Abstract
As for lineages of known methanogens, several lineages of uncultured archaea were recurrently retrieved in freshwater sediments. However, knowledge is missing about how these lineages might be affected and structured according to depth. In the present study, the vertical changes of archaeal communities were characterized in the deep sediment of the freshwater meromictic Lake Pavin. For that purpose, an integrated molecular approach was performed to gain information on the structure, composition, abundance and vertical stratification of archaeal communities thriving in anoxic freshwater sediments along a gradient of sediments encompassing 130 years of sedimentation. Huge changes occurred in the structure and composition of archaeal assemblages along the sediment core. Methanogenic taxa (i.e. Methanosaeta and Methanomicrobiales) were progressively replaced by uncultured archaeal lineages (i.e. Marine Benthic Group-D (MBG-D) and Miscellaneous Crenarchaeal Group (MCG)) which are suspected to be involved in the methane cycle.
Introduction
The high densities of viable prokaryotes in marine or freshwater sediments (between 109 and 1010 cells.cm−3 of sediment) have prompted microbiologists to consider the metabolic roles of the sediment microbiota in the cycling of nutrient elements [1]: oxidation of deposited organic matter, regeneration of inorganic nutrients and transformation of those inorganic materials [2]. In those environments, the complete mineralization of complex organic matter involves a variety of anaerobic prokaryotes operating in close interactions [3]. These microbial interactions are a classic example of microbial interdependency following the concept of the “anaerobic food chain” borrowed from ruminant microbiology [4]. In anaerobic sediments, these processes may be viewed as sequential in space or in time, as recently deposited material moves deeper into the sediments and through different “microbial zones” successively dominated by a terminal-electron-accepting process (i.e. denitrification, iron reduction, sulfate-reduction and methanogenesis [5]).
Whereas these global patterns remain valid, research performed in the last two decades has extended our knowledge of the diversity and function of the sedimentary microbiota, particularly those of the “most enigmatic of life’s three domains”, the Archaea. A striking example is the discovery of the anaerobic methane oxidation process (AOM) performed by a microbial consortium involving the ANaerobic MEthanotrophs (ANME) archaea in marine sediments [6]–[8], and probably other archaeal groups in freshwater systems [9]. Another major advance is the identification of number of uncultured archaeal lineages with unknown functions in ecosystems with moderate environmental conditions, including marine and freshwater sediments [10].
In comparison to marine sediments, archaeal community structure and composition were less documented in freshwater lake sediments and studies were mainly focused on the methanogenic archaea of the surficial sediment layer [11]–[14]. While a general view of the dominant archaeal lineages inhabiting freshwater sediments has begun to emerge from these studies, knowledge is missing about how these lineages might be affected and structured according to depth and changing environmental factors. Among freshwater systems, meromictic lakes are rare and of considerable interest to microbial ecologists because their permanent anoxic layers exhibit potential undisturbed climax microbial communities and because of their relationship to an earlier biosphere [15]. Lake Pavin in France provides such an environment. The sedimentary compartment of Lake Pavin, permanently surmounted by a 30 m anoxic water column, is thus unusual and provides a special opportunity for investigation of the sediment microbiota, e.g. stability of physical parameters (temperature, sedimentation rates), steady state of the above anoxic water column and low availability of inorganic electron acceptors [16]–[19].
The present study is an exploratory work to investigate the structure and composition of archaeal communities thriving in anoxic freshwater sediments along a gradient of sediments encompassing 130 years of sedimentation. The issues raised in this study are as follows: (i) is archaeal communities important component (quantitative) of microbial communities inhabiting this environment, (ii) are methanogenic groups dominating the Archaea? and if not, which archaeal groups are retrieved? (iii) is Archaea exhibiting a spatial pattern consistent with changes in environmental factors (e.g. organic matter). To these aims, an integrated fine-scale microbial community structure analysis was performed, using multiple molecular approaches including fingerprint patterns, quantitative polymerase chain reaction (qPCR) and 16S rRNA analyses.
Materials and Methods
Site Description
No specific permits were required for the described field studies, as the location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species.
Lake Pavin, located at 45°55 N and 2°54 E, is the youngest volcano crater lake in the French Massif Central (6,000 years BP). Lake Pavin has a circular shape, an area of 0.44 km2 and a maximum depth of 92 m, at an elevation of 1,197 m above sea level. Lake Pavin is the unique meromictic lake in France and is characterized by the presence of two permanent stratified layers. The upper layer (mixolimnion) extends from the surface to 60 m depth and is affected by mixing during fall and spring. The deepest layer (monimolimnion) extends from 60 to 92 m depth and includes the chemocline (60- to 70-m). The conductivity in the monimolimnion increases from 40 to 340 µS.cm−1 [17] and is in the range of that measured in freshwater lakes. Depth profiles of nutrients, oxygen, temperature, conductivity, methane, carbon dioxide, sulfate, nitrate, iron, and trace element concentrations in the anoxic water column have been published previously [16]–[20]. Two papers were also related to bacterial counts, structure and phylogenetic diversity of bacterial and archaeal communities in the anoxic zone of Lake Pavin [15], [20].
Sampling Procedure
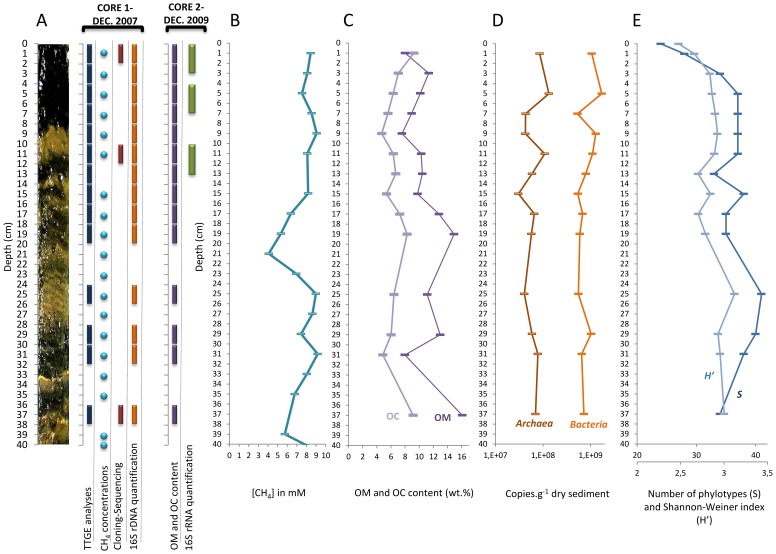
Two 40 cm-sediment cores, representing approximately 130 years of sedimentation history of the lake [21], were sampled in December 2007 (sediment core 1) and in December 2009 (sediment core 2), at the maximum depth zone of the lake, using an Uwitech gravity corer. The sediment cores were sectioned into segments and homogenized aseptically under N2 flux to preserve anaerobic conditions. Sub-samples of the homogenized sections were stored at −20°C for DNA-based analyses. On sediment core 2, subsamples were pooled and were frozen in the field and kept at −80°C until RNA extraction. The sampling strategy and the experiments conducted on the different sediment-cores are presented in the Figure 1A. Although no temporal study has been conducted on Lake Pavin sediments, the steady state of the anoxic water column [16]–[19] suggests that temporal variations in the physicochemical composition of the sedimentary compartment are limited. Accordingly, temporal changes in the stratification of benthic microbial communities are expected to be low. This assumption is supported by a study on the meromictic Lake Kaiike which has reported minor seasonal changes in the stratification of benthic bacterial communities [22]. Physicochemical and biological differences between the two sediment cores analyzed in the present study are obviously not excluded. However, in the particular context of meromictic lakes and considering that sampling was performed in the same season (December), data from the two sediment cores were interpolated.
Figure 1. Sampling strategy and stratification of abiotic and biotic features in Lake Pavin sediments.
(A) Sampling strategy: the depths where analyses were performed on each sediment core are indicated by circles or rectangles. (B) Methane profile (concentration in mM) along the sediment core 1. (C) Organic matter (OM) and organic carbon (OC) content determined from Lake Pavin sediment core 2. (D) Abundance of Bacteria and Archaea along the sediment core 1, each symbol represents the average value of duplicate quantifications. (E) Vertical changes of the number of archaeal phylotypes (richness, S, on the horizontal axis 20 to 40) and diversity (Shannon diversity index, H’, on the horizontal axis 2 to 3.5) along the sediment core 1. The number of phylotypes corresponds to the number of bands on the TTGE gel. The diversity was calculated according to the number of TTGE bands and their relative intensity.
