Abstract
Prevalence of hypogonadism in men with cancer has been reported between 40% and 90%, which is significantly higher than in the general population. Hypogonadism is likely to affect the quality of life in these patients by contributing to non-specific symptoms, including decreased energy, anorexia, sarcopenia, weight loss, depression, insomnia, fatigue, weakness, and sexual dysfunction. Pathogenesis of hypogonadism in cancer patients is thought to be multi-factorial. Inflammation may play an important role, but leptin, opioids, ghrelin, and high-dose chemotherapy through different mechanisms have all been implicated as the cause. Hypogonadism is also associated with poor survival in cancer patients. Data looking into the treatment of hypogonadal male cancer patients with testosterone are limited. However, improvements in body weight, muscle strength, lean body mass, and quality of life have been shown in hypogonadal men with other chronic diseases on testosterone replacement therapy. Prospective and interventional trials are needed to test the efficacy and safety of testosterone treatment in improving quality of life of these patients.
Keywords: Inflammation, Leptin, Ghrelin, Cachexia, Sarcopenia
Introduction
Approximately 800,000 men are diagnosed with cancer each year in the USA [1], and cancer treatments account for more than $41 billion in spending each year [2]. Cancer patients suffer from significant morbidity caused by the primary disease directly or by the administration of chemotherapy or radiotherapy. They often present with non-specific symptoms, including decreased energy, anorexia, sarcopenia, weight loss, depression, insomnia, fatigue, weakness, and sexual dysfunction. Some of these symptoms are known to be associated with hypogonadism in patients with advanced cancer [3] and have a profound impact in these individuals’ survival, quality of life, and return to productivity. Here, we review the evidence of the potential contribution of hypogonadism to symptom burden in male patients with cancer, its pathophysiology, and the potential therapeutic role of testosterone replacement in this setting.
Prevalence of hypogonadism in non-cancer and cancer patients
The prevalence of hypogonadism in non-cancer patients increases with age. For example, in the Baltimore Longitudinal Study of Aging, the incidence of hypogonadism was approximately 20% in men over 60, 30% in men over 70, and 50% in men over 80 years of age using total testosterone levels <325 ng/dl as a criterion [4]. In the Massachusetts Male Aging Study, a population-based cohort of men between ages 40 and 69 years, the estimated crude prevalence of androgen deficiency at baseline and follow-up were 6% and 12%, respectively [5]. In the Hypogonadism in Male (HIM) study which included 2,162 men older than 45 years of age in the primary care setting, the crude prevalence of hypogonadism (defined as total testosterone <300 ng/dl) was 39% [6].
Prevalence of hypogonadism in cancer patients ranges from 40% to 90% [7, 8]. In an early study, total testosterone levels in patients with pancreatic adenocarcinoma were similar to the levels in control subjects [9]. Another study that evaluated gonadal hormonal function of 44 adult males with disseminated cancer found a prevalence of hypogonadism of 43% and 66% on the basis of total testosterone and free testosterone, respectively. However, this study included primarily malnourished cancer patients, in which 82% of patients were less than 90% of ideal body weight [10]. Sex hormone binding globulin (SHBG) levels are increased in cancer patients, so measuring free and bioavailable testosterone is a more reliable and accurate way of determining the gonadal status of male patients than total testosterone level. Strasser et al. [3] reported a prevalence of hypogonadism of 64% in advanced incurable cancer patients based on free testosterone levels <35 ng/ml. Our group published data showing that cancer patients had mean total testosterone levels similar to the levels in an age-matched non- cancer control group but significantly lower levels of free and bioavailable testosterone [7]. Recently, Fleishman et al. reported the prevalence of hypogonadism in cancer patients based on total testosterone (TT, <300 ng/dl), free testosterone (FT, <52 pg/dl), and bioavailable testosterone (BT, <95 ng/dl) to be 48%, 78%, and 66%, respectively [11].
