Abstract
Increasing the multi-drug resistance Aeromonas hydrophila creates a health problem regularly thus, an urgent needs to develop and screen potent antibiotics for controlling of the infections. There are many studies have focused on interactions between specific drugs, little is known about the system properties of a full drug interaction in gene network. Thus, an attractive approach for developing novel antibiotics against DNA gyrase, an enzyme essential for DNA replication, transcription, repair and recombination mechanisms which is important for bacterial growth and cell division. Homology modeling method was used to generate the 3-D structure of B subunit of DNA gyrase (gyrB) using known crystal structure. The active amino acids in 3-D structure of gyrB were targeted for structure based virtual screening of potent drugs by molecular docking. Number of drugs and analogs were selected and used for docking against gryB. The drugs Cinodine I, Cyclothialidine and Novobiocin were found to be more binding affinity with gyrB-drug interaction. The homology of gyrB protein sequence of A. hydrophila resembles with other species of Aeromonas closely showed relationship in phylogenetic tree. We have also demonstrated the gene network interactions of gyrB with other cellular proteins which are playing the key role in gene regulation. These findings provide new insight to understand the 3-D structure of gyrB which can be used in structure-based drug discovery; and development of novel, potent and specific drug against B subunit of DNA gyrase.
Electronic supplementary material
The online version of this article (doi:10.1007/s11693-012-9093-z) contains supplementary material, which is available to authorized users.
Keywords: Aeromonas hydrophila, gyrB, Docking, Phylogenetic tree, Gene network, Drug
Introduction
Aeromonas hydrophila is a ubiquitous pathogen involved in gastroenteritis, meningitis, skin and soft tissue infections with a variety of clinical syndromes in immunocompromised patients globally (Holmes et al. 1996; Janda and Abbott 1998). It is associated with hemorrhagic septicemia in cold blooded animals including fish, reptiles and amphibians (Janda 1991; Austin and Austin 1999). Hemorrhagic septicemia in fish fin and tail rot due to A. hydrophila infection resulted in high rate of mortality in aquaculture systems (Chakraborty et al. 1987; Hickman-Brenner et al. 1987; Barghouthi et al. 1989; Janda et al. 1994). A. hydrophila is an emergent human pathogen which caused serious health problem regularly around the globe (Janda and Abbott 1998; Abbott et al. 1998; Joseph and Carnahan 2000).
The most widely used method for controlling A. hydrophila infections in aquaculture is using antimicrobial drugs. Extensive use of antibiotic has resulted in rapid spread of multi-drug resistant pathogens (Rathore et al. 2006). There is an essential for controlling A. hydrophila infection using different antibiotics which is targeted the specific protein/enzyme of A. hydrophila. The unique ability of gyrase B to introduce negative supercoiling into DNA and itself controls DNA supercoiling. Gyrase catalyzes the conversion of relaxed, closed circular duplex DNA to negatively superhelical form, which is more favorable for recombination. The mechanism of supercoiling involves in wrapping of gyrase around a region of DNA, double strand breaks in that region, passing a second region of DNA through the breaking and rejoining of broken strands. The gyrB encodes B-subunit of DNA gyrase, a type-II DNA topoisomerase which have been reported as a suitable phylogenetic marker for bacterial identification (Yamamoto and Harayama 1996, 1998; Venkateswaran et al. 1998; Yamamoto et al. 1999, 2000).
There are several reports available on generation of three dimensional (3-D) structure of unknown protein using homology modeling. However, the 3-D structure of gyrB from A. hydrophila is not yet determined. Homology modeling has been used for generation of 3-D structures of 3-oxoacyl-acyl carrier protein synthase II of Mycobacterium tuberculosis (Singh and Somvanshi 2009a) and aerolysin as well as hemolysin proteins in A. hydrophila (Singh and Somvanshi 2009b; Singh et al. 2009). Therefore, several antibiotics and its derivative have been used against DNA gyrase for controlling the cell viability. Cyclothialidine is a potent drug against DNA gyrase which has been isolated from Streptomyces filipinensis (Goetschi et al. 1993). The amino substituted coumarins have synthesised and evaluated in vitro as inhibitors of DNA gyrase which is also showed antibacterials activity. Novobiocin like coumarins, 4-(dialkylamino)-methylcoumarins and 4-((2- alkylamino)ethoxy)coumarins have been discovered as gyrase B inhibitors with promising antibacterial activity (Laurin et al. 1999). However, single drug against particular protein is not very effective to control multidrug resistance A. hydrophila. Therefore, we need to understand the complete gene network involves in cellular activity.
