Abstract
Amphetamine-like compounds are commonly used to enhance cognition and to treat attention deficit/hyperactivity disorder, but they also function as positive reinforcers and are self-administered at doses far exceeding clinical relevance. Many of these compounds (including methamphetamine) are substrates for dopamine reuptake transporters, elevating extracellular dopamine by inhibiting uptake and promoting reverse transport. This produces an increase in extracellular dopamine that inhibits dopamine neuron firing through autoreceptor activation and consequently blunts phasic dopamine neurotransmission, an important learning signal. However, these mechanisms do not explain the beneficial behavioral effects observed at clinically useful concentrations. In the present study, we have used patch-clamp electrophysiology in slices of mouse midbrain to show that, surprisingly, low concentrations of methamphetamine actually enhance dopamine neurotransmission and increase dopamine neuron firing through a dopamine transporter-mediated excitatory conductance. Both of these effects are reversed by higher concentrations of methamphetamine, which inhibit firing through dopamine D2 autoreceptor activation and decrease the peak amplitude of dopamine-mediated synaptic currents. These competing, concentration-dependent effects of methamphetamine suggest a mechanistic interplay by which lower concentrations of methamphetamine can overcome autoreceptor-mediated inhibition at the soma to increase phasic dopamine transmission.
Keywords: G protein-coupled inward rectifier K+ channel, mouse, dopamine transporter, psychostimulant, D2 receptor
methamphetamine is a psychomotor stimulant that is commonly abused but is also administered clinically at low concentrations to treat attention deficit/hyperactivity disorder, enhance cognition, and decrease hunger. Acutely, methamphetamine acts centrally as a substrate for biogenic amine uptake transporters (Han and Gu 2006; Sulzer et al. 2005). Most of the central reinforcing properties of methamphetamine are mediated through action at the dopamine transporter (DAT) and the vesicular monoamine transporter VMAT2 (Sulzer et al. 2005). Effects at these uptake transporters promote increased extracellular levels of dopamine at both cell bodies and axon terminal fields, a hallmark observation produced by most, if not all, classes of abused drugs (Campbell et al. 1996; Chen et al. 1993; Di Chiara and Imperato 1988; Kalivas and Duffy 1988; Klitenick et al. 1992; Rahman et al. 2003). The methamphetamine-induced increase in extracellular dopamine presumably activates somatodendritic dopamine D2 autoreceptors, hyperpolarizing the cells through a G protein-coupled inward rectifier K+ (GIRK; Kir3) channel-mediated potassium conductance (Beckstead et al. 2004; Grace and Bunney 1984; Lacey et al. 1987). This hyperpolarization would decrease phasic dopamine release both by decreasing neuronal firing rate and by directly inhibiting dopamine release at the axon terminals. However, because the phasic release of dopamine has been strongly implicated in error prediction and reward learning (Phillips et al. 2003; Schultz 2006; Zweifel et al. 2009), it remains unclear how DAT substrates can improve cognition while presumably decreasing phasic dopamine release through autoreceptor activation.
Although methamphetamine is known to act on the dopaminergic system that originates in the ventral midbrain, its precise effects on dopamine neuron excitability and firing have not been thoroughly investigated. Electrophysiological investigations of this nature have been previously hindered by the lack of a measurable dopamine-mediated synaptic potential. Our recent discovery of a slow inhibitory postsynaptic current (IPSC) mediated by D2 autoreceptors (Beckstead et al. 2004) has now made it possible to directly test the effects of methamphetamine on dopamine-mediated synaptic transmission. In addition to activation of autoreceptors, prior work describing electrophysiological recordings of cultured cells suggests that the DAT substrates dopamine, d-amphetamine, and methamphetamine also produce an excitatory conductance that is not coupled to uptake (Erreger et al. 2008; Goodwin et al. 2009; Ingram et al. 2002; Sonders et al. 1997). The physiological consequences of this conductance have not been determined because it has not been described in an ex vivo brain slice preparation.
In this study we hypothesized that dopamine neuron excitability would be differentially affected by low and high concentrations of methamphetamine. We thus performed patch-clamp electrophysiology of dopamine neurons in slices of mouse midbrain and examined the acute effects of methamphetamine on dopamine neuron firing rate and dopamine-mediated synaptic transmission. Our observations suggest that low concentrations of methamphetamine produce a DAT-mediated excitation, increasing cell firing while enhancing dopamine-mediated synaptic transmission. Conversely, high concentrations produce the opposite effects, decreasing firing through tonic activation of D2 autoreceptors while inhibiting the amplitude of dopamine-mediated synaptic currents.
MATERIALS AND METHODS
Animals and electrophysiological recordings.
Procedures were approved a priori by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. Male C57Bl6J mice (minimum six-wk old) were maintained and used for all experiments. Mice were anesthetized with isoflurane and killed by decapitation. Brains were quickly removed, blocked with a razor blade, and placed on a vibrating microtome for slicing as described previously (Beckstead et al. 2009). Horizontal slices (180–200 μm thick) of the ventral midbrain containing the substantia nigra and ventral tegmental area (VTA) were obtained. Slices were incubated at 34°C for 30–60 min and then maintained at room temperature in a modified Krebs solution containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, 11 d-glucose, and 0.01 MK-801.
