Abstract
A major pathway for B cell acquisition of lymph-borne particulate antigens relies on antigen capture by subcapsular sinus macrophages of the lymph node. Here we tested whether this mechanism is also important for humoral immunity to inactivated influenza virus. By multiple approaches, including multiphoton intravital imaging, we found that antigen capture by sinus-lining macrophages was important for limiting the systemic spread of virus but not for the generation of influenza-specific humoral immunity. Instead, we found that dendritic cells residing in the lymph node medulla use the lectin receptor SIGN-R1 to capture lymph-borne influenza virus and promote humoral immunity. Thus, our results have important implications for the generation of durable humoral immunity to viral pathogens through vaccination.
Vaccines can protect against viral and bacterial pathogens1. An important component of an effective vaccine against a virus such as influenza is the induction of a strong neutralizing antibody response2. Therefore, understanding how the immune system first recognizes the vaccine and processes it for durable humoral immunity is of fundamental importance. Vaccines, which are administered intra-muscularly or subcutaneously, drain into the regional lymph nodes, where circulating T cells and B cells scan for their cognate antigen3. The highly ordered architecture of the lymph node maximizes the chance that a lymphocyte will encounter cognate antigen. B cells acquire antigen from specialized stromal cells called ‘follicular dendritic cells’ (FDCs)4, which are a chief source of B cell chemokines and survival factors5. These cells promote the formation of germinal centers, where B cells differentiate into memory and antibody- producing cells6.
Published imaging studies have provided insight into how B cells acquire antigen7–11. Particulate antigen and large immune complexes are captured from the lymph by subcapsular sinus macrophages (SSMs)7,8,10,12. Antigens are shuttled across the SSM surface to the underlying B cell compartment, where cognate B cells bind the antigen and migrate to the outer follicular zone for presentation of the antigen to T cells. Alternatively, immune complexes that are bound by complement C3 are captured by naive B cells through the CD21–CD35 receptor8,11 and are subsequently transported to FDCs. Notably, small soluble antigens access the B cell compartment via conduits11,13 or gaps in the floor of the subcapsular sinus14.
Macrophages lining the medulla also filter draining lymph. Medullary macrophages are phenotypically similar to SSMs but are unique in their expression of the surface marker F4/80 (refs. 15,16). Although their role in the capture of B cell antigen is less well known, medullary macrophages are more phagocytic than are SSMs16,17 and are phenotypically similar to SIGN-R1+F4/80+ marginal-zone macrophages of the spleen18. SIGN-R1 is a C-type lectin that shares homology with the human dendritic cell (DC)-specific intercellular adhesion molecule DC-SIGN19. SIGN-R1 is expressed by macrophages in the marginal zone of the spleen and medullary region of lymph nodes and is known to bind carbohydrate structures such as dextran20 and capture pathogens from the blood such as yeast21 and encapsulated bacteria such as Streptococcus pneumoniae22. In addition to their well-described role in the uptake and processing of antigen for presentation to T cells, DCs are possible transporters of B cell antigen9,23–25. A subset of CD11b+ DCs with mannose receptor–binding activity (CR-Fc+ cells) has been found to localize in splenic follicles, which suggests that they may transfer antigen to B cells24.
We sought to determine how a model influenza vaccine is handled in the draining lymph node and whether sinus-lining macrophages are required for humoral immunity. We found that virus was opsonized by mannan-binding lectin (MBL) in lymph and was rapidly endocytosed by SSMs and medullary macrophages. Notably, macrophages were not required for an effective humoral response. Instead, we found that capture of influenza virus strain A/PR/8/34 (PR8) by DCs residing in the lymph node medulla was required. This capture was SIGN-R1 dependent.
RESULTS
Sinus-lining macrophages capture lymph-borne influenza virus
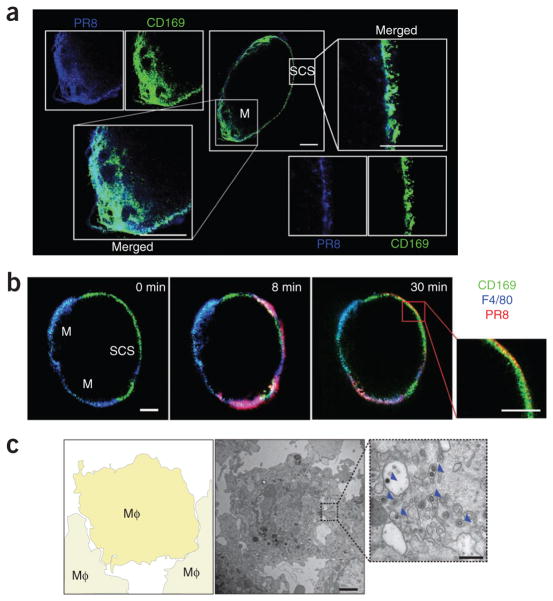
To identify the mechanism by which lymph-borne influenza virus is captured, we injected mice in the footpad with fluorescence-labeled PR8 inactivated by ultraviolet irradiation. Cryosections of popliteal lymph nodes showed localization of PR8 together with CD169+ SSMs and medullary macrophages15,16 within 30 min of injection (Fig. 1a). We confirmed rapid uptake of virus in real time by multiphoton intra-vital microscopy (MP-IVM; Fig. 1b and Supplementary Movie 1). Electron microscopy of the subcapsular sinus confirmed that PR8 was indeed internalized by resident macrophages (Fig. 1c and Supplementary Fig. 1). Thus, as reported before, influenza virus was captured from draining lymph by SSMs but, in contrast to published studies of vesicular stomatis virus7, the influenza virus was rapidly internalized.
Figure 1.
Lymph-borne influenza virus is captured by macrophages in the subcapsular sinus and medulla of the draining lymph node. (a) Confocal microscopy of the localization of PR8 (blue) in the popliteal lymph node 30 min after footpad injection, in a lymph node cryosection stained with anti-CD169 (green) to identify medullary (M) and subcapsular sinus (SCS) macrophages. Scale bars, 200 μm. Results are representative of four independent experiments with four mice. (b) MP-IVM of the entry of PR8 (red) into the popliteal lymph node after pretreatment with anti-CD169 (green; subcapsular sinus and medulla) and anti-F4/80 (blue; medulla). Numbers in top right corners indicate time after virus injection. Scale bars, 200 μm. Additional images, Supplementary Movie 1. Results are representative of six independent experiments with six mice. (c) Transmission electron microscopy (middle, right) of PR8 in the subcapsular sinus of a popliteal lymph node 30 min after injection. Left, diagram of middle image (boxed area is enlarged at right). Arrowheads (right) indicate phagocytosed virus particles. Scale bars, 2 μm (middle) or 500 nm (right). Mφ, macrophage. Results are representative of three independent experiments with three mice.
