous reflexes (Jones and Yang 1994; Zehr and Loadman 2012; Zehr et al. 1998).
Recent observations from studies of background muscle activation and reflex modulation during rhythmic arm cycling provide evidence for the existence of similar neural control for rhythmic arm and leg movements in neurologically intact participants (Zehr et al. 2004a, 2009). Cutaneous reflexes in arm muscles are modulated with the phase of the movement cycle (Zehr and Chua 2000), depend on the motor task (Carroll et al. 2005; Zehr and Hundza 2005), are nerve-specific (Zehr and Kido 2001), and produce functionally relevant corrective responses (Klimstra et al. 2011). Further, reflexes evoked by stimulating different cutaneous fields in one hand generate coordinated patterns of suppression and facilitation in muscles of both arms (Zehr and Kido 2001). Progress has been made in the understanding of the neural control of rhythmic arm movement (for reviews see Zehr and Duysens 2004; Zehr et al. 2004a, 2009), which has been suggested to parallel that for rhythmic leg movements (Haridas and Zehr 2003; Zehr and Duysens 2004; Zehr and Haridas 2003). A contribution from subcortical and presumed spinal pattern generating networks (e.g., locomotor central pattern generators [CPGs]) is implicit in these observations (Zehr et al. 2004a).
Byblow and colleagues have provided compelling evidence concerning the neural control of rhythmic wrist movement after stroke (Lewis and Byblow 2004a,b,c; Stinear and Byblow 2004a,b,c). Currently there has been little study of the neural control of whole-arm rhythmic movements after stroke. The purpose of the present study was to evaluate the extent to which the characteristic pattern of muscle activity and cutaneous reflex modulation is expressed after interruption of descending input arising from stroke. Since rhythmic arm cycling relies on contributions from subcortical circuits (Carroll et al. 2006), we hypothesized partial preservation of patterns of reflex modulation during arm cycling after stroke. As such, background electromyographic (EMG) and reflex modulation serve as proxies for the changes in neural function resulting either from the initial lesion or from the ensuing neuroplastic pathologic adaptations after stroke.
METHODS
Twenty-five participants (18 males, 7 females; average age 65.84 ± 14.12 y, range = 32–86) who were at least 6 mo postinfarction of a single CVA and clinically stable participated with informed, written consent. Participants were selected if they had sufficient residual function to perform low-intensity seated arm cycling and had no significant cardiovascular comorbidities contraindicating the performance of light exercise (Zehr 2011). All participants were screened with the PAR Q (CSEP 2002) and a family physician's confirmation of the ability to participate was obtained as appropriate. Details about the site and extent of their lesion can be found in Table 1. Control data from 33 neurologically intact (NI) participants (average age: 43.7 ± 21.6 y, range 22–82) were compiled from three previous investigations (Carroll et al. 2005; Zehr and Hundza 2005; Zehr and Kido 2001). Experimental procedures were approved by the Human Research Ethics Board at the University of Victoria and performed in accordance with the Declaration of Helsinki.
Table 1.
Summary of physical characteristics, lesion location, and clinical assessments for stroke participants
| Subject | Age (y) | Sex | Infarct | Most Affected Side | Time Post Stroke (mo) | Brunnstrom (arm) | Brunnstrom (hand) | Ashworth (arm) | Locomotor Scale | Semmes - Weinstein hand/arm (gm) | Crank Length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | L BG | R | 10 | 6 | 6 | 0 | 5 | 2.052 (DPS) | 19.5 |
| 2 | 66 | M | RCVA | L | 195 | 2 | 1 | 1 | 4 | 447.0 (LPS) | 19.5 |
| 3 | 75 | M | LCVA | R | 24 | 6 | — | — | 4 | — | 19.5 |
| 4 | 47 | M | L Te | R | 33 | 5 | 5 | — | 4 | 0.0677 (N) | 14 |
| 5 | 67 | M | L PA | R | 78 | — | — | 0 | 3 | — | 3.8 |
| 6 | 86 | M | LCVA | R | 12 | 7 | 7 | 0 | 5 | — | 19.5 |
| 7 | 55 | M | R Temp | L | 14 | 1 | 1 | 2 | 2 | 447.0 (LPS) | 3.8 |
| 8 | 81 | M | RCVA | L | 66 | 3 | 0 | — | 5 | 2.052 (DPS) | 3.8 |
| 9 | 67 | M | L Th | R | 15 | 7 | 6 | 0 | 5 | 447.0 (LPS) | 19.5 |
| 10 | 63 | M | L Occ | R | 76 | 6 | 6 | 0 | 4 | 3.632 (LPS) | 19.5 |
| 11 | 53 | M | R MCA | L | 31 | 6 | 6 | 0 | 5 | 0.4082 (DLT) | 19.5 |
| 12 | 79 | F | R BG | L | 41 | 5 | 5 | — | 3 | 2.052 (DPS) | 14 |
| 13 | 53 | M | L TP | R | 134 | 6 | 6 | 0 | 5 | 3.632 (LPS) | 19.5 |
| 14 | 56 | F | RMCA | L | 36 | 6 | 6 | 0 | 5 | 447.0 (LPS) | 16.5 |
| 15 | 32 | F | R Th | L | 132 | 6 | 5 | 2 | 5 | 0.4082 (DLT) | 19.5 |
| 16 | 83 | M | R TP | L | 20 | 6 | 6 | 0 | 5 | 0.4082 (DLT) | 19.5 |
| 17 | 57 | M | R PA | L | 71 | 3 | 4 | 3 | 5 | 447.0 (LPS) | 14 |
| 18 | 78 | F | L BG | R | 27 | 7 | 7 | 0 | 5 | 0.0677 ((N) | 19.5 |
| 19 | 76 | F | cerebral atrophy | R | 43 | 3 | 2 | 4 | 5 | 0.4082 (DLT) | 19.5 |
| 20 | 76 | M | R IC | L | 42 | 4 | 5 | 1 | 5 | 0.4082 (DLT) | 19.5 |
| 21 | 86 | F | L IC & BG | L | 104 | 7 | 7 | 3 | 5 | 447.0 (LPS) | 16.5 |
| 22 | 53 | F | R TP | L | 45 | 3 | 1 | 4 | 4 | > T6.65 (U) | 3.8 |
| 23 | 50 | M | L MCA | R | 41 | 3 | 2 | 3 | 4 | 0.4082 (DLT) | 6.5 |
| 24 | 79 | M | RMCA | L | 265 | 7 | 7 | 0 | 5 | 0.4082 (DLT) | 19.5 |
| 25 | 71 | M | LCVA | R | 28 | 7 | 7 | 0 | 5 | 3.632 g (LPS) | 19.5 |
| n = 25 | 65.84 ± 14.12 | M18:F7 | L13:R12 | 63.32 ± 61.57 | 5.08 ± 1.84 | 4.7 ± 2.30 | 1.10 ± 1.48 | 4.48 ± 0.82 | 15.6 ± 6 |
*Averages are with SD of the mean. M, male; F, female. Semmes-Weinstein: DLT, diminished light touch; DPS, diminished protective sensation; LPS, loss of protective sensation; U, untestable (unable to feel any filament); —, not tested; MCA, middle cerebral artery; IC, internal capsule; BG, basal ganglia; TP, temporal parietal; TH, thalamic; PA, parietal; CVA, cerebrovascular accident; L, left; R, right.