For electrophysiological experiments, slices were maintained at 34°C and continuously superfused with 1.5–2.0 ml/min Krebs solution. Dopamine cells in the substantia nigra and VTA were identified visually by size and location in relation to the medial terminal nucleus of the accessory optic tract, the interpeduncular fossa, and the medial lemniscus (Ford et al. 2006). Recordings were divided evenly between the substantia nigra and the VTA, and because no obvious differences were noted between these two brain areas, the data were combined. Further verification of cell identity was provided by examining the neurons' electrical properties, which included high capacitance, an input resistance <250 MΩ, a >100 pA hyperpolarization-activated current, spontaneous pacemaker firing between 1.5 and 4 Hz, wide (>1.2 ms) extracellular spike waveforms, and sensitivity to dopamine D2 receptor and/or DAT ligands. Pipettes for whole cell voltage-clamp recordings (resistance 1.5–2.0 MΩ) were filled with a solution containing (in mM) 115 K-methylsulfate or K-gluconate, 20 NaCl, 1.5 MgCl2, 10 BAPTA, 10 HEPES, 2 ATP, and 0.4 GTP, pH 7.35–7.40, 267–275 mosmol/l. Cells were typically voltage clamped at −55 mV, below the threshold for spontaneous action potential formation. A calculated liquid junction potential of 14 mV was not corrected. Gramicidin D (Sigma-Aldrich, St. Louis, MO) for perforated-patch experiments was dissolved each day in DMSO at a stock concentration of 10 mg/ml, vortexed, and added fresh to the pipette solution at a final concentration of 100 μg/ml. Pipettes for gramicidin perforated-patch experiments (5–10 MΩ) were also filled with (in mM) 115 K-gluconate, 20 NaCl, 1.5 MgCl2, 0.4 EGTA, and 10 HEPES. Series resistance was monitored carefully throughout each experiment, and recordings were discarded if there was a sudden or substantial change in access. Electrodes (5–10 MΩ) for cell-attached (firing) experiments contained a solution made up almost entirely of Na-HEPES plus 20 mM NaCl, pH 7.40, 290 mosmol/l (Beckstead and Phillips 2009).
Dopamine-mediated IPSCs were obtained as described previously (Beckstead et al. 2009). Briefly, a bipolar platinum stimulating electrode (FHC, Bowdoin, ME) was placed a few hundred micrometers caudal to the cell being recorded. A train of five 0.5-ms stimulations was applied at 40 Hz once every 50 s in the presence of the following neurotransmitter receptor blockers: picrotoxin (100 μM, GABAA), MK-801 (10 μM, NMDA), hexamethonium (100 μM, nicotinic acetylcholine), 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM, AMPA), and CGP 55845 (100 nM, GABAB). Other antagonists used at times to block dopamine-sensitive receptors included sulpiride (200–500 nM, D2-type), eticlopride (100 nM, D2-type), SKF 83566 (500 nM, D1-type), and prazosin (100 nM, α1-adrenergic). Drugs were applied by bath perfusion with the exception of dopamine, which was usually applied by iontophoresis (Dagan Instruments, Minneapolis, MN). Dopamine (1 M) was loaded into a high-resistance (∼60 MΩ) electrode and placed 10–20 μm caudal to the soma of the cell being recorded. Dopamine was ejected as a cation with a pulse of +150 nA for 2–3 s. Leakage was prevented between pulses by applying a backing current of −25 nA. Because high concentrations of methamphetamine, cocaine, and dopamine incompletely wash out of brain slices, only one cell was recorded per slice.
Drugs.
Dopamine hydrochloride, MK-801, DNQX, picrotoxin, hexamethonium, prazosin, sulpiride, eticlopride, fluoxetine, nomifensine, Mg-ATP, Na-GTP, Na-HEPES, K-HEPES, EGTA, and gramicidin D were obtained from Sigma-Aldrich. Isoflurane was purchased from Henry Schein (Melville, NY). CGP 55845, SKF 83566, and GBR 12909 were obtained from Tocris Bioscience (Ellisville, MO). BAPTA tetrapotassium salt was obtained from Invitrogen (Carlsbad, CA). Cocaine and methamphetamine (hydrochloride salts) were generous gifts from the National Institute on Drug Abuse drug supply program (Bethesda, MD).
Statistical analyses.
One- and two-way analyses of variance (ANOVAs) were used to analyze data, and within-cell designs were employed wherever feasible. Tukey's or Dunnett's post hoc tests were performed subsequent to significant ANOVAs. Data are presented as means ± SE. In all cases α was set a priori at 0.05.