Prevalence of hypogonadism is even higher in cancer patients with a history of cachexia (described as weight loss over the previous 6–12 months greater than 5%). In a Dutch study, male cancer patients who had lost less than 10% of their body weight had higher serum testosterone levels than those with weight loss greater than 10% [12]. Our group recently reported a strong association between BT and SHBG and appetite levels [7]. Post-hoc analyses showed a higher prevalence of hypogonadism defined as a BT < 70 ng/ml in cachectic subjects when compared to non-cachectic subjects in this cohort.
Pathogenesis of hypogonadism in cancer
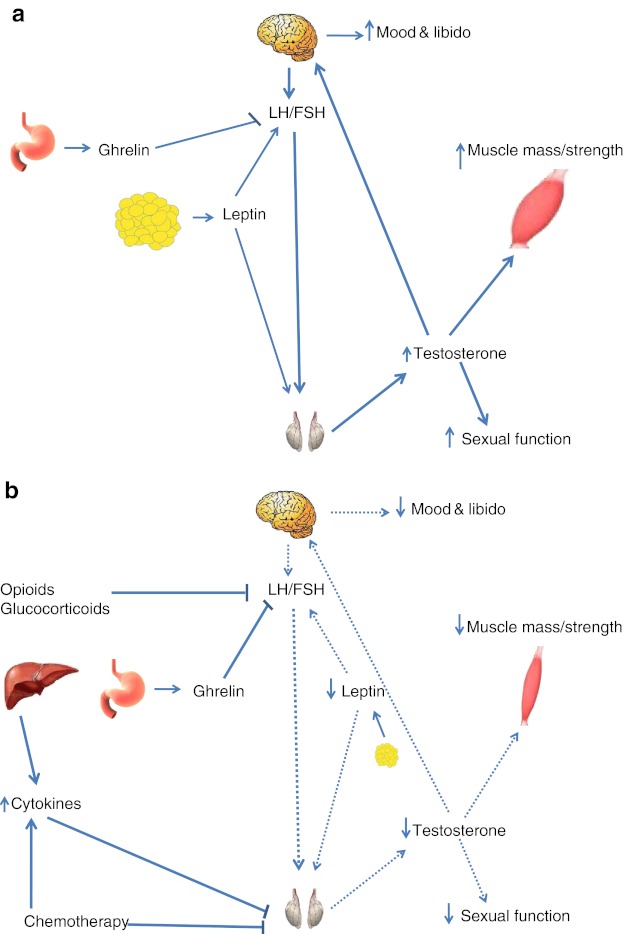
Although the exact mechanism of hypogonadism in cancer patients is not entirely known, it is thought to be multi-factorial. Patients with cancer may have a component of both types of hypogonadism: primary (testicular, hypergonadotrophic, or with elevated luteinizing hormone—LH—and follicular stimulating hormone—FSH) and secondary (central, hypogonadotrophic, or with low or inappropriately normal FSH and LH). Factors associated with primary hypogonadism in this patient population include inflammatory cytokines and chemotherapeutic agents. On the other hand, factors responsible for the secondary/central hypogonadism include opioids, glucocorticosteroids, low leptin levels, and/or high concentrations of ghrelin (Fig. 1).
Fig. 1.
Illustration of pituitary–gonadal axis in healthy individuals (a) and in cancer patients (b). Increased ghrelin, decreased leptin, and medications (opioids and glucocorticoids) suppress LH production, thereby causing central hypogonadism. Increased inflammation, certain chemotherapeutic agents, and changes in ghrelin and leptin may also contribute to hypogonadism by downregulating testicular production of testosterone directly. Dashed lines indicate a decrease in the pathway
Inflammation
Inflammation is thought to play an important role in the pathogenesis of hypogonadism in cancer patients. Cancer like other chronic diseases is a pro-inflammatory state with elevated levels of inflammatory cytokines [13, 14]. It has been postulated that cytokines may have a direct effect on the hypothalamic–pituitary–gonadal axis. This was supported by a study of 15 healthy male subjects who were injected with subcutaneous recombinant IL-6. Results showed that testosterone levels were suppressed acutely in these healthy individuals after IL-6 administration without apparent changes in gonadotrophin levels [15]. Also, animal data have suggested that inflammatory cytokines may have an inhibitory effect on Leydig cell steroidogenesis, thereby affecting the hypothalamic–pituitary–gonadal axis at the level of testes [16–18]. The mechanism by which IL-6 inhibits the Leydig cell steroidogenesis is unclear, but it has been postulated that it may negatively affect the activity of 3β-hydroxysteroid dehydrogenase, one of the key enzymes for testosterone synthesis [17].