Organic Carbon and Methane Analyses
Organic carbon and organic matter were measured using a modification of the procedure outlined in Buckley et al. [23]. Subsample of homogenized sediment (3 mL) was dried to constant mass for analysis of bulk density, water content, and porosity using standard methods. Dried sediment aliquots were placed in an acid fumer for 24 h to remove inorganic carbon and subsequently dried. These samples were split for organic carbon analysis using a Carlo Erbo elemental analyzer, or combusted at 375°C for 24 h in air for the oxidation of heat-labile organic matter components. The latter fraction is operationally termed “organic matter” as described previously [23]. Methane concentrations were determined as described by Lopes et al. [17].
DNA and RNA Extraction
Genomic DNA was extracted using the Ultra clean® soil DNA extraction kit (MOBio Laboratories, Inc) according to the manufacturer’s instructions. Total RNA was extracted and separated from DNA as previously described by Purdy et al. [24]. Residual DNA was digested with Ambion® TURBO DNA-free kit (Applied Biosystems) according to rigorous DNase treatment protocol. According to this procedure the step of DNA digestion was repeated one more time and the volume of DNase inactivation reagent was doubled.
Clone Library Construction and Phylogenetic Analyses
Archaeal 16S rRNA genes (hereafter referred to as “16S rDNA” for a better distinction with 16S rRNA transcripts) were amplified using the set of primers 21f and 1386r (Table 1, [25], [26]). The PCR reaction mixture (50 µl) contained 5 µl of 10 X reaction buffer, 2.5 mM MgCl2, 200 µl of each deoxyribonucleotide triphosphate (dATP, dCTP, dGTP, dTTP; Eurobio), 200 nM of each primer, 250 ng/µl of bovine serum albumin (BSA), 1.5 U of Taq Hotstar (Qiagen) and 1 µl of genomic DNA.
Table 1. Primers and amplification features used in this study.
| Approach | Targeted group | Primer set | Sequence (5′ –>3′) | Amplicon size | Annealing T°C | Reference |
| Cloning | Archaea | 21f | TTCCGGTTGATCCYGCCGGA | 1360 | 60 | [25] |
| 1386r | GCGGTGTGTGCAAGGAGC | [26] | ||||
| TTGE | Archaea | 934fGC* | GAATTGGCGGGGGAGCAC | 490 | 60 | [26] |
| 1386r | GCGGTGTGTGCAAGGAGC | [26] | ||||
| qPCR | Bacteria | BAC338f | ACTCCTACGGGAGGCAG | 470 | 60 | [41] |
| 515r | ATTACCGCGGCTGCTGGCA | [39] | ||||
| Archaea | 934f | GAATTGGCGGGGGAGCAC | 105 | 60 | [26] | |
| 1040r | GGCCATGCACCWCCTCTC | [40] | ||||
| Crenarchaeota | 771f | ACGGTGAGGGATGAAAGCT | 227 | 60 | [38] | |
| 957r (modified) | CGGCGTTGACTCCAATTR | This study ** | ||||
| Methanomicrobiales | MMB282f | ATCGRTACGGGTTGTGGG | 506 | 60 | [41] | |
| MMB832r | CACCTAACGCRCATHGTTTAC | [41] | ||||
| Methanosaetaceae | Mst702f | TAATCCTYGARGGACCACCA | 164 | 60 | [41] | |
| Mst862r | CCTACGGCACCRACMAC | [41] | ||||
| MBG-D | 490f | GAGAGTAAGRGCTGGGTA | 348 | 58 | This study | |
| 818r | ACTAACATCAAGCRAGCAG | This study |
Amplifications were performed with a PTC-200 thermal cycler (MJ Research) using the following program: a 15 min hot start at 95°C, followed by 35 cycles consisting of denaturation (1 min at 95°C), annealing (1 min at 58°C) and extension (1 min at 72°C), with a final extension for 10 min at 72°C. PCR products were cloned using a TOPO TA cloning Kit according to the manufacturer’s instructions (Invitrogen). Cloned inserts were PCR amplified using the M13 forward and reverse primers and amplicons were digested with the restriction endonuclease HaeIII (Qbiogene) at 37°C for 12 h. Digests were analyzed by gel electrophoresis using 2.5% (wt/vol) Nusieve 3∶1 agarose (Tebu-Bio) gels containing ethidium bromide (0.5 mg.L−1) normalized with a 100 bp size marker (Invitrogen). Visual analyses of restriction fragment length polymorphism (RFLP) patterns were performed and banding patterns were grouped according to similarity. Plasmid DNAs from representatives of each RFLP pattern were isolated using the QIAprep plasmid purification kit (Qiagen, Chatsworth, Calif.). Clones were sequenced by Eurofins MWG Operon (Ebersberg, Germany) using M13 forward and reverse primers, and clone libraries were screened for chimeric sequences with the Bellerophon program available at http://foo.maths.uq.edu.au~/huber/bellerophon.pl [27].
Sequences were compared to available databases using the BLAST network service to determine approximate phylogenetic affiliations. Sequences were aligned with CLUSTAL W [28], and phylogenetic trees were computed using neighbor-joining approaches with MEGA 5 software (available at http://www.megasoftware.net/ [29]). The robustness of inferred topologies was tested by bootstrap analysis and 1000 resamplings of trees. Sequences exhibiting more than 97% of similarity were grouped into the same Operational Taxonomic Unit (OTU). Coverage values of clone libraries were calculated as previously described [30]. Sequences were deposited in Genbank under accession No. GU135459-GU135502. Sorensen similarity (Cs) and Bray Curtis dissimilarity indices were calculated as previously described [31].
Temporal Temperature gel Gradient Electrophoresis (TTGE) Analyses
Archaeal 16S rDNA were amplified using the set of primers 934f-GC/1386r (Table 1, [26], [32]). The reaction mixture (50 µl) contained the same component as described above (see clone library construction). A touch-down PCR was performed using the following program: a 15 min hot start at 95°C, 5 cycles consisting of denaturation (1 min at 95°C), annealing (1 min at 65°C) with a decrease of 1°C per cycle, and extension (1 min at 72°C), followed by 30 cycles consisting of denaturation (1 min at 95°C), annealing (1 min at 60°C), and extension (1 min at 72°C), with a final extension for 10 min at 72°C. The PCR products were quantified with the DNA quantitation kit fluorescence assay (Sigma-Aldrich) and 300 ng of each sample were electrophoresed through a 8% polyacrylamide gel (TAE 1.25 x, urea 7 M, Temed 0.06%, ammonium persulfate 0.0625%) as previously described [33]. Band patterns were analyzed using the GelCompare 4.6 software package (Applied Maths, Kortrijk, Belgium). A 1% band position tolerance (relative to total length of the gel) was applied in band assignment, which indicates the maximal shift allowed for two bands in different TTGE tracks to be considered as identical. Pairwise similarity matrices were calculated using the Jaccard equation from presence/absence data. Dendrograms were generated using UPGMA method [34]. A distance of 40% was used to separate clusters in the hierarchical classification. Relationships among samples were visualized using the ordination technique multidimensional scaling (MDS) using a standardized stress with 1000 iterations computed with XLSTAT version 6.01. Analysis of similarity (ANOSIM, [35]) was used to test the hypothesis that communities within each cluster were more similar to each other than to communities in other clusters. The number of bands in a profile was expressed as the phylotype richness (S). The Shannon diversity index (H’) was calculated from the number of bands and their relative intensity.