RESULTS
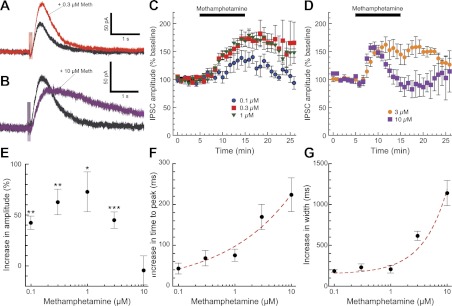
We sought to investigate the effects of different concentrations of methamphetamine on two distinct components of dopamine neuron physiology: dopamine-mediated synaptic transmission and dopamine neuron firing. We obtained whole cell voltage-clamp recordings (−55 mV) of substantia nigra and VTA dopamine neurons in horizontal slices of mouse midbrain. We first examined the acute effects of methamphetamine on dopamine-mediated IPSCs. As we described previously, dopamine neurons exhibit an electrically evoked slow dopamine IPSC that is mediated through activation of somatodendritic D2 receptors and GIRK channels (Beckstead et al. 2004). Bath perfusion of low concentrations of methamphetamine (0.1–1.0 μM) persistently increased the amplitude of dopamine IPSCs (Fig. 1, A and C), consistent with a decrease in dopamine uptake through DAT. A higher concentration of methamphetamine (10 μM) briefly enhanced IPSC amplitudes as the drug washed into the brain slice and then decreased IPSC amplitudes to slightly below baseline levels (Fig. 1, B and D). Thus methamphetamine exhibited bidirectional effects on dopamine IPSC peak amplitudes (Fig. 1E). In addition, low concentrations of methamphetamine produced a slight increase in the time to peak and half-width of dopamine IPSCs. Higher concentrations of methamphetamine dramatically slowed IPSC kinetics (Fig. 1, F and G).
Fig. 1.
Methamphetamine has bidirectional, concentration-dependent effects on dopamine inhibitory postsynaptic current (IPSC) amplitudes. We obtained whole cell patch-clamp recordings of dopamine-mediated IPSCs in substantia nigra and ventral tegmental area (VTA) dopamine neurons (Beckstead et al. 2004) in the presence of GABA, glutamate, and nicotinic acetylcholine receptor blockers. Bath perfusion of a low concentration of methamphetamine (0.3 μM, red trace) significantly enhanced the amplitude and slightly prolonged the duration of dopamine IPSCs (A and C; n = 6–13 cells from 3–9 mice). Perfusion of a high concentration of methamphetamine (10 μM, purple trace) briefly enhanced IPSC amplitudes but subsequently decreased IPSC amplitudes to baseline levels or slightly below (B and D; n = 6–12 cells from 3–6 mice). Thus methamphetamine exhibited an inverted U-shaped concentration-effect curve on dopamine IPSC amplitudes, determined as the average amplitude 10–12 min after the beginning of methamphetamine perfusion (E; paired t-tests for each concentration: *P < 0.05, **P < 0.01, ***P < 0.001). Methamphetamine effects on IPSC kinetics were examined by measuring time to peak (F) and width at 50% maximum amplitude (G). The slowing of IPSC kinetics was progressively enhanced by higher methamphetamine concentrations and was modeled well by a single exponential increase (red dashed lines).
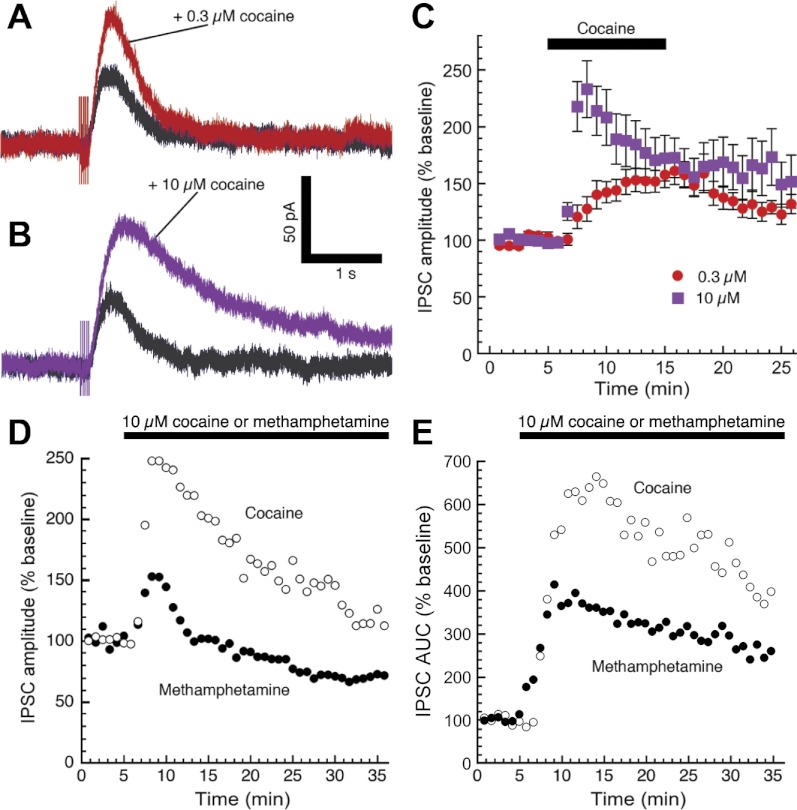
We next examined the effects of cocaine, which is an abused drug that inhibits DAT but (unlike methamphetamine) is not a DAT substrate. A low concentration of cocaine (0.3 μM) produced similar effects to the low concentration of methamphetamine, enhancing IPSC amplitudes while producing a minor slowing in kinetics (Fig. 2A). However, a high concentration of cocaine (10 μM) produced a greater enhancement of IPSC peak amplitudes while substantially prolonging IPSC kinetics (Fig. 2, B and C). A separate experiment was performed to better mimic the prolonged exposure to psychostimulants that occurs in the brains of drug abusers. Thirty-minute perfusions of either cocaine or methamphetamine (10 μM) indicated that cocaine was more effective than methamphetamine at enhancing the peak amplitude (Fig. 2D) and area under the curve (Fig. 2E) of dopamine IPSCs. The prolonged exposure substantially decreased IPSC amplitudes while only modestly decreasing total charge transfer. As a DAT substrate, methamphetamine can gain access to the inside of the cell, where it can decrease vesicular dopamine content by acting as a VMAT2 substrate and/or disrupting vesicular pH gradients (Sulzer 2011). It is likely that the differences in the effects of methamphetamine vs. cocaine on the kinetics of the dopamine IPSC are due to intracellular actions that cocaine does not share.