Protocol
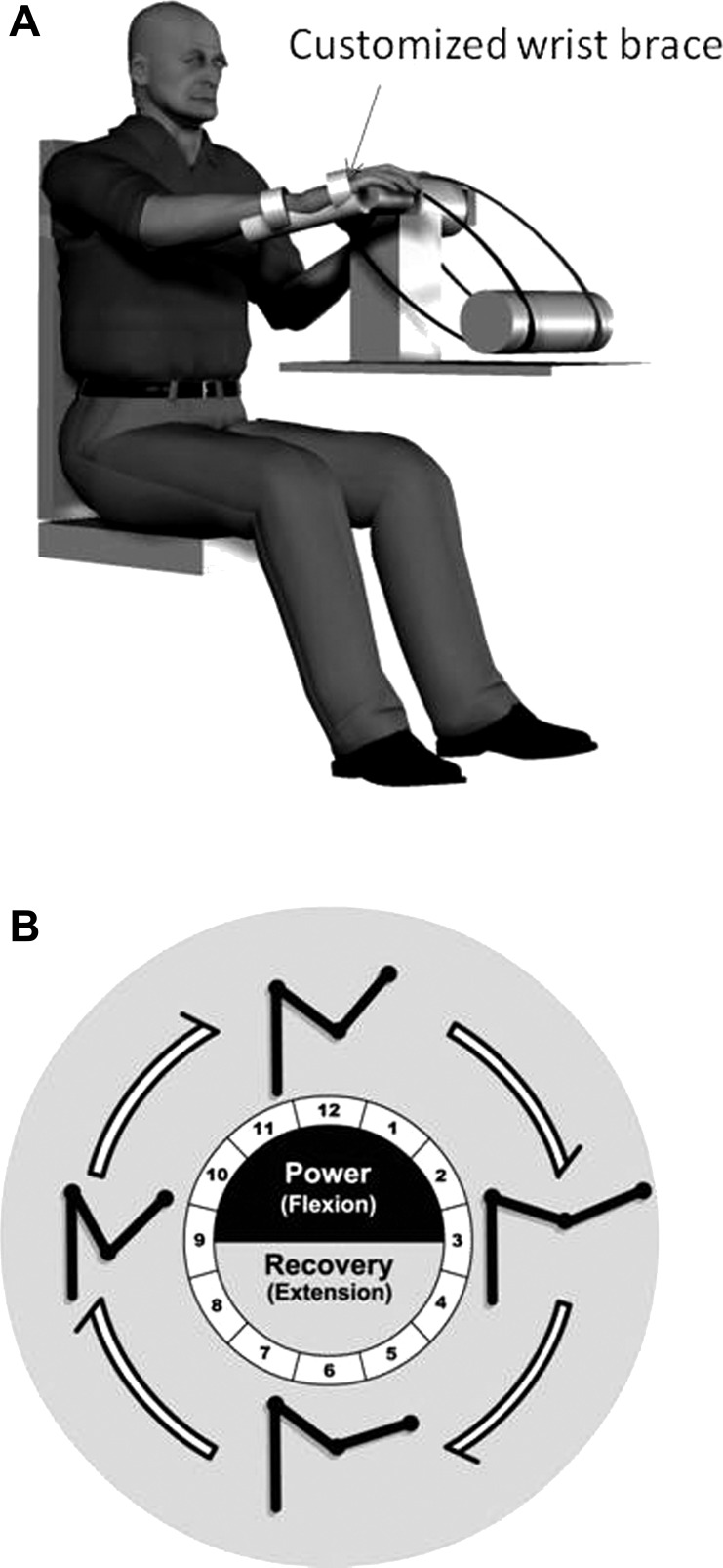
The experimental methodology is similar to that described previously (Carroll et al. 2005; Hundza and Zehr 2006; Zehr and Hundza 2005; Zehr and Kido 2001). Participants performed trials of bilateral rhythmic arm cycling at a comfortable rate of about 1 Hz (stroke: 0.91 ± 0.05; control: 1.1 ± 0.12 Hz) on a previously described hydraulic arm ergometer (Zehr et al. 2003) while the superficial radial (SR) nerve of either the more affected (MA) or less affected (LA) of stroke participants or the right (R) or left (L) arm of control was stimulated. The MA limb was operationally defined as the contralesional and the LA as the ipsiliesional arm. Each cycling trial (120–140 stimulated cycles interleaved with unstimulated cycles) lasted between 5 and 10 min and all participants performed at least one trial of movement with rest breaks as required. In contrast to our previous experiments in NI participants who had full range of motion (ROM) in both shoulder and elbow joints and who used the standard 19.5-cm-long crank arms, flexion contractures, or shoulder subluxation often limited full ROM and precluded stroke participants from cycling with the arm crank at full length on the MA side. In those cases (see Table 1), the lengths of the arm cranks were incrementally adjusted (from 19.5 to 3.8 cm) until a comfortable ROM was reached for each subject. A previous study confirmed that this does not significantly alter the motor task, or the reflex modulation of arm cycling (Hundza and Zehr 2006). Additionally, to facilitate holding the cycle handgrips, molded forearm and hand supports were used. The participant placed his or her hand into the molded support and the hand and forearm were then secured with Velcro straps. The entire hand support interface was then attached to the cycle handgrip as shown in Fig. 1A.
Fig. 1.
Schematic cartoon illustration of (A) stroke participant performing arm cycling with modified wrist brace and handgrip interface and (B) clockface showing 12 phases of movement and stick figure of the arm (adapted from Klimstra et al. 2011).
During rhythmic arm cycling, the position of the ergometer crank arms in the movement cycle was determined from a linear continuous turn potentiometer connected in series with the chain and was reset with each complete 360° movement cycle. The movement cycle is defined here relative to a clockface with the position where the ipsilateral crank is pointing straight up, defined as 12 o'clock and phase definitions continuing clockwise (see Fig. 1, A and B). All references to position in the movement cycle are thus given with respect to the hour hand on a clockface. Offline, responses occurring at a given position in the movement cycle were partitioned together with reference to these definitions. In correspondence with this, the movement cycle was partitioned into 12 separate phases. Accordingly, phase 1 is that part of the movement cycle that covers the excursion from 12 to 1 o'clock, phase 2 from 1 to 2 o'clock, and so on. This is illustrated in Fig. 1B, which includes the additional depiction of the general mechanical arrangement of the arm cycling movement into power (or shoulder flexion; equivalent to stance phase of walking in legs) from 9 until 3 o'clock and recovery (or shoulder extension; equivalent to swing phase of walking in legs) from 3 to 9 o'clock (Klimstra et al. 2011) when the clockface is viewed from the right.
Clinical evaluations.
Clinical classification of all stroke participants was carried out by a registered physiotherapist. Muscle tone was measured using the Modified Ashworth Scale (5 points) at the elbow for the upper limb (Bohannon and Smith 1987). This is a graded rating of elbow flexion spasticity scored from 0 to 4, with 0 being flaccid and 4 being rigid. Motor impairment was measured using the Brunnstrom Stroke Scale (Stages 1–7) for both the arm and hand (Brunnstrom 1966) and a functional measure of gait was derived using the 6-point Functional Ambulation Categories Scale (FACS, 0–5 points) (Holden et al. 1984). The Brunnstrom stages of motor recovery after stroke is a 7-point scale ranging from: 1) flaccid paralysis with no reflexes; 2) some spastic tone, but no voluntary movement and muscle synergies are elicited through facilitation; 3) spasticity is marked and synergistic movements may be elicited voluntarily; 4) spasticity decreases and synergistic movements predominate; 5) spasticity wanes and there is an ability to move out of synergies, although synergies are still present; 6) coordination and movement patterns are near normal, but there is trouble with more rapid complex movements; and 7) normal. The FACS rates walkers on a 0 to 5 scale (Collen et al. 1990) from an inability to walk (score 0), to dependence requiring continuous contact (score 1), intermittent contact (score 2), or verbal supervision (score 3) of another person, to independence on either even (score 4) or uneven (score 5) surfaces.
The ability of participants to discern both cutaneous light touch and pressure was measured in the hand and arm using the Semmes-Weinstein esthesiometer (Hage et al. 1995). Data from these evaluations along with other characteristics of the stroke participants are found in Table 1.
Nerve Stimulation
Because the MA arm of stroke participants was the main limb of interest, it was always stimulated in the first arm cycling trial to ensure that these data were collected at a minimum. If the participant was able, the less affected (LA) arm was subsequently stimulated in a separate trial. Stimulation was applied pseudorandomly across the cycle of movement with trains of 5 × 1-ms pulses at 300 Hz via a Grass S88 stimulator (Astro-Med Grass Instrument, West Warwick, RI) connected in series with an SIU5 isolator and a CCU1 constant-current unit. The SR nerve was stimulated just proximal to the anatomical snuff box close to the distal end of the radius using bipolar surface electrodes. Appropriate stimulation location was verified by confirming that a radiating sensation was evoked in the innervation distribution of the SR nerve. The intensity of stimulation was approximately twofold the threshold for radiating paraesthesia (RT) in both groups (stroke MA: mean 2.11 ± 0.72 [range 1.1–4], stroke LA: 2.19 ± 0.54 [range 1.6–4]; control RSR: 2.2 ± 0.60 [range 1.5–4], control LSR: 2.3 ± 0.56 [range 1–4]) and was not significantly different between groups [F(2,58) = 2.164, P = 0.124 with Lavene's correction statistic P = 0.238]. Since MA and LA (stroke) and left and right (control) arms were both stimulated in separate trials reference is made to limbs based on the relative site of stimulation: ipsilateral (i) or contralateral (c) to the site of stimulation. In one stroke participant with sensory impairments we were not able to determine the RT; therefore, stimulation intensity in that subject was determined by the presence of a minimal reflex response in the static position.
Electromyography
For all participants bipolar surface electromyographic (EMG) recordings were made from shoulder (anterior [AD] and posterior [PD] deltoid), elbow (biceps [BB] and triceps [TB] brachii), and wrist (flexor carpi radialis [FCR]) muscles. Ground electrodes were placed on the skin overtop of bony landmarks. EMG signals were preamplified (×1,000–5,000) and bandpass filtered at 100–300 Hz (P511 Astro-Med Grass Instrument).