On the other hand, Malkin et al. showed that 1 month of testosterone replacement given to 27 male patients with hypogonadism resulted in a statistically significant reduction in inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β [19], and Kalinchenko et al. showed that testosterone replacement decreased C-reactive protein (CRP), IL-1β, and TNF-α in hypogonadal men with the metabolic syndrome [20]. Similar immunosuppressive and anti-inflammatory actions of androgens were seen in study of patients with rheumatoid arthritis who were treated with oral testosterone [21]. In light of these studies, inflammation is likely to play a very important role in the pathogenesis of hypogonadism in cancer patients, and low testosterone can, in turn, exacerbate or maintain the pro-inflammatory state forming a vicious circle.
Leptin
Leptin is a hormone secreted by adipocytes in proportion to fat mass, and it is responsible for regulating energy homeostasis (food intake and energy expenditure) at the level of the hypothalamus. Also, it is required for normal LH and FSH secretion centrally and production of testosterone in the gonads, providing the link between nutrition and reproduction. It has been proposed that low leptin levels (as seen in starvation or in the setting of cachexia) function as a nutrient sensor and provide a peripheral signal to the brain that the environment may not be favorable for reproduction due to unavailability of food, leading to a decrease in sex hormones. Both animal and human models have shown that restoring normal levels of leptin results in normalization of the hypothalamic–pituitary–gonadal axis and reversal of infertility and delayed puberty [22, 23], providing further support to this hypothesis. Leptin replacement also has resulted in normalization of starvation-induced fall in reproductive hormones in male subjects [24]. As leptin is secreted by adipocytes, fat atrophy can potentially cause hypogonadism by decreasing leptin levels [25]. This could potentially explain the higher prevalence of hypogonadism in cancer cachexia subjects as they suffer from significant fat mass loss and have been shown to have lower leptin levels than subjects with cancer but without cachexia [26].
Ghrelin
Ghrelin is a 28-amino acid peptide which is primarily involved in the control of food intake and growth hormone secretion. Its levels increase in anticipation of a meal and with weight loss correlating inversely with leptin levels. Based on this, it also has been proposed as a nutrient signal that links reproduction and nutrition. Animal data show that systemic or intracerebral administration of ghrelin causes significant inhibitory responses in the secretion of LH and to a lesser extent FSH [27–29]. In a male rat model, chronic ghrelin administration resulted in a significant drop in serum LH and testosterone levels [30]. In a healthy human study of ten male subjects, ghrelin administration was associated with significantly lower mean plasma levels of both LH and testosterone than placebo [31]. Similar effects of ghrelin on LH were published by Lanfranco et al. [32]. We published that cancer patients had significantly higher active ghrelin levels than healthy controls, and the ghrelin levels correlated inversely with free and bioavailable testosterone [7]. All of these studies suggest that higher levels of ghrelin through its inhibitory effects on LH can contribute to hypogonadism in the setting of negative energy balance as seen in patients with advanced cancer.
Opioids
Use of opioids in cancer patients is very common as these patients suffer from significant pain from their primary disease. It has been shown that opioids have the potential of causing secondary hypogonadism in non-cancer patients with pain [33–35]. The exact mechanism by which opioids cause hypogonadism is not entirely known; however, a few theories have been suggested. Opioids cause the disruption of the normal pulsatility of gonadotrophin releasing hormone (GnRH) secretion through δ-receptors present on GnRH neurons [36]. They are also occasionally reported to increase prolactin levels, thereby reducing testosterone secretion [37]. Moreover, testosterone concentrations drop more than 50% within a few hours of taking an opioid, usually returning to baseline within 24–72 h after withdrawal [38]. In a small observational study of 54 men taking oral opioids including methadone for non-malignant pain, levels of free and total testosterone were subnormal in 56% and 74% of patients, respectively [39]. Similar results have been seen in cancer patients. Fraser et al. reported a hypogonadism prevalence of 75% in male cancer subjects receiving oral opioids for chronic pain [40]. In another study, 18 out of 20 of male cancer survivors who had received high-dose opioids orally had lower total testosterone level compared to 8 out of 20 matched cancer survivors who did not receive opioids [8]. Taken together, the data strongly suggest that opioids play an important role in the pathogenesis of hypogonadism in cancer patients.