Fig. 2.
Unlike methamphetamine, high concentrations of cocaine increase dopamine IPSC peak amplitudes. Perfusion of 0.3 μM cocaine [a dopamine transporter (DAT) inhibitor but not a DAT substrate] enhanced the amplitude and slightly prolonged the duration of dopamine IPSCs (A), similar to the effects of 0.3 μM methamphetamine shown in Fig. 1A. However, IPSC amplitudes were further elevated by high concentrations of cocaine (B and C; n = 9–13 cells from 6 mice), whereas the kinetics of the IPSC were substantially prolonged. In a separate experiment, either cocaine or methamphetamine (10 μM) was continuously perfused for 30 min. Summarized data indicate that during prolonged application of methamphetamine, the peak amplitude of dopamine IPSCs decreased (D) to a greater extent than the total charge transferred (area under the curve, E). Differences in the effects of the two psychostimulants were already evident during the first few minutes of perfusion. Error bars are omitted for clarity (n = 4–6 cells from 4–6 mice).
While conducting the experiments for Figs. 1 and 2, we observed that only the highest concentration of methamphetamine (10 μM) produced a sufficient rise in tonic levels of extracellular dopamine to produce a significant outward change in holding current (17.6 ± 11.0 pA, n = 7, data not shown). This increase in steady-state extracellular levels of dopamine could decrease dopamine-mediated currents by occluding D2 receptors or producing postsynaptic receptor desensitization. To investigate this possibility, we coupled episodic, maximally effective iontophoresis of dopamine with bath perfusion of methamphetamine (Fig. 3A). Methamphetamine (10 μM) did produce a statistically significant decrease in maximal D2 receptor-mediated currents (Fig. 3B). However, currents were only reduced by an average of 12.2%. This suggests that (under these recording conditions) the combined effects of occlusion and desensitization do not substantially decrease the maximum possible dopamine-mediated GIRK current.
Fig. 3.
Bath perfusion of methamphetamine produces a slight reduction in maximal response to D2 receptor activation. A brief, maximal activation of D2 receptors was produced by a 2- to 3-s iontophoresis of exogenous dopamine (A; 1 M), repeated once every 5 min. Methamphetamine (10 μM) was bath perfused for 15 min. Summarized data (B; n = 8 cells from 6 mice) suggest that methamphetamine produced a significant but small (10–15%) reduction of maximal D2 receptor-mediated currents [1-way repeated-measures ANOVA (P = 0.042) followed by Dunnett's post hoc test: *P < 0.05, **P < 0.01].