Data Acquisition and Analysis
All data were sampled at 1 kHz with a 12-bit A/D converter connected to a computer running custom-written LabVIEW (National Instruments, Austin, TX) virtual instrument applications. Offline, the data were partitioned into 12 movement phases based on a division of the cycle of rhythmic arm movement into 12 equidistant portions that represent one “hour” as described earlier. Evoked responses to nerve stimulation in each phase were averaged. The same was done for EMG recorded without stimulation (unstimulated data) in each phase. Subtracted traces (∼10–20 observations per phase) of reflex EMG were created by subtracting unstimulated data from data on which evoked responses were superimposed.
Analysis of background muscle activation.
As described earlier, “background” EMG amplitudes were calculated for each phase of the movement cycle from the unstimulated data. All amplitudes were normalized to the maximum (max) background EMG detected in a given muscle for each subject during arm cycling. The background EMG data were analyzed in three different ways. First, we analyzed the amplitude of background EMG occurring at each cycling position (phase). Second, to quantify the rhythmic activation of muscles during arm cycling, a modulation index (MI = [(EMGmax − EMGmin)/EMGmax] × 100) was calculated for each muscle across the movement cycle. This measure provides a means of comparing the extent to which muscles varied between phasic bursts of activity (large MI) to alternatively tonic activity (small MI) throughout the movement cycle. Third, we calculated coactivation ratios for antagonist muscles of the same arm and homologous muscles between the arms. The ratio within an arm yields a quantified index of the extent to which antagonist muscles were coactive during rhythmic arm movement and was applied to AD/PD and BB/TB. It was also applied between the arms to create ratios of activity in homologous muscles bilaterally for AD, PD, BB, and TB, yielding an index relating the extent to which coordination of muscle activity was maintained between the arms after stroke. In this way a high ratio is indicative of less contralateral coactivation. All ratios were compared with the normative values taken from the neurologically intact control participants.
Cutaneous reflex analysis.
Subtracted reflex EMG responses were analyzed at early (∼50–80 ms to peak) and middle (∼80–120 ms to peak) latencies. During analysis of individual participant data, all 12 phases of subtracted traces for a given muscle were plotted. Reflex amplitudes were considered significant and included in the analysis if they exceeded a 2SD band calculated on the residual prestimulus subtracted EMG. A 10-ms window centered on the peak of each reflex response was averaged at early and middle latency. All subtracted EMG amplitudes were then normalized to the maximum background EMG recorded during the cycling trial for the nerve stimulated.
Statistics
ANOVA was conducted separately on background EMG, and early and middle latency reflexes to determine significant main effects for phase of movement in separate analyses for control neurologically intact (NI) and stroke data (Statistica, Statsoft). Tukey's honestly significant difference (HSD) post hoc test was used to evaluate significant main effects. Pearson's correlation coefficients (r) were calculated between reflex amplitudes and background control EMG for each muscle and tested for significance. Descriptive statistics included mean ± SE and coefficient of variation (CV) of means. To evaluate the extent to which a stroke altered the muscle activation and reflex output, data were compared with pooled results in control NI participants obtained over several years in different projects. Differences between stroke and NI participant group data were evaluated using t-tests with Lavene's F-test corrections for unequal variance. Any differences between MA and LA arms and NI data were evaluated by subtracting the data for the NI mean from the single participant data and calculating sums of squares on the residual. This was then tested statistically using t-ratios and a hypothesized mean difference of “zero” (see (Zehr et al. 1998)). Cochran's Q statistic was used for statistical quantification of numbers of differences from control within the LA and MA arms. Statistical significance was set at P ≤ 0.05.
RESULTS
Background EMG
Phase-dependent modulation of background EMG amplitude was noted for all muscles in control and in the LA arm of stroke participants. In stroke participants, background EMG in iBB and iTB muscles acting at the elbow of the MA arm were the only ones to show significant modulation across the movement cycle. In Table 2, a summary of phase-dependent modulation of background EMG and reflex amplitude for stroke and control participants is provided. Cells filled with asterisks (*) indicate a significant main effect for movement phase for that muscle, arm, and participant group. For example, taking the first row of Table 2, background EMG along with both early and middle latency reflexes were significantly phase-modulated in the control iAD muscle. However, for the stroke MA arm only early-latency reflexes were phase modulated, whereas for the LA arm background EMG and early latency reflexes showed significant effects.
Table 2.
Details of statistical analyses for phase-modulation of background rhythmic EMG and cutaneous reflex amplitudes
| Background EMG |
Early Latency Reflexes |
Middle Latency Reflexes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | MA | LA | Control | MA | LA | Control | MA | LA | |
| iAD | * | ns | * | * | * | * | * | ns | ns |
| iPD | * | ns | * | * | ns | * | * | * | ns |
| iBB | * | * | * | * | ns | * | * | ns | * |
| iTB | * | * | * | * | ns | * | * | ns | ns |
| iFCR | * | ns | * | ns | ns | * | * | ns | ns |
| cAD | * | * | * | * | ns | ns | * | * | ns |
| cPD | * | * | * | ns | ns | ns | ns | * | ns |
| cBB | * | * | * | ns | * | ns | ns | ns | ns |
| cTB | * | * | * | * | ns | ns | * | ns | ns |
| cFCR | * | * | ns | * | ns | ns | ns | ns | ns |
Cells filled with an asterisk (*) indicate a significant main effect for movement phase from ANOVA at P < 0.05. Cells (ns) indicate those without a significant main effect.
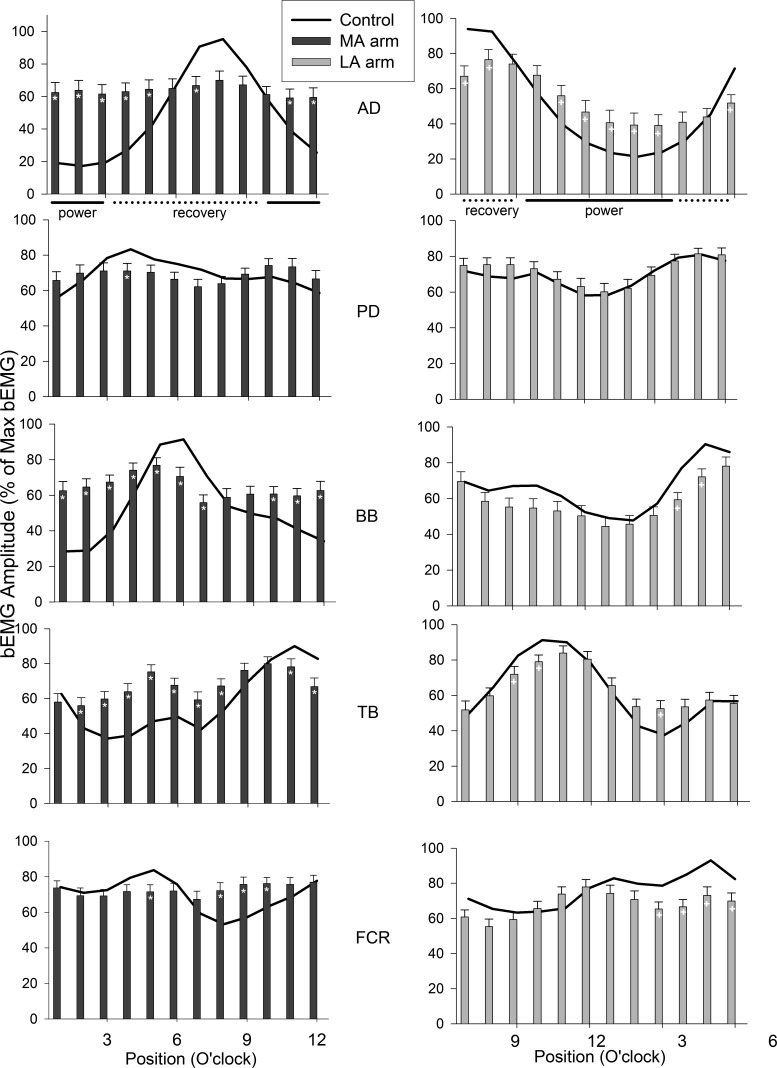
The bilateral patterns of background EMG amplitudes across all 12 phases of movement for all muscles in the MA (dark bars, left side panels) and LA (light bars, right side panels) arms in stroke participants and in the R (ipsi) and L (contra) arms of control participants (black lines) are shown in Fig. 2. Generally, in the MA arm after stroke the background EMG pattern differed from control. Overall, there appears to be a “flattening” of the modulation pattern across the movement cycle. This is observed as excess activity during periods when muscles are normally quiescent as well as reduced amplitudes when muscles are normally active. This can be detected in the number of differences in a phasewise comparison between the MA and control arms. The largest number of differences across cycle positions were seen in AD (8), BB (10), and TB (9). In contrast, FCR (4) and PD (1) had fewer differences from control. For the stroke LA arm there were fewer differences from the pattern in the control subjects. However, just as in the MA arm, less affected AD had many (8) differences compared with control. The patterns in BB (2), TB (3), and FCR (4) also differed from control, whereas PD showed no differences at all. It is interesting to note that all the muscles showed differences during the recovery phases (4–9 o'clock).
Fig. 2.