Quantitative PCR and RT-qPCR Analyses
PCR primer design and modification
The primers 490f and 818r targeting MBG-D were designed in this study (Table 1, Figure S1) and were analyzed with Beacon Designer program (available at http://www.premierbiosoft.com/qOligo/Oligo.jsp?PID=1) in order to avoid hairpins, self- and heterodimers. Specificity of primers 490f and 818r was checked in silico using ProbeCheck ([36], available at http://www.microbial-ecology.net/probecheck) querying RDP II database version 9.61 [37]. In situ analysis, performed by sequencing clone libraries constructed with the set of primers 490f and 818r (according to experimental procedure described above), confirmed primer specificity. The primer 957r targeting Crenarchaeota [38] was modified (R instead of G at the 3′ end) to remove one mismatch with most of the Crenarchaeota sequences retrieved in clone libraries constructed in this study (Table 1). With this modification, the sequences of the main euryarchaeotal lineages found in Lake Pavin kept, at least, 4 mismatches with the two primers targeting the Crenarchaeota. In order to estimate the consequences of this modification, qPCR were performed with both modified and unmodified 957r primers on serial dilutions of plasmids containing 1380-bp partial 16S rDNA sequences of Methanosaetaceae, Methanomicrobiales and MBG-D. Aspecific amplifications were above the detection limit of the method only when concentrations of Methanosaeta, Methanomicrobiales and MBG-D were at least one order of magnitude above those detected in Lake Pavin samples.
16S rDNA qPCR
Archaea, Bacteria, Crenarchaeota, Methanosaetaceae, Methanomicrobiales and MBG-D were quantified by real-time PCR using a specific set of primers (Table 1, [26], [38]–[41]). Real-time quantitative PCR analysis was conducted with a Mastercycler ep realplex (Eppendorf) using MESA green qPCR Master mix Plus for SYBR assay (Eurogenetec). In each qPCR run, besides community DNA and negative controls, a recombinant plasmid, linearized with the endonuclease XbaI, containing the 1380-bp partial 16S rDNA sequence was quantified using Quant-iT™ PicoGreen® dsDNA (Invitrogen) and was 10-fold serially diluted in triplicates ranging from 102 to 109 genome equivalents and used as templates to determine the standard curves by plotting the threshold cycle (CT) value against the logarithm of copy numbers (log Co) of 16S rDNA in each dilution. Amplification reactions contained the following: 12.5 µL of MESA green qPCR Master mix Plus for SYBR® assay (Eurogenetec), 400 mM of each primers, 250 ng.µL−1 of BSA, 2 µL of DNA template and water to a 25 µl final reaction volume. Runs were performed under the following conditions: a 5 min denaturation at 95°C, followed by 40 cycles of consisting of denaturation (30 s at 95°C), annealing (20 s at various temperatures (Table 1)), extension (30 s at 72°C), detection of SYBR green I signal measurement at (20 s at 80°C) and a final extension for 10 min at 72°C. Melting curve analysis was performed at the end of 40 cycles to ensure the proper amplification of targeted fragments and to investigate the differentiation of the 16S rDNA retrieved from the environmental samples. Fluorescence readings were consecutively collected during the melting process from 60 to 95°C. Fluorescence data were converted into melting peaks. All data were analyzed using Mastercycler ep realplex software (Eppendorf). CT values were used to determine the copy numbers of 16S rDNA in the environmental samples based on the standard curve. Quantifications were performed in duplicate. The number of 16S rDNA copies was converted into cell number assuming 2 and 3.8 copies of the 16S rDNA per archaeal and bacterial cell respectively [42].
16S rRNA RT-qPCR
RNA extracts were diluted (1, 10 and 100 fold) and complementary DNA (cDNA) of archaeal 16S rRNA was obtained by using 1386r primer and the superscript III reverse transcriptase kit (Invitrogen) according to manufacturer’s instructions. Control amplifications with 21f and 1386r primers were carried out on samples treated with DNase before and after reverse transcription to check the purity of cDNA. Conditions for qPCR were similar as previously described for 16S rDNA (see above). Quantifications were performed in triplicate. The average 16S rDNA copy numbers from 0–4 cm, 4–8 cm and 10–14 cm in sediment core 1 were used to calculate the 16S rRNA to 16S rDNA copy number ratios.
Statistical Analyses
Box plots and Spearman’s rank correlations were performed using Past software available at http://folk.uio.no/ohammer/past/ [43]. Significance was tested by one way-ANOVA.
Results
Archaeal Community Structure and Composition along the Sediment Core
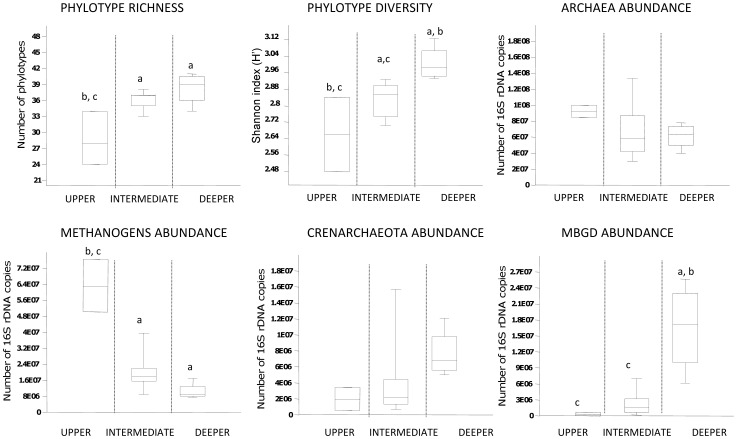
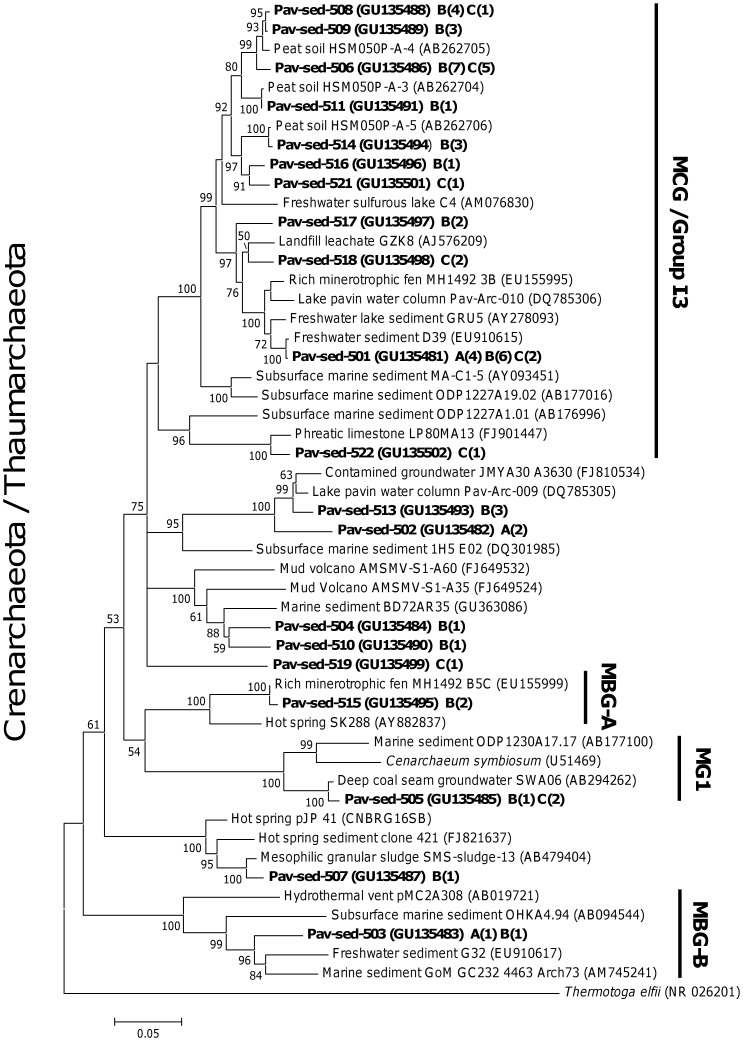
Archaea exhibited gradual changes in community structure along the sediment core as profiled by TTGE of amplified 16S rDNA fragments (Figures 2 and S2). Groupings obtained from hierarchical cluster and from MDS analyses discriminated communities of the upper (0–4 cm depth) from those of the intermediate (4–20 cm depth) and of the deeper (24–38 cm depth) sediment layers (Figures 2 and S2A). These groupings were confirmed with ANOSIM statistics (Figure S2B). Both richness and diversity of the archaeal community increased with depth, particularly within the first centimeters (Figure 1E). Intermediate and deeper layers supported a significantly higher diversity of archaeal community than the surface layer (Figures 1E and 3). The large dissimilarity (>60%, Figure S2A) between clusters suggests that archaeal populations in deeper sediment layers clearly differed from those at the sediment surface. To confirm this observation and to identify archaeal taxa, the archaeal community composition was assessed by creating 16S rDNA clone libraries from samples at 0–2 cm, 10–12 cm and 36–38 cm depth of the sediment core (Figure 1A). A total of 199 clones were obtained and were grouped into different operational taxonomic units (OTU) according to their RFLP patterns. One representative clone of each OTU was sequenced and a total of 32 distinct archaeal sequences, according to a cut-off value of 97%, were subjected to phylogenetic analyses. The number of clones analyzed represented 91%, 64% and 74% coverage for clone libraries of the uppermost, intermediate and deep sediment layers, respectively. Sequences retrieved in this study were mainly affiliated to the two archaeal phyla, Euryarchaeota (∼ 70% of total sequences) and Crenarchaeota (∼ 30% of total sequences) (Figure 4A, Table S1), and one third of the sequences exhibited less than 95% of similarity with sequences available in public databases. The methanogenic lineages of Methanomicrobiales and Methanosaetaceae, and uncultured lineages Marine Benthic Group D (MBG-D) and Miscellaneous Crenarchaeal Group (MCG) accounted for 93% of archaeal clone sequences (Figure 4A, Table S1). Other lineages were detected but at low relative proportions (Figures 4A, 5 and 6): the RC-V [44], the Val-III [45], the Deep Sediment Euryarchaeotal Group (DSEG, [46], the Marine Benthic Groups A and B (MBG-A, MBG-B [47], the Marine Group-I (MGI [25]), and an unidentified lineage related to MCG. It should be noticed that the affiliation of several lineages detected in this study (MCG and MBG-B) within the “Crenarchaeota” is debated [48] as they could also be assigned to the base of the archaeal phylum “Thaumarchaeota” [49] or related to a more recently proposed archaeal phylum named “Aigarchaeota” [50]. Herein, they were referred as crenarchaeotal lineages. These lineages, and especially MCG, are phylogenetically diversified in Lake Pavin sediments and largely contributed to the high richness and diversity of the overall archaeal community in the intermediate and deeper layers of the sediment (Figures 1E and 6, Table S1).