Macrophages are dispensable for humoral immunity to influenza
Given published studies showing that MBL binds influenza virus26 and our own work showing direct binding of mouse MBL to PR8 (Supplementary Fig. 2a–c), we examined the capture of PR8 by lymph node macrophages in MBL-deficient mice (Mbl2−/−; called ‘Mbl−/−’ here)27. Imaging by MP-IVM demonstrated that virus drained into the lymph nodes in Mbl−/− mice, but in contrast to results obtained with control mice, it was not retained in the subcapsular sinus region (Supplementary Fig. 2d). This finding indicated that opsonization of PR8 by MBL was required for capture by SSMs. To determine whether this population was important for eliciting humoral immunity to influenza, we evaluated the antibody response to PR8 after immunization of Mbl−/− mice. We found that Mbl−/− mice responded in a manner similar to that of wild-type mice after immunization with PR8 (Supplementary Fig. 2e–g), which suggested that virus capture by SSMs was not required for the humoral response to PR8. We also evaluated the capture of PR8 by SSMs using a mannose- functionalized dendrimer, which blocks the binding of MBL to influenza virus (Supplementary Fig. 2b,c). The administration of mannose- dendrimer 1 h before injection of PR8 resulted in a pattern of uptake similar to that of Mbl−/− mice, in which virus rapidly associated with medullary macrophages in the draining lymph nodes (Fig. 2a–c, Supplementary Fig. 2h and Supplementary Movie 2). Notably, blocking virus uptake by SSMs with dendrimer resulted in higher titers of immunoglobulin M (IgM) but had no effect on the titers of IgG2a and IgG2b antibody to PR8 (anti-PR8; Fig. 2d–f). Similarly, enzyme-linked immunospot analysis of popliteal lymph nodes from dendrimer-treated mice showed more antibody-secreting cells, consistent with the higher total immunoglobulin serum concentrations (Fig. 2g). Notably, mannose-dendrimer did not impart a general adjuvant effect, as its administration did not alter the antibody response to phycoerythrin immune complexes relative to the response obtained with antigen alone (Supplementary Fig. 2i). Additionally, naive B cells did not seem to transport PR8 as immune complexes in this model (Supplementary Fig. 2j). Moreover, the virus response remained localized to the draining lymph node despite the lack of virus binding by SSMs (Fig. 2h,i).
Figure 2.
SSMs are not required for humoral immunity to influenza virus. (a–c) MP-IVM of PR8 (red) in a popliteal lymph node after treatment with dendrimer and anti-CD169 (green) and anti-CD35 (blue). Numbers in top right corners indicate time after virus injection. Scale bar, 200 μm. Results are representative of four independent experiments with four mice. (d–f) Serum titers of PR8-specific IgG2a (d), IgG2b (e) or IgM (f) at 10 d in mice pretreated with PR8 alone (PR8) or dendrimer and PR8 (Den + PR8) or injected with PBS alone (PBS). Results are representative of three experiments with five mice per group. (g–i) Antibody-secreting cells (ASC) in the popliteal lymph nodes (LN; g), lumbar lymph nodes (h) and spleen (per 1 × 106 spleen cells; i) at 10 d after injection as described in d–f. *P = 0.005 (unpaired t-test). Results are representative of three experiments with five mice per group. In d–i, each symbol represents an individual mouse; small horizontal lines indicate the mean. (j) Frequency of B1–8 B cells (B220+IgMa+ immunoglobulin-λ1-positive (Igλ1+) cells) that bound NP-conjugated PR8 in dendrimer-treated mice at 2 h and 8 h after injection of virus. Each symbol represents an individual mouse. Results are from one experiment (mean of two mice per group at each time point).
In published reports, SSMs have been shown to capture viral and particulate antigens and shuttle these to B cells in the underlying follicle7,10. To determine whether the capture of virus by SSMs was important for uptake by PR8-specific B cells, we pretreated B1–8 mice (which have a targeted knock-in replacement of their Igh locus with a nitrophenyl (NP)-specific heavy-chain gene) with dendrimer and injected them with NP-conjugated-labeled PR8. Flow cytometry demonstrated a similar extent of NP-virus uptake by hapten-specific B cells in dendrimer-treated and untreated mice (Fig. 2j).
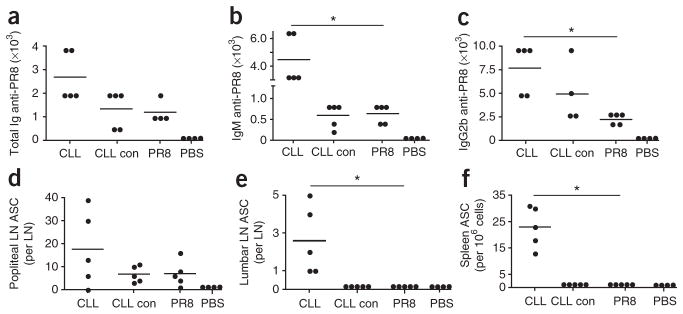
To determine whether the antibody response to influenza virus was dependent on medullary macrophages, we injected mice with clodronate liposomes (CLLs), which eliminate macrophages from the lymphoid compartment7. Using confocal microscopy to analyze lymph nodes at day 5 after CLL injection, we confirmed that mice were depleted of SSMs as well as medullary macrophages (Supplementary Fig. 3a–d). Notably, we found that a more robust humoral response developed in CLL-treated mice than in mice that received PR8 alone (Fig. 3a–c). In contrast to control mice, in which the response remained localized to draining lymph nodes, CLL-treated mice had virus-specific antibody-secreting cells in downstream lumbar lymph nodes and the spleen (Fig. 3d–f). Together, these results indicate that SSMs and medullary macrophages function to contain PR8 within the draining lymph node but are dispensable for humoral immunity to inactivated influenza virus.