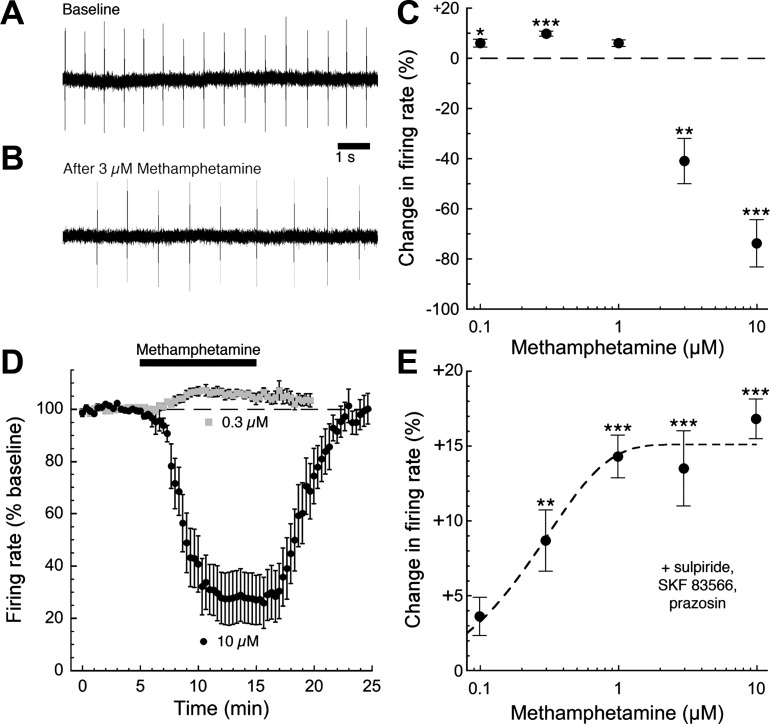
Having determined the effects of methamphetamine on electrically evoked dopamine-mediated neurotransmission, we next examined the effects of methamphetamine on basal firing of dopamine neurons. Dopamine neurons in brain slices lack active glutamatergic input and exhibit a slow, tonic pacemaker firing pattern produced by intrinsic conductances. We obtained loose cell-attached recordings of dopamine neurons in slices of mouse midbrain to examine pacemaker firing without disrupting the cell membrane and dialyzing intracellular contents (Fig. 4A). As expected, bath perfusion of high concentrations of methamphetamine (3–10 μM) slowed the firing rate of dopamine neurons, suggesting that methamphetamine increases tonic levels of extracellular dopamine and indirectly activates inhibitory somatodendritic D2 autoreceptors (Fig. 4B). Interestingly, low concentrations of methamphetamine (0.1–0.3 μM) had the opposite effect, producing a subtle and unexpected increase in firing rate (Fig. 4, C and D). We next examined firing in the presence of dopamine receptor antagonists to isolate the excitatory effect of methamphetamine. Under these conditions, methamphetamine produced a concentration-dependent increase in firing that occurred at concentrations an order of magnitude lower than the indirect D2 receptor-mediated inhibition (Fig. 4E).
Fig. 4.
Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron firing rate. The rate of dopamine neuron pacemaker firing was monitored using the loose cell-attached patch technique (A). As expected, bath perfusion of high concentrations of methamphetamine (3–10 μM) reduced the firing rate of dopamine neurons, a consequence of increased extracellular concentrations of dopamine producing an activation of somatodendritic D2 autoreceptors (B). Surprisingly, however, lower concentrations of methamphetamine did not inhibit cell firing but actually produced a modest but significant increase in firing rate (C; n = 5–15 cells from 3–7 mice). D shows the time course of the excitatory and inhibitory effects of methamphetamine on firing rate (n = 12–15 cells from 6–7 mice). To eliminate the contribution of dopamine receptors to the effects of methamphetamine, we repeated the same experiment in the presence of the dopamine D2-type receptor antagonist sulpiride (200–500 nM), the D1/D5 receptor antagonist SKF 83566 (500 nM), and the α1-adrenergic receptor antagonist prazosin (100 nM). Under these conditions, bath perfusion of methamphetamine produced a concentration-dependent increase in pacemaker firing rate that was nearly maximal at 1 μM (E; n = 5–11 cells from 3–5 mice; 1-way ANOVA followed by Dunnett's post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001).
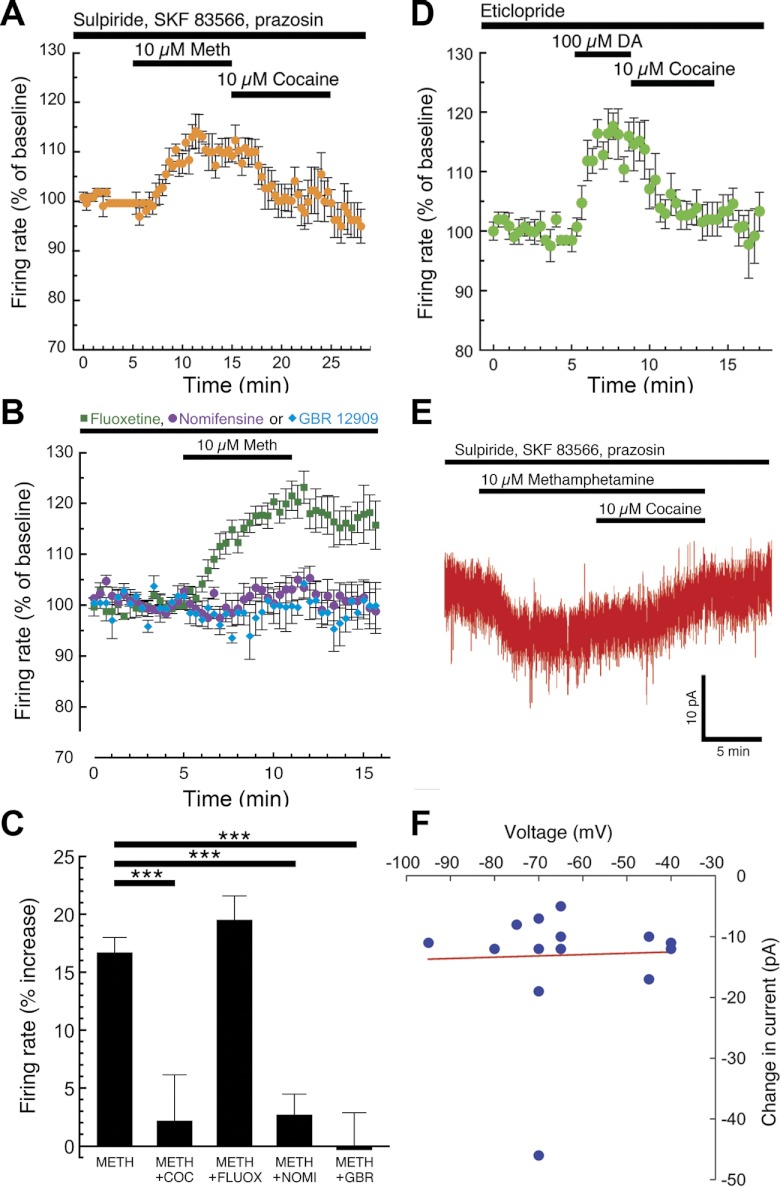