Figure 2. Multidimensional scaling plot (MDS) of the archaeal community based on TTGE profiling of archaeal 16S rRNA genes.
This plot corresponds to a two-dimensional visualization of the Jaccard distance matrix. The ellipses designate clusters exhibiting more than 55% similarity and delineate the upper layer (0–3 cm depth), the intermediate layer (4–20 cm depth) and the deeper layer (24–38 cm depth) of the sediment core 1.
Figure 3. Box plots of richness, diversity and abundance for the Archaea, methanogens, Crenarchaeota and MBG-D.
The three layers were discriminated from TTGE profile analyses. a, b, c indicate significant differences (p<0.05). Richness and diversity were calculated from the number and the relative peak area of bands on TTGE profiles. Average abundances were determined from 16S rDNA q-PCR data. The abundance of the methanogens was estimated by addition of the abundance of Methanosaetaceae and Methanomicrobiales. All the data were obtained on sediment core 1.
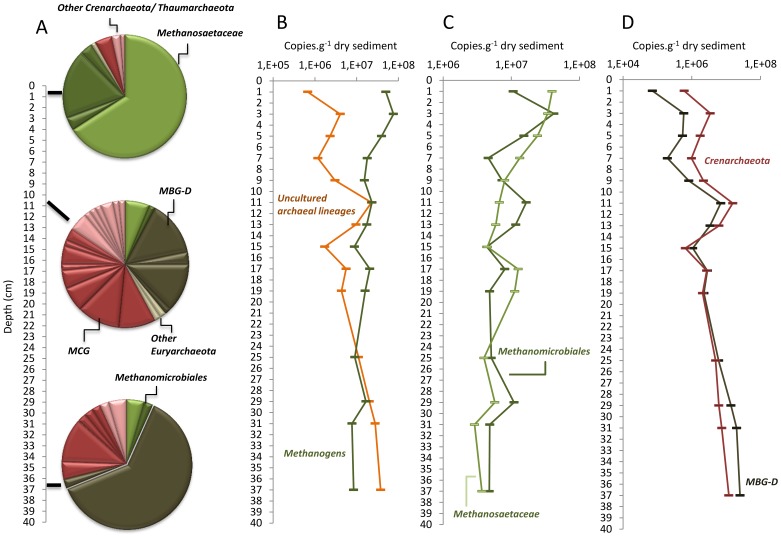
Figure 4. Frequency and abundance of the archaeal lineages.
(A) Frequency of archaeal lineages in clone libraries constructed from samples collected at 0–2 cm, 10–12 cm and 36–38 cm in the sediment core 1. Segments in the circles correspond to OTUs grouping sequences with >97% similarity. (B) Abundance of the methanogenic (Methanosaetaceae+Methanomicrobiales) and uncultured archaeal lineages (MBG-D+Crenarchaeota). (C) Abundance of the Methanomicrobiales and Methanosaetaceae; (D) Abundance of the MBG-D and Crenarchaeota. Each symbol represents the average value of the duplicate of 16S rDNA quantification from samples collected in the sediment core 1. MBG-D: Marine Benthic Group-D, MCG: Miscellaneous Crenarchaeotal Group.
Figure 5. Neighbor-joining tree of the euryarchaeotal 16S rRNA genes.
Bootstrap values (in percent) are based on 1000 replicates and are indicated at nodes for branches values ≥50% bootstrap support. The number of sequences within each OTU is shown in parentheses, and the depths of samples used for clone library construction is indicated by a letter: A, B, C for 0–2 cm, 10–12 cm and 36–38 cm depth, respectively. The scale bar represents a 5% sequence difference, and Thermotoga elfii was used as an out-group. Sequences were obtained from sediment core 1. MBG-D: Marine Benthic Group D, LDS: Lake Dagow Sediments, RCIII: Rice Cluster III, RCV: Rice Cluster V, DSEG: Deep Sediment Euryarchaeotal Group, Val-III: Valkea-III.
Figure 6. Neighbor-joining tree of the crenarchaeotal/thaumarchaeotal 16S rRNA genes.
Bootstrap values (in percent) are based on 1000 replicates and are indicated at nodes for branches values ≥50% bootstrap support. The number of sequences within each OTU is shown in parentheses, and the depths of samples used for clone library construction is indicated by a letter: A, B, C for 0–2 cm, 10–12 cm and 36–38 cm depth, respectively. Data were obtained from sediment core 1. The scale bar represents a 5% sequence difference, and Thermotoga elfii was used as an out-group. Sequences were obtained from sediment core 1. MCG: Miscellaneous Crenarchaeotal Group, MBG-A: Marine Benthic Group A, MBG-B: Marine Benthic Group B, MGI: Marine Group I.
The archaeal community composition exhibited a large heterogeneity with depth, with only 20% similarity between archaeal 16S rDNA sequences of 0–2 cm and 36–38 cm depths (Figure 4A). The uppermost layer was dominated by methanogenic lineages (66% of acetoclastic Methanosaetaceae and 25% of hydrogenotrophic Methanomicrobiales), whereas deeper in the sediment core, clone libraries were mainly composed of uncultivated archaeal lineages (62% of MBG-D and 26% of MCG, Figure 4A, Table S1). According to Bray-Curtis dissimilarity index, MBG-D and MCG lineages also exhibited different populations between samples of the intermediate and the deeper layers (33% and 57% of dissimilarity, respectively). Groupings of sediment depths with similar archaeal community structure, revealed by multivariate analysis of TTGE patterns, were then further supported by differences in phylogenetic composition of Archaea.