Figure 3.
Medullary macrophages are not required for humoral immunity to influenza virus. (a–c) Serum titers of PR8-specific total immunoglobulin (Ig; a), IgM (b) or IgG2b (c) at 10 d after injection of PR8 into mice treated with CLLs (CLL), empty liposomes (CLL con) or PR8 alone (PR8) or injected with PBS only (PBS). (d–f) Antibody-secreting cells in the popliteal lymph nodes (d), lumbar lymph nodes (e) and spleen (per 1 × 106 spleen cells; f) at 10 d after injection of PR8 into mice treated as described in a–c. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P = 0.005 (unpaired t-test). Results are representative of three experiments with five mice per group.
Lymph node DCs capture influenza in the medulla
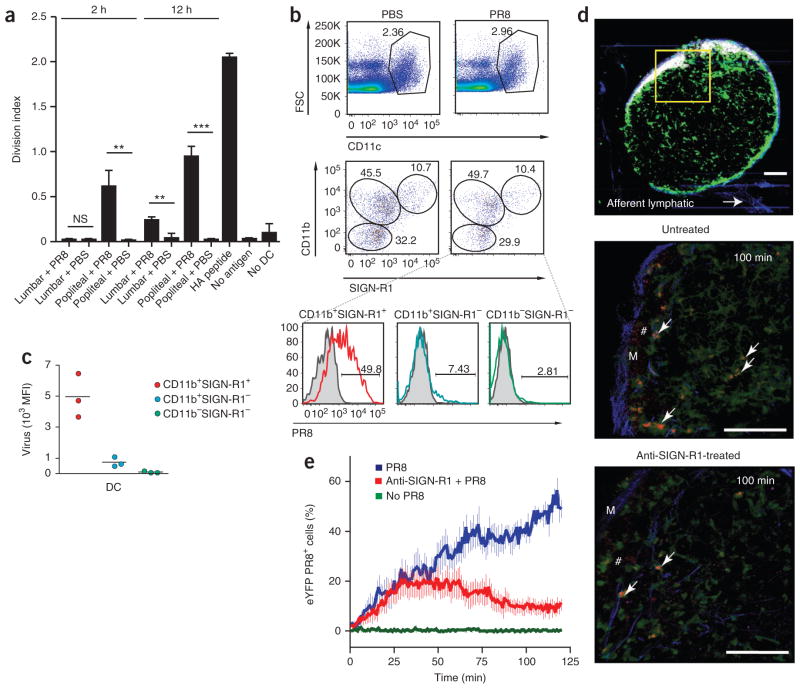
The finding that macrophages were not required for an efficient humoral response to PR8 suggested that other cell types, such as DCs, might be involved in handling PR8 and possibly delivering virus to the B cell compartment28,29. To determine whether DCs capture and process PR8, we isolated lymph node CD11chi cells at various times after PR8 immunization and cultured them together with naive hemagglutinin-specific CD4+ T cells30. DCs from the draining lymph nodes of PR8-immunized mice were indeed able to stimulate proliferation of the virus-specific T cells as early as 2 h after injection and became even more stimulatory by 12 h after injection (Fig. 4a). These results demonstrate that DCs have access to viral antigen at very early time points after immunization.
Figure 4.
CD11b+SIGN-R1+ lymph node–resident DCs bind lymph-borne PR8 in the medulla. (a) Proliferation of naive hemagglutinin-specific CD4+ T cells labeled with the cytosolic dye CFSE and cultured alone (No DC), with DCs purified from the popliteal or lumbar lymph nodes at 2 h and 12 h after PR8 or PBS injection or with DCs left untreated (No antigen) or pulsed with hemagglutinin (HA) peptide, assessed by CFSE dilution after 72 h (division index). *P < 0.05, **P < 0.005 and ***P < 0.0005 (unpaired t-test). Results are representative of four independent experiments (error bars, s.d. of three mice). (b) Cytofluorometry of the surface expression of CD11b and SIGN-R1 (middle) by CD11c+ cells (top) in popliteal lymph nodes 2 h after injection of PBS or PR8, identifying three distinct DC populations (bottom). Numbers adjacent to outlined areas indicate percent CD11c+ cells (top row), or percent CD11b−SIGN-R1− cells (bottom left, middle row), CD11b+SIGN-R1− cells (top left, middle row) or CD11b+SIGN-R1+ cells (top right, middle row); numbers above bracketed lines (bottom) indicate DC populations that captured PR8 (solid lines) relative to those of PBS-injected mice (gray filled histograms). Results are representative of five independent experiments with five mice. (c) Virus uptake by DC subsets, presented as mean fluorescence intensity (MFI). Each symbol represents an individual mouse; small horizontal lines indicate the mean. Data are representative of three experiments with three mice per condition. (d) MP-IVM of the capture of PR8 by DCs in the medulla of popliteal lymph nodes from untreated and anti-SIGN-R1-treated mice at 100 min after virus injection (additional images, Supplementary Movie 3). Arrows indicate virus-bearing eYFP+ DCs; #, virus not bound by eYFP+ DCs. Scale bars, 200 μm (top) or 100 μm (middle and bottom). Results are representative of four independent experiments with eight mice. (e) Quantification of PR8 capture by DCs in the medulla of popliteal lymph nodes from mice left untreated (No PR8) or injected with PR8 alone (PR8) or after anti-SIGN-R1 treatment (Anti-SIGN-R1 + PR8). Results are representative of two independent experiments with two mice (untreated) or four independent experiments with four mice (PR8 and PR8 plus anti-SIGN-R1; error bars, s.e.m.).
Next, we sought to determine by flow cytometry which subset(s) of DCs captured the virus. We distinguished three distinct subsets of CD11c+ cells on the basis of expression of CD11b and SIGN-R1 (Fig. 4b). All three subsets were associated with PR8 at 2 h after injection; however, the CD11b+SIGN-R1+ DC subset seemed to have the predominant role in capturing lymph-borne virus, as ~50% of cells in this population contained PR8, whereas only a small percentage of CD11b+SIGN-R1− and CD11b−SIGN-R1− DCs contained virus (Fig. 4b). Moreover, CD11b+SIGN-R1+ DCs contained much more PR8 per cell, with 7-fold more virus than CD11b+SIGN-R1− DCs and 40-fold more virus than CD11b−SIGN-R1− DCs (Fig. 4c). Notably, injection of PR8 did not alter the abundance of CD11c+ cells in the draining lymph nodes during this 2-hour time window (PBS, 2.37% ± 0.8%; PR8, 3.06% ± 0.1%; Fig. 4b), which suggested that the virus-bearing DCs represented lymph node–resident DCs rather than newly emigrated tissue-derived DCs.