A DAT-mediated excitatory conductance has been reported previously in expression systems and cell culture, but never before in an ex vivo brain slice preparation (Erreger et al. 2008; Goodwin et al. 2009; Ingram et al. 2002; Sonders et al. 1997). Thus we next tested the hypothesis that the methamphetamine-induced excitation of dopamine neurons in brain slices was due to a DAT-mediated conductance. We examined pacemaker firing of dopamine neurons in the presence of α1-adrenergic and dopamine receptor (D1–D5) blockers. Under these conditions, dopamine neuron firing rate was increased by methamphetamine (10 μM) and was rapidly reversed by bath perfusion of cocaine (10 μM; Fig. 5A). The methamphetamine-induced increase in firing was also blocked by preincubation with the more selective DAT inhibitors GBR 12909 (1 μM) and nomifensine (1–3 μM) but not the serotonin transporter inhibitor fluoxetine (800 nM), suggesting that methamphetamine-induced excitation is mediated through DAT (Fig. 5, B and C). In the presence of the D2-type receptor antagonist eticlopride, firing rate was also increased by the endogenous DAT substrate dopamine, an effect that was also quickly reversed by cocaine (Fig. 5D). We next sought to determine the voltage dependence of the methamphetamine-induced excitation by performing voltage-clamp experiments using the gramicidin perforated-patch technique. At a variety of negative holding voltages, bath perfusion of methamphetamine consistently produced an inward current of ∼10 pA that was reversed by cocaine (Fig. 5, E and F). This suggests that the DAT-mediated excitation is not strongly voltage dependent and is not produced by inhibition of a potassium conductance.
Fig. 5.
DAT inhibitors block the dopamine neuron excitation produced by DAT substrates. Pacemaker firing of dopamine neurons was recorded in the presence of dopamine receptor antagonists (200–500 nM sulpiride, 500 nM SKF 83566, and 100 nM prazosin). Bath perfusion of methamphetamine (10 μM) increased firing rate, an effect that was quickly reversed by the nonselective DAT inhibitor cocaine (A; n = 5 cells from 2 mice). The methamphetamine-induced increase in firing was blocked by preincubation with either the norepinephrine/dopamine transporter inhibitor nomifensine (1–3 μM) or the selective DAT inhibitor GBR 12909 (1 μM), but not by the serotonin transporter inhibitor fluoxetine (800 nM; B). Summarized data are illustrated in C (1-way ANOVA followed by Dunnett's post hoc test: ***P < 0.001). In the presence of the D2 receptor antagonist eticlopride (100 nM), bath perfusion of the endogenous DAT substrate dopamine (100 μM) also increased dopamine neuron firing rate, an effect that was also rapidly reversed by cocaine (D; n = 6 cells from 3 mice). To investigate the voltage dependence of the DAT-mediated excitation, we obtained whole cell voltage-clamp recordings using the gramicidin perforated-patch technique in the presence of pharmacological blockers of dopamine receptors (sulpiride, SKF 83566, and prazosin). Under these conditions, bath perfusion of methamphetamine (10 μM) consistently produced a small inward current that was reversed by the DAT inhibitor cocaine (E). Methamphetamine produced inward currents at holding voltages ranging from −40 to −95 mV, suggesting that DAT-mediated excitation is not voltage dependent at subthreshold voltages (F). Each data point represents an individual perforated-patch experiment performed at the indicated holding voltage.
DISCUSSION
Acute effects of low concentrations of methamphetamine.