16S rDNA and 16S rRNA Quantification
The prokaryotic community in the Lake Pavin sediment was dominated by Bacteria, and Archaea accounted for 3 to 12% of the total prokaryotic 16Sr DNA copy number (Figure 1D). According to the average 16S rDNA copy number in archaeal and bacterial genomes [42], Archaea accounted for 5 to 18% of the total prokaryotic cells. No significant trend was noted for archaeal abundance according to depth (Figures 1D and 3). Abundance of the dominant archaeal lineages detected in clone libraries, the euryarchaeotal lineages Methanosaetaceae, Methanomicrobiales, MBG-D and of the Crenarchaeota, was determined through the quantification of their 16S rDNA by qPCR (Figures 3 and 4B to D). The acetotrophic (Methanosaetaceae) and hydrogenotrophic (Methanomicrobiales) methanogens dominated the archaeal community in the uppermost layer (4×107 and 1×107 copies g−1 dry sediment at 0–2 cm depth, respectively) and decreased with depth (3.7×106 and 4.7×106 copies g−1 dry sediment at 36–38 cm, respectively, Figure 4B). Consistent with cloning and TTGE analyses, qPCR results showed a clear difference in archaeal community structure along the sediment core. The methanogenic populations were replaced by the MBG-D (2.6×107 copies g−1 dry sediment at 36–38 cm depth, Figure 4D) and the Crenarchaeota (1.2×107 copies g−1 dry sediment at 36–38 cm depth, Figure 4D). A strong correlation was noted between the abundances of these two lineages (r = 0.901, p<0.001, Table S2). The abundances of Methanosaetaceae and MBG-D groups were strongly negatively correlated (r = −0.835, p<0.001, Table S2) and exhibited a positive and slightly negative correlation with OM and OC content of the sediment, respectively (Table S2).
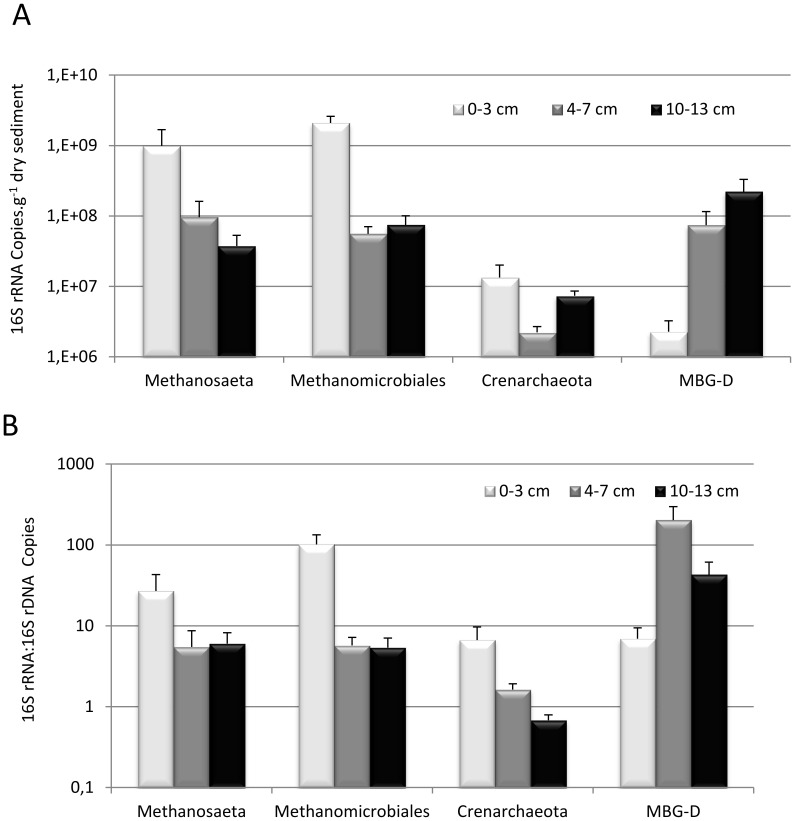
As RNA content is generally correlated to cell activity [51], the 16S rRNA of dominant archaeal lineages (Methanosaetaceae, Methanomicrobiales, MBG-D lineages and Crenarchaeota) were quantified and compared to their number of 16S rDNA in order to estimate how their activity changed with depth (Figure 7). The trends were consistent with those of 16S rDNA quantification for Methanosaetaceae, Methanomicrobiales and MBG-D but not for Crenarchaeota (Figures 4D and 7A). The ratios of 16S rRNA to 16S rDNA copy number were consistent with those of the 16S rRNA quantification (Figures 7A and B).
Figure 7. Activity of the main archaeal lineages.
(A) Activity of the methanogens, Crenarchaeota and MBG-D was estimated by the reverse transcription and PCR quantification of their 16S rRNA; (B) 16S rRNA to 16S rDNA ratio of the methanogens, Crenarchaeota and MBG-D. Quantifications of 16S rRNA were performed on the sediment core 2, whereas those of 16S rDNA were performed on sediment core 1. MBG-D: Marine Benthic Group-D.
Discussion
Archaea are not the Dominant Component of the Prokaryotic Community in Freshwater Sediments
Archaea is most likely the dominant microbial domain of the deep marine subsurface [10], [52]. In lake sediments, archaea account from less than 1% [13] to 99% [53] of the prokaryotic community. According to available data, archaea were found to be dominant in sediments of saline lakes [e.g. Lake Chaka [54], Lake Qinghai [55]] and to be a minority component of the prokaryotic community in freshwater sediments [13], [56], [57]. Agreeing with these previous observations, our q-PCR analysis demonstrated that archaea accounted for 5–18% of the prokaryotic community throughout the 40-cm core of the freshwater sediments of Lake Pavin. Whereas few studies reported quantification of archaea in lake sediments, discrepancies in the relative proportion of archaea according to salinity might be explained, in part, by Valentine’s hypotheses [58]. In this interesting opinion paper, the author postulated that “adaptation to chronic energy stress is the primary factor differentiating archaeal and bacterial ecology”. In freshwater sediments, such as in Lake Pavin, where energetic stress is not “chronic” compared to saline lakes, the high degree of adaptability and metabolic diversification of bacteria would allow them to dominate archaea.
Archaea Exhibited a Dichotomic Distribution
Whereas, no significant vertical variations in the abundance of the overall archaea were noted, important depth-related changes occurred in the composition and structure of the archaeal community. Such vertical changes in the structure of the archaeal community were reported from freshwater lake sediments according to fingerprint analyses [53], [59], but without clear determination of how each archaeal lineage is affected by depth in the sediment. In our study, the gradual shift of archaeal community composition along the Lake Pavin sediment core was supported by TTGE patterns, phylogenetic and quantification analyses.
Changes in archaeal community composition were accompanied by an increase in both richness and diversity between the upper and the deeper layers of the sediment. Compared to available data, the archaeal richness in Lake Pavin sediments (35 phylotypes in average) was high (e.g. Lake Hovsgol-14 phylotypes [60]; Lake Biwa-10 phylotypes [59], Lake Taihu-19 phylotypes [53], Lake Kivu-12 phylotypes [61]). Deep sediment layers, although with binding conditions, supported a diverse archaeal community. In undisturbed ecosystems like deep lake sediments, inactive cells may accumulate and persist during a long period after growth, depending on their resistance to starvation [62]. In these cold anoxic environments, the majority of DNA is extracellular and may be preserved for thousands of years [63], [64]. However, two observations show that the high diversity of archaea in the deep layers was not artefactual: (1) a clear dichotomy in the distribution of archaeal populations with methanogenic archaea progressively replaced by other archaeal clades (putatively non-methanogenic), (2) 16S rRNA quantification revealed that a large proportion of targeted communities are seemingly active (with the exception of Crenarchaeota).
All of these observations (diversity, composition, and activity patterns of archaeal communities along the sediment core) suggest that more ecological niches, clearly different from those in the surficial sediment, are available for archaeal taxa in the deep sediment layers. Notably, the clear inverse trend between methanogenic and uncultured archaeal lineage (putatively non-methanogenic) abundances with depth suggests they have contrasted ecological niches within the sediment. Several studies on archaeal communities inhabiting lake sediments also reported an inverse correlation between the relative proportions of Methanosaeta and Methanomicrobiaceae, and those of MBG-D [9], [12], [13], [55], [57], [59], [61], [65].
Which Archaeal Groups Occurred and What can they do?