The gene networks are a complex interaction of biological parts such as DNA, RNA and proteins contributed in biological process. In depth knowledge of biological process seems that we need to explore the relationship between network structure and the dynamics of genes, proteins and other biomolecules. There are number of reports available on gene and protein interaction and other gene networks which lead to target site for drug discovery (Samal and Jain 2008). It can also be helpful to repress and activate the gene networks for control of cellular activity. Some proteins serve to activate and repress other genes which are also known as transcription factors are playing key role in gene regulation. It binds with operator site on promoter region which is initiated the turn off and on process. The aim of present study is to develop homology based model of DNA gyrase B subunit in A. hydrophila and structure based potent drugs screening; and demonstrated the gene network of B subunit of DNA gyrase.
Materials and methods
Collection of sequences
The complete protein sequence of DNA gyrase B subunit of A. hydrophila and other species of Aeromonas were retrieved from NCBI (http://www.ncbi.nlm.nih.gov). The relatedness of sequence deposited in databases was evaluated by BLAST (Altschul et al. 1997) which is implemented in NCBI (http://www.ncbi.nim.nih.gov/blast). We performed BLASTP for searching of protein structural similarity with protein databank (PDB). The alignment was also done for query protein sequence with Protein Data Bank (PDB: 1EI1) template using CLUSTAL X.
Homology modeling for generation of 3-D structure
The crystal structure of DNA gyrase B of Escherichia coli was available at 2.30 Å resolution in PDB (PDB: 1EI1) and used as a template from protein data bank (http://www.rcsb.org/pdb/) to generate 3-D models. The homology modeling was used to generate the 3-D structure of gyrB using Modeller9v6 (Sali and Blundell 1993) and was visualized in PYMOL (Delano 2002). Evaluation of generated 3-D structure of gyrB was considered minimum model score and dope score. The 3-D structure of gyrB was validated with PROCHECK (Laskowski et al. 1993) which generates Ramachandran plot (RP); where the amino acid residues present in allowed, disallowed region and overall G- factor were considered.
Virtual screening of drugs by molecular docking
All drugs were taken from NCBI Pubchem compounds in SDF which is converted into 3-D structure using Open Babel 2.0.2 software. The generated 3-D structure of gyrB and drugs were used for molecular docking with AutoDock3.0.5 (Morris et al. 1998). The docking parameters were as follows: 100 docking trials, population size 150, random starting position and conformational translation step ranges of 1.5 Ǻ, rotation step ranges 35, elitism of 1, mutation rate 0.02, cross over rate of 0.8, local search rate of 0.06 and 25 million energy evaluations. Distance-dependent function of the dielectric constant was used for the calculation of the energetic maps and all other parameters were used by default value. We selected the drugs bind to 3-D of gyrB with highest binding affinity.
Construction and analysis of phylogenetic tree
The protein sequence of gyrB of A. hydrophila was used to search for homologous sequences by using BLASTP and homologous sequences were retrieved from NCBI-GenBank. The retrieved sequences were aligned in CLUSTALX (Thompson et al. 1997) and using the passion correction which is implemented in MEGA4.0 (Tamura et al. 2007) software for construction of phylogenetic tree by neighbor-joining (NJ) method. Total 100 bootstrapped values were sampled to determine a measure of the support for each node on the consensus tree.
Protein–protein interaction
We used STRING database of known and predicted protein interaction (http://string-db.org/). The interactions include direct (physical) and indirect (functional) associations; they are derived from four sources: (1) genomic context, (2) High-throughput experiments, (3) conserved coexpression and (4) literature knowledge. In the STRING quantitatively integrates interaction data from these sources for a large number of organisms and transfers information between organisms. It is currently covers 5,214,234 proteins from 1,133 organisms during protein–protein interaction.
Protein structure accession number
The homology model of 3-D structure of DNA gyrase B subunit of A. hydrophila subsp. hydrophila ATCC 7966 was submitted to Protein Model Database (http://mi.caspur.it/PMDB/) and assigned under PM0075580.