Background electromyographic (EMG) patterns across the movement cycle for stroke (bars) and neurologically intact control (lines) participants. Data for the more affected (MA) arm in dark gray (right) and less affected arms (LA) are in light gray (right). Data are means ± SE. *Differences between stroke and control at P < 0.05. Anterior (AD) and posterior (PD) deltoid; biceps (BB) and triceps (TB) brachii; flexor carpi radialis (FCR).
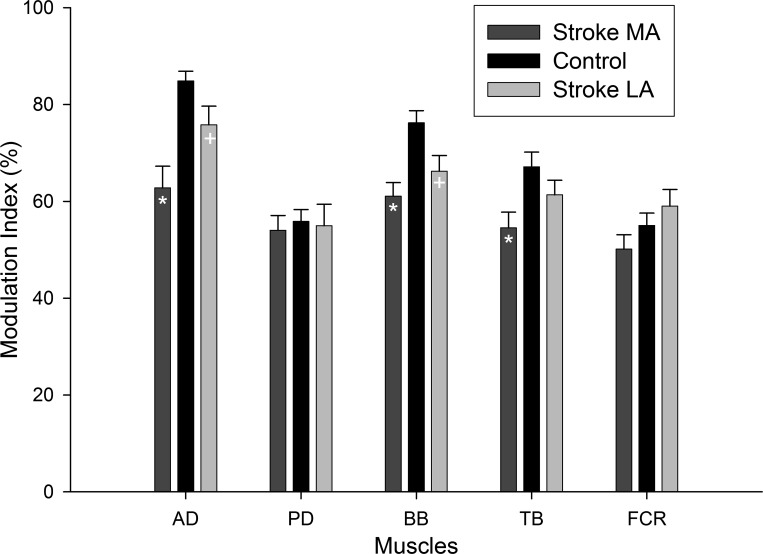
An additional way to appreciate the “flattening” of the background EMG patterns across the movement cycle can be seen in the modulation indices for all muscles. A lower modulation index indicates that the EMG amplitude moved through a narrower range across the movement cycle. These indices are plotted in Fig. 3. For ease of comparison the data are shown as bars for MA, control, and then LA arms. For the MA arm, the MIs in AD, BB, and TB were all significantly (P < 0.05) reduced compared with control. For the LA arm, AD and BB were also significantly reduced compared with control.
Fig. 3.
Background EMG modulation indices across muscles for stroke (bars) and neurologically intact control (black bars) participants. Data for the less affected arms (LA) are in light gray and more affected (MA) in dark gray. Data are means ± SE. * and + indicate differences between stroke and control at P < 0.05. Abbreviations are as in Fig. 2.
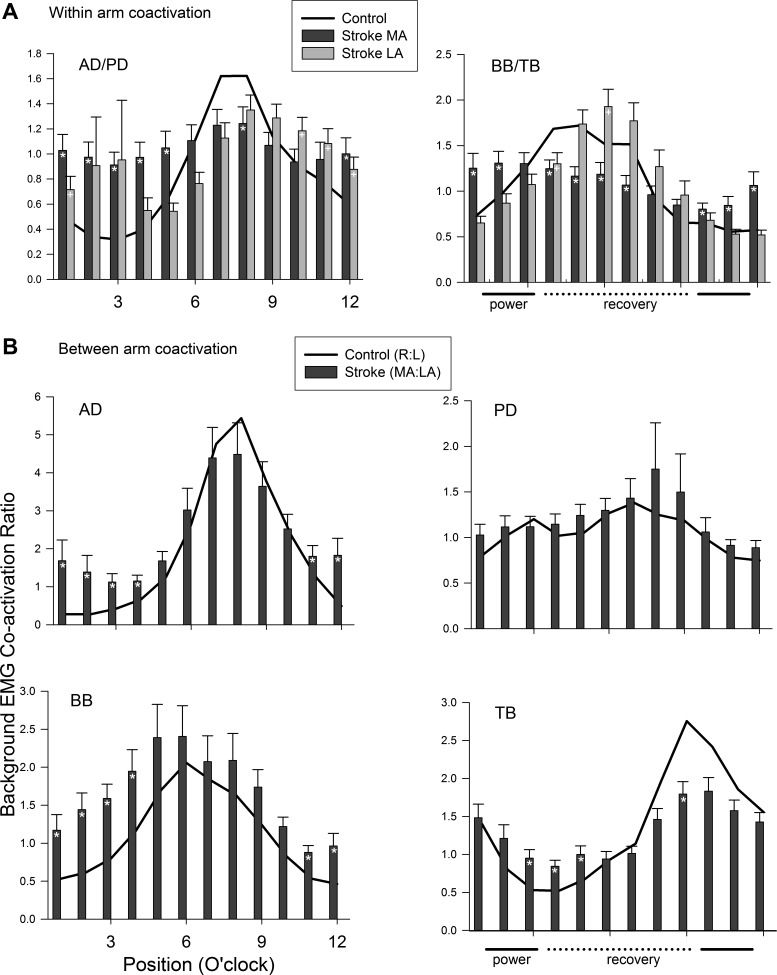
To gauge the extent that changes in muscle activation may functionally alter coordinated muscle activation patterns within and between the arms, coactivation ratios of background EMG were calculated (see Fig. 4). Flexor/extensor background EMG ratios between functional antagonists AD/PD (particularly during the power phases) and BB/TB (across all phases) within the MA and LA and control arms are shown in Fig. 4A. In Fig. 4A the excess coactivation at the shoulder (AD/PD ratio) can be clearly seen, particularly for the MA arm. More than half (7 of 12) of the bins show altered coactivation patterns in the MA arm compared with control. For the LA arm, one third (4 of 12) of the phases show altered activation ratios. A similar result was also seen in the MA arm for the muscles acting at the elbow. For the BB/TB ratio, three quarters (9 of 12) of the phases differed in the MA arm, whereas only 2 phases showed differences for the LA arm.
Fig. 4.
Coordination ratios for background EMG across the movement cycle. A: within-limb ratios. B: between-limb ratios. Data for the less affected arms (LA) are in light gray and more affected (MA) in dark gray. Data are means ± SE. *Differences between stroke and control at P < 0.05. Ipsilateral to site of stimulation (i); contralateral to site of stimulation (c). Other abbreviations are as in Fig. 2.
Figure 4B displays the traces for coactivation ratios in homologous muscle pairs calculated between the arms. The between arm (MA/LA for stroke and R/L for control) coactivation ratios show that for the flexors AD and BB and the extensor TB, there was exaggerated coactivity in the MA arm compared with the coactivation seen for control. This was especially evident during the power (clock positions 11–4) phases of the movement cycle for AD and BB.
Phase-Dependent Modulation of Cutaneous Reflexes
In control NI participants, significant phase-dependent modulation of early and middle latency reflexes was seen in all ipsilateral muscles except for FCR at early latency. Contralateral reflexes were significantly phase-modulated in all muscles at early latency and in cAD and cPD at middle latency. Early-latency reflexes in the LA arm of stroke participants were all phase-modulated in muscles ipsilateral to stimulation, but no contralateral muscles showed significant main effects. At middle latency, only the iBB muscle showed a main effect for phase in the LA arm. Fewer observations of phase dependence were seen in the MA arm. Significant main effects were seen at early latency in iAD and cBB and at middle latency in iPD, cAD, and cPD muscles. Table 2 presents a summary of the main effects for phase arising from the ANOVA analyses for all muscles and both groups.