As outlined above, methanogenic archaea were progressively replaced along the sediment core by archaeal clades with unknown metabolisms. It is exciting as well as frustrating to detect so many archaeal clades for which no information about their metabolic function currently exists.
Methanogenic archaea
According to our data, it is clear that the main archaeal metabolic function in the surface sediment layer (0–4 cm) of Lake Pavin is methane production. Methanogen dominance in this layer is consistent with Lake Pavin meromicticity, as the reduction of the inorganic electron acceptors (i.e. sulfate, ferric iron, nitrate…) occurs in the anoxic water column [17]. According to the high methane concentration in the sediment (Figure 1B) and to the high abundance and activity of the methanogens in the superficial sediment layer, where mineralization is the most intensive, methanogenesis is expected to be the most important terminal mineralization pathway in this ecosystem. This terminal-electron-accepting pathway accounts, in freshwater system, for 10 to 50% of organic matter mineralization [66].
In Lake Pavin sediments, methanogenesis is performed by two known methanogenic lineages: the acetotrophic Methanosaetaceae and the putatively hydrogenotrophic Methanomicrobiales. These lineages are frequently observed in superficial zone of freshwater sediments [13], [53], [57], [59], [67]. The genus Methanosaeta and the family Methanoregulaceae have been also found to dominate freshwater sediments [68]. The abundance of Methanosaetaceae was significantly correlated with both OM and OC content along the sediment core (Table S2). In contrast with the abundance of Methanosaetaceae, the content in OM and OC did not decrease significantly with depth (Figure 1C). This suggests that another factor, probably the organic matter lability, is responsible for the decrease in the abundance of Methanosaetaceae. This hypothesis is supported by the decrease of the 16S rRNA to 16S rDNA ratio (Figure 7B). Methanosaeta members are only able to grow on acetate, and considering that the organic matter reactivity decreases with sediment age and depth [69], and that dissolved labile compounds only diffuse on a limited surface layer [70], cells detected in the deepest layers are probably not growing but rather surviving. The abundance of Methanomicrobiales is less negatively correlated to depth than that of Methanosaetaceae (Table S2). Chan et al. [57] hypothesized that this differential response of Methanomicrobiales and Methanosaetaceae to increasing depth may be due to a differential death (starvation) rather than a differential growth.
Another surprising observation is the detection of high methane concentrations in the deep layer of the sediment core where known methanogenic archaea were detected at low relative abundances and proportions. We cannot exclude that methane detected in the deep layer is an older CH4 that is physically entrapped but, the methane profile, with concentrations decreasing in the intermediate layer (Figure 1B), suggests that archaeal groups other than known methanogenic lineages might be involved in the methane cycle. This hypothesis is discussed further in the sections below.
Uncultured archaeal clades
Uncultured archaeal lineages are ubiquitous in freshwater sediments (Table S3, [61], [71], [72]) suggesting that they are adapted to this type of environment where they might play key functional roles. In Lake Pavin, the relative proportion and abundance of MBG-D and MCG increased with depth and these lineages dominated the archaeal community in the deeper layers of the sediment. A similar trend was also reported, for MBG-D, according to clone libraries from marine [73], freshwater [9] and hypersaline [65] sediments, and from a peatland [74]. According to their environmental distribution, mainly in sub-surfaces (Table S3), these archaeal lineages are probably able to use intriguing metabolic pathways and probably have special physiological adaptations allowing them to thrive with the low energetic flux available in buried sediment layers. But, these adaptations might reduce the competiveness of these archaea in dynamic and energetic environments as they are less abundant in surface sediment layer where labile organic matter is seasonally delivered to the sediment by dead algal deposition.
Sequences belonging to the euryarchaeotal the Marine benthic Group-D, a Thermoplasmatales-related group, were found in a variety of freshwater and marine habitats [9], [74]–[76]. Along with Rice Cluster V (RC-V) and Lake Dagow Sediment (LDS) lineages [72], MBG-D is the most widely encountered uncultured lineage in freshwater lake sediments (Table S3). Although the metabolism of MBG-D representatives remains unknown, several hypotheses may be postulated according to their environmental repartition and their phylogenetic affiliation. The high methane concentration in the deeper layer might be consistent with a methanogenic activity of MBG-D, although this group was previously identified as non-methanogenic [55]. MBG-D represented the unique archaeal members in a clone library from the bottom of the AOM zone of the Lake Cadagno [9] and were detected in several other marine environments and cultures where AOM occurs [76]–[78]. Beal et al., [79] also noted that MBG-D clones represented up to 40% of archaea in a clone library of their AOM enrichments from methane-seep sediment. These observations might imply the involvement of MBG-D members in AOM. This hypothesis is consistent with the high activities of MBG-D in the intermediate layer of Pavin sediments where methane concentrations decreased (Figure 1B and 7). However MBG-D also represents a high fraction of the prokaryotic community in hypersaline sediments where methane concentrations are extremely low [55], [65], suggesting that some MBG-D members are not involved in AOM processes but instead, would benefit from waste-products, intermediates or dead cells produced in sedimentary environments. As proposed for members of two lineages related to MBG-D, the Thermoplasmatales [80] and RC-III (a sister group of MBG-D, [81]), representatives of MBG-D might be scavengers in their environment and may use components from decaying microorganisms (such as oligopeptides [82]) as carbon and/or energetic sources for growth. For example, the decay of methanogenic microorganisms with depth might provide building elements for some representatives of uncultured archaeal lineages (as isoprenoids moieties of lipids) that may minimize their energy expenditure for growth and maintenance [83].
The Miscellaneous Crenarchaeal Group is a cosmopolitan group, frequently retrieved in anoxic habitats [10], [84] and have been considered as heterotrophic anaerobes based on their capability to take up organic carbon in buried sediments [84]. However, their huge cosmopolitan distribution in a wide range of biogeochemically distinct sedimentary settings [84] and their complex phylogeny suggest a metabolic diversity and an ecosphysiological flexibility larger than assumed previously. Current evidence suggests that some members of the MCG lineage may obtain energy from the anaerobic oxidation of methane but use a “dissimilatory” methane-oxidizing process and do not assimilate its carbon [84]. This hypothesis fits with the decrease of methane concentrations in the intermediate layers of the sediment core of Lake Pavin where MCG dominated the archaeal community. However, the achievement of other metabolic pathways by MCG occurring in Lake Pavin sediments is not excluded.
Conclusion
This study revealed that important changes in the archaeal structure occurred in the sediment of Lake Pavin along the 40 cm sediment core. The opposite trends between methanogens and uncultured lineage abundances may be explained by differing or opposite optimum growth conditions. It could also be hypothesized that the decay of methanogenic microorganisms with depth furnishes building elements for some representatives of uncultured archaeal lineages. One of these uncultured archaeal groups, MBG-D, is generally associated with marine and hypersaline environments, however, its high abundance in Lake Pavin sediments and its common occurrence in freshwater lake sediments demonstrate that it can be an important group in sedimentary environments regardless of the salinity. The common association of MBG-D with communities performing AOM, in a number of environments and/or culture enrichments, presents the intriguing hypothesis that AOM also occurs in Lake Pavin sediments, though this needs further study. The new primers designed herein and the probes recently designed [9] would be helpful to clarify the role MBG-D plays in this significant ecological process.
Supporting Information
Specificity and binding sites (shading) of primers 490f and 818r targeting MBG-D. These features are shown on a partial alignment of representative archaeal 16S rDNA sequences from positions 490 to 508 and from 818 to 837 (E. coli numbering), respectively.
(DOC)
Hierarchical cluster analysis and ANOSIM statistics on TTGE profiles. (A) Hierarchical cluster analysis, performed from TTGE banding patterns for each sample of the sediment core 1 (Fig. 1A), using the Jaccard coefficient and the UPGMA method. The dashed vertical line indicates the distance that was chosen for cluster separation. (B) ANOSIM statistics for comparisons of communities using TTGE similarity values. Upper, Intermediate and Deeper refers to clusters defined in Figure 2.
(DOC)
Number and affiliation of archaeal 16S rRNA gene sequences identified in clone libraries. Data were obtained on samples from sediment core 1. MBG-D: Marine Benthic Group D; RC-V: Rice Cluster V; DSEG: Deep Sediment Euryarchaeotal Group; Val-III: Valkea-III; MCG: Miscellaneous Crenarchaeotal Group; MGI: Marine Group I; MBG-B, -A: Marine Benthic Group B and A.