Results and discussion
DNA gyrase B subunit (NCBI accession number: ABK37959) of A. hydrophila was used for generation of molecular model using known 3-D crystal structure. The N-terminal region of gyrB of A. hydrophila contains ATP binding site which was confirmed from NCBI conserved domain database. It was resembled with DNA gyrase B subunit of E. coli. There are few drugs earlier reported against the inhibition of gyrase activity. Sometimes due to mutations in the active amino acid residues, bacteria showed the resistance to antibiotics. There is an important requirement to develop; screen and potent target based specific antibiotics for controlling of A. hydrophila infection. Total 25 isolates of A. hydrophila have been selected for antibiotic sensitivity assay. All these isolates were resistant to Cephalothin, Ampicillin, Novobiocin and Nitrofurazone while sensitive to Gentamicin (80 %), Co-trimaxazole (92 %), Chloramphenicol and Ciprofloxacin (Rathore et al. 2006).
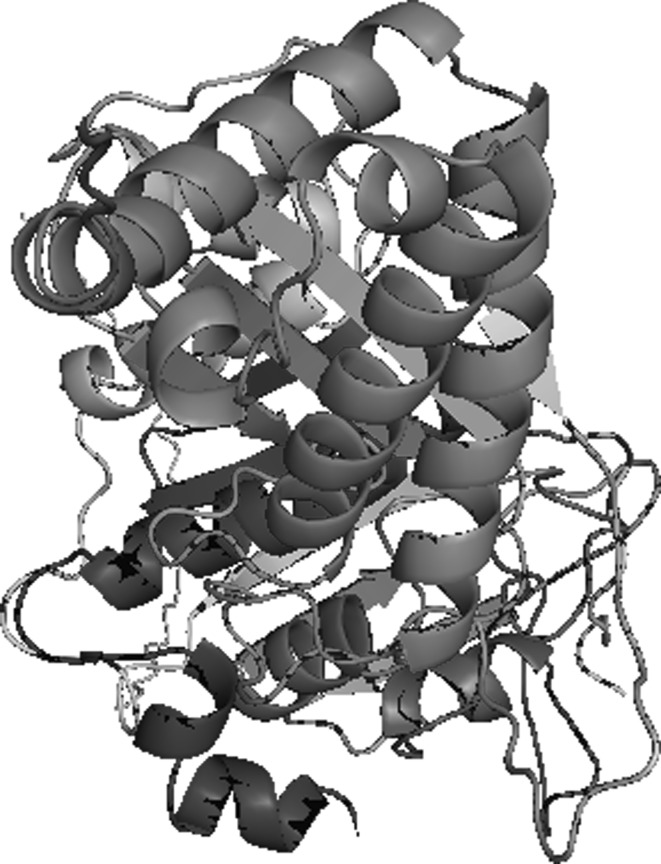
However, there is major difficulty for selection of suitable antibiotics for controlling of infection. Here, we were performed the homology modeling for generation of conserved protein DNA gyrase B subunit which showed 36 % identity with crystal structure of DNA gyrase B of E. coli. Both the protein sequences of gyrB of A. hydrophila and E. coli (PDB: 1EI1) were aligned. Total 5 models were generated by Modeller9v6 and the Gibbs free energy of gyrB of A. hydrophila was almost similar with template. The 3-D structure of gyrB of A. hydrophila was shown (Fig. 1) and it restrains alpha helix rich in the structure. Ramachandran plot (RP) for gyrB of A. hydrophila was shown (Supplementary Fig. S1) in the allowed and disallowed regions the amino acid residues were 80.9 and 1.6 % respectively. All these above properties of gyrB showed the good quality of 3-D structure.
Fig. 1.
The 3-D structure of DNA gyrase B subunit of Aeromonas hydrophila showing α + β sheets
There is recent report on the homology modeling has been used to fabricate the 3-D structure of aerolysin and hemolysin of A. hydrophila using known protein crystal structure (Singh and Somvanshi 2009b; Singh et al. 2009). The 3-D structure of gyrB of A. hydrophila was taken for screening of suitable antibacterial drugs based on highest binding affinity. The gyrB specific antibacterial drugs such as common name, IUPAC name, molecular formula, molecular weight and 2-D structure of each drugs used in this study were given (Supplementary Table 1). Eight suitable drugs were selected for molecular docking against whole 3-D structure of gyrB and three drugs Cinodine I, Cyclothialidine and Novobiocin were found highest binding affinity. On the basis of docking, the interaction of drugs with gyrB shown several energy such as docking energy, inter molecular energy, torsional energy, RMSD and internal energy were given (Table 1).
Table 1.
The interaction energy (kcal/mol) of DNA gyrase B and drugs obtained from the molecular docking
| S no. | Drugs | Binding energy (kcal/mol) | Docked energy (kcal/mol) | Inter molecular energy (kcal/mol) | Torsional energy (kcal/mol) | Internal energy (kcal/mol) | RMSD |
|---|---|---|---|---|---|---|---|
| 1. | Albamycin | −04.45 | −02.26 | −06.94 | 2.49 | 04.68 | 133.15 |
| 2. | Coumermycin | −07.13 | 19.55 | −10.87 | 3.74 | 30.42 | 149.55 |
| 3. | Cyclothialidine | −16.24 | −16.20 | −18.53 | 2.49 | 02.53 | 123.77 |
| 4. | Cinodine I | −11.51 | −09.14 | −17.11 | 5.60 | 07.97 | 145.20 |
| 5. | Novobiocin | −08.46 | −05.79 | −10.64 | 2.18 | 04.85 | 125.65 |
| 6. | Clerocidin | −08.67 | 07.95 | −13.96 | 5.29 | 21.91 | 131.25 |
| 7. | Nalidixic acid | −00.59 | −00.81 | −01.22 | 0.62 | 00.41 | 138.00 |
| 8. | Cathomycin | −03.02 | −03.94 | −04.57 | 1.56 | 00.63 | 136.03 |
In the present study, total 10 docking experiment were performed with the entire 3-D structural protein of gyrB which is considered lowest free energy of docked complex with hydrogen bonds. The docking energy of the Cinodine I, Cyclothialidine and Novobiocin were −09.14, −16.20 and −05.79 kcal/mol, respectively. The design and synthesis of a series of novel 2, 3-dihydroisoindol-1-ones structurally related to cyclothialidine 2 with DNA gyrase showed inhibition activity (Lübbers et al. 2007). The fragment-based design of potent DNA gyrase inhibitors has been reported. Using the virtual screening and NMR spectroscopy analysis identified the binding of two low-molecular weight fragments (2-aminobenzimidazole and indolin-2-one) to the 24 kDa N-terminal fragment of DNA gyrase B. In silico optimization of indolin-2-one led to the discovery of potent DNA gyrase inhibitors (Oblak et al. 2005).
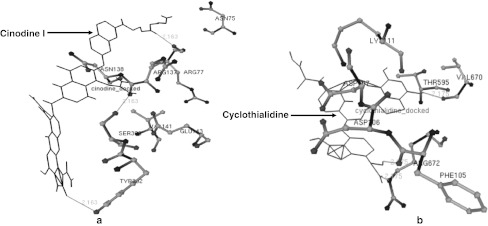
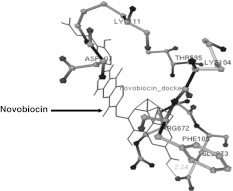
In the present study, several amino acids residue in 3-D structure of gyrB during the interaction with drug were given in Table 2. Amino acid residues such as Asn75, Arg137, Arg77, Asn138, Ser303, Val141, Glu143 and Tyr302 in 3-D structure of gyrB of A. hydrophila were observed with interaction of Cinodine I. The drug was bound with these amino acids of gyrB and three hydrogen bond (HB) were generated between UNK1:O-Ser303:OG, UNK1:O-Tyr302 UNK1:H-Arg77:O atoms with distance these 2.163, 2.163 and 2.163 Ǻ respectively (Fig. 2a). In second interaction, amino acid residues viz. Phe105, Arg672, Asp106, Asp107, Thr595, Lys111 and Val670 in gyrB of A. hydrophila were observed with interaction of Cyclothialidine. The drug was bound with these amino acids of gyrB and three HB were formed between drug and amino acids UNK0:H—Phe105:O, UNK0:H—Val670:O, UNK0:H-Arg672:O atoms with distance 2.175, 2.175 and 2.175 Ǻ respectively (Fig. 2b). The amino acid residues viz. Lys111, Thr595, Lys104, Phe105, Glu673, Asp107, Arg672 in gyrB of A. hydrophila were observed in the interaction with Novobiocin which was bound with these amino acids of gyrB and single hydrogen bond (HB) was formed in between UNK1:H—Glu673:OE1 atom with 2.24 Ǻ distance (Fig. 3). The amino acids of the 3-D structure of aerolysin of A. hydrophila have been targeted by saponin drug which could be useful for prevention of oligomerization on the surface of RBCs. Molecular docking has been performed against the aerolysin with the saponin and its analogs which showed to be potent for inhibition of oligomerzation (Singh and Somvanshi 2009b).