As SIGN-R1+ DCs have not been well characterized in popliteal lymph nodes, we next wanted to determine where these cells normally reside. Analysis of immunostained cryosections indicated that SIGN-R1+ cells, including SIGN-R1+CD11c+ DCs, resided almost exclusively in the medullary region of the popliteal lymph node (Supplementary Fig. 4a). These data suggest that SIGN-R1+ DCs have an important role in capturing lymph-borne influenza virus from the lymph node medulla within the first few hours after immunization.
SIGN-R1 mediates influenza binding by lymph node DCs
Published studies have determined that SIGN-R1 expressed on DCs, splenic marginal zone macrophages and medullary macrophages is a major polysaccharide receptor20,22. Given our results on the binding of MBL to influenza and our finding that SIGN-R1+ DCs bound virus in draining lymph nodes, we reasoned that SIGN-R1 might also recognize PR8. Thus, we sought to test the function of SIGN-R1 in the capture of PR8 by lymph node DCs by pretreating the cells with a SIGN-R1-specific blocking antibody (22D1)31 24 h before PR8 administration. The antibody 22D1 induces SIGN-R1 down-regulation but not the elimination of resident DCs or macrophages, as described before22. By flow cytometry, we found that treatment with 22D1 resulted in substantially less capture of PR8 by lymph node DCs (Supplementary Fig. 4b). The residual PR8 binding observed in 22D1-treated mice probably represented virus bound via other receptors, as found with SSMs or incomplete SIGN-R1 downregulation before vaccination.
Next, we used MP-IVM to evaluate whether SIGN-R1 mediates the capture of influenza virus by DCs residing in the lymph node medulla. We used CD11c–enhanced yellow fluorescent protein (CD11c-eYFP) mice, in which DCs can be imaged by MP-IVM because of their high expression of eYFP. For this, we injected CD11c-eYFP mice with fluorescence-labeled PR8 and visualized virus binding in popliteal lymph nodes. PR8 associated with eYFP+ cells (eYFP fluorescence corresponds to CD11chi DCs32) and eYFP− cells (Fig. 4d,e) within 10 min of injection, and tracking of the virus-bearing eYFP+ cells demonstrated that the binding was stable, as DCs retained virus for at least 2 h (Fig. 4e). Treatment with 22D1 decreased the percentage of DC-bound virus in the lymph node medulla from approximately 60% to 13% at 2 h after injection (Fig. 4e and Supplementary Movie 3). Together, these results suggest that lymph node–resident medullary DCs use SIGN-R1 to capture influenza virus.
DCs are required for humoral immunity to influenza virus
Next, we sought to determine whether DCs promote the humoral response to PR8 using the CD11c–diphtheria toxin receptor (CD11c-DTR) mouse model, in which conventional DCs can be ablated after administration of diphtheria toxin. We prepared bone marrow–chimeric mice using bone marrow from CD11c-DTR–transgenic or wild-type C57BL/6 (B6) mice and transferred the bone marrow into irradiated wild-type B6 mice. We allowed the chimeric mice to ‘rest’ for 6 weeks and then injected them once with diphtheria toxin to deplete CD11c+ cells from lymph node and spleen 24 h before injection with virus33. Under these conditions, conventional DCs are eliminated from spleen and lymph nodes, along with ablation of marginal-zone and metallophilic macrophages34. Evaluation of serum titers of anti-PR8 immunoglobulin at day 10 indicated a negligible total immunoglobulin and IgM response in diphtheria toxin–treated B6 mice that received CD11c-DTR bone marrow compared with that of B6 mice that received B6 bone marrow (Supplementary Fig. 5a,b). Similarly, the number of total immunoglobulin antibody–secreting cells in draining lymph nodes of diphtheria toxin–treated B6 mice that received CD11c-DTR bone marrow was almost undetectable relative to that of B6 control mice that received B6 bone marrow (Supplementary Fig. 5c). Given the normal robust T cell–independent response to influenza35,36, our finding that local IgM titers were also impaired in DC-depleted mice suggested that DCs are important not only for activating virus-specific T cells but also for eliciting the PR8-specific humoral response.
Medullary DCs carry influenza to FDC area
Expression of the ligand for the cysteine-rich domain of the mannose receptor identifies a subset of DCs that transports antigen into B cell follicles24. In our studies, staining of CD11c+ cells with a fusion protein of the cysteine-rich domain and Fc (CR-Fc) identified ~65% of SIGN-R1+ DC in untreated mice (data not shown). Notably, the abundance of this subset was unaffected by PR8 immunization, and approximately 50% of virus-bearing CD11b+SIGN-R1+ DCs bound CR-Fc compared with control staining with a mutant form (W117A) of CR-Fc that diminishes CR-Fc binding37 (Supplementary Fig. 4c). This result raised the possibility that a subset of lymph node–resident DCs involved in capturing lymph-borne PR8 may deliver virus from the medulla to B cell follicles.
Resident DCs that line the medullary sinus adjacent to medullary macrophages are thought to be relatively sessile32. To determine whether binding of virus allows DCs to gain motility, we used CD11c-eYFP mice to monitor by MP-IVM DC motility in the popliteal lymph nodes before and after immunization. For this, we pretreated CD11c-eYFP mice with anti-CD35 to identify the FDC region and imaged the lymph nodes by MP-IVM for 60 min before subcutaneous injection of fluorescence-labeled PR8. In agreement with earlier reports32, we found that DCs were relatively immobile during this time. Similarly, after PR8 injection, the virus-negative DCs remained relatively non-motile. In contrast, the motility of virus-bearing DCs was over fourfold higher than that of DCs that had not bound virus (Fig. 5a). To ascertain whether virus-bearing DCs migrated toward the FDC area, we randomly selected DCs from the medullary region adjacent to B cell follicles and tracked them over 60 min (Supplementary Fig. 6a–d). We calculated a net vector of migration for all tracks38,39. The results indicated that virus-bearing DCs migrated toward the FDC area in a nonrandom manner, whereas virus-negative DCs and DCs tracked before virus injection migrated randomly (Fig. 5b–d, Supplementary Fig. 6e,f and Supplementary Movie 4). In further support of the idea of directed migration, the virus-bearing DCs demonstrated greater displacement and a lower angle of migration than those of the other two groups (Fig. 5e,f). Thus, DCs lining the medullary sinus captured lymph-borne virus either from medullary macrophages or directly from lymph via SIGN-R1 (Supplementary Fig. 7a,b) and subsequently migrated in a directional manner toward FDCs.
Figure 5.
CD11c+ cells bind lymph-borne influenza virus in the medulla of the lymph node and increase their velocity toward the FDC area. (a) Velocity of eYFP+ DCs in the medulla of popliteal lymph nodes 60 min before (Pre-virus) and after injection of PR8, for eYFP+ DCs that bound virus (Virus+ DCs) or did not bind virus (Virus– DCs). Each symbol represents an individual cell; small horizontal lines indicate the mean. *P < 0.001 (unpaired t-test). Results are representative of four independent experiments with 200 cells per group. (b–f) Vector analysis of the net and angular displacement of DCs in the medulla of popliteal lymph nodes 60 min before and after injection of PR8. (b–d) Displacement of 50 randomly selected eYFP+ DCs per group from their point of origin (x,y = 0,0); blue lines indicate mean vector. Scale bar, 200 μm. (e,f) Summary of net displacement (e) and angular displacement (f) for the DCs described in a. Each symbol represents an individual cell; small horizontal lines indicate the mean. *P < 0.001 (unpaired t-test). Results are representative of four independent experiments with four mice (b–d) or four mice and 200 cells per group (e,f).
Humoral immunity to influenza requires SIGN-R1
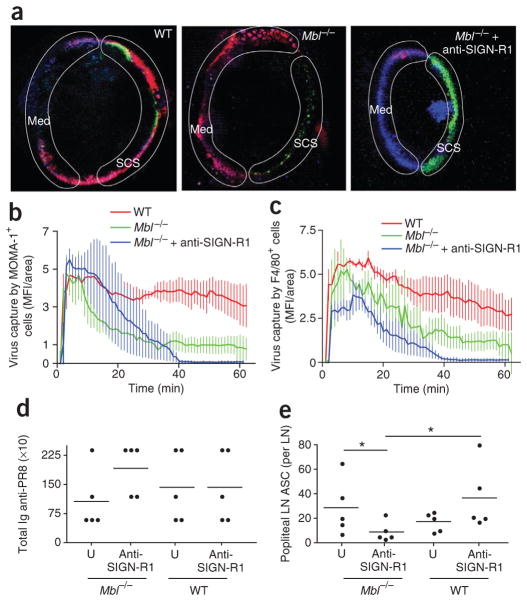
The finding that both macrophages and DCs lining the subcapsular and medullary sinuses captured PR8 via SIGN-R1 suggested an important role for this receptor in local humoral immunity. To directly determine whether SIGN-R1 is required for the humoral response to PR8, we pretreated Mbl−/− mice with anti-SIGN-R1 and then immunized them subcutaneously the next day with fluorescence-labeled PR8. As expected, PR8 uptake in Mbl−/− mice was limited to the medullary region, whereas there was negligible binding in the popliteal lymph nodes in Mbl−/− mice treated with anti-SIGN-R1 (Fig. 6a–c and Supplementary Movie 5). Thus, most uptake of lymph-borne PR8 is mediated by MBL and SIGN-R1. Characterization of the humoral response of the treated mice at day 10 after immunization showed no substantial difference in overall immunoglobulin titers among the four groups (Fig. 6d). In contrast, enzyme-linked immuno-spot analysis of the number of antibody-secreting cells in the local popliteal lymph nodes identified a negligible response in the nodes of Mbl−/− mice treated with anti-SIGN-R1 relative to that of Mbl−/− mice without treatment (untreated, 29 ± 23; anti-SIGN-R1-treated, 9 ± 7; Fig. 6e). In summary, our results have demonstrated that SIGN-R1-mediated capture of influenza virus by medullary DCs is essential for humoral immunity in draining lymph nodes.
Figure 6.
MBL is required for PR8 capture in the lymph node subcapsular sinus, whereas SIGN-R1 mediates PR8 capture in the lymph node medulla. (a) MP-IVM of the capture of PR8 (red) at 45 min in wild-type mice (WT), Mbl−/− mice and anti-SIGN-R1-treated Mbl−/− mice after pretreatment with antibody to the metallophilic macrophage–specific marker MOMA-1 (green), anti-F4/80 (blue) and anti-CD35 (8C12; blue). Results are representative of three independent experiments per treatment. (b,c) Quantification of PR8 capture by MOMA-1+ cells (b) or F4/80+ cells (c) in the subcapsular sinus or medullary area, respectively, over the first 60 min after PR8 injection. Results are representative of three independent experiments with four mice. (d) Serum titers of PR8-specific total immunoglobulin at 10 d after injection of PR8 into untreated (U) and anti-SIGN-R1-treated Mbl−/− and wild-type mice. Results are representative of QQ experiments with five mice per group. (e) Antibody-secreting cells in the popliteal lymph node 10 d after footpad injection of PR8 into untreated and anti-SIGN-R1-treated Mbl−/− and wild-type mice. *P < 0.05 (unpaired t-test). Results are representative of one experiment with five mice per group.
DISCUSSION
Published studies have identified a pathway by which SSMs of the draining lymph node and marginal zone sinus in the spleen capture particulate antigen and immune complexes for transfer to the B cell compartment. Our finding that SSMs were not required for humoral immunity to influenza virus was unexpected, given published observations that they can transfer particulate antigen from the lymph to cognate B cells7,10. One explanation for the difference is that in our model, PR8 was opsonized with MBL and possibly complement C3 (ref. 40). Thus, the combination of MBL, complement and viral surface antigen may trigger internalization by SSMs. In contrast, in published models, immune complexes alone or coated with C3 do not promote internalization but are shuttled to the underlying B cell compartment16.
SSMs and medullary macrophages have been linked to the prevention of systemic dissemination of virus such as vesicular stomatitis virus7. Similarly, we found that PR8 also reached organs beyond the lymph nodes draining the site of immunization after CLL treatment. Here we demonstrated that depletion of lymph node macrophages with CLLs resulted in an enhanced (local or systemic) humoral response to PR8. Furthermore, we showed that PR8 was not disseminated systemically when viral uptake by SSMs was selectively blocked, leaving medullary macrophage function intact. The administration of dendrimer to prevent PR8 uptake by SSMs did not affect IgG titers but did result in much higher IgM titers than those of mice immunized without dendrimer. Although dendrimer did not demonstrate a direct adjuvant effect (data not shown), inhibiting viral uptake by SSMs could result in greater antigen abundance in the medulla and consequently an improved humoral response. Together, our results have identified an essential role for medullary macrophages as scavengers of the lymphatic system. Furthermore, the viral uptake via MBL suggests a distinct role for SSMs in response to PR8.
Our findings that lymph-borne influenza virus was trapped in the lymph node medulla but that medullary macrophages were dispensable for humoral immunity to PR8 raised the possibility that another medullary cell was involved in promoting the antibody response. Our observations that DCs resided in the lymph node medulla and captured lymph-borne virus within hours of immunization suggested that lymph node–resident DCs might snare virus in the medulla and in turn promote humoral immunity. DCs in lymph nodes can be sub-categorized into two types on the basis of tissue origin41. One type is migratory DCs, which sample antigen in the periphery and transport it to draining lymph nodes; the other is lymph node–resident DCs, whose life history is restricted to one lymphoid organ. The understanding of antigen sampling by lymph node–resident DCs is relatively limited. A CD11b+ subset of lymph node–resident DCs residing in close proximity to FRCs in the paracortex has been found to sample small lymph-borne antigens arriving via conduits42,43. The CD8+ DC subset, which also resides mainly in the paracortex, seems to acquire antigen from tissue-derived DCs once they migrate into the lymph node. Our studies have identified a previously unknown population of DCs that reside in the lymph node medulla adjacent to medullary macrophages and rapidly capture lymph-borne influenza virus. These findings suggest that medullary DCs have a function complementary to that of conduit-lining DCs localized in the paracortex. As CLL treatment did not alter humoral immunity in the draining lymph node, this suggests that this subset of DCs captures PR8 independently of local macrophages, possibly by directly sampling the lymph. However, we cannot rule out the possibility of transfer of antigen from medullary macrophages to DCs.
Published work has shown not only that resident DCs localized in the lymph node paracortex can sample soluble antigen from lymph shortly after administration into the footpad but also that presentation of this early wave of antigen results in tolerance42. In contrast, DCs that migrate into the lymph node from the site of antigen administration (within 12 h) present antigen in an immunogenic manner42. In our study, sampling of lymph-borne virus by SIGN-R1+ medullary resident DCs occurred almost immediately after the arrival of antigen in the lymph node and led to an efficient humoral response. The ability of lymph node DCs to trigger activation of hemagglutinin-specific CD4+ T cells was detectable by 2 h and increased over the next 10 h. This enhanced stimulatory capacity could have been due to greater exposure time of lymph node–resident DCs to PR8 or to the arrival of migratory DCs contributing viral antigen from the periphery.
Published studies using MP-IVM have observed migrating DCs as they travel into lymph nodes and concentrate around high endothelial venules, where they transfer antigen to arriving cognate B cells9. Although those studies support the idea of a role for DCs, they do not show actual capture of antigen from draining lymph or migration into B cell follicles. In our model, up to 5% of cognate B cells specific for NP-PR8 contained virus; however, the amount of virus per cell was negligible compared with that of lymph node–resident DCs. Therefore, it remains to be elucidated if such transfer happens and, if so, whether it leads to B cell transport of viral material or results in only extrafollicular accumulation and subsequent helper response, as seen before9.
On the basis of published observations that SSMs shuttle immune complexes to naive B cells7, we expected that if DCs were involved, their role would be limited to the transfer of virus to B cells. The importance of immune-complex transfer by naive lymph node follicular B cells has been shown to occur in a CD21- and CD35-dependent manner8. That report and others have shown that splenic marginal zone B cells similarly transport immune complexes into the follicles and off-load them to FDCs by a mechanism that is complement dependent44–47. Thus, B cell transport of immune complexes coated with activated C3 is a well-characterized pathway for targeting antigen to FDCs. In contrast, we did not observe substantial uptake of virus by naive B cells even in mice in which the MBL lectin pathway was intact. Instead, we found that a population of relatively sessile DCs residing in the medullary region rapidly took up PR8 from the draining lymph via SIGN-R1. SIGN-R1 has been identified as the main receptor for Streptococcus pneumonia, as pretreatment of mice intraperitoneally with anti-SIGN-R1 results in a much lower humoral response and diminished survival after intravenous infection22,31. In our model, pretreatment of mice subcutaneously with anti-SIGN-R1 resulted in much less uptake of PR8 by popliteal lymph node–resident DCs, as shown by various techniques, including flow cytometry of lymph node DCs and in situ MP-IVM analysis of popliteal lymph nodes of CD11c-eYFP+ and Mbl−/− mice. Notably, blockade of SIGN-R1 in Mbl−/− mice resulted in a much lower local humoral response to PR8. Although the overall serum immunoglobulin titer was not lower, this result was expected, as the virus was no longer retained in the popliteal lymph nodes, and subcutaneous treatment with anti-SIGNR-1 would be unlikely to efficiently block PR8 binding in the spleen. Further support for the idea of a role for DCs in the transport of PR8 to the B cell follicles was the finding that elimination of DCs resulted in much lower serum IgM and total immunoglobulin responses to PR8. Moreover, the lower serum titers correlate with negligible PR8-specific antibody–secreting cells in the popliteal lymph nodes. Thus, as a large fraction of the IgM response is T cell independent35,36, the impaired primary response in diphtheria toxin–treated B6 chimeras given CD11c-DTR bone marrow was most probably due to blockade of viral transport into the B cell follicles.
Our results have demonstrated that SIGN-R1-mediated capture of influenza virus triggered a substantial increase in the motility of virus-bearing DCs and endowed these cells with the ability to migrate in a directed manner toward FDCs. Thus, the uptake of PR8 by resident DCs via SIGN-R1 represents a previously unknown pathway for capture of lymph-borne viral pathogens and delivery to the B cell follicles. It remains to be elucidated whether these migratory virus-containing cells are identical to the CR-Fc+ DCs found in the follicle in published studies24. Future experiments should also determine whether SIGN-R1 binds directly to specific carbohydrate ligands exposed on the inactive virus or binds indirectly after opsonization in the lymph and whether this binding is sufficient to induce directed migration. A slowing of the migration of cognate B cells has been seen before after injection of vesicular stomatitis virus into the footpad7, even in CLL-treated mice. The authors of that study7 proposed that the pan-follicular effect is due to uptake of soluble vesicular stomatitis virus protein exposed to the B cell follicle by gaps in the sinus-lining floor, as described earlier14. An alternative explanation for the effect in CLL-treated mice is that intact virus is delivered to the follicles by lymph node–resident DCs.
In summary, we found that SSMs and medullary macrophages were important for binding inactive influenza virus in draining lymph nodes and preventing the dissemination of virus to distal tissues. However, lymph node macrophages were dispensable for the development of humoral immunity to influenza virus. In contrast, uptake of influenza virus via SIGN-R1 promoted the migration of DCs from the lymph node medulla toward B cell follicles and was essential for local humoral immunity. Our results have implications for the generation of durable humoral immunity to viral pathogens through vaccination.
ONLINE METHODS
Mice
Mice were bred in house or were from Jackson Laboratories or Charles River Laboratory. The following strains were used: wild type (C57BL/6 and BALB/c), CD11c-eYFP32, B1–8 heavy-chain knock-in48, CD11c-DTR49 and Mbl−/− (ref. 27). All mice were on the C56BL/6 background. Mice were maintained in specific pathogen–free facilities at Immune Disease Institute, Dana Farber Cancer Institute or Harvard Medical School. All animal experiments were in accordance with protocols approved by the Subcommittee on Research Animals Care at Harvard Medical School and The Immune Disease Institute, and were in accordance with guidelines set by the National Institutes of Health.
Growth and purification of PR8
Influenza A/PR/8/34 was provided by A. Garcia-Sastre. Virus was grown and purified as described50. All PR8 was inactivated by 15 min of ultraviolet irradiation at 25 °C. Additional information is in the Supplementary Methods.
Antibodies
The following antibodies were from BD Bioscience: anti-immunoglobulin-λ1 (R11–153) and anti-DC (33D1). The following antibodies were from BioLegend: anti-IgMa (DS-1), anti-B220 (RA3-6B2), anti-CD11c (N418), anti-CD11b (M1/70), anti-CD8α (53-6.7), anti-CD4 (RM4–5 and GK1.5), anti-CD40 (1C10), anti-CD80 (1610-A1), anti-NK1.1 (PK136), anti-CD19 (6D5) and anti-CD3e (145-2C11). Anti-LYVE-1 (223322) was from R&D Systems, and CD68 (FA-11) and CD169 (36.112 and MOMA-1) were from AbD Serotec. Anti-CD35 (8C12), anti-SIGN-R1 (22D1), anti-CD11b (M1/70), anti-F4/80 (HB-198; American Type Culture Collection), anti-IgD (1.3–5) and anti-hemagglutinin (H36-4-5-2) were produced in-house and were purified by affinity chromatography51. Secondary antibodies, including streptavidin–Alexa Fluor 488 (S11223), streptavidin–Alexa Fluor 568 (S11226), streptavidin–Alexa Fluor 633 (S21375) and Alexa Fluor 633–conjugated anti-rat (A21094; all from Invitrogen); and DyLight 488–conjugated anti–Armenian hamster (127–485-160), indocarbocyanine-conjugated anti–Armenian hamster (127–165-160) and tetramethylrhodamine isothiocyanate–conjugated anti-human (709–026–098; all from Jackson Immunoresearch). Purified antibodies were labeled with Alexa Fluor 488 (A10235), Alexa Fluor 568 (A10238), Alexa Fluor 633 (A20170), Alexa Fluor 680 (A20172) or Pacific blue (P30012) according to the manufacturer’s instructions (Invitrogen).
Mouse pretreatment
For dendrimer studies, dendrimer was prepared as described52 and 40 μg in a volume of 10 μl was injected into the footpad at 1 h before experimentation. For depletion of lymph node macrophages, C57BL/6J mice were injected with clodronate-encapulated liposomes (CLLs) or empty liposomes (Encapsula NanoSciences) 5 d before experimentation. BALB/c mice required a further injection at 3 d before PR8 injection. For anti-SIGN-R1 treatment, 50 μg of 22D1 was injected into footpads 1–2 d before experimentation22. For labeling of FDCs, mice received 5 μg anti-CD35 intraperitoneally 24 h before MP-IVM. For labeling of SSMs in vivo, 1 μg fluorescence-labeled CD169 or 1 μg CR-Fc reagent, followed by 1 μg fluorescence- labeled secondary anti-human (A568), was injected into the footpad 3–5 h before MP-IVM.
Enzyme-linked immunosorbent and immunospot assays
Mice were immunized with 1 × 105 PR8 virions subcutaneously. At day 10, blood was collected and serum was obtained. Enzyme-linked immunosorbent assays were done as described50. For enzyme-linked immunospot assays, popliteal lymph nodes, lumbar lymph nodes and spleens were removed aseptically and disrupted by passage through 70-μm mesh, and antibody-secreting cells were quantified as described53.
Isolation and proliferative responses of hemagglutinin-specific transgenic CD4+ T cells
Lymph nodes were collected from T cell antigen receptor–transgenic mice (6.5) on a recombination-activation gene 1–deficient background with expression of an αβ T cell antigen receptor specific for influenza hemagglutinin peptide (amino acids 110–120) presented by I-Ed, and single-cell suspensions were prepared. After lysis of red blood cells by ammonium chloride–potassium bicarbonate buffer (BioWhittaker), cells were labeled for 10 min with 5 mM CFSE (carboxyfluorescein diacetate succinimidyl ester; Invitrogen) and washed thoroughly. Hemagglutinin-specific CD4+ T cells (2 × 105) were cultured for 72 h together with DCs (5 × 104) and CD4+ T cell proliferation was measured by flow cytometry (presented as division index).
Isolation of DCs
DCs were isolated from lymph node by digestion for 20 min with collagenase or Liberase (Roche Diagnostics) and DNAse I (Sigma) and were sieved to produce single-cell suspensions. In some experiments, samples were enriched for CD11c+ cells by magnetic separation with CD11c microbeads and LS25 columns (Miltenyi Biotech). Purity was typically higher than 85%.
Flow cytometry, data analysis and statistics
A FACSCanto II, FACSCalibur or FACSAria (BD Biosciences) was used for flow cytometry. Dead cells were excluded by Hoechst 33342 dye or 7-amino-actinomycin D (Invitrogen). Data were analyzed with FlowJo software (Tristar), including calculation of division indices. Prism 4 (Graphpad Software) was used for statistical analyses.
Virus injection
Anesthetized mice were injected with approximately 1 × 106 to 4 × 106 labeled PR8 virions per footpad (in a volume of 10 μl). At various time points, draining and nondraining lymph nodes were isolated for analysis of virus trafficking and PR8-specific immune responses.
Histology and microscopy
Cryosections of lymph nodes embedded in optimal cutting temperature compound (TissueTek) were prepared, then sections were washed with Hank’s balanced salt solution and incubated with anti-FcR (2.4G2) before incubation with antibody as described11. Transmission electron microscopy of lymph nodes injected with influenza virus was done as described11. MP-IVM was done as described54. For all mouse pretreatments, footpads were injected with a volume of no more than 10 μl. Additional information is available in the Supplementary Methods.
Generation of bone marrow chimeras
Recipient mice were irradiated two doses of 650 Gy, and 24 h later, 2 × 106 bone marrow cells were injected intravenously. Before injection, bone marrow was depleted of T cells, natural killer cells and B cells with anti-CD3e (145-2C11), anti-CD19 (6D5) and anti-NK1.1 (PK136) by magnetic-activated cell sorting. Recipient mice received antibiotic (Baytril) for the first 4 weeks after irradiation and were used for experimentation 6–8 weeks after receiving bone marrow.
Supplementary Material
Acknowledgments
We thank J. Nolting (Dana Farber Cancer Institute) for hemagglutinin–T cell antigen receptor–transgenic mice; M. Nussenzweig (Rockefeller University) for CD11c-eYFP mice; R. Steinman (Rockefeller University) for the anti-SIGN-R1 hybridoma; K. Rajewsky (Harvard Medical School) for B1.8 mice; W. Gerhard (Wistar Institute) for the anti-hemagglutinin hybridoma; A. Garcia-Sastre (Mount Sinai School of Medicine) for influenza A/PR/8; J. Jensenius (Aarhus University) and S. Thiel (Aarhus University) for anti-MBL and technical advice; T. Mempel for the use of microscopy facilities and technical assistance; and H. Leung, E. Marino, M. Ericsson, H. Kim and A. Gillmore for technical assistance. Supported by the US National Institutes of Health (5 R01 AI039246, 1 P01 AI078897 and 5 R01 AI067706 to M.C.C., RO1 GM62444 to M.J.C. and RO1 DK074500 to S.J.T.) and the Seventh Framework Programme of the European Union (Marie Curie International Outgoing Fellowship 220044 to S.F.G.).
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS
S.J.T. and M.C.C. directed the study, designed experiments, analyzed and interpreted results and wrote the manuscript; S.F.G., V.L.-K., M.P.K., L.A.P., S.E.D. and Y.-A.K. designed experiments, analyzed and interpreted results; M.J.C. prepared dendrimer; and L.M.-P. and S.G. contributed reagents and helped to interpret results.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 3.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 4.Kosco-Vilbois MH, Gray D, Scheidegger D, Julius M. Follicular dendritic cells help resting B cells to become effective antigen-presenting cells: Induction of B7/BB1 and upregulation of major histocompatibility complex class II molecules. J Exp Med. 1993;178:2055–2066. doi: 10.1084/jem.178.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 7.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 8.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 9.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Roozendaal R, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Pomares L, Gordon S. Antigen presentation the macrophage way. Cell. 2007;131:641–643. doi: 10.1016/j.cell.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Bajenoff M, Germain RN. B cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood. 2009;114:4989–4997. doi: 10.1182/blood-2009-06-229567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 16.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor PR, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 18.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 19.Geijtenbeek TB, et al. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 20.Kang YS, et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, et al. The role of SIGNR1 and the β-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol. 2004;172:1157–1162. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 22.Kang YS, et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 24.Berney C, et al. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 26.Kawai T, et al. Anti-influenza A virus activities of mannan-binding lectins and bovine conglutinin. J Vet Med Sci. 2007;69:221–224. doi: 10.1292/jvms.69.221. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 29.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 30.Bot A, Casares S, Bot S, von Boehmer H, Bona C. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR receptor specific for class II hemagglutinin peptide in CD4+ and CD8+ T cells. J Immunol. 1998;160:4500–4507. [PubMed] [Google Scholar]
- 31.Kang YS, et al. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 32.Lindquist RL, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 33.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst HC, et al. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sha Z, Compans RW. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BO, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PR, et al. Development of a specific system for targeting protein to metallophilic macrophages. Proc Natl Acad Sci USA. 2004;101:1963–1968. doi: 10.1073/pnas.0308490100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwickert TA, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 39.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 41.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 42.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 43.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 46.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 47.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 48.Sonoda E, et al. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 49.Gack MUSY, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez SF, Jayasekera JP, Carroll MC. Complement and natural antibody are required in the long term memory response to influenza virus. Vaccine. 2008;26:I86–I93. doi: 10.1016/j.vaccine.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Pomares L, et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woller EK, Cloninger MJ. The lectin-binding properties of six generations of mannose-functionalized dendrimers. Org Lett. 2002;4:7–10. doi: 10.1021/ol016568+. [DOI] [PubMed] [Google Scholar]
- 53.Barrington RA, Borde M, Rao A, Carroll MC. Involvement of NFAT1 in B cell self-tolerance. J Immunol. 2006;177:1510–1515. doi: 10.4049/jimmunol.177.3.1510. [DOI] [PubMed] [Google Scholar]
- 54.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.