Abstract
There is pressing need to understand the aging process to better cope with its associated physical and societal costs. The age-related muscle wasting known as sarcopenia is a major contributor to the problems faced by the elderly. By hindering mobility and reducing strength, it greatly diminishes independence and quality of life. In studying the factors that contribute to the development of sarcopenia, the focus is shifting to the study of disordered muscle anabolism. The abnormal response of muscle to previously well-established anabolic stimuli is known as anabolic resistance, and may be a key factor in the development and progression of sarcopenia. Factors such as age, obesity, inflammation, and lipotoxicity contribute to anabolic resistance, and have been studied either directly or indirectly in cell systems and whole animals. Understanding the physiologic and mechanistic basis of anabolic resistance could be the key to formulating new and targeted interventions that would ease the burden currently borne by the world’s aged population.
Keywords: Sarcopenia, Anabolic resistance, Skeletal muscle
Introduction
The rapid growth of the world’s aging population has sparked many discussions of the public health implications of aging. The WHO has estimated that the expansion of the population aged 60 and over will more than triple from 600 million to greater than two billion between 2000 and 2050 [1]. With a projected global population of approximately nine billion by the year 2050, between one fifth and one quarter of the world would fall into this category. Increasingly, older adults play an active role in caregiving, volunteerism, and many other integral social facets. Consider, too, the susceptibility of the elderly to disease and the accompanying debilitation. Taken together, these circumstances highlight the importance of understanding the etiology of the aging process and the underlying metabolic alterations that occur.
In the past decades, substantial research has revealed that large sections of the older population suffer from loss of muscle mass and function, which has been shown to contribute to functional limitations and disability [2, 3]. This age-related muscle wasting has been termed sarcopenia [4]. Recently it has been observed that sarcopenia incurs a significant cost to the American Health System, so a better understanding of the disease etiology as well as development of interventional therapy cannot only have a profound impact on the individual level but on a much larger scale as well [5].
Mechanistically, muscle loss as a result of sarcopenia is better understood now than it was some 15 years ago. Preferential loss of type II muscle fibers is characteristic and seen in individuals as early as the age of 25, but the loss of muscle function to actual tissue loss seems disproportionate: strength diminishes more than muscle loss would predict [6, 7]. Recently, studies have implicated a derangement of equilibrium between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) as a major contributor to this phenomenon. In particular, alterations in MPS during anabolic conditions in aged populations have been implicated as a significant contributor to this imbalance [6, 8, 9].
Anabolic resistance, the impaired rate of cellular anabolism in response to various anabolic stimuli, is one potential mechanism of disordered MPS [8]. The inability of muscle to regulate maintenance of protein homeostasis in response to feeding and exercise is characteristic in older individuals. Investigation into both normal and disordered muscle anabolism has provided unique insight to this problem [9, 10].
Anabolic stimuli and signaling
Anabolic stimulators such as insulin, branched chain amino acids (BCAA), and insulin-like growth factors (IGF-1) increase skeletal MPS in young healthy individuals. Anabolic stimulation in skeletal muscle causes MPS to exceed MPB, resulting in muscle hypertrophy. Insulin has been well described as a stimulator of MPS and has also been seen to participate in muscle amino acid uptake [11–14]. However, more recent findings have highlighted insulin as permissive rather than modulatory in MPS. Insulin acts to prime the muscle for protein synthesis, but MPS is stimulated in a dose-dependent fashion by BCAA in young men. So availability of BCAA rather than insulin appears to regulate rate of protein synthesis observed [15]. Additionally, IGF-1 has also been shown to induce hypertrophy in muscle [16]. While these stimuli constitute the most commonly studied and well-characterized dietary and homeostatic drivers of MPS, exercise has also been shown to affect the dynamics of muscle equilibrium.
For years now exercise has been studied in the context of sarcopenic muscle loss [17, 18]. Aerobic exercise, while having no observable impact on muscle protein turnover, has been shown to increase MPS in older individuals [19]. Muscle contraction by resistance exercise increases the overall rate of muscle protein turnover, but does so disproportionately in favor of MPS [12]. Other studies have reported that an intense single bout of resistance training can increase MPS well above baseline in the post-exercise time period that extends from 1 to 72 h [20–22]. Similar to BCAA availability, acute bouts of resistance training have been shown to affect MPS in a dose-dependent manner [23].
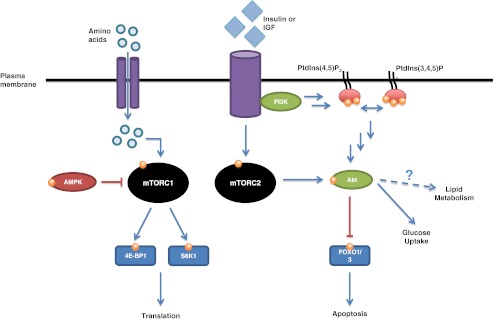
Anabolic signals provoke MPS by activating a highly diverse signaling pathway in skeletal muscle cells. The mammalian/mechanistic target of rapamycin (mTOR) is a member of a family of signaling proteins that serve a myriad of roles in stress and survival pathways. Two distinct mTOR complexes, mTORC1 and mTORC2, act as nutrient sensors that are activated under specific conditions. In response to anabolic stimuli, mTORC1 targets and activates downstream kinases such as S6 kinase 1 (S6K1), or binding proteins like eIF4E-binding protein 1 (4E-BP1), as seen in Fig. 1. The action of mTOR and its downstream mediators heightens the efficiency of ribosomal biogenesis and ultimately translation [24–26]. Additionally, the energy sensor AMP-activated kinase (AMPK) holds a measure of control over mTORC1, and the two together exert antagonistic control over muscle cell size [27].
Fig. 1.
Anabolic stimuli in the form of amino acids, growth factors (insulin, IGF-1), and exercise (not shown) act through the mTOR and Akt signaling pathways. mTORC1 is responsible for the phosphorylation and activation of S6K1 and phosphorylation and inactivation of 4E-BP1. It results in ribosome biogenesis, increase in translational efficiency, and heightened MPS. The energy sensor AMPK inhibits this pathway, and is active when the AMP/ATP ratio is high. Growth factor stimulates activation of the PI3K pathway as well as mTORC2; both eventually activate Akt, which can inhibit stress signaling and apoptosis through inactivation of FOXO1/O3 transcription factors. Akt also plays a role in carbohydrate metabolism. P phosphorylation, activation or inactivation; PI3K phosphoinositide 3 kinase; Akt protein kinase B; FOXO forkhead protein box O; AMPK AMP-activated kinase; 4E-BP1 eIF4E-binding protein 1; S6K1 S6 kinase 1
Protein kinase B, also known as Akt, plays an important role in skeletal muscle homeostasis. A target of mTORC2, Akt is a member of the phosphoinositide 3 kinase (PI3K) family and involved in stress and survival signaling. It acts through control over the forkhead box protein O (FOXO) 1 and FOXO3 transcription factors, which mediate cellular stress responses such as apoptosis [28]. Akt has also been observed to play a role in lipid metabolism, meaning it may have additional culpability in disordered anabolic signaling [29, 30]. Zoncu et al. and Rivas et al. have written detailed reviews on these signaling pathways and their function in skeletal muscle homeostasis; they should be referenced for additional details [28, 34].
Anabolic resistance and aging
As aging is seen to be the root cause of sarcopenia, it has been studied as a potential root of the underlying problem, anabolic resistance. The study of anabolic resistance in both human and rodent models has allowed for a translational approach in determining the mechanism of disordered skeletal muscle metabolism. Initially, a study by Guillet and colleagues showed that the ability of insulin and BCAA to initiate protein translation was hampered in older individuals when compared with young subjects as well [31]. Rassmussen and colleagues corroborated these findings while also pointing out that a blunted vasodilatory response of older muscle to insulin may play a role in nutrient deficiency and unavailability [32]. It was also revealed that the ability of BCAA to foster MPS was blunted in old rats compared with their younger adult counterparts [10]. This observed decrease in MPS in response to anabolic stimulus with aging is thought to be a result of diminished mTOR signaling [26].
Precedent for diminished mTOR signaling in response to anabolic stimulus was established through experimentation with rapamycin, an immunosuppressant and inhibitor of mTOR phosphorylation [33–35]. Cuthbertson and colleagues have observed decreased mTOR phosphorylation in older individuals as compared to young individuals in response to BCAA [15]. Others have seen attenuation of the mTOR signaling pathway despite muscle contraction or overload in aged animals [36–39]. Overall, there seems to be consensus that age-induced anabolic resistance is grounded in the inability of hypertrophy controlling signaling pathways to successfully coordinate the proper response to their normal stimuli.
Diet, obesity, and diminished anabolic signaling
Approximately one decade ago, a study by Baumgartner revealed that older individuals categorized as both sarcopenic and obese were at the highest risk among those suffering from disorders of body composition [40]. Further investigation of this “sarcopenic obesity” would indicate that the two are pathophysiologically linked, perhaps even at a mechanistic level [41]. It comes as no surprise then that anabolic resistance has been observed to arise in response to obesity and high-fat feeding. Diet-induced obesity is often implicated in insulin resistance when studied as a contributor to metabolic syndrome [42]. A study in mice conducted by Anderson and colleagues showed that diet-induced obesity reduced skeletal MPS in response to nutrient intake [43]. Yet another study was able to find a link between impaired protein metabolism and reduced post-absorptive protein turnover in obese individuals [44].
In spite of compelling evidence, it is yet unclear how obesity and insulin resistance are linked mechanistically. It has been shown that increased activation of the mTOR pathway can diminish the ability of Akt to function properly in fat-fed obese rats [45]. The downstream effectors of the mTOR pathway have also been implicated as contributors to insulin resistance stemming from obesity [46]. Some mechanistic insight into anabolic signaling has been gleaned through the study of β-hydroxy-β-methylbutyrate (HMB), an active leucine metabolite. HMB has been shown to increase MPS through activation of the mTOR pathway and to attenuate MPB through various anticatabolic actions, such as inhibition of caspase-8 signaling [47–49]. Still, the lack of concrete mechanisms for the roles of obesity and anabolic signaling in anabolic resistance makes this a rich area for study moving forward.
Inflammation and lipotoxicity
Obesity aside, lipid accumulation may have another important role in anabolic signaling and resistance. Accumulation of lipids in nonfatty tissue sites has been linked with activation of proinflammatory mediators such as toll-like receptor 4, tumor necrosis factor alpha (TNFα), and nuclear factor kappa-light-chain enhancer of activated B cells (NFκB) [50]. Several studies have shown that inflammation, mediated by these same intermediaries, may play a key role in the development of sarcopenia, especially through anabolic resistance [51, 52]. Cuthbertson and colleagues have suggested that increased basal expression of NFκB, as well as TNFα and interleukin-6, in older individuals is an inhibitor of muscle anabolism by interference with mTOR signaling [15]. Inflammation like this is viewed as chronic in older individuals, characterized by heightened production of cytokines and acute-phase proteins; the phenomenon has come to be called “inflammaging” by some [53–56]. Furthermore, recent findings have linked lipid accumulation to proinflammatory macrophage infiltration in muscle tissue and intermuscular adipose tissue; this is certainly detrimental, as fatty acids can trigger macrophage secretion of factors that would further impair anabolic stimulation [57–59].
Lipotoxicity contributes to anabolic resistance through the action of ceramides, sphingolipids composed of a sphingosine backbone and fatty acid chain. Ceramides function as regulators of cellular stress and are mechanistic modulators of metabolic diseases, especially insulin/anabolic resistance [60–62]. Hyde and colleagues reported that ceramide diminished intracellular amino acid availability and reduced phosphorylation of translational regulators downstream of mTOR in vitro [63]. Others have gone on to show that ceramide activates protein phosphatase 2A, which targets and inhibits Akt and S6K1 by dephosphorylation [64–66]. It has been hypothesized that ceramides are mediators of TNFα inhibition of anabolic signaling; but as of yet, there has been no mechanistic link between the two [67, 68].
Ceramide is not the only lipid species implicated in lipotoxicity; triglycerides have also been seen as detrimental to anabolic signaling in skeletal muscle. Recently, we were able to show that age-induced intramuscular triglyceride infiltration is associated with an increase in several lipogenic regulators including: sterol regulatory element-binding protein, fatty acid synthase, acetyl CoA carboxylase, and stearoyl CoA desaturase. Additionally, we demonstrated that AMPK activation and concentration are heightened in aged skeletal muscle, even in animals that were subjected to anabolic stimulus in the form of chronic muscle overload [69]. This would suggest that intramuscular lipid accumulation, especially of triglycerides, is somehow involved in the blunting of anabolic signaling pathways. Further investigation in this vein is warranted, as elucidation of the mechanistic underpinnings of lipotoxicity could present new and better ways to intervene in anabolic resistance at the protein or genetic level, stemming sarcopenia at the source.
Future directions
Clearly, the story of anabolic resistance is compelling, but still being written. Further exploration of the mechanistic similarities between aging and obesity would clarify whether or not there is a link between resistances observed in both cases. From an interventional standpoint, diet and exercise could be better tailored to sarcopenia of specific origin to optimize recovery of muscle mass and function. But where intervention in diet and with exercise may be less effective, as in the oldest of individuals, development of therapeutic or pharmaceutical agents is warranted. A better idea of the role metabolic or age-related inflammation plays in anabolic resistance would be necessary to develop targeted countermeasures. Similarly, understanding how lipid infiltration and lipotoxicity adversely impact anabolic signaling at the genetic, protein, and metabolic levels is an area ripe for study. Luckily, this field of study is relatively new, so developments in all these areas will come in time. And with an improved understanding of anabolic resistance, we can better apply ourselves to the treatment of sarcopenia, thereby reducing the subsequent burden of disability from this condition.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [70].
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.WHO. Ageing (online). 2008. http://www.who.int/topics/ageing/en. Accessed 9 Aug 2011.
- 2.Melton LJ, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 3.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12(7):452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 7.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 8.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34(3):377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prod’homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, et al. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol. 2005;563(Pt 1):235–248. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol. 1990;259(2 Pt 1):E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- 12.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95(2):811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin’s effect to stimulate protein synthesis in the human forearm. Am J Physiol. 1998;274(6 Pt 1):E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 14.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52(6):1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 16.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81(6):2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 17.Fielding RA. Effects of exercise training in the elderly: impact of progressive- resistance training on skeletal muscle and whole-body protein metabolism. Proc Nutr Soc. 1995;54(3):665–675. doi: 10.1079/PNS19950066. [DOI] [PubMed] [Google Scholar]
- 18.Chin AV, Robinson DJ, O’Connell H, Hamilton F, Bruce I, Coen R, et al. Vascular biomarkers of cognitive performance in a community-based elderly population: the Dublin Healthy Ageing study. Age Ageing. 2008;37(5):559–564. doi: 10.1093/ageing/afn144. [DOI] [PubMed] [Google Scholar]
- 19.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 20.MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20(4):480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- 21.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 22.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567(Pt 3):1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(Pt 1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 25.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 27.Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Foretz M, Viollet B. Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle. 2011;10(16):2640–2646. doi: 10.4161/cc.10.16.17102. [DOI] [PubMed] [Google Scholar]
- 28.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, et al. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006;4(1):89–96. doi: 10.1016/j.cmet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Rivas DA, Yaspelkis BB, 3rd, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside. J Endocrinol. 2009;202(3):441–451. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18(13):1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vary TC, Anthony JC, Jefferson LS, Kimball SR, Lynch CJ. Rapamycin blunts nutrient stimulation of eIF4G, but not PKCepsilon phosphorylation, in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293(1):E188–E196. doi: 10.1152/ajpendo.00037.2007. [DOI] [PubMed] [Google Scholar]
- 34.Rivas DA, Lessard SJ, Coffey VG. mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab. 2009;34(5):807–816. doi: 10.1139/H09-073. [DOI] [PubMed] [Google Scholar]
- 35.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1080–R1086. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- 37.Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64(6):618–628. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;97(1):243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 39.Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574(Pt 1):291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 41.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn CR. Banting lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43(8):1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 43.Anderson SR, Gilge DA, Steiber AL, Previs SF. Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metabolism. 2008;57(3):347–354. doi: 10.1016/j.metabol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, et al. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94(8):3044–3050. doi: 10.1210/jc.2008-2216. [DOI] [PubMed] [Google Scholar]
- 45.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146(3):1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 46.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117(2):387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. beta-Hydroxy-beta-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R701–R715. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem. 2009;330(1–2):171–179. doi: 10.1007/s11010-009-0130-5. [DOI] [PubMed] [Google Scholar]
- 49.Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007;293(4):E923–E931. doi: 10.1152/ajpendo.00314.2007. [DOI] [PubMed] [Google Scholar]
- 50.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21(2):253–263. doi: 10.1152/physiolgenomics.00249.2004. [DOI] [PubMed] [Google Scholar]
- 52.Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1485–R1495. doi: 10.1152/ajpregu.00467.2009. [DOI] [PubMed] [Google Scholar]
- 53.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82(2):428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 54.Paolisso G, Rizzo MR, Mazziotti G, Tagliamonte MR, Gambardella A, Rotondi M, et al. Advancing age and insulin resistance: role of plasma tumor necrosis factor-alpha. Am J Physiol. 1998;275(2 Pt 1):E294–E299. doi: 10.1152/ajpendo.1998.275.2.E294. [DOI] [PubMed] [Google Scholar]
- 55.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–M26. doi: 10.1093/gerona/53A.1.M20. [DOI] [PubMed] [Google Scholar]
- 56.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296(6):E1300–E1310. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr. 2010;13(4):382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- 60.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277(29):25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 61.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes. 2005;54(3):591–602. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- 62.Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci. 2002;967:329–335. doi: 10.1111/j.1749-6632.2002.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 63.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19(3):461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 64.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50(10):2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, et al. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PloS One. 2010;5(5):e10578. doi: 10.1371/journal.pone.0010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin associated protein. Proc Natl Acad Sci U S A. 1999;96(8):4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50(11):2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 68.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Rivas DA, Morris EP, Fielding RA. Lipogenic regulators are elevated with age and chronic overload in rat skeletal muscle. Acta Physiol (Oxf) 2011;202(4):691–701. doi: 10.1111/j.1748-1716.2011.02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1(1):7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]