Table 2.
The summary of the active amino acid residues and hydrogen bond formed between the drugs and DNA gyrase B obtained through molecular docking
| S. No. | Protein designation | Active amino acids in gyrB | Drugs | Interaction of gyrB and drugs | Distance of hydrogen bonds (Ǻ) |
|---|---|---|---|---|---|
| 1. | gyrB | Asn75, Arg137, Arg77, Asn138, Ser303, Val141, Glu143, Tyr302 | Cinodine I | UNK1:O—Ser303:OG UNK1:H—Arg77:O UNK1:O—Tyr302 |
2.163 2.163 2.163 |
| 2. | gyrB | Phe105, Arg672, Asp106, Asp107, Thr595, Lys111, Val670 | Cyclothialidine | UNK0:H—Phe105:O UNK0:H—Val670:O UNK0:H—Arg672:O |
2.175 2.175 2.175 |
| 3. | gyrB | Lys111, Thr595, Lys104, Phe105, Glu673, Asp107, Arg672 | Novobiocin | UNK1:H—Glu673:OE1 | 2.240 |
Fig. 2.
The interaction of high affinity potent antibacterial drugs with gyrB of A. hydrophila showing the hydrogen bonds. a Cinodine I and b: Cyclothialidine
Fig. 3.
The interaction of high affinity potent Novobiocin antibacterial drugs with gyrB of A. hydrophila showing the hydrogen bonds
The 3-oxoacyl-acyl carrier protein synthase II of M. tuberculosis catalyses the initiation of fatty acid synthesis pathway by condensation of acyl CoA and mycolic acid during the elongation phase. The homology modeling has been used to generate and validate 3-D protein structure of 3-oxoacyl-acyl carrier protein synthase II. Thiolactomycin, Thiophenone and multidrug Cerulenin Isoniazed have been found potent for inhibition against M. tuberculosis docking experiment (Singh and Somvanshi 2009a). As we know that the evolutionary relationship of A. hydrophila based on DNA gyrase protein which is also present in other bacterial pathogen.
In this study, the phylogenetic tree was constructed using gyrB sequence of A. hydrophila and other bacteria. Total 3 major clades like Enterobacteriaceae, Vibrio and Aeromonas species were observed in phylogeny of gyrB protein sequences. A. hydrophila and A. salmonicida were observed in same clades; it indicates the similar antibacterial drugs such as Cinodine I, Cyclothialidine and Novobiocin can be used to inhibit the gyrase activity (Supplementary Fig. S2). Vibrio species were also closed with Aeromonas species; the same drugs might be helpful for inhibition of gyrase activity. The phylogeny indicates the gyrB is a stable and potential drug target for Aeromonas species especially A. hydrophila and A. salmonicida. The phylogenetic relationships of Aeromonas species have been reported using the sequences of gyrB. The nucleotide sequences of gyrB has been determined from 53 Aeromonas strains including some new strains, which were also characterized using 16S rDNA regions (Yanez et al. 2003). A. bestiarum and A. salmonicida have been reported for phylogenetic relationships on the basis of 70 strains using rpoD sequence. Whereas, the sequences of gyrB has already been proven for determining the phylogenetic relationship. Nucleotide sequences of rpoD and gyrB showed that both genes are similar substitution rates and a similar number of variable positions (Soler et al. 2004). The phylogenetic tree has been constructed using the aerolysin and hemomysin protein sequences of A. hydrophila and showed the homology with Aeromonas and other pathogenic bacteria (Singh and Somvanshi 2009b; Singh et al. 2009). In the above point, we have observed one protein targeted by single drug; sometime there is problem of drug activity because of high rate of mutation in gyrB. Therefore, we further move forward to understand the gene regulation.
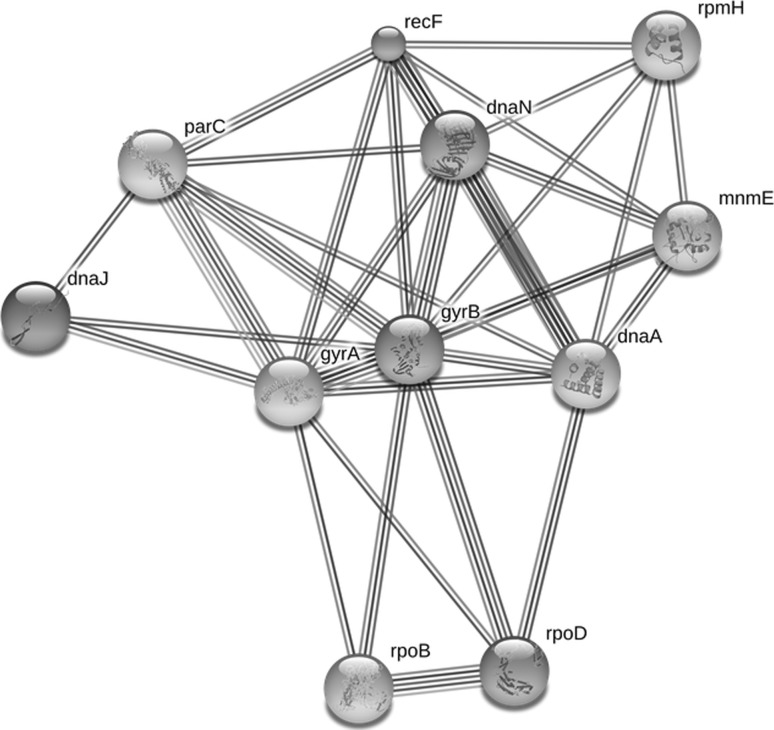
In the present study, we have shown role of DNA gyrase B in cellular system and also how to interact with other proteins/enzymes. B subunit; DNA gyrase negatively supercoils closed circular double- stranded DNA in an ATP-dependent manner and also catalyzes the inter conversion of other topological isomers of double-stranded DNA rings including catenanes and knotted rings. In the gyrB gene network, we found closely related proteins such as gyrA, dnaN, parC, recF and dnaA with >0.9 score (Table 3). We showed herein combinatorial influence of these newly constructed gene network of A. hydrophila that can use for regulation of gyrB gene for controlling cell physiology. Moreover we have shown protein–protein interaction in gyrB gene network (Fig. 4). In the Protein–protein interactions (PPI) occur when the two or several proteins/enzymes bind to each other often to carry out their biological function. Many of the molecular processes in cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein components organised by their protein–protein interactions. There is a global analysis of 2,709 interactions between proteins of Saccharomycescerevisiae has been performed and facilitated the establishment of a single large network of 2,358 interactions among 1,548 proteins (Schwikowski et al. 2000). The first step is needed to specifically define PPI which is commonly involved as physical interactions with molecular docking between proteins within a cellular systems. In several PPI repositories, it is a straightforward process to obtain all the proteins that interact with a given query protein which builds a corresponding network of molecular interactions (De Las Rivas and Montanillo 2010). To illuminate the architecture and dynamics of large scale genetic regulatory networks of cells is an important goal in systems and synthetic biology. The system level dynamical properties of the gene network of Escherichiacoli that regulates metabolism and show how its design leads to biologically useful cellular properties (Samal and Jain 2008). It is a new approach for targeting any gene regulation for the cellular mechanism by knowing the expected proteins involve in gene network.
Table 3.
Protein–protein interaction in gene network
| Functional proteins | Score | Potential functions |
|---|---|---|
| gyrA | 0.999 | DNA gyrase, A subunit |
| dnaN | 0.995 | DNA polymerase III, beta subunit; DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria |
| parC | 0.990 | DNA topoisomerase IV |
| recF | 0.987 | DNA replication and repair protein RecF; The recF protein is involved in DNA metabolism; it is required for DNA replication and normal SOS inducibility |
| dnaA | 0.977 | Chromosomal replication initiation protein; Plays an important role in the initiation and regulation of chromosomal replication. Binds to the origin of replication |
| rpoD | 0.875 | RNA polymerase sigma factor RpoD; Sigma factors are initiation factors that promote the attachment of RNA polymerase to specific initiation sites and are then released |
| rpoB | 0.741 | DNA-directed RNA polymerase subunit beta; DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates |
| dnaJ | 0.738 | Chaperone protein DnaJ; Participates actively in the response to hyperosmotic and heat shock by preventing the aggregation of stress-denatured proteins and by disaggregating proteins, also in an autonomous, dnaK-independent fashion |
| mnmE | 0.726 | tRNA modification GTPase TrmE; Exhibits a very high intrinsic GTPase hydrolysis rate. Involved in the addition of a carboxymethylaminomethyl (cmnm) group at the wobble position (U34) of certain tRNAs, forming tRNA- cmnm(5)s(2)U34 |
| rpmH | 0.706 | 50S ribosomal protein L34 |
Fig. 4.
Protein–protein Interaction of DNA gyrase B subunit (gyrB) of A. hydrophila with other proteins cellular activity in gene network
Conclusions
The present work was carried out to develop the 3-D structure of B subunit of DNA gyrase of A. hydrophila. The 3-D structure based screening for appropriate drugs was performed using docking and three antibacterial drugs such as Cinodine I, Cyclothialidine and Novobiocin could be found highest binding affinity with gyrB. The phylogeny of gyrB of A. hydrophila indicates that homologous gyrB protein may serve as a better target for the same drug which can also inhibit the growth of other bacteria. This study provides a new insight to control superfluous use of drugs in vitro trials. Construction of novel gyrB gene network of A. hydrophila plays a key role in gene regulation which provides a new conceptual framework for understanding the functional mechanisms of drugs and their cellular targets.
Electronic supplementary material
Acknowledgments
Authors are grateful to Anand Kumar Singh, Pritee Singh and Reena for providing the suggestions, encouragement and fruitful discussion during preparation of the manuscript.
Conflict of interest
There is no competing interest.
Contributor Information
Vijai Singh, Phone: +33-169475399, FAX: +33-169474437, Email: vijaisingh15@gmail.com.
Dharmendra Kumar Chaudhary, Phone: +33-169475399, FAX: +33-169474437, Email: chaudharydk12@gmail.com.
References
- Abbott SL, Seli LS, Catino M, Jr, Hartley MA, Janda JM. Misidentification of unusual Aeromonas species as members of the genus Vibrio: a continuing problem. J Clin Microbiol. 1998;36:1103–1104. doi: 10.1128/jcm.36.4.1103-1104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Thomas LM, Alejandro AS, Jinghui Z, Zheng Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B, Austin DA. Bacterial fish pathogens: diseases in farmed and wild fish. Chichester: Praxis Publishing; 1999. [Google Scholar]
- Barghouthi S, Young R, Olson MO, Arceneaux JE, Clem LW, Byers BR. Amonabactin a noval trypophan or phenylalanine- containing phenolate siderophore in Aeromonas hydrophila. J Bacteriol. 1989;171:1811–1816. doi: 10.1128/jb.171.4.1811-1816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T, Huhle B, Hof H, Bergbauer H, Goebel W. Marker exchange mutagenesis of aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in Aeromonas hydrophila associated systematic infection. Infect Immunol. 1987;55:2274–2280. doi: 10.1128/iai.55.9.2274-2280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las RivasJ, Montanillo C. Protein–protein interactions essentials: key concepts to building and analyzing interactome networks. PLoS Comput Biol. 2010;6:e1000807. doi: 10.1371/journal.pcbi.1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano WL. The PYMOL molecular graphics system. Palo Alto: DeLano Scientific; 2002. [Google Scholar]
- Goetschi E, Angehrn P, Gmuender H, Hebeisen P, Link H, Masciadri R, Nielsen J. Cyclothialidine and its congeners: a new class of DNA gyrase inhibitors. Pharmacol Therap. 1993;60(2):367–380. doi: 10.1016/0163-7258(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Hickman-Brenner FW, Mvdonald KL, Steiferwatl AG, Faning FR, Brenner DJ, Farmer JJ. Aeromonas veronii, a new ornithine decarboxylase positive species the may cause diarrhea. J Clin Microbiol. 1987;25:900–906. doi: 10.1128/jcm.25.5.900-906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes P, Niccolls LM, Sartory DP, et al. The ecology of mesophilic Aeromonas in the aquatic environment. In: Austin B, et al., editors. The genus Aeromonas. New York: Wiley; 1996. pp. 127–150. [Google Scholar]
- Janda JM. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. 1991;4:397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- Janda JM, Abbott SL, Cheung WK, Hanson DF. Biochemical identification of citrobacteria in the clinical laboratory. J Clin Microbiol. 1994;32:1850–1854. doi: 10.1128/jcm.32.8.1850-1854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SW, Carnahan AM. Update on the genus Aeromonas. ASM News. 2000;66:218–223. [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structure. J Appl Crystallogr. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- Laurin P, Ferroud D, Schio L, Klich M, Dupuis-Hamelin C, Mauvais P, Lassaigne P, Bonnefoy A, Musicki B. Structure-activity relationship in two series of aminoalkyl substituted coumarin inhibitors of gyrase B. Bioorg Medicinal Chem Lett. 1999;9:2875–2880. doi: 10.1016/S0960-894X(99)00492-8. [DOI] [PubMed] [Google Scholar]
- Lübbers T, Angehrn P, Gmünder H, Herzig S. Design, synthesis, and structure-activity relationship studies of new phenolic DNA gyrase inhibitors. Bioorg Medicinal Chem Lett. 2007;17:4708–4714. doi: 10.1016/j.bmcl.2006.12.065. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comp Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- Oblak M, Grdadolnik SG, Kotnik M, Jerala R, Filipič M, Šolmajer T. In silico fragment-based discovery of indolin-2-one analogues as potent DNA gyrase inhibitors. Bioorg Medicinal Chem Lett. 2005;15:5207–5210. doi: 10.1016/j.bmcl.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Rathore G, Singh V, Kumar G, Swaminathan TR, Mahanta PC. Antibiotic Sensitivity and characterization of Aeromonas hydrophila isolated from fish and water samples. J Ecophysiol Occup Health. 2006;6:41–43. [Google Scholar]
- Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restrains. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Samal A, Jain S. The regulatory network of E. coli metabolism as a Boolean dynamical system exhibits both homeostasis and flexibility of response. BMC Syst Biol. 2008;2:21. doi: 10.1186/1752-0509-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwikowski B, Uetz P, Fields S. A network of protein–protein interactions in yeast. Nat Biotechnol. 2000;18(12):1257–1261. doi: 10.1038/82360. [DOI] [PubMed] [Google Scholar]
- Singh V, Somvanshi P. Homology modeling of 3-oxoacyl-acyl carrier protein synthase II (KAS II) from Mycobacterium tuberculosis H37Rv and molecular docking for exploration of drugs. J Mol Model. 2009;15:453–460. doi: 10.1007/s00894-008-0426-5. [DOI] [PubMed] [Google Scholar]
- Singh V, Somvanshi P. Inhibition of oligomerization of aerolysin from Aeromonashydrophila: homology modeling and docking approach for exploration of hemorrhagic septicemia. Lett Drug Des Discov. 2009;6(3):215–223. doi: 10.2174/157018009787847864. [DOI] [Google Scholar]
- Singh V, Somvanshi P, Rathore G, Kapoor D, Mishra BN. Gene cloning, expression and homology modeling of hemolysin gene from Aeromonas hydrophila. Protein Expr Purif. 2009;65:1–7. doi: 10.1016/j.pep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Soler L, Yanez MA, Chacon MR, Aguilera-Arreola MG, Catalan V, Figueras MJ, Martınez-Murcia AJ. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int J Syst Evol Microbiol. 2004;54:1511–1519. doi: 10.1099/ijs.0.03048-0. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTALX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran K, Dohmoto N, Harayama S. Cloning and nucleotide sequence of the gyrB gene of Vibrioparahaemolyticus and its application in detection of this pathogen in shrimp. Appl Environ Microbiol. 1998;64:681–687. doi: 10.1128/aem.64.2.681-687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Harayama S. Phylogenetic analysis of Acinetobacter strains based on the nucleotide sequences of gyrB genes and on the amino acid sequences of their products. Int J Syst Bacteriol. 1996;46:506–511. doi: 10.1099/00207713-46-2-506. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Harayama S. Phylogenetic relationships of Pseudomonasputida strains deduced from the nucleotide sequences of gyrB, rpoD and 16S rRNA genes. Int J Syst Bacteriol. 1998;48:813–819. doi: 10.1099/00207713-48-3-813. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Bouvet PJM, Harayama S. Phylogenetic structures of the genus Acinetobacter based on gyrB sequences: comparison with the grouping by DNA–DNA hybridization. Int J Syst Bacteriol. 1999;49:87–95. doi: 10.1099/00207713-49-1-87. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kasai H, Arnold DL, Jackson RW, Vivian A, Harayama S. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiol. 2000;146:2385–2394. doi: 10.1099/00221287-146-10-2385. [DOI] [PubMed] [Google Scholar]
- Yanez MA, Catalan V, Apraiz D, Figueras MJ, Martınez-Murcia AJ. Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. Int J Syst Evol Microbiol. 2003;53:875–883. doi: 10.1099/ijs.0.02443-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.