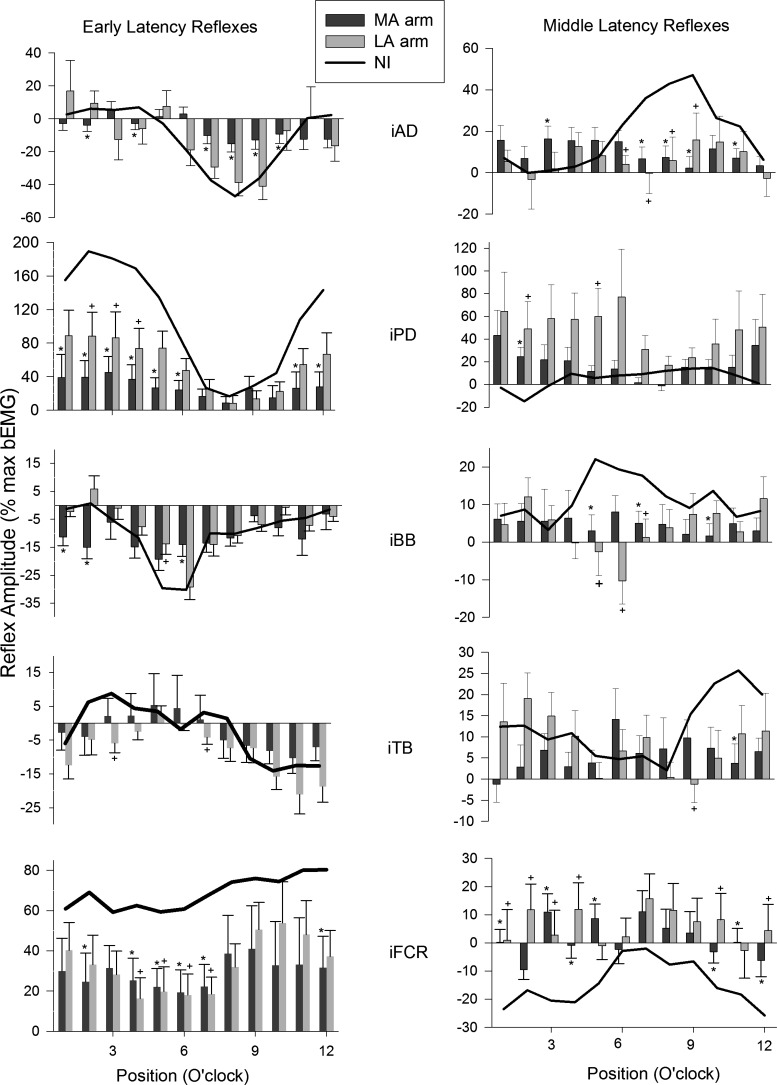
Reflex Amplitudes for Muscles in the Arm Ipsilateral to the Site of Stimulation
Figure 5 shows single participant reflex traces across the full cycle of movement for the shoulder flexor muscle AD, ipsilateral to the site of stimulation in the MA and LA arms of a stroke and the right arm of a control participant. Note that early- and middle-latency reflexes (both of which are contained in the boundary outlined by the gray rectangles) can be seen across all three sets of traces spanning MA, LA, and control. Group averages for early (left) and middle (right) latency reflexes across all subjects are shown in Figs. 6 and 7. Figure 6 shows responses in muscles of the control, MA, and LA arms when they are the stimulated limb (ipsilateral muscles). These data show that, although there are some similarities in the overall shape across the movement cycle between stroke and control at early latency for shoulder (AD, PD) and elbow (BB, TB) muscles, there are considerable differences in the actual reflex amplitudes (e.g., PD). Furthermore, the responses in the wrist flexor FCR in the MA and LA arms differ from control.
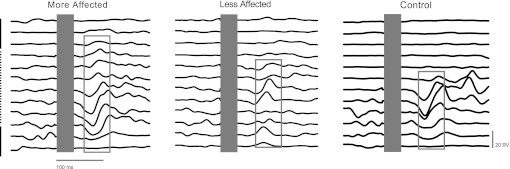
Fig. 5.
Single subject examples of reflexes evoked in shoulder extensor AD muscle by superficial radial (SR) nerve stimulation in the MA and LA arms after stroke and in control. Single horizontal lines represent each of the 12 phases of the arm cycle beginning at top dead center (12 o'clock) and progressing clockwise to the bottom. The blank area at time zero (shown as a gray rectangle in each set of sweeps) shows the elimination of the stimulus artifact for data analysis. The gray rectangles highlight the early and middle latency responses. The vertical dashed line represents recovery and the solid line power.
Fig. 6.
Early (left panels) and middle (right panels) latency reflex amplitudes evoked by SR nerve stimulation and plotted across the movement cycle for stroke (bars) and neurologically intact control (lines) participants. Data are from the stimulated (ipsilateral) limb. Data are means ± SE. *Differences between stroke and control at P < 0.05. +, Differences between the MA arm and control and LA arm and control. Other abbreviations are as in Fig. 2.
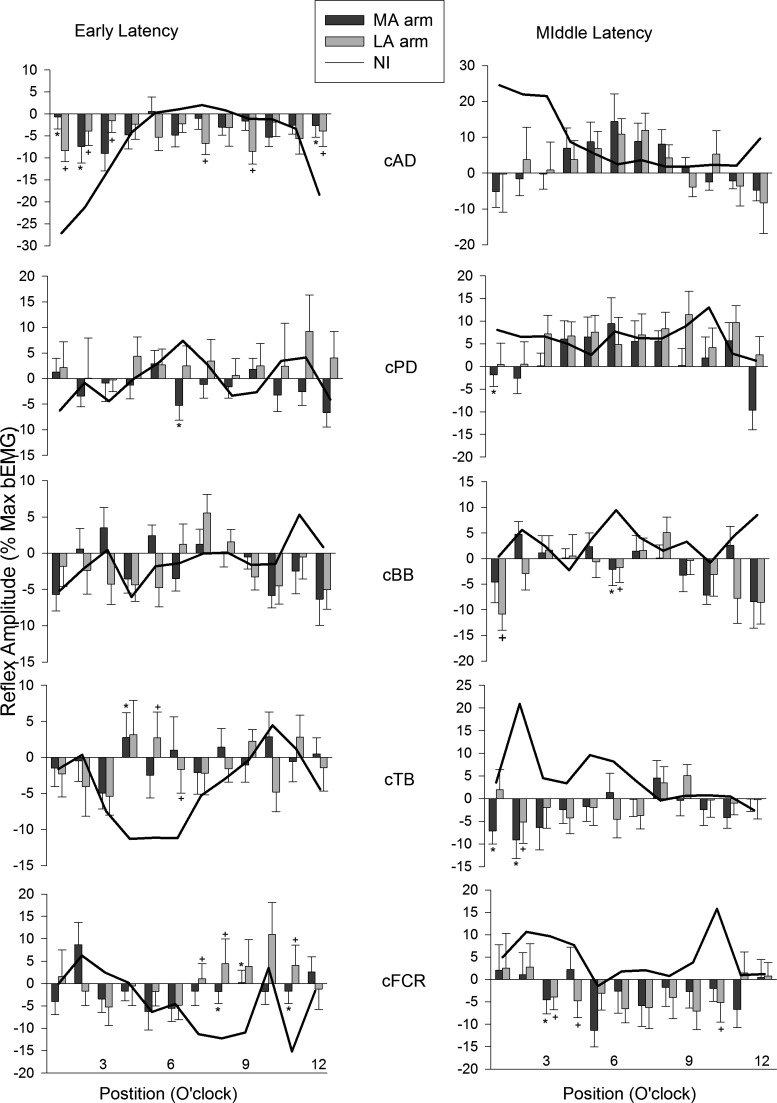
Fig. 7.
Early (left panels) and middle (right panels) latency reflex amplitudes evoked by SR nerve stimulation and plotted across the movement cycle for stroke (bars) and neurologically intact control (lines) participants. Data are from the unstimulated (contralateral) limb. Data are means ± SE. *Differences between stroke and control at P < 0.05. Abbreviations are as in Figs. 2 and 6.
Although it is only descriptive, when considering the MA and LA arms at early latency there are more differences from control in the proximal shoulder muscles AD (MA = 6, LA = 0) and PD (MA = 8, LA = 3) and the elbow flexor BB (MA = 3, LA = 1). Fewer differences are noted for the elbow extensor TB (MA = 0, LA = 2). However, at the wrist for FCR muscle, many differences from control were seen (MA = 6, LA = 4). At middle latency, the shoulder muscle AD shows the most differences (MA = 5, LA = 3), PD a few (MA = 1, LA = 2), and again elbow flexor BB shows 3 for each arm. Just as with early latency, few differences are seen in TB (MA and LA = 1) and the middle-latency reflexes in FCR show many differences (MA = 7, LA = 6). An additional feature to note is that for both MA and LA arms every muscle shows a difference from control NI participants during the recovery phase.
Reflex Amplitudes for Muscles in the Arm Contralateral to the Site of Stimulation
Data plotted in Fig. 7 are reflexes in muscles of the LA and MA arms, contralateral (c) to the stimulation. As is typical with crossed-limb cutaneous reflexes, the EMG amplitudes here were generally much smaller than those in the stimulated limb. This is especially evident at the shoulder (compare the y-axis scaling for AD in Fig. 5 and between Figs. 6 and 7). Interestingly, unlike the reflexes in the ipsilateral LA and MA arms, there is a fairly even distribution of differences for both reflex latencies. The only obvious exceptions are at the shoulder and wrist where AD (MA = 3, LA = 6) and FCR (MA = 3, LA = 3) show many differences at early latency. Otherwise, few differences were seen for cPD (MA = 1, LA = 0), cBB (no differences), and cTB (MA = 1, LA = 2) muscles at early latency. At middle latency, differences from control were seen in cAD (no differences), cPD (MA = 1, LA = 0), cBB (MA = 1, LA = 2), cTB (MA = 2, LA = 1), and cFCR (MA = 1, LA = 3).
In an effort to quantify statistically the number of differences from control within the LA and MA arms we used Cochran's Q statistic. The result showed no significant differences in the departure from control in both arms overall. That is, although both arms differed from control as indicated earlier, the extent of that difference was not different between the two arms.