Chemotherapy
Alkylating and other chemotherapeutic agents at high doses are known to cause hypogonadism in cancer patients. This is believed to be related to the dose-dependent direct toxic effect of these agents on the gonads especially on Leydig cell function [41]. There have been multiple studies showing elevated LH levels and low to normal testosterone levels in cancer patients receiving chemotherapy, suggesting damage of the hypothalamic–pituitary–gonadal axis at the testicular level [42, 43]. Howell et al. [41] measured testosterone and LH levels in 135 men treated for lymphomas. They found 44 men (31%) with significantly higher LH level compared to a cohort of age-matched controls in the presence of a testosterone levels in the lower half of the normal range or frankly low. However, it is important to note that non-standard, high cumulative doses of chemotherapy are required to cause significant and persistent impairment of Leydig cell function and subsequent hypogonadism in cancer patients.
Hypogonadism, symptom burden, and quality of life in cancer patients
It has been suggested that androgen deficiency is an important component of the Cancer Anorexia–Cachexia Syndrome (CACS) [44]. Hypogonadism due to any cause significantly affects quality of life in affected individuals and is associated with symptoms such as decreased appetite, decreased energy level and fatigue, insomnia, low libido, sexual dysfunction, depressed mood, fatigue, sarcopenia, and weakness [45, 46]. Appetite and body weight are affected in cancer patients with hypogonadism. In our previous work, we found a direct correlation between appetite scores and free and bioavailable testosterone in cancer patients [7]. Skipworth et al. showed that hypogonadal cancer male patients experienced greater percentage weight loss than eugonadal cancer patients (16.7% vs. 11.3%) [47]. Chlebowski et al. reported a 66% prevalence of hypogonadism in metastatic cancer patients, where >80% of the patients were at <90% of ideal body weight [10]. Todd and colleagues reported a negative correlation of total testosterone with BMI and mid-arm circumference in men with advanced cancer [9].
Hypogonadism also affects the functional and emotional well-being in cancer subjects. In a recently published study, cancer survivors with hypogonadism had lower scores in short-term health survey and functional assessment of chronic illness—fatigue (FACIT-F) questionnaire suggesting poor quality of life and increased fatigue in cancer patients [48]. Fleishman and colleagues [11] showed similar results in hypogonadal cancer patients. They used Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire to measure different aspects of quality of life and found that hypogonadal cancer patients scored worse in the total as well as physical and functional well-being component of the questionnaire when compared to eugonadal cancer subjects. Strasser et al. showed that hypogonadal patients with cancer (on the basis of low free testosterone) had higher scores in anxiety and depression measured by Hospital Anxiety and Depression Scale, lower functional and emotional well-being measured by Functional Assessment of Cancer Therapy, and higher fatigue measured by FACIT-F [3].
Sexual dysfunction is another problem that hypogonadal cancer patients encounter. Most of the data on sexual dysfunction in cancer patients come from prostate cancer studies [49, 50], which may not be a representative subgroup to study given that part of the treatment of prostate cancer includes induction of profound hypogonadism. Common factors that affect sexual function in cancer patients other than hypogonadism include pelvic surgery, resulting in damage to nerve/blood supply and radiation/chemotherapy-induced peripheral nerve damage. Greenfield and colleagues reported greater degree of sexual dysfunction in cancer patients with testosterone levels in lower quartiles compared to patients with testosterone in high quartiles [48]. Similar results were published by Fleishman et al. who reported lower scores in FACT-P sexual function questionnaire in hypogonadal cancer patients compared to eugonadal cancer patients [11].
Few other general symptoms have been linked to hypogonadism. Del Fabbro et al. [51] recently showed that hypogonadism in cancer patients is associated with insomnia. This was attributed to the disruption of normal circadian rhythm and sleep. In the same study, the investigators also found that lower testosterone levels were associated with dyspnea. It was postulated that lower levels of testosterone resulted in loss of lean body mass and wasting of respiratory muscles, causing dyspnea.
Hypogonadism and cancer survival
To date, there has been only one study that looked into survival of hypogonadal patients with cancer. The result of this single center study showed that survival of male patients with total testosterone levels <185 ng/dl was decreased compared to patients with level of testosterone >185 ng/dl (93 vs. 433 days) [51]. In another study, there was a trend towards lower overall survival in patients with baseline low testosterone who were receiving anti-androgen therapy for prostate cancer [52]. Whether hypogonadism contributes to a decrease in survival by a not well-characterized mechanism or is simply a marker of severity of disease is not known.
Treatment of hypogonadism in cancer patients
In non-cancer, hypogonadal men, testosterone replacement has a positive effect on several parameters that are also impaired in the setting of cancer, and treatment of hypogonadism is clinically indicated in symptomatic men with low testosterone [53]. Furthermore, testosterone administration has been shown to increase lean body mass and bone mineral density, to improve quality of life, and to decrease fatigue in this setting [54, 55]. Sexual function and mood parameters improve rapidly and are maintained throughout testosterone treatment [56, 57]. Also, testosterone replacement reduces cytokine levels in hypogonadal men [19, 20] and induces a reduction in inflammatory markers and clinical remission in other hypogonadal states such as arthritis [21].
Treatment of hypogonadism in HIV patients with wasting has been beneficial. Previous studies have shown improvement in body weight, muscle strength, lean body mass, and improved quality of life in HIV patients on testosterone replacement therapy [58–60]. Whether similar benefits will apply to hypogonadal cancer patients is not known. Chlebowski et al. showed decreased severity of weight loss in cancer patients who were treated with nandrolone [10]. However, Loprinzi and colleagues did not show any benefit of fluoxymesterone, an anabolic corticosteroid which was found to be inferior to dexamethasone and megestrol with respect to appetite enhancement [61]. Certainly, there is paucity of data when it comes to interventional studies looking at the safety and efficacy of testosterone in this patient population, although several studies are ongoing using testosterone or selective androgen receptor modulators (SARMs) which would potentially present the advantage of good oral bioavailability and decreased prostatic and virilizing side effects. Recently, a phase II study using the SARM ostarine was reported to cause a dose-dependent increase in lean body mass and functional performance [62].
Conclusion
The development of therapies for the prevention or treatment of cancer-related symptoms is desperately needed because they significantly reduce quality of life in these patients. The prevalence of hypogonadism is higher in cancer patients than in non-cancer patients. It is associated with poor quality of life, and it likely contributes to weight loss, sexual dysfunction, fatigue, and weakness. Hypogonadism is also associated with poor survival in cancer patients. Data regarding the effectiveness and safety of testosterone treatment in this population are scant, and more prospective trials are needed to determine if testosterone replacement therapy will improve muscle mass and strength, quality of life, sexual function, and fatigue in this setting.
Acknowledgments
Dr. Garcia received research support from MERIT grants from the Dept. of Veterans Affairs (I01-BX000507, CX000174) and from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (R21HD060870). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health. The authors of this manuscript certify that they comply with the principles of ethical publishing in the Journal of Cachexia, Sarcopenia, and Muscle [63].
Financial Disclosure
JMG receives research support from and is a consultant with Abbott.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jemal A, Siegel R, Xu R, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG EM, Kosary CL, Hankey BF, Miller MBA, Clegg L,, al. e. SEER cancer statistics review, 197–2000. Bethesda, (MD): National Cancer Institute. 2003.