Here we report that the acute effects of methamphetamine on dopamine neurons are multifaceted and concentration dependent. Low concentrations of methamphetamine directly increase dopamine neuron firing through a DAT-mediated excitation that occurs independently of dopamine receptor activation. Low concentrations also enhance the amplitude of dopamine-mediated IPSCs. The fact that both of these effects peak at high nanomolar concentrations suggests that DAT, which has a reported affinity for methamphetamine of 470 nM in mouse (Han and Gu 2006), could be a common molecular target. Low concentrations of methamphetamine do not produce sufficient D2 receptor activation to counteract the increase in pacemaker firing rate produced by the DAT-mediated excitatory conductance. A recently published electrochemical study (Ford et al. 2010) suggests that the time course of dopamine release and the effects of DAT inhibition in the striatum and the VTA are remarkably similar. Thus a methamphetamine-induced increase in firing at the soma could work in synergy with DAT inhibition at the terminal to enhance dopamine-mediated synaptic transmission. This subsequently would enhance the phasic dopamine signals that have been implicated in error prediction and reward learning (Floresco et al. 2003; Phillips et al. 2003; Schultz 2006; Zweifel et al. 2009) and could conceivably explain the cognitive enhancement and increased focus produced by clinical concentrations of DAT substrates. Based on previous pharmacokinetic studies in mice, the low range of methamphetamine doses that cause locomotor stimulation (0.5–1 mg/kg) produce peak brain concentrations of a few micromolar with a half-time of ∼1 h (Brien et al. 1978; Kamens et al. 2005; Zombeck et al. 2009). Thus the concentrations of methamphetamine that increase dopamine neuron firing and enhance dopamine-mediated IPSCs are at or below the levels that produce a gross increase in forward locomotion.
Acute effects of high concentrations of methamphetamine.
In addition to its effects on DAT, methamphetamine is an intracellular substrate for the vesicular monoamine transporter VMAT2 (Chu et al. 2010; Sulzer et al. 1993). Once inside the cell, methamphetamine displaces vesicular dopamine with a reported IC50 of 13.8 μM in vesicles from rat striatum (Sulzer et al. 2005; Yasumoto et al. 2009). Elevated levels of cytoplasmic dopamine promote reverse transport through DAT (Sulzer et al. 2005), which elevates standing (or tonic) extracellular dopamine levels in both terminal fields and somatodendritic regions (Di Chiara and Imperato 1988; Kalivas and Duffy 1988). Increased extracellular dopamine can activate somatodendritic D2 receptors, inhibiting neuronal activity by activating GIRK channels (Beckstead et al. 2004; Lacey et al. 1987). This mechanism is likely responsible for the decreased dopamine neuron firing we observed at higher methamphetamine concentrations. Pacemaker firing completely ceased in 2 of 11 neurons exposed to 3 μM methamphetamine and in 9 of 15 neurons exposed to 10 μM methamphetamine. Exposure to 10 μM methamphetamine also produced sufficient dopamine release to induce a modest but significant outward current in voltage-clamp experiments.
Although 10 μM methamphetamine appeared to increase tonic levels of dopamine, it inhibited the peak amplitude and slowed the kinetics of electrically evoked dopamine IPSCs. These effects most likely occurred through a combination of three mechanisms. First, sustained activation could desensitize postsynaptic D2 receptors or their G protein-mediated signaling pathway. Second, increased extracellular dopamine could occlude D2 receptor activation. Third, methamphetamine could decrease dopamine vesicular content. The concentration of 10 μM methamphetamine undoubtedly produces occlusion to some extent, as suggested by the outward current observed in voltage-clamp experiments. However, the outward current averaged 17.6 pA, or only 6.5% of the average maximal current produced during the iontophoresis experiment. Exposure to 10 μM cocaine also produced a comparable outward current (15.7 ± 1.7 pA, n = 7, not shown) but more effectively enhanced IPSC amplitudes, suggesting that occlusion cannot explain the differences between the effects of cocaine and methamphetamine. Furthermore, the iontophoresis experiment indicated that the combined reduction produced by occlusion and desensitization was just 12%. Therefore, it is likely that the decrease in vesicular content produced by methamphetamine is responsible for the disparities between the two psychostimulants. Methamphetamine and similar compounds reduce quantal content of dopamine release, probably by acting as VMAT2 substrates and/or disrupting vesicular pH gradients (Sulzer 2011). This could decrease synaptic dopamine concentrations, slowing the synchronous receptor activation produced by evoked release (Ford et al. 2009) and decreasing IPSC amplitudes to a greater extent than slower currents induced by iontophoresis. A rapid disruption of vesicular content is consistent with the rapid decrease in stimulated synaptic overflow by d-amphetamine reported in striatal brain slices (Jones et al. 1998). Thus, although all three mechanisms probably contribute to the effects of methamphetamine, decreased vesicular dopamine content probably contributes most to the reduction in IPSC amplitude.
A fourth plausible mechanism that we do not believe contributes substantially to our results is the decrease in DAT function and/or surface expression produced by DAT substrates (Gulley and Zahniser 2003; Sandoval et al. 2001). Methamphetamine application in this study was typically limited to 10 min, and we made no subsequent attempt to completely wash methamphetamine out of our slices. Thus any analysis of dopamine clearance would be confounded by the continued presence of the uptake inhibitor and would be uninterpretable. Although we do not know how sensitive DAT-mediated excitation is to changes in surface expression, there does not appear to be any tachyphylaxis represented in Fig. 5, A and B, but there may be a small bit indicated in Fig. 5E.
DAT-mediated excitation of dopamine neurons.