Relationship Between Reflex and Background EMG Amplitudes
Results from the linear regression analyses are shown in Table 3. Data from control participants are shown at the top, with LA and MA arm data from stroke participants in the middle and bottom of the table, respectively. The Pearson r value is given for early- and middle-latency reflexes, along with the n for each comparison (which varied for control), and the corresponding critical value of r. Correlation coefficients significant at P ≤ 0.05 are shown in bold type. In control NI subjects there was significant correlation between early-latency reflex amplitudes and background EMG bilaterally for AD and ipsilaterally for BB. At middle latency only in iAD was there a significant correlation. Thus, in only 4 cases of a possible 20 did control participants show significant linear relationships between background EMG and reflex amplitudes. The most striking result for the data in stroke participants was that for both MA and LA arms there were no significant correlations for any muscles at any latency.
Table 3.
Correlation analysis between reflex amplitudes and background EMG
| Control | Early | Middle | n | Critical value of r |
|---|---|---|---|---|
| iAD | −0.688* | 0.517* | 33 | 0.343 |
| iPD | 0.174 | 0.019 | 33 | 0.343 |
| iBB | −0.466* | 0.229 | 33 | 0.343 |
| iTB | −0.255 | 0.183 | 33 | 0.343 |
| iFCR | 0.116 | −0.327 | 33 | 0.343 |
| cAD | −0.580* | 0.321 | 29 | 0.367 |
| cPD | −0.123 | −0.001 | 29 | 0.367 |
| cBB | −0.063 | −0.059 | 15 | 0.514 |
| cTB | −0.212 | 0.010 | 15 | 0.514 |
| cFCR | −0.170 | −0.101 | 15 | 0.514 |
| Stroke LA Stimulated | ||||
| iAD | −0.199 | −0.064 | 22 | 0.423 |
| iPD | 0.089 | 0.130 | 22 | 0.423 |
| iBB | −0.355 | −0.048 | 22 | 0.423 |
| iTB | −0.342 | −0.114 | 22 | 0.423 |
| iFCR | 0.111 | −0.220 | 22 | 0.423 |
| cAD | −0.123 | −0.133 | 22 | 0.423 |
| cPD | −0.163 | −0.051 | 22 | 0.423 |
| cBB | −0.103 | −0.096 | 21 | 0.413 |
| cTB | −0.064 | −0.151 | 22 | 0.423 |
| cFCR | 0.087 | −0.111 | 22 | 0.423 |
| Stroke MA Stimulated | ||||
| iAD | −0.320 | 0.151 | 25 | 0.396 |
| iPD | 0.173 | −0.025 | 25 | 0.396 |
| iBB | −0.156 | 0.043 | 25 | 0.396 |
| iTB | −0.071 | 0.041 | 25 | 0.396 |
| iFCR | 0.096 | −0.128 | 25 | 0.396 |
| cAD | −0.184 | 0.056 | 25 | 0.396 |
| cPD | −0.084 | −0.056 | 25 | 0.396 |
| cBB | −0.100 | −0.114 | 25 | 0.396 |
| cTB | −0.036 | −0.137 | 25 | 0.396 |
| cFCR | −0.130 | −0.080 | 25 | 0.396 |
Bold font and * indicate a significant correlation.
DISCUSSION
Here we examined the modulation of background EMG and cutaneous reflexes in both the MA and LA upper limbs of stroke participants during rhythmic arm cycling and compared them with observations taken from neurologically intact participants. There are three main new observations. First, background EMG activity patterns were rhythmically modulated in both the LA and MA arms of stroke participants. However, EMG amplitudes were blunted in many muscles and the range of EMG amplitudes (depth of modulation) was reduced in both arms after stroke compared with control. Related to this, reciprocal coactivation patterns between antagonist and crossed limb homologous muscles were altered in both MA and LA arms after stroke. Second, although cutaneous reflexes were altered after stroke compared with control, they remained phase-modulated in some muscles in both MA and LA arms. Overall, the data suggest that reflex patterns are partially retained even in the MA limb during rhythmic arm cycling poststroke. Finally, measures of background motor output and reflex modulation in both arms after stroke differed from control contributing to the concept that, in a neurophysiologic context, there is no truly “unaffected” side after stroke.
Rhythmic Background Motor Output During Arm Cycling After Stroke
Taken together, the overall modulation patterns for background EMG and reflex amplitudes provide insight into the status of neural control circuits in the intact and damaged nervous system (Burke 1999; Zehr et al. 2004a). Our results add to existing evidence that portions of the neural circuitry regulating rhythmic motor output for the arms remain accessible after stroke (Ferris et al. 2006; Zehr and Loadman 2012; Zehr et al. 2004a, 2009). This preservation of neural regulation is seen for both the MA and LA arms; however, the regulation of the MA arm is reduced compared with the LA arm or to the control data. Generally this can be described as “blunting” involving reduced amplitudes and reduced modulation ranges that are most prominent in the MA arm. A functional outcome of this blunting effect in MA and LA arms is that there is more coactivation within the arm as well as between the arms. This can be seen in terms of the differences across the movement cycle in background EMG and reflex amplitudes as well as the change in coactivation ratios (see Fig. 4).
There are some interesting comparisons in rhythmic background motor output between our data for arm cycling and observations from other experiments during walking and leg cycling after stroke. With walking, changes in burst durations and cocontractions during stance (Shiavi et al. 1987) and reduced modulation of EMG amplitude across the full walking cycle (Burridge et al. 2001) have been noted. In a recent study of walking after stroke, we showed that the depth of modulation for background EMG activity was similar between stroke and control participants (Zehr and Loadman 2012).
Leg cycling has been a very useful experimental paradigm for the study of bilateral neuronal linkages after stroke and there are detailed analyses of patterns of muscle activation (Alibiglou and Brown 2011; Brown and Kautz 1998; Brown et al. 1997; Schindler-Ivens et al. 2004). Input from the LA leg potentiates dysfunctional muscle activation in the MA leg during leg cycling (Kautz and Patten 2005). It was also demonstrated that leg cycling could help induce rhythmic motor output in the contralateral leg, even when it was stationary (Kautz et al. 2006). These observations highlight the potential for interlimb coupling effects to facilitate bilateral motor output during rhythmic leg cycling after stroke. Currently, this type of systematic investigation of the effect of the movement state of the arms during arm cycling is not available.
Organization of Cutaneous Reflex Pathways After Stroke
Our data show alterations in neural control of rhythmic arm movement such that cutaneous reflex amplitudes and modulation patterns in both MA and LA arms were modified relative to arms of the neurologically intact. Changes in the MA arm are certainly expected but still show some remnants of normal control. The alterations in the LA arm add to the much earlier suggestion of Thilmann et al. (1990) that the stroke ipsilesional side is not to be taken as a normal or unaffected limb. More recently, Mirbagheri et al. (2008) used finely controlled torque motor perturbations to reveal that, compared with control, ankle reflex stiffness was exaggerated in both legs after hemiparetic stroke. Our data add to this research and support the position that the ipsilesional limb is not a neurophysiologically valid reference as a control or “unaffected” limb.
Based on our data we suggest that cutaneous reflex patterns are partially retained during rhythmic arm cycling after stroke. Of particular interest is the MA arm, where the observed residual phase modulation is on the contralateral side, suggesting access to spinal cord circuits with nerve stimulation applied to either side. Transmission in crossed cutaneous reflex pathways from the MA to LA arm is altered compared with transmission from the LA to the MA arm when stimulation was applied to the MA arm (compare data and number of differences from control in Figs. 6 and 7). As such, cutaneous inputs and associated modulation of reflex amplitudes could serve as probes to identify alterations in neural function on either side, resulting either from the initial lesion or from the ensuing neuroplastic adaptations occurring with spontaneous recovery or targeted rehabilitation (Wolpaw 2010). Such neural plastic adaptations in the MA arm resulting from a variety of processes, including timed pruning, changes in connectivity, functional networking with unmasking silent pathways, and neurogenesis, have potential to both ameliorate and degrade functional plasticity (Cramer et al. 2011; Grefkes and Fink 2011). Changes in the LA arm may result from aberrant neuroplasticity after the injury or from the initial lesion, an issue that is not possible to resolve with our data.
Although there are no strictly specific studies on cutaneous reflex pathways during arm cycling after stroke, there are related studies from walking and leg cycling. Previously we showed extensive bilateral cutaneous reflex activity evoked during stationary (Zehr et al. 2001) and locomotor (Haridas and Zehr 2003; Zehr et al. 2007b) tasks in neurologically intact participants. Recently, in stroke, we showed that contralateral stimulation of the cutaneous superficial peroneal nerve innervating the foot dorsum could evoke significant crossed reflexes in the MA leg (Zehr and Loadman 2012). Overall, results in walking were consistent with dysfunctional reflex control, particularly during the swing phase. It was speculated that changes in interneuronal excitability during swing could arise from reduced supraspinal regulation of alphamotoneuronal and interneuronal activity caused by the stroke lesion (Dobkin 2005; Dobkin et al. 2004). Interestingly, in this study, the largest differences between stroke and control arms were seen in the recovery phases of arm cycling. This part of the arm cycling movement has been functionally considered as equivalent to swing phase for the legs during walking (Klimstra et al. 2011). Swing phase was also the portion of the step cycle during which many differences were noted between control and the MA leg during walking after stroke (Zehr and Loadman 2012). These observations may suggest a similar effect of the stroke lesion on the mechanisms regulating rhythmic arm and rhythmic leg movement. Currently, we are not able to determine the overall effect that a larger reliance on supraspinal regulation of spinal cord excitability during rhythmic arm movement (Barthelemy and Bo Nielsen 2010) than during rhythmic leg movement (Bo Nielsen 2002; Zehr and Duysens 2004) may have after stroke.