- 3.Strasser F, Palmer JL, Schover LR, Yusuf SW, Pisters K, Vassilopoulou-Sellin R, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer. 2006;107:2949–2957. doi: 10.1002/cncr.22339. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jc.86.2.724. [DOI] [PubMed] [Google Scholar]
- 5.Araujo AB, O’Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–2591. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100:851–858. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 9.Todd BD. Pancreatic carcinoma and low serum testosterone; a correlation secondary to cancer cachexia? Eur J Surg Oncol. 1988;14:199–202. [PubMed] [Google Scholar]
- 10.Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982;42:2495–2498. [PubMed] [Google Scholar]
- 11.Fleishman SB, Khan H, Homel P, Suhail MF, Strebel-Amrhein R, Mohammad F, et al. Testosterone levels and quality of life in diverse male patients with cancers unrelated to androgens. J Clin Oncol. 2010;28:5054–5060. doi: 10.1200/JCO.2010.30.3818. [DOI] [PubMed] [Google Scholar]
- 12.Simons JP, Schols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci (Lond). 1999;97:215–223. doi: 10.1042/CS19990021. [DOI] [PubMed] [Google Scholar]
- 13.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 14.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–1216. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 15.Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. J Interferon Cytokine Res. 1999;19:1271–1276. doi: 10.1089/107999099312948. [DOI] [PubMed] [Google Scholar]
- 16.Watson ME, Newman RJ, Payne AM, Abdelrahim M, Francis GL. The effect of macrophage conditioned media on Leydig cell function. Ann Clin Lab Sci. 1994;24:84–95. [PubMed] [Google Scholar]
- 17.Afane M, Dubost JJ, Sauvezie B, Issoual D, Dosgilbert A, Grizard G, et al. Modulation of Leydig cell testosterone production by secretory products of macrophages. Andrologia. 1998;30:71–78. doi: 10.1111/j.1439-0272.1998.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology. 1997;138:1008–1013. doi: 10.1210/en.138.3.1008. [DOI] [PubMed] [Google Scholar]
- 19.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 20.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf).73:602-12. [DOI] [PubMed]
- 21.Cutolo M, Balleari E, Giusti M, Intra E, Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1–5. doi: 10.1002/art.1780340102. [DOI] [PubMed] [Google Scholar]
- 22.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 24.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 26.Smiechowska J, Utech A, Taffet G, Hayes T, Marcelli M, Garcia JM. Adipokines in patients with cancer anorexia and cachexia. J Investig Med. 2010;58:554–559. doi: 10.231/JIM.0b013e3181cf91ca. [DOI] [PubMed] [Google Scholar]
- 27.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–785. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, et al. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245–255. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett. 2004;362:103–107. doi: 10.1016/j.neulet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Sirotkin AV, Chrenkova M, Nitrayova S, Patras P, Darlak K, Valenzuela F, et al. Effects of chronic food restriction and treatments with leptin or ghrelin on different reproductive parameters of male rats. Peptides. 2008;29:1362–1368. doi: 10.1016/j.peptides.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–3205. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- 32.Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93:3633–3639. doi: 10.1210/jc.2008-0049. [DOI] [PubMed] [Google Scholar]
- 33.Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126–131. doi: 10.1016/0885-3924(94)90166-X. [DOI] [PubMed] [Google Scholar]
- 34.Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–2222. doi: 10.1210/jc.85.6.2215. [DOI] [PubMed] [Google Scholar]
- 35.Finch PM, Roberts LJ, Price L, Hadlow NC, Pullan PT. Hypogonadism in patients treated with intrathecal morphine. Clin J Pain. 2000;16:251–254. doi: 10.1097/00002508-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Pimpinelli F, Parenti M, Guzzi F, Piva F, Hokfelt T, Maggi R. Presence of delta opioid receptors on a subset of hypothalamic gonadotropin releasing hormone (GnRH) neurons. Brain Res. 2006;1070:15–23. doi: 10.1016/j.brainres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Delitala G, Grossman A, Besser GM. The participation of hypothalamic dopamine in morphine-induced prolactin release in man. Clin Endocrinol (Oxf). 1983;19:437–444. doi: 10.1111/j.1365-2265.1983.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 38.Mendelson JH, Ellingboe J, Judson BA, Goldstein A. Plasma testosterone and luteinizing hormone levels during levo-alpha-acetylmethadol maintenance and withdrawal. Clin Pharmacol Ther. 1984;35:545–547. doi: 10.1038/clpt.1984.75. [DOI] [PubMed] [Google Scholar]