(DOC)
Spearman rank correlations between the abundance of prokaryotic groups, depth, OM and OC. Only correlations with p value <0.05 are shown. *p value <0.01. **p value <0.001. OM, Organic matter content, OC, Organic carbon content, Mst: Methanosaetaceae, MM: Methanomicrobiales, Cre: Crenarchaeota, Arc: Archaea, Bac: Bacteria; MBG-D: Marine Benthic Group D.
(DOC)
Occurrence of the main uncultured euryarchaeotal and crenarchaeotal/thaumarchaeotal lineages in freshwater lake sediments. 16S rRNA gene sequences used for this table were previously published. The affiliation was based on published trees and new construction of trees using archaeal sequences from lake sediments retrieved from NCBI. “×” : detected; “−” : undetected; “?” ambiguous affiliation. MBG-D: Marine Benthic Group D, LDS: Lake Dagow Sediments, RCIII: Rice Cluster III, RCV: Rice Cluster V, DSEG: Deep Sediment Euryarchaeotal Group, Val-III: Valkea-III, MCG: Miscellaneous Crenarchaeotal Group, MBG-A: Marine Benthic Group A, MBG-B: Marine Benthic Group B, MGI: Marine Group I.
(DOC)
Acknowledgments
We thank Jonathan Colombet and Lionel Jouve for their help during sampling fields. We are also grateful for the useful advices of Marion Sabart for the qPCR, and Isabelle Batisson for the TTGE. We greatly thank Kevin Purdy for his invitation at the Department of Biological Sciences of University of Warwick and Franck Carbonero for his careful teaching of RNA extractions from sediment.
Funding Statement
This work was funded by the Programme EC2CO of the French Centre National de la Recherche Scientifique (Projects Methanolac and Interlac). The work was also supported by a grant to GB from the region Auvergne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Oppenheimer CH (1960) Bacterial activity in sediments of shallow marine bays. Geochim Cosmochim Acta 19: 244–246. [Google Scholar]
- 2.Fenchel T, Blackburn TH (1979) Bacteria and mineral cycling. London: Academic Press. 225 p.
- 3.Schink B (1988) Principles and limits of anaerobic degradation: environmental and technological aspects. In: Zehnder AJB, editor. Biology of anaerobic microorganisms. New York: John Wiley & Sons. 771–846.
- 4. Wolin M (1974) Metabolic interactions among intestinal microorganisms. Am J Clin Nutr 27: 1320–1328. [DOI] [PubMed] [Google Scholar]
- 5. Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and freshwater sediments - contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33: 725–749. [Google Scholar]
- 6. Hinrichs KU, Hayes JM, Sylva SP, Brewer PG, DeLong EF (1999) Methane-consuming archaebacteria in marine sediments. Nature 398: 802–805. [DOI] [PubMed] [Google Scholar]
- 7. Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, et al. (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407: 623–626. [DOI] [PubMed] [Google Scholar]
- 8. Orphan VJ, House CH, Hinrichs KU, McKeegan KD, DeLong EF (2001) Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 293: 484–487. [DOI] [PubMed] [Google Scholar]
- 9. Schubert CJ, Vazquez F, Lösekann-Behrens T, Knittel K, Tonolla M, et al. (2011) Evidence for anaerobic oxidation of methane in sediments of a freshwater system (Lago di Cadagno). FEMS Microbiol Ecol 76: 26–38. [DOI] [PubMed] [Google Scholar]
- 10. Teske A, Sorensen KB (2008) Uncultured archaea in deep marine subsurface sediments: have we caught them all? ISME J 2: 3–18. [DOI] [PubMed] [Google Scholar]
- 11. Purdy KJ, Nedwell DB, Embley TM (2003) Analysis of the sulfate-reducing bacterial and methanogenic archaeal populations in contrasting Antarctic sediments. Appl Environ Microbiol 69: 3181–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nüsslein B, Chin K-J, Eckert W, Conrad R (2001) Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ Microbiol 3: 460–470. [DOI] [PubMed] [Google Scholar]
- 13. Schwarz JIK, Eckert W, Conrad R (2007) Community structure of Archaea and Bacteria in a profundal lake sediment Lake Kinneret (Israel). Syst Appl Microbiol 30: 239–254. [DOI] [PubMed] [Google Scholar]
- 14. Conrad R, Klose M, Claus P, Enrich-Prast A (2010) Methanogenic pathway, C-13 isotope fractionation, and archaeal community composition in the sediment of two clear-water lakes of Amazonia. Limnol Oceanogr 55: 689–702. [Google Scholar]
- 15. Lehours AC, Evans P, Bardot C, Joblin K, Gerard F (2007) Phylogenetic diversity of archaea and bacteria in the anoxic zone of a meromictic lake (Lake Pavin, France). Appl Environ Microbiol 73: 2016–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aeschbach-Hertig W, Hofer M, Kipfer R, Imboden DM, Wieler R (1999) Accumulation of mantle gases in a permanently stratified volcanic lake (Lac Pavin, France). Geochim Cosmochim Acta 63: 3357–3372. [Google Scholar]
- 17. Lopes F, Viollier E, Thiam A, Michard G, Abril G, et al. (2011) Biogeochemical modelling of anaerobic vs. aerobic methane oxidation in a meromictic crater lake (Lake Pavin, France). Appl Geochem 26: 1919–1932. [Google Scholar]
- 18. Viollier E, Jezequel D, Michard G, Pepe M, Sarazin G, et al. (1995) Geochemical Study of a Crater Lake (Pavin Lake, France) - Trace-Element Behavior in the Monimolimnion. Chem Geol 125: 61–72. [Google Scholar]
- 19. Viollier E, Michard G, Jezequel D, Pepe M, Sarazin G (1997) Geochemical study of a crater lake: Lake Pavin, Puy de Dôme, France. Constraints afforded by the particulate matter distribution in the element cycling within the lake. Chem Geol 142: 225–241. [Google Scholar]
- 20. Lehours AC, Bardot C, Thenot A, Debroas D, Fonty G (2005) Anaerobic microbial communities in Lake Pavin, a unique meromictic lake in France. Appl Environ Microbiol 71: 7389–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schettler G, Schwab MJ, Stebich M (2007) A 700-year record of climate change based on geochemical and palynological data from varved sediments (Lac Pavin, France). Chem Geol 240: 11–35. [Google Scholar]
- 22. Koizumi Y, Kojima H, Oguri K, Kitazato H, Fukui M (2004) Vertical and temporal shifts in microbial communities in the water column and sediment of saline meromictic Lake Kaiike (Japan), as determined by a 16S rDNA-based analysis, and related to physicochemical gradients. Environ Microbiol 6: 622–637. [DOI] [PubMed] [Google Scholar]
- 23. Buckley DR, Rockne KJ, Li A, Mills WJ (2004) Soot deposition in the Great Lakes: Implications for semi-volatile hydrophobic organic pollutant deposition. Environ Sci Technol 38: 1732–1739. [DOI] [PubMed] [Google Scholar]
- 24. Purdy KJ, Embley TM, Takii S, Nedwell DB (1996) Rapid extraction of DNA and rRNA from sediments by a novel hydroxyapatite spin-column method. Appl Environ Microbiol 62: 3905–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89: 5685–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skillman LC, Evans PN, Naylor GE, Morvan B, Jarvis GN, et al. (2004) 16S ribosomal DNA-directed PCR primers for ruminal methanogens and identification of methanogens colonising young lambs. Anaerobe 10: 277–285. [DOI] [PubMed] [Google Scholar]
- 27. Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20: 2317–2319. [DOI] [PubMed] [Google Scholar]
- 28. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al.. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol: doi: 10.1093/molbev/msr1121. [DOI] [PMC free article] [PubMed]
- 30. Hill TCJ, Walsh KA, Harris JA, Moffett BF (2003) Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol 43: 1–11. [DOI] [PubMed] [Google Scholar]
- 31.Magurran AE (1988) Ecological diversity and its measurement. Princeton: Taylor & Francis. 179 p.