Independence of Reflex and Background EMG Amplitudes After Stroke
Significant linear correlation between cutaneous reflex amplitude and background EMG has been observed for static, postural tasks but not for rhythmic locomotor activity in both the arms and legs (Hundza and Zehr 2006; Zehr et al. 2007a). Additionally, discrete tasks, such as nonrhythmic leg extension or flexion, show strong correlation as well (Zehr et al. 2007a). These robust correlations normally seen during static or discrete actions have been ascribed to automatic gain compensation (Matthews 1986) arising from voluntary alterations in motoneuronal excitability. In contrast, the separation between reflex and background EMG amplitudes during rhythmic motor output has previously been ascribed to the action of locomotor central pattern generator (CPG) output modulating transmission in cutaneous pathways by premotoneuronal gating (Duysens 1998; Duysens and Van de Crommert 1998). Descending supraspinal inputs can therefore regulate reflex output either through modulation of excitability in the interneuronal reflex pathways or through the interneuronal networks that are part of the CPG itself (pattern formation layer; see McCrea and Rybak 2008; Zehr 2005). The present observations of complete lack of significant correlation of background EMG and reflex amplitudes during rhythmic arm cycling in stroke participants can be inferred as an outcome of decreased supraspinal regulation during rhythmic arm movement arising after stroke (and see preceding text for discussion, Rhythmic Background Motor Output During Arm Cycling After Stroke). That is, the supraspinal lesion has further weakened the small effect normally seen in some muscles, such as AD, in control subjects. This could arise from weakened direct supraspinal regulation of interneuronal reflex pathways. Additionally, the observation of few correlations in this large sample of control subjects further supports the concept that during rhythmic motor output, cutaneous reflex amplitudes are typically uncoupled from the background EMG amplitudes.
Methodologic Considerations
After stroke, there is often reduced range of motion at the shoulder and elbow in the MA arm. In a prior study we used three variations in crank length (CL) (Long = 19.5, Medium = 13.5, and Short = 3.8 cm) to create an asymmetrical cycling pattern (Hundza and Zehr 2006). These variations in CL produced significant changes in the range of motion of the shoulder and elbow joints. Angular displacement at the shoulder joint for crank lengths of 19.5, 13.5, and 3.8 cm were approximately 64°, 38°, and 13°, respectively. The corresponding ROM at the elbow was about 103°, 66°, and 25°, respectively. Thus, this issue could have been a confounder in the effects we observed here in the LA arm. Since our prior study showed that background EMG amplitude in muscles of the arm opposite that with the altered CL (in the case of stroke this means the LA arm) increased significantly as CL decreased. As a result, at any phase in the movement cycle, the background EMG was different between three CL trials and was typically higher on the opposite side. This did not have a major effect on the pattern of EMG modulation during arm cycling but rather induced a scaling effect. If this were a significant factor at play in the current study we would predict an increase in EMG amplitude generally in the muscles in the LA arm of stroke. Yet the background EMG graphs show that this effect is not present. Additionally, despite the effect on the amplitudes of background EMG, amplitudes of early- and middle-latency cutaneous reflexes in muscles of both arms were essentially invariant at each phase of the movement cycle between the different CLs. We concluded that variations of arm cycling that primarily yield significant changes in the amplitude of muscle activity (like crank length manipulations) do not require significant task-specific change in neural control.
We therefore cannot completely exclude a contribution from this effect of altered crank length and joint range of motion. Despite that, with reference to Table 1, it can be seen that 11 of our 25 stroke participants required an adjusted crank length to modify the MA ROM. Of those 11, 5 had modest adjustments that put them between the long and medium CL and 5 at the short CL. Given the results in the current article, taken together with Hundza and Zehr (2006), we do not think this had a major methodologic impact on our results.
Translational Implications for Rehabilitation
The current observations help to further characterize the integrity of neural circuits regulating rhythmic arm movement after stroke. Understanding the status of the nervous system function after stroke is crucial in advancing effective rehabilitative practice. We previously showed that rhythmic arm cycling can activate descending pathways to modulate lumbar spinal cord activity (Dragert and Zehr 2009; Frigon et al. 2004; Hundza and Zehr 2009; Loadman and Zehr 2007; Zehr et al. 2004b). Recently this observation was extended to stroke participants where a similar but reduced effect was observed even in those with spasticity (Barzi and Zehr 2008). This suggested that rhythmic cycling with the arms could activate descending effects on the lumbar cord and implied the presence of similar neural control of the arms as in neurologically intact participants. This includes a regulation from subcortical and presumed spinal pattern generating networks (e.g., locomotor CPGs) contributing to rhythmic arm cycling (Zehr et al. 2004a, 2009). The present result adds to the picture of neural control of rhythmic arm movement after stroke and shows a partial preservation of the linkages seen in the neurologically intact state. This may include the use of the arms in functional stabilization of gait through the cancellation of horizontal angular momentum produced by the legs (see Zehr et al. 2009).
Of particular interest are the earlier results of Diserens et al. (2007) that rhythmic arm cycling could reduce spasticity in arm muscles. We suggest our data support that such cycling training could help engage the neural mechanisms regulating rhythmic arm movement. The overall implication of this result is that the putative spinal contributions to rhythmic human arm movement remain accessible after stroke, which has implications for rehabilitative interventions. Notably, this access is maintained despite differences in range of motion at the arm in stroke, as confirmed previously for neurologically intact participants (Hundza and Zehr 2006). Engaging these mechanisms could lead to enhanced and retrained strength and mobility for arm function as it has in the retraining of leg function after stroke and spinal cord injury (Barbeau and Visintin 2003; Ferris et al. 2006; Moseley et al. 2008). Previously, the use of discrete arm extension and flexion actions cued with auditory stimuli has been shown to dramatically improve motor function after stroke (Whitall et al. 2000; also see review in Cauraugh et al. 2010). This form of training has been shown to generalize to other motor tasks like reaching and pointing (Senesac et al. 2010) and to lead to extensive bilateral reorganization of cortical activation (Luft et al. 2004). Currently, the relationship between the whole arm cycling action explored here, ascribed as a functional proxy to arm swing during walking (Zehr et al. 2004a), and the planar extension and flexion action established by Whitall et al. (2000) is uncertain but warrants exploration.
We conclude that circuits regulating rhythmic motor output remain accessible but have an altered “operating range” after stroke. It remains to examine the extent to which activity in these circuits can be reinforced by habitual activity in rehabilitation.
GRANTS
This work was supported by a Grant-in-Aid of Research from the Heart and Stroke Foundation of Canada (HSF, BC and Yukon) (to E.P.Z.) and by a Focus-on-Stroke Fellowship from HSF (BC and Yukon) (to S.H.), and the Canadian Institutes for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.P.Z. and S.R.H. conception and design of research; E.P.Z., P.M.L., and S.R.H. performed experiments; E.P.Z., P.M.L., and S.R.H. interpreted results of experiments; E.P.Z. drafted manuscript; E.P.Z., P.M.L., and S.R.H. edited and revised manuscript; E.P.Z. approved final version of manuscript; P.M.L. and S.R.H. analyzed data; P.M.L. prepared figures.
REFERENCES
- Alibiglou L, Brown DA. Impaired muscle phasing systematically adapts to varied relative angular relationships during locomotion in people post-stroke. J Neurophysiol 105: 1660–1670, 2011 [DOI] [PubMed] [Google Scholar]
- Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil 84: 1458–1465, 2003 [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Bo Nielsen JB. Corticospinal contribution to arm muscle activity during human walking. J Physiol 588: 967–979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzi Y, Zehr EP. Rhythmic arm cycling suppresses hyperactive soleus H-reflex amplitude after stroke. Clin Neurophysiol 119: 1443–1452, 2008 [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res 131: 305–319, 2000 [DOI] [PubMed] [Google Scholar]
- Bohannon R, Smith M. Interrater reliability on a modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207, 1987 [DOI] [PubMed] [Google Scholar]
- Bo Nielsen J. Motoneuronal drive during human walking. Brain Res Rev 40: 192–201, 2002 [DOI] [PubMed] [Google Scholar]
- Brown DA, Kautz SA. Increased workload enhances force output during pedaling exercise in persons with poststroke hemiplegia. Stroke 29: 598–606, 1998 [DOI] [PubMed] [Google Scholar]
- Brown DA, Kautz SA, Dairaghi CA. Muscle activity adapts to anti-gravity posture during pedalling in persons with post-stroke hemiplegia. Brain 120: 825–837, 1997 [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46: 357–375, 1966 [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res 128: 263–277, 1999 [DOI] [PubMed] [Google Scholar]
- Burridge JH, Wood DE, Taylor PN, McLellan DL. Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys 23: 427–434, 2001 [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, O'Dwyer NJ. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J Neurol Sci 176: 45–56, 2000 [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Baldwin ER, Collins DF, Zehr EP. Corticospinal excitability is lower during rhythmic arm movement than during tonic contraction. J Neurophysiol 95: 914–921, 2006 [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Zehr EP, Collins DF. Modulation of cutaneous reflexes in human upper limb muscles during arm cycling is independent of activity in the contralateral arm. Exp Brain Res 161: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Lodha N, Naik SK, Summers JJ. Bilateral movement training and stroke motor recovery progress: a structured review and meta-analysis. Hum Move Sci 29: 853–870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain 123: 940–953, 2000 [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, et al. Harnessing neuroplasticity for clinical applications. Brain 134: 1591–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diserens K, Perret N, Chatelain S, Bashir S, Ruegg D, Vuadens P, Vingerhoets F. The effect of repetitive arm cycling on post stroke spasticity and motor control: repetitive arm cycling and spasticity. J Neurol Sci 253: 18–24, 2007 [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Rehabilitation after stroke. N Engl J Med 352: 1677–1684, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage 23: 370–381, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragert K, Zehr EP. Rhythmic arm cycling modulates Hoffmann reflex excitability differentially in the ankle flexor and extensor muscles. Neurosci Lett 450: 235–238, 2009 [DOI] [PubMed] [Google Scholar]
- Duysens J. From cat to man: basic aspects of locomotion relevant to motor rehabilitation of SCI. Neurorehabilitation 10: 107–118, 1998 [DOI] [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HW. Neural control of locomotion. Part 1: The central pattern generator from cats to humans. Gait Posture 7: 131–141, 1998 [DOI] [PubMed] [Google Scholar]
- Ferris DP, Huang HJ, Kao P-C. Moving the arms to activate the legs. Exerc Sport Sci Rev 34: 113–120, 2006 [DOI] [PubMed] [Google Scholar]
- Frigon A, Collins DF, Zehr EP. Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J Neurophysiol 91: 1516–1523, 2004 [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134: 1264–1276, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage JJ, van der Steen LP, de Groot PJ. Difference in sensibility between the dominant and nondominant index finger as tested using the Semmes-Weinstein monofilaments pressure aesthesiometer. J Hand Surg Am 20: 227–229, 1995 [DOI] [PubMed] [Google Scholar]
- Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol 90: 2850–2861, 2003 [DOI] [PubMed] [Google Scholar]
- Hundza S, Zehr E. Cutaneous reflexes during rhythmic arm cycling are insensitive to asymmetrical changes in crank length. Exp Brain Res 168: 165–177, 2006 [DOI] [PubMed] [Google Scholar]
- Hundza SR, Zehr EP. Suppression of soleus H-reflex amplitude is graded with frequency of rhythmic arm cycling. Exp Brain Res 193: 297–306, 2009 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Rev 40: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Jones CA, Yang JF. Reflex behavior during walking in incomplete spinal-cord-injured subjects. Exp Neurol 128: 239–248, 1994 [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol 93: 2460–2473, 2005 [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol 95: 3154–3163, 2006 [DOI] [PubMed] [Google Scholar]
- Kim CM, Eng JJ. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: relationship to walking speed. Gait Posture 20: 140–146, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra MD, Thomas E, Zehr PE. Biomechanical outcomes and neural correlates of cutaneous reflexes evoked during rhythmic arm cycling. J Biomech 44: 802–809, 2011 [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Bimanual coordination dynamics in poststroke hemiparetics. J Mot Behav 36: 174–188, 2004a [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. The effects of repetitive proprioceptive stimulation on corticomotor representation in intact and hemiplegic individuals. Clin Neurophysiol 115: 765–773, 2004b [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil 18: 48–59, 2004c [DOI] [PubMed] [Google Scholar]
- Loadman PM, Zehr EP. Rhythmic arm cycling produces a non-specific signal that suppresses soleus H-reflex amplitude in stationary legs. Exp Brain Res 179: 199–208, 2007 [DOI] [PubMed] [Google Scholar]
- Luft A, McCombe-Waller S, Whitall J, Forrester L, Macko R, Sorkin J, Schulz J, Goldberg A, Hanley D. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. J Am Med Assoc 292: 1853–1861, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374: 73–90, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke. J Neuroeng Rehabil 5: Art. 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 1: CD002840, 2005 [DOI] [PubMed] [Google Scholar]
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture 4: 136–148, 2001 [Google Scholar]
- Richards C, Olney SJ. Hemiparetic gait following stroke. Part II: Recovery and physical therapy. Gait Posture 4: 149–162, 2001 [Google Scholar]
- Rymer WZ, Dewald J, Given PTJ, Beer R. Abnormal muscle synergies in hemiparetic stroke: origins and implications for movement control. In: Progress in Motor Control: Berstein's Traditions in Movement Studies, edited by Latash ML. Champaign, IL: Human Kinetics, 1998, vol. 1, p. 191–202 [Google Scholar]
- Schindler-Ivens S, Brown DA, Brooke JD. Direction-dependent phasing of locomotor muscle activity is altered post-stroke. J Neurophysiol 92: 2207–2216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesac CR, Davis S, Richards L. Generalization of a modified form of repetitive rhythmic bilateral training in stroke. Hum Move Sci 29: 137–148, 2010 [DOI] [PubMed] [Google Scholar]
- Shiavi R, Bugle HJ, Limbird T. Electromyographic gait assessment, Part 2: Preliminary assessment of hemiparetic synergy patterns. J Rehabil Res Dev 24: 24–30, 1987 [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol 21: 426–434, 2004a [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Modulation of human cervical premotoneurons during bilateral voluntary contraction of upper-limb muscles. Muscle Nerve 29: 506–514, 2004b [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol 21: 124–131, 2004c [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Fellows SJ, Garms E. Pathological stretch reflexes on the “good” side of hemiparetic patients. J Neurol Neurosurg Psychiatry 53: 208–214, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 31: 2390–2395, 2000 [DOI] [PubMed] [Google Scholar]
- Whitlock JA. Neurophysiology of spasticity. In: The Practical Management of Spasticity in Children and Adults, edited by Glen MB, Whyte J. Philadelphia: Lea & Febiger, 1990, p. 8–33 [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010 [DOI] [PubMed] [Google Scholar]
- Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev 33: 54–60, 2005 [PubMed] [Google Scholar]
- Zehr EP. Evidence-based risk assessment and recommendations for physical activity clearance: stroke and spinal cord injury. Appl Physiol Nutr Metab 36: S214–S231, 2011 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Balter JE, Ferris DP, Hundza SR, Loadman PM, Stoloff RH. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J Physiol 582: 209–227, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Carroll TJ, Chua R, Collins DF, Frigon A, Haridas C, Hundza SR, Kido A. Possible contributions of spinal CPG activity to rhythmic human arm movement. Can J Physiol Pharmacol 82: 556–568, 2004a [DOI] [PubMed] [Google Scholar]
- Zehr EP, Chua R. Modulation of human cutaneous reflexes during rhythmic cyclical arm movement. Exp Brain Res 135: 241–250, 2000 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140: 495–504, 2001 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Frigon A, Hoogenboom N. Neural control of rhythmic human arm movement: phase dependence and task modulation of Hoffmann reflexes in forearm muscles. J Neurophysiol 89: 12–21, 2003 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist 10: 347–361, 2004 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Fujita K, Stein RB. Reflexes from the superficial peroneal nerve during walking in stroke subjects. J Neurophysiol 79: 848–858, 1998 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Haridas C. Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res 149: 260–266, 2003 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hoogenboom N, Frigon A, Collins DF. Facilitation of soleus H-reflex amplitude evoked by cutaneous nerve stimulation at the wrist is not suppressed by rhythmic arm movement. Exp Brain Res 159: 382–388, 2004b [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hundza SR. Forward and backward arm cycling are regulated by equivalent neural mechanisms. J Neurophysiol 93: 633–640, 2005 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hundza SR, Vasudevan EV. The quadrupedal nature of human bipedal locomotion. Exerc Sport Sci Rev 37: 102–108, 2009 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537: 1033–1045, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Klimstra M, Dragert K, Barzi Y, Bowden MG, Javan B, Phadke C. Enhancement of arm and leg locomotor coupling with augmented cutaneous feedback from the hand. J Neurophysiol 98: 1810–1814, 2007b [DOI] [PubMed] [Google Scholar]
- Zehr EP, Loadman PM. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol 123: 796–807, 2012 [DOI] [PubMed] [Google Scholar]