Our findings suggest that methamphetamine produces an excitatory conductance in dopamine neurons that is mediated through DAT. Importantly, this excitation can be observed at concentrations an order of magnitude lower than mass inhibition through D2 receptor activation and thus can be observed even when D2 receptors are not blocked. DAT-mediated excitation may contribute to the effects of low methamphetamine doses that are observed clinically (such as increased focus) or in the early stages of drug abuse (such as euphoria and reinforcement). Previous studies suggest that other DAT substrates can produce similar effects. Seutin et al. (1991) reported in the text, but did not show, that amphetamine increases the firing of dopamine neurons in brain slices in the presence of a D2 receptor antagonist. Data obtained using expression systems and cell culture later identified DAT-mediated excitation produced by dopamine, d-amphetamine, and methamphetamine, including one report in cultured dopamine neurons (Erreger et al. 2008; Goodwin et al. 2009; Ingram et al. 2002; Sonders et al. 1997). In vivo, amphetamine can also excite dopamine neurons through an α1-adrenergic receptor-mediated circuit mechanism (Shi et al. 2000, 2007). Like amphetamine, methamphetamine is a substrate for both DAT and VMAT2 and can increase extracellular dopamine concentrations. The two compounds do differ somewhat in their abuse liability as well as their central effects (Goodwin et al. 2009; National Institute on Drug Abuse 2006; Shoblock et al. 2003a, 2003b).
In summary, the acute effects of methamphetamine on dopamine neuron excitability and output are concentration dependent and bidirectional. At low concentrations, methamphetamine increases dopamine neuron firing and enhances stimulated dopamine neurotransmission. At higher concentrations, both effects are reversed. Movement of dopamine out of the vesicles and into the extracellular space slows dopamine neuron firing while decreasing the peak amplitude of electrically evoked dopamine neurotransmission. These observations could explain the therapeutic window produced by low concentrations of methamphetamine. DAT-mediated excitation of dopamine neuron firing could work in concert with enhanced neurotransmission in terminal regions to produce the beneficial behavioral effects (i.e., increased attention and cognition) observed with low doses of DAT substrates.
GRANTS
This work was funded by National Institute of Drug Abuse Grant K01 DA21699.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.Y.B. and M.J.B. performed experiments; S.Y.B. and M.J.B. analyzed data; S.Y.B. and M.J.B. interpreted results of experiments; S.Y.B. and M.J.B. drafted manuscript; S.Y.B. and M.J.B. approved final version of manuscript; M.J.B. conception and design of research; M.J.B. prepared figures; M.J.B. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge Drs. Amanda L. Sharpe and Carlos A. Paladini for helpful contributions.
REFERENCES
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PEM, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology 34: 1926–1935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939–946, 2004 [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Phillips TJ. Mice selectively bred for high- or low-alcohol-induced locomotion exhibit differences in dopamine neuron function. J Pharmacol Exp Ther 329: 342–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JF, Kitney JC, Peachey JE, Rogers BJ. Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res Commun Chem Pathol Pharmacol 22: 313–328, 1978 [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13: 569–574, 1996 [DOI] [PubMed] [Google Scholar]
- Chen J, Marmur R, Pulles A, Paredes W, Gardner EL. Ventral tegmental microinjection of delta 9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain Res 621: 65–70, 1993 [DOI] [PubMed] [Google Scholar]
- Chu PW, Hadlock GC, Vieira-Brock P, Stout K, Hanson GR, Fleckenstein AE. Methamphetamine alters vesicular monoamine transporter-2 function and potassium-stimulated dopamine release. J Neurochem 115: 325–332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Grewer C, Javitch JA, Galli A. Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function. J Neurosci 28: 976–989, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973, 2003 [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci 30: 6975–6983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26: 2788–2797, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Phillips PE, Williams JT. The time course of dopamine transmission in the ventral tegmental area. J Neurosci 29: 13344–13352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem 284:2978–2989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur J Pharmacol 479: 139–152, 2003 [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci 5: 971–978, 2002 [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18: 1979–1986, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem 50: 1498–1504, 1988 [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav 4: 110–125, 2005 [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci 12: 2623–2632, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392: 397–416, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse NIDA Research Report: Methamphetamine Abuse and Addition (NIH Publication No. 06-4210) Bethesda, MD: National Institute on Drug Abuse, 2006, p. 1–8 [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618, 2003 [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett 348: 61–64, 1992 [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci 21: 1413–1419, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57: 87–115, 2006 [DOI] [PubMed] [Google Scholar]
- Seutin V, Verbanck P, Massotte L, Dresse A. Acute amphetamine-induced subsensitivity of A10 dopamine autoreceptors in vitro. Brain Res 558: 141–144, 1991 [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of d-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci 20: 3504–3511, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Zhang XY, Pun CL, Bunney BS. Clozapine blocks d-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology 32: 1922–1928, 2007 [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 170: 150–156, 2003a [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 165: 359–369, 2003b [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci 17: 960–974, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69: 628–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem 60: 527–535, 1993 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75: 406–433, 2005 [DOI] [PubMed] [Google Scholar]
- Yasumoto S, Tamura K, Karasawa J, Hasegawa R, Ikeda K, Yamamoto T, Yamamoto H. Inhibitory effect of selective serotonin reuptake inhibitors on the vesicular monoamine transporter 2. Neurosci Lett 454: 229–232, 2009 [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 201: 589–599, 2009 [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 106: 7281–7288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]