- 32. Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Batisson I, Pesce S, Besse-Hoggan P, Sancelme M, Bohatier J (2007) Isolation and characterization of diuron-degrading bacteria from lotic surface water. Microb Ecol 54: 761–770. [DOI] [PubMed] [Google Scholar]
- 34. Ward JH (1963) Hierarchical grouping to optimize an objective function. J Amer Statistical Assoc 58: 236–244. [Google Scholar]
- 35.Clarke K, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation (PRIMER-E). Plymouth: Plymouth Marine Laboratory.
- 36. Loy A, Arnold R, Tischler P, Rattei T, Wagner M, et al. (2008) probeCheck–a central resource for evaluating oligonucleotide probe coverage and specificity. Environ Microbiol 10: 2894–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cole J, Chai B, Farris R, Wang Q, Kulam-Syed-Mohideen A, et al. (2006) The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 35: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5: 787–797. [DOI] [PubMed] [Google Scholar]
- 39. Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, et al. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy of Sciences 82: 6955–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reysenbach A, Pace N (1995) Reliable amplification of hyperthermophilic archaeal 16S rRNA genes by the polymerase chain reaction. In: Robb FT, Place AR, editors. Archaea: a laboratory manual. New York: Cold Spring Harbor press. 101–107.
- 41. Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89: 670–679. [DOI] [PubMed] [Google Scholar]
- 42. Klappenbach JA, Dunbar JM, Schmidt TM (2000) rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol 66: 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol Electron 4: 1–9. [Google Scholar]
- 44. Grosskopf R, Stubner S, Liesack W (1998) Novel euryarchaeotal lineages detected on rice roots and in the anoxic bulk soil of flooded rice microcosms. Appl Environ Microbiol 64: 4983–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jurgens G, Glockner FO, Amann R, Saano A, Montonen L, et al. (2000) Identification of novel Archaea in bacterioplankton of a boreal forest lake by phylogenetic analysis and fluorescent in situ hybridization. FEMS Microbiol Ecol 34: 45–56. [DOI] [PubMed] [Google Scholar]
- 46. Takai K, Moser DP, DeFlaun M, Onstott TC, Fredrickson JK (2001) Archaeal diversity in waters from deep South African gold mines. Appl Environ Microbiol 67: 5750–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vetriani C, Jannasch HW, MacGregor BJ, Stahl DA, Reysenbach AL (1999) Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl Environ Microbiol 65: 4375–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pester M, Schleper C, Wagner M (2011) The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr Opin Microbiol 14: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6: 245–252. [DOI] [PubMed] [Google Scholar]
- 50. Nunoura T, Takaki Y, Kakuta J, Nishi S, Sugahara J, et al. (2011) Insights into the evolution of Archaea and eukaryotic protein modifier systems revealed by the genome of a novel archaeal group. Nucleic Acids Res 39: 3204–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kerkhof L, Kemp P (1999) Small ribosomal RNA content in marine Proteobacteria during non steady state growth. FEMS Microbiol Ecol 30: 253–260. [DOI] [PubMed] [Google Scholar]
- 52. Lipp JS, Morono Y, Inagaki F, Hinrichs KU (2008) Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 454: 991–994. [DOI] [PubMed] [Google Scholar]
- 53. Ye WJ, Liu XL, Lin SQ, Tan J, Pan JL, et al. (2009) The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiol Ecol 70: 263–276. [DOI] [PubMed] [Google Scholar]
- 54. Jiang H, Dong H, Yu B, Liu X, Li Y, et al. (2007) Microbial response to salinity change in Lake Chaka, a hypersaline lake on Tibetan plateau. Environ Microbiol 9: 2603–2621. [DOI] [PubMed] [Google Scholar]
- 55. Jiang HC, Dong HL, Yu BS, Ye Q, Shen J, et al. (2008) Dominance of putative marine benthic Archaea in Qinghai Lake, north-western China. Environ Microbiol 10: 2355–2367. [DOI] [PubMed] [Google Scholar]
- 56. Falz KZ, Holliger C, Grosskopf R, Liesack W, Nozhevnikova AN, et al. (1999) Vertical distribution of methanogens in the anoxic sediment of Rotsee (Switzerland). Appl Environ Microbiol 65: 2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan OC, Claus P, Casper P, Ulrich A, Lueders T, et al. (2005) Vertical distribution of structure and function of the methanogenic archaeal community in Lake Dagow sediment. Environ Microbiol 7: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 58. Valentine DL (2007) Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol 5: 316–323. [DOI] [PubMed] [Google Scholar]
- 59. Koizumi Y, Takii S, Fukui M (2004) Depth-related change in archaeal community structure in a freshwater lake sediment as determined with denaturing gradient gel electrophoresis of amplified 16S rRNA genes and reversely transcribed rRNA fragments. FEMS Microbiol Ecol 48: 285–292. [DOI] [PubMed] [Google Scholar]
- 60. Nam YD, Sung Y, Chang HW, Roh SW, Kim KH, et al. (2008) Characterization of the depth-related changes in the microbial communities in Lake Hovsgol sediment by 16S rRNA gene-based approaches. J Microbiol 46: 125–136. [DOI] [PubMed] [Google Scholar]
- 61.Bhattarai S, Ross KA, Schmid M, Anselmetti FS, Bürgmann H (in press) Local Conditions Structure Unique Archaeal Communities in the Anoxic Sediments of Meromictic Lake Kivu. Microb Ecol. [DOI] [PubMed]
- 62. Koizumi Y, Kojima H, Fukui M (2003) Characterization of depth-related microbial community structure in lake sediment by denaturing gradient gel electrophoresis of amplified 16S rDNA and reversely transcribed 16S rRNA fragments. FEMS Microbiol Ecol 46: 147–157. [DOI] [PubMed] [Google Scholar]
- 63. Danovaro R, Dell’anno A, Pusceddu A, Fabiano M (1999) Nucleic acid concentrations (DNA, RNA) in the continental and deep-sea sediments of the eastern Mediterranean: relationships with seasonally varying organic inputs and bacterial dynamics. Deep Sea Res Part I 46: 1077–1094. [Google Scholar]
- 64. Coolen MJL, Overmann J (2007) 217 000-year-old DNA sequences of green sulfur bacteria in Mediterranean sapropels and their implications for the reconstruction of the paleoenvironment. Environ Microbiol 9: 238–249. [DOI] [PubMed] [Google Scholar]
- 65. Swan BK, Ehrhardt CJ, Reifel KM, Moreno LI, Valentine DL (2010) Archaeal and bacterial communities respond differently to environmental gradients in anoxic sediments of a California hypersaline lake, the Salton Sea. Appl Environ Microbiol 76: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bastviken D, Cole JJ, Pace ML, Van de Bogert MC (2008) Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J Geophys Res 113: G02024 doi:02010.01029/02007JG000608. [Google Scholar]
- 67. Glissmann K, Chin KJ, Casper P, Conrad R (2004) Methanogenic pathway and archaeal community structure in the sediment of eutrophic Lake Dagow: Effect of temperature. Microb Ecol 48: 389–399. [DOI] [PubMed] [Google Scholar]
- 68. Borrel G, Jézéquel D, Biderre-Petit C, Morel-Desrosiers N, Morel J-P, et al. (2011) Production and consumption of methane in freshwater lake ecosystems. Res Microbiol 162: 832–847. [DOI] [PubMed] [Google Scholar]
- 69. Burdige DJ (2007) Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem Rev 107: 467–485. [DOI] [PubMed] [Google Scholar]
- 70. Schwarz JIK, Eckert W, Conrad R (2008) Response of the methanogenic microbial community of a profundal lake sediment (Lake Kinneret, Israel) to algal deposition. Limnol Oceanogr 53: 113–121. [Google Scholar]
- 71. Auguet JC, Barberan A, Casamayor EO (2010) Global ecological patterns in uncultured Archaea. ISME J 4: 182–190. [DOI] [PubMed] [Google Scholar]
- 72. Barberán A, Fernández-Guerra A, Auguet J-C, Galand PE, Casamayor EO (2011) Phylogenetic ecology of widespread uncultured clades of the Kingdom Euryarchaeota. Mol Ecol 20: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 73. Roussel EG, Sauvadet AL, Chaduteