Abstract
This single-institution phase II study was performed to estimate the response rate to lapatinib in neurofibromatosis type 2 (NF2) patients with progressive vestibular schwannoma (VS). Twenty-one eligible patients were enrolled. Brain and spine MRIs, including 3-dimensional volumetric tumor analysis, and audiograms were performed once at baseline and again every 12 weeks. The primary response end point was evaluable in 17 patients and defined as ≥15% decrease in VS volume. Hearing was evaluable as a secondary end point in 13 patients, with responses defined as an improvement in the pure tone average of at least 10 dB or a statistically significant increase in word recognition scores. Four of 17 evaluable patients experienced an objective volumetric response (23.5%; 95% confidence interval [CI], 10%–47%), with median time to response of 4.5 months (range, 3–12). In responders, reduction in VS volumes ranged from −15.7% to −23.9%. Four of 13 patients evaluable for hearing met hearing criteria for response (30.8%; 95% CI, 13%–58%). One sustained response exceeded 9 months in duration. Median time to overall progression (ie, volumetric progression or hearing loss) was 14 months. The estimated overall progression-free survival and volumetric progression-free survival at 12 months were 64.2% (95% CI, 36.9%–82.1%) and 70.6% (95% CI, 43.1%–86.6%), respectively. Toxicity was generally minor, and no permanent dose modifications were required. Lapatinib carries minor toxicity and has objective activity in NF2 patients with progressive VS, including volumetric and hearing responses. Future studies could explore combination therapy with other molecular targeted agents such as bevacizumab.
Keywords: acoustic neuroma, lapatinib, phase II trial, neurofibromatosis type 2, vestibular schwannoma
Neurofibromatosis type 2 (NF2)–related tumors, such as vestibular schwannoma (VS), are deficient in the NF2 protein Merlin, which is believed to act as a tumor suppressor. Although the mechanism through which Merlin controls cell proliferation remains poorly understood, recent studies have suggested that abnormal activation of receptor tyrosine kinases (RTKs), such as those in the epidermal growth factor receptor (EGFR or ErbB) family, may be of key importance.1–5 We have recently shown that both EGFR and ErbB2 are overexpressed and activated in VS and that lapatinib, an EGFR/ErbB2 inhibitor, has antitumor activity in a preclinical schwannoma model.6 Lapatinib reversibly inhibits EGFR and ErbB2, blocking phosphorylation and activation of Erk1/2 and Akt in EGFR- and/or ErbB2-expressing tumor cell lines and animal xenografts, with cytostatic or cytotoxic antitumor effects, depending on cell type.7 Lapatinib has been shown to have limited, predictable, and manageable side effects, such as diarrhea, rash, fatigue, and cardiotoxicity, and was approved by the FDA in 2007 for use in the treatment of ErbB2 (HER2) overexpressing advanced or metastatic breast cancer in combination with chemotherapy.8 Based on these encouraging preclinical data and a favorable safety profile, we designed a prospective phase II clinical trial to assess the antitumor activity of lapatinib in NF2 patients with progressive VS. To our knowledge, this study represents the first completed prospective phase II clinical trial evaluating an antitumor drug specifically in NF2 patients.
Patients and Methods
Patient Eligibility and Enrollment
Adult and pediatric patients (age >3 years) with a clinical diagnosis of NF2 according to the revised NIH criteria9 and at least one progressive VS (tumor growth or hearing progression within the past 12 months) were eligible. Histological confirmation was not required, because tumor biopsies are rarely indicated in this disease. For eligibility, tumor growth was defined as increase in tumor size of at least 2 mm in greatest diameter on conventional MRI10 or an increase in tumor volume of ≥15% as measured by 3-dimensional (3D) volumetric analysis. Progressive hearing loss was defined as a drop in pure tone average (PTA) of ≥10 dB at ≥2 consecutive or nonconsecutive frequencies or a drop in word recognition score (WRS) of ≥12% compared with the prior audiogram. Key eligibility criteria also included volumetrically measurable VS on MRI with size ≥0.5 cc, Karnofsky/Lansky performance score ≥50%, normal cardiac left ventricular ejection fraction by transthoracic echocardiogram, and adequate bone marrow, renal, and hepatic function. Exclusion criteria included any surgery within 4 weeks prior to enrollment, prior therapy with agents targeting EGFR or ErbB2, known preexisting cardiac disease, and concurrent therapy with cytochrome P450 inducers or inhibitors.