- 39.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3:377–384. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- 40.Fraser LA, Morrison D, Morley-Forster P, Paul TL, Tokmakejian S, Larry Nicholson R, et al. Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp Clin Endocrinol Diabetes. 2009;117:38–43. doi: 10.1055/s-2008-1076715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howell SJ, Radford JA, Ryder WD, Shalet SM. Testicular function after cytotoxic chemotherapy: evidence of Leydig cell insufficiency. J Clin Oncol. 1999;17:1493–1498. doi: 10.1200/JCO.1999.17.5.1493. [DOI] [PubMed] [Google Scholar]
- 42.Palmieri G, Lotrecchiano G, Ricci G, Spiezia R, Lombardi G, Bianco AR, et al. Gonadal function after multimodality treatment in men with testicular germ cell cancer. Eur J Endocrinol. 1996;134:431–436. doi: 10.1530/eje.0.1340431. [DOI] [PubMed] [Google Scholar]
- 43.Stuart NS, Woodroffe CM, Grundy R, Cullen MH. Long-term toxicity of chemotherapy for testicular cancer—the cost of cure. Br J Cancer. 1990;61:479–484. doi: 10.1038/bjc.1990.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Hijazi RA, Cunningham GR. Andropause: is androgen replacement therapy indicated for the aging male? Annu Rev Med. 2005;56:117–137. doi: 10.1146/annurev.med.56.082103.104518. [DOI] [PubMed] [Google Scholar]
- 46.Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61:162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- 47.Skipworth RJ, Moses AG, Sangster K, Sturgeon CM, Voss AC, Fallon MT et al. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer.19:391–401. [DOI] [PubMed]
- 48.Greenfield DM, Walters SJ, Coleman RE, Hancock BW, Snowden JA, Shalet SM et al. Quality of life, self-esteem, fatigue, and sexual function in young men after cancer: a controlled cross-sectional study. Cancer.116:1592–601. [DOI] [PubMed]
- 49.Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008;179:S40–S44. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 50.Deliveliotis C, Liakouras C, Delis A, Skolarikos A, Varkarakis J, Protogerou V. Prostate operations: long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urol Res. 2004;32:283–289. doi: 10.1007/s00240-004-0411-0. [DOI] [PubMed] [Google Scholar]
- 51.Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, et al. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39:1016–1024. doi: 10.1016/j.jpainsymman.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Taira AV, Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, et al. Pretreatment serum testosterone and androgen deprivation: effect on disease recurrence and overall survival in prostate cancer patients treated with brachytherapy. Int J Radiat Oncol Biol Phys. 2009;74:1143–1149. doi: 10.1016/j.ijrobp.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 54.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 55.Jockenhovel F, Minnemann T, Schubert M, Freude S, Hubler D, Schumann C, et al. Comparison of long-acting testosterone undecanoate formulation versus testosterone enanthate on sexual function and mood in hypogonadal men. Eur J Endocrinol. 2009;160:815–819. doi: 10.1530/EJE-08-0830. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 57.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 58.Rabkin JG, Rabkin R, Wagner G. Testosterone replacement therapy in HIV illness. Gen Hosp Psychiatry. 1995;17:37–42. doi: 10.1016/0163-8343(94)00062-I. [DOI] [PubMed] [Google Scholar]
- 59.Coodley GO, Coodley MK. A trial of testosterone therapy for HIV-associated weight loss. AIDS. 1997;11:1347–1352. doi: 10.1097/00002030-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Corcoran C, Grinspoon S. Treatments for wasting in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1999;340:1740–1750. doi: 10.1056/NEJM199906033402207. [DOI] [PubMed] [Google Scholar]
- 61.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 62.Evans WJ, Smith M, Morley JE, et al. Ostarine increases lean body mass and improves physical performance in healthy elderly subjects: implications for cancer cachexia patients. J Clin Oncol. 2007;25:522S. [Google Scholar]
- 63.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. [DOI] [PMC free article] [PubMed]