The study was conducted under a protocol approved by the institutional review board of NYU Langone Medical Center and registered at ClinicalTrials.gov (NCT00973739). Informed consent was obtained from the patients and guardians in accordance with institutional policies. All consecutive patients who met study entry criteria and who consented to participate were enrolled.
Study Design
This study was a prospective, open label, 2-stage phase II study. The primary and secondary end points were volumetric response and hearing response, respectively. To test the null hypothesis that the response rate is <5% versus the alternative that the response rate is ≥25%, a 2-stage Simon design was used.11 Nine patients were to be enrolled in stage 1. If at least 1 patient of these 9 had a volumetric response in stage 1 at any given evaluation point, an additional 8 patients were to be enrolled in stage 2. The overall alpha level for this design was 0.05, with a power of 80%. Lapatinib was to be considered effective and of interest for further study if, after successful completion of both stages, there were at least 3 responses in the combined stages.
Treatment
Lapatinib was supplied by GlaxoSmithKline and administered in continuous 4-week courses. Pediatric patients <18 years of age received 900 mg/m2 twice daily, up to a maximum dose of 750 mg twice daily, according to published phase I data.12 Patients ≥18 years of age received the standard recommended adult dose of 1500 mg once daily. For drug-induced diarrhea, a weight-based dose of loperamide was administered as needed. For treatment-related acneiform rash, clindamycin and benzoyl peroxide topical gel were prescribed as needed. Clinical evaluations, including a complete physical and neurological exam, complete blood count with differential, comprehensive metabolic panel, and serum pregnancy test (for females of child-bearing potential), were performed at baseline and every 4 weeks thereafter. To monitor for potential cardiotoxicity, echocardiograms and electrocardiograms were obtained at baseline and every 3 courses thereafter. Patients were allowed to remain on study unless volumetric progression, objective hearing deterioration, or unacceptable toxicity occurred. Adverse events were graded using version 3.0 of the NCI Common Toxicity Criteria (CTCAE). For treatment interruptions due to adverse events, therapy had to be held until toxicity was sufficiently improved, to grade ≤2 or ≤baseline.
Response Evaluation
Baseline MRIs were required within 30 days and baseline audiograms within 14 days prior to starting lapatinib. A target tumor was defined as any volumetrically measurable VS. Volumetric response (primary end point) and hearing response (secondary end point) were assessed at the end of every third 4-week course and compared with baseline. On-study imaging consisted of MRIs of the brain and entire spine, and 3D tumor volumetrics were obtained on postcontrast, T1-weighted magnetization-prepared rapid acquisition with gradient echo sequences, a 1-mm slice thickness, and no gap, using semi-automated segmentation software (Vitrea platform). As volumetric measurements are superior to traditional tumor measurements in regard to sensitivity, reliability, and reproducibility,13 volumetrics have become the modality of choice for defining and assessing imaging response in NF2 clinical trials.14,15 We defined volumetric response or progression as a ≥15% decrease or increase, respectively, in VS volume compared with baseline. The ≥15% threshold was determined after intraobserver variability was found to be negligible compared with the 15% change of interest for tumor volumes >0.5 cc in a pilot study (data not shown). Interobserver variability was also found to be low but was eliminated by assigning each patient to a specific radiology technician for volumetric measurement. Other NF2-related tumors, such as additional cranial nerve schwannomas and intracranial and intraspinal meningiomas and ependymomas, were also monitored radiologically and clinically.
Serial audiological evaluations were used to assess hearing response, including determination of pure tone thresholds and WRS. WRS was tested using the 50-item recorded CID (Central Institute for the Deaf)-W22 monosyllable word list. Hearing response or progression was defined as a clinically significant increase or decrease, respectively, in the WRS. WRS represents the most clinically relevant objective measure of hearing quality in NF2 patients and has therefore been suggested as a trial end point.14 PTA was calculated by the mean of the individual threshold frequencies at 500, 1000, 2000, and 4000 Hz and was recorded for each ear. An increase of ≥10 dB in the PTA between any follow-up assessment and the baseline value was considered hearing deterioration, while an improvement of ≥10 dB indicated a clinically significant improvement, as previously suggested.14
Pharmacokinetics
Blood samples for measurement of lapatinib plasma concentration were collected from pediatric patients immediately prior to a scheduled lapatinib dose, as well as at 2, 4, and 6 hours (optional) after, as previously described.16
Statistical Analysis
Progression-free survival (PFS) was measured from date of enrollment to date of volumetric or hearing progression. PFS was analyzed using the Kaplan–Meier method in terms of overall PFS (volumetric or hearing progression), volumetric progression, and hearing progression. Point estimates for PFS with 95% confidence intervals (CIs) were calculated from Kaplan–Meier curves.
Results
Patients
Twenty-one patients were enrolled between October 2009 and March 2011. There were 13 males (61.9%) and 8 females (38.1%), and participants were a median age of 28 years at enrollment (range, 10–51), including 4 pediatric patients <18 years. Three patients (patients 2, 3, and 5) had familial NF2; the remainder were sporadic NF2 patients. All patients or their legal representatives provided written informed consent for treatment. Four patients were nonevaluable (NE). Two patients were found to be ineligible after consenting to participate: patient NE3 had a volumetrically measured tumor size <0.5 cc but remained on protocol therapy as per the family's wish. Patient NE4 developed rapid tumor progression and was diagnosed with a malignant peripheral nerve sheath tumor arising within the VS. That patient's past medical history was remarkable for childhood medulloblastoma treated with surgery, chemotherapy, and radiation therapy. Two patients were nonevaluable due to coming off study prior to the first scheduled response evaluation: one was diagnosed with sarcoidosis (patient NE1) and one withdrew from the study for personal reasons (patient NE2). Patient NE3 elected to remain on study medication despite being found ineligible due to small tumor size <0.5 cc. Stage 2 of the study was opened for enrollment after the first response was observed on stage 1. Accrual to this study was closed after the planned enrollment of 17 evaluable subjects was reached, who had a total of 22 measurable (ie, target) VS tumors. Characteristics of all evaluable patients are summarized in Table 1.
Table 1.
Summary of general patient characteristics at enrollment (measurable tumor in eligible patients)
| Patient | Age (y) | Sex | Target Tumor Size Left/Right (mL) | Prior Treatment of Left/Right Tumors | Baseline WRS of Target Left/Right Tumor (%) | Baseline PTA of Target Left/Right Tumor (dB) |
|---|---|---|---|---|---|---|
| 1 | 22 | F | NA/7.72 | S/S | NA | NA |
| 2 | 21 | M | NA/26.80 | S/None | 4 | 46.25 |
| 3 | 13 | M | 1.54/0.61 | None/None | 84/NA | 28.75/115.00 |
| 4 | 30 | M | NA/51.51 | S/None | NA | NA |
| 5 | 51 | F | 0.64/NA | None/GK, S | 4 | 55.00 |
| 6 | 28 | M | 15.39/NA | None/RT, S | 72 | 38.75 |
| 7 | 22 | M | 13.96/6.10 | S/S | NA/NA | NA/NA |
| 8 | 10 | M | 5.77/NA | None/None | 96 | 26.25 |
| 9 | 51 | M | 3.21/3.18 | S, GK/S | NA/92 | NA/27.50 |
| 10 | 20 | M | NA/3.74 | RT/RT | NA | 76.25 |
| 11 | 28 | M | NA/0.90 | S/None | 84 | 33.75 |
| 12 | 19 | F | 1.06/NA | None/None | 100 | 2.50 |
| 13 | 35 | M | NA/1.08 | S/None | 100 | 12.50 |
| 14 | 29 | F | 2.19/NA | None/S | 96 | 21.25 |
| 15 | 46 | F | NA/1.13 | S/S | NA | NA |
| 16 | 19 | F | 1.10/0.59 | None/None | 36/NA | 66.25/NA |
| 17 | 16 | M | 2.62/1.28 | None/None | 100/92 | 11.25/47.50 |
Abbreviations: F, female; M, male; S, surgery; GK, gamma knife; RT, radiation therapy; PTA, pure tone average; WRS, word recognition score; NA, not applicable (for WRS, NA indicates complete deafness).
Treatment
The 17 evaluable patients received a total of 190 four-week courses of lapatinib. The median number of courses received was 12 (range, 3–21). Five patients did not complete the planned 12 months of therapy, all due to volumetric progression. One of these patients (patient 4) came off study for a tumor volume increase of >15%, although this initial measurement was revised down to +10.4% after a secondary review performed on all patients prior to publication disclosed a technical error in the volumetric measurement for this patient only. He therefore would have been eligible to continue on study in retrospect but remained in the progressive disease category for study analysis. Two patients progressed after receiving the 12 months of therapy. The estimated volumetric PFS at 12 months was 70.6% (95% CI, 43.1%–86.6%). Of note, 2 patients on lapatinib (patients 6 and 10) with stable VS and hearing suffered from progressively growing meningioma requiring surgical resection. Both patients continued on study with a brief interruption of lapatinib perisurgically.
The initial study design limited lapatinib to 12 cycles, but the protocol was subsequently amended to allow further treatment for patients with evidence of continued clinical benefit. Seven patients chose to continue on lapatinib beyond the 12th course, but all were eventually taken off study due to either MRI progression (n = 1), hearing deterioration (n = 1), increased size of the tumor's cystic component (n = 1), prolonged adverse event (delayed postsurgical wound healing; n = 1), or patient and/or family preference (n = 3).
Toxicity
All 21 enrolled participants were available for toxicity monitoring. Observed toxicity was generally minor (CTCAE 3.0 grades 1 and 2) and most commonly included rash (53%) and less commonly diarrhea, fatigue, nail changes, headache, and elevation of alanine aminotransferase and aspartate aminotransferase. A single patient (4.8%) experienced a grade 3 toxicity (ie, delayed wound healing possibly related to lapatinib after surgery for progressive meningioma). No cardiotoxicity and no grade 4 toxicity were observed.
Volumetric and Hearing Responses
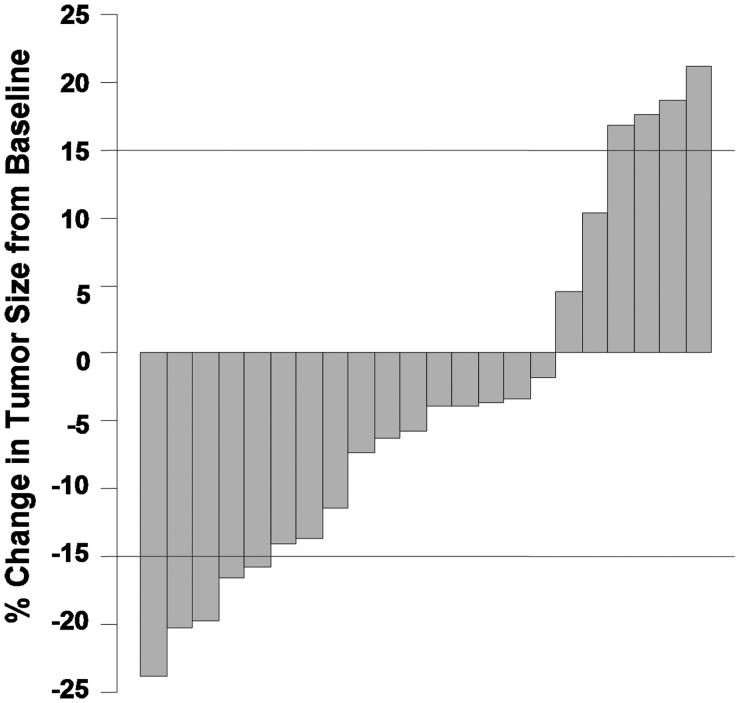
Baseline patient characteristics and responses to treatment are summarized in Tables 1 and 2, respectively, with additional details included in the online supplementary Table S1. Considering all target tumors, best volumetric change ranged from −23.9% (shrinkage) to +17.57% (progression) compared with the baseline measurement (see Fig. 3). We observed a volumetric response in 4 subjects (patients 6, 9, 10, and 13), with a median time to response of 4.5 courses (range, 3–12). The total volumetric response rate in evaluable patients was therefore 23.5% (95% CI, 10%–47%). In responders, reduction in VS volumes ranged from −15.74% to −23.9%. In addition, one subject (patient 9) had a significant volume reduction in both target VS tumors. Three of the responders had follow-up neuroimaging studies that showed a small increase in their tumor volumes, although none reached baseline tumor size.
Table 2.
Summary of evaluable patient treatment outcomes on study
| Patient | Treatment Duration (courses) | Best Reduction in Target Left/Right Tumor Volume (% baseline volume) | Progression of Target Left/Right Tumor Volume (% baseline volume) | Best Change in WRS in Target Left/Right ear (absolute score in %; difference from baseline) | Best Change in PTA (dB) for Target Tumor in Left/Right (absolute PTA; difference from baseline) | Reason for Treatment Discontinuation |
|---|---|---|---|---|---|---|
| 1 | 21 | −13.73 | NA | NA | NA | Patient preference |
| 2 | 3 | No reduction | +17.57 | 0 (−4) | 56.25 (+10.00) | R VS growth |
| 3 | 12 | −3.90/−11.48 | +20.78/+19.67 | 100/NA (+16) | 28.75/NA (0) | R and L VS growth |
| 4 | 3 | NA | +10.41 | NA | NA | R VS growth |
| 5 | 15 | −6.25 | NA | 44 (+40) | 51.25 (−3.75) | Patient preference |
| 6 | 21 | −16.57 | NA | 80 (+8) | 48.75 (+10.00) | Progressive hearing loss |
| 7 | 6 | No reduction | +10.89/+16.89 | NA/NA | NA/NA | R VS growth |
| 8 | 6 | No reduction | +21.14 | 100 (+4) | 21.25 (−5.00) | L VS growth |
| 9 | 12 | −23.90/−20.25 | NA/NA | NA/100 (+8) | NA/23.75 (−3.75) | Growth of R VS cystic component |
| 10 | 15 | −19.79 | NA | NA | NA | Delay in wound healing |
| 11 | 9 | −3.33 | NA | 96 (+12) | 31.25 (−2.50) | Progressive hearing loss |
| 12 | 12 | −14.15 | NA | 100 (0) | 3.75 (+1.25) | Completed 12 cycles |
| 13 | 12 | −15.74 | NA | 100 (0) | 10.00 (−2.50) | Completed 12 cycles |
| 14 | 16 | −7.31 | NA | 100 (+4) | 21.25 (0) | Patient preference |
| 15 | 12 | −1.77 | +22.12 | NA | NA | R VS growth |
| 16 | 3 | −3.64/NA | NA/+18.64 | 32/NA (−4) | 63.75/NA (−2.50) | R VS growth |
| 17 | 12 | −5.73/−3.91 | NA/NA | 100/96 (0/+4) | 10.00/40.00 (−1.25/−7.50) | Completed 12 cycles |
Boldface values signify that the value met the clinical definition for response.
Abbreviations: R, right; L, left; VS, vestibular schwannoma; WRS, word recognition score; PTA, pure tone average; NA, not applicable.
Fig. 3.
Waterfall plot of the percentage of change in tumor size, from baseline, for each evaluable ear. Each column represents a volumetrically measurable vestibular schwannoma. For each tumor, the best response while on study is shown. For tumors that did not show any volume reduction, the largest percentage of volumetric growth during therapy is indicated.
The distribution of the PTA and WRS for each ear is shown in the online supplementary Table S2. For all ears, the mean baseline PTA and WRS were 66.93 dB (SD ± 36.41) and 36.14% (SD ± 46.12), respectively. Four patients were deaf bilaterally at study enrollment and were therefore not evaluable for hearing response. Four patients (patients 3, 5, 9, and 11) experienced an improvement in their WRS sufficient to meet the definition of a clinical response, as established a priori. The responses were observed after a median of 3 courses (range, 3–9). Considering 4 responders of 13 evaluable patients, excluding deaf patients, the hearing response rate was 30.8% (95% CI, 12.7%–57.6%). Improvement in WRS was sustained in patient 3 only and exceeded 9 months in duration. The serial audiological measurements for each individual patient are available in the online supplementary Table S3. Of note, a combined volumetric and WRS hearing response of a tumor was observed in patient 9 only.
Regarding PTA, no patient had an improvement of ≥10 dB, indicative of a response. However, 2 patients reached the threshold of ≥10 dB deterioration in their PTAs, patient 3 (who experienced a concomitant deterioration in WRS) and patient 6 (who later suffered from a drop in WRS); the data are summarized in the online supplementary Table S4.
Progression-free Survival and Median Time to Progression
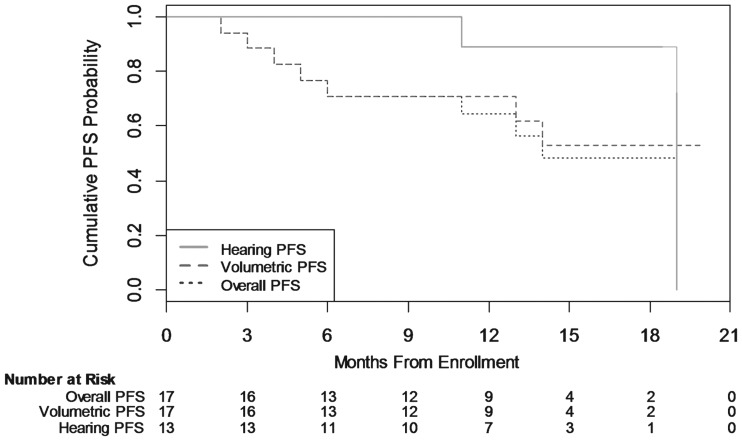
Seven patients experienced volumetric progression, and 2 patients experienced hearing progression. At 12 months, estimated PFS was 70.6% (95% CI, 43.1%–86.6%) for volumetrics, 88.9% (95% CI, 43.3%–98.4%) for hearing, and 64.2% (95% CI, 36.9%–82.1%) for overall progression (ie, volumetric or hearing progression). The median time to progression (hearing or volumetric) was 14 months (see Fig. 1).
Fig. 1.
Cumulative progression-free survival (PFS) probability. This figure illustrates the volumetric, hearing, and overall PFS probability using the Kaplan–Meier method, as measured from date of enrollment to date of progression for all evaluable patients. “Overall PFS” is defined as either volumetric or hearing progression.
Pharmacokinetics
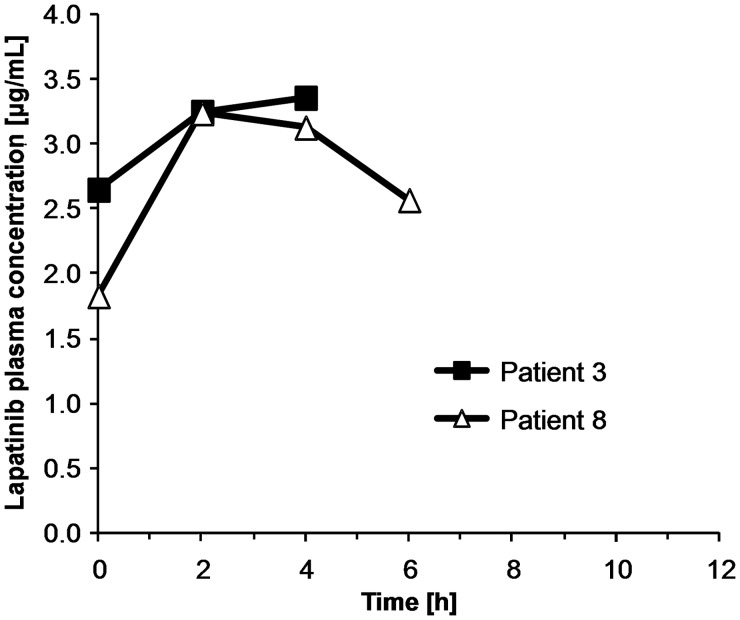
Informed consent/assent was required for pharmacokinetic blood sampling and was provided by 2 of 3 evaluable pediatric patients. The maximum plasma concentrations observed were 3.24 and 3.36 μg/mL (see Fig. 2), similar to prior pharmacokinetic data from a pediatric phase I trial, which showed a median peak plasma concentration at steady state of 6.2 μg/mL (range, 3.1–10.3) at the same dose level used in our study (ie, 900 mg/m2 twice daily).12
Fig. 2.
Pharmacokinetic profiles of lapatinib in plasma from 2 pediatric patients receiving doses of 900 mg/m2 twice daily.
Discussion
NF2 is an autosomal-dominant genetic disease with an incidence of approximately 1 per 30 000.17 The majority of NF2 patients develop progressive hearing loss in adolescence or young adulthood due to unilateral or bilateral VS.18 Additional intracranial and spinal tumors, including meningiomas, schwannomas, and ependymomas, are also highly prevalent.19 NF2-related tumors, although mostly slow growing, cause considerable morbidity and mortality, particularly when diagnosed at a young age, and result in a significantly reduced life expectancy. The available treatment options for these neoplasms, which often occur at multiple sites simultaneously, are noncurative and mostly limited to surgery and/or radiation therapy. Surgical resection of VS often leads to complete deafness and facial nerve injury, while radiation therapy may increase the risk for developing secondary tumors.20 Therefore, effective medical treatment options are urgently needed for this disease.
Although chemotherapy is effective for low-grade brain tumors, such as astrocytomas, no such therapy has been validated in NF2. However, many conventional chemotherapeutic agents are unsuitable for NF2 patients due to neuro- and/or ototoxicity, and clinicians are reluctant to use mutagenic chemotherapy in patients with loss of tumor suppressor function, such as NF2 patients. Presently, no known effective chemotherapy option exists for the treatment of schwannomas. However, recent retrospective studies have shown that bevacizumab, a monoclonal anti–vascular endothelial growth factor (VEGF) antibody, may result in tumor shrinkage and hearing improvement in patients with NF2 and VS,21,22 although “rebound” tumor growth has been observed after discontinuation of the drug.23 Based on encouraging preclinical data6 and a favorable safety profile, we conducted a phase II clinical trial using lapatinib in NF2 patients with progressive VS.
Lapatinib was generally well tolerated in this patient population, with toxicities that were usually mild and manageable.
Our volumetric and audiological response rates of 23.5% and 30.8%, respectively, appear superior to a recently published retrospective series of 10 patients treated with erlotinib, an EGFR inhibitor.24 In that series, no objective volumetric responses in the primary target tumors were observed, although 3 patients experienced stable disease volumetrically, with maximum tumor shrinkage of −14% in 1 patient. Prolonged stable disease was observed in 3 patients, and only 1 transient hearing response by WRS was observed. The median time to progression for either tumor growth or hearing loss in our study was 14 months, which is preferred over the 7.1 months in the erlotinib series.
Suggested response criteria for NF2 clinical trials14 published after the conception of this study have proposed to define a response as a reduction of ≥20% in tumor volume and to consider a reduction between 5% and <20% as a minor response. According to these response definitions, we would have obtained major and minor response rates of 5.9% (1 of 17 evaluable patients) and 52.9% (9 of 17 evaluable patients), respectively.
We did not observe any imaging responses in meningiomas, and these tumors continued to progress in many of our patients during the study period. These observations are consistent with recently published results of a phase II trial using the EGFR inhibitors erlotinib and gefitinib in patients with progressive meningiomas, where no response was seen.25
The only other antitumor drug reported in the literature leading to tumor shrinkage and hearing improvement in NF2 patients is bevacizumab. The first and, to date, largest retrospective series of NF2 patients treated with bevacizumab showed a reduction in VS size in 9 of 10 patients (90%), with a range of −5% to −44% in tumor volume.21 Applying our volumetric response criterion of ≥15% in volume reduction, 7 of 10 patients (70%) were responders in that study, which appears superior to the 23.5% observed in our prospective trial. In the bevacizumab series, 4 of 7 patients (57.1%) had significant hearing improvement, which also appears superior to our results with lapatinib.
One possible limitation of lapatinib's activity in our study might be suboptimal drug delivery to tumor tissue. Although responses to lapatinib have been observed in some HER2-positive breast cancer patients with brain metastases,26,27 it is not known whether VS is protected by the blood–brain barrier, the blood–nerve barrier, or the blood–cerebrospinal fluid barrier. The achievable tissue concentration of lapatinib in VS may be lower than that in tumors outside the CNS, limiting efficacy. Clinical data on brain metastasis show tissue lapatinib concentrations averaging approximately 7-fold higher than plasma concentrations.28 To determine the achievable intratumoral lapatinib concentration in VS and the effects on EGFR/ErbB2 signaling, we are currently conducting a lapatinib “phase 0” or pharmacodynamic trial in patients undergoing VS surgery (ClinicalTrials.gov identifier NCT00863122).15
In summary, our study indicates that lapatinib is well tolerated and promotes antitumor activity, including hearing responses, in a subset of NF2 patients with progressive VS. Our study results serve as a valuable benchmark for comparison with future efficacy trials in this patient population. Further studies will be needed to better define the role of lapatinib in the treatment of VS in NF2 patients, and possible combination therapies with other molecular targeted agents should be explored. In a variety of preclinical models, the EGFR/ErbB2 signaling pathway and VEGF-dependent angiogenesis are functionally linked, and VEGF may play a role in the acquired resistance to ErbB receptor antagonists.29 Combination therapy with bevacizumab and lapatinib showed activity in a recent phase II study for HER2-overexpressing metastatic breast cancer in a heavily pretreated patient population30 and should be investigated in NF2 patients as well.
Supplementary Material
Acknowledgments
We are grateful to Erin Hartnett and Carole W. Mitchell for excellent study-related patient care. This study was presented in part at the 14th International Symposium on Pediatric Neuro-Oncology in Vienna, Austria, June 2010, the Pediatric Neuro-Oncology Basic and Translational Research Conference, New Orleans, LA, May 2011, and the Children's Tumor Foundation NF Conference, Jackson Hole, WY, June 2011.
Conflict of interest statement. M. A. K. received funding for this study, as well as for a separate pharmacokinetic/pharmacodynamic study with lapatinib from GlaxoSmithKline under institutional clinical trial agreements. K. M. K. is an employee of GlaxoSmithKline and has stock ownership in the company. All other authors declare that they have no relevant conflicts of interest.
Funding
This study was supported in part by GlaxoSmithKline, Inc. and National Institutes of Health (grant P30 CA16087 to J. D. G and Cancer Center Support Grant to NYU School of Medicine).
References
- 1.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004;23(8):1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Suzuki K. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp Cell Res. 1996;226(1):214–222. doi: 10.1006/excr.1996.0221. [DOI] [PubMed] [Google Scholar]
- 3.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty JK, Ongkeko W, Crawley B, Andalibi A, Ryan AF. ErbB and Nrg: potential molecular targets for vestibular schwannoma pharmacotherapy. Otol Neurotol. 2008;29(1):50–57. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- 5.Evans DG, Kalamarides M, Hunter-Schaedle K, et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin Cancer Res. 2009;15(16):5032–5039. doi: 10.1158/1078-0432.CCR-08-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12(8):834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon S, Wagstaff AJ. Lapatinib. Drugs. 2007;67(14):2101–2108. doi: 10.2165/00003495-200767140-00008. discussion 2109–2110. [DOI] [PubMed] [Google Scholar]
- 9.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278(1):51–57. [PubMed] [Google Scholar]
- 10.Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27(4):547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 11.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 12.Fouladi M, Stewart CF, Blaney SM, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(27):4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurg. 2008;62(6):1314–1319. doi: 10.1227/01.neu.0000333303.79931.83. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin SR, Halpin C, Blakeley JO, et al. Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol. 2009;93(1):61–77. doi: 10.1007/s11060-009-9867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2011;158A(1):24–41. doi: 10.1002/ajmg.a.34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai F, Freeman BB, 3rd, Fraga CH, Fouladi M, Stewart CF. Determination of lapatinib (GW572016) in human plasma by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831(1–2):169–175. doi: 10.1016/j.jchromb.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 18.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603–618. [PubMed] [Google Scholar]
- 19.Mautner VF, Tatagiba M, Lindenau M, et al. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol. 1995;165(4):951–955. doi: 10.2214/ajr.165.4.7676998. [DOI] [PubMed] [Google Scholar]
- 20.Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43(4):289–294. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, II, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12(1):14–18. doi: 10.1093/neuonc/nop010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mautner VF, Nguyen R, Knecht R, Bokemeyer C. Radiographic regression of vestibular schwannomas induced by bevacizumab treatment: sustain under continuous drug application and rebound after drug discontinuation. Ann Oncol. 2010;21(11):2294–2295. doi: 10.1093/annonc/mdq566. [DOI] [PubMed] [Google Scholar]
- 24.Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG., 2nd. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 28.Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2011;29(3):770–781. doi: 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nature Clin Prac. 2008;5(9):521–530. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 30.Rugo HS, Jo Chien A, Franco SX, et al. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. doi: 10.1007/s10549-011-1918-z. [published online ahead of print December 24, 2011] doi:10.1007/s10549-011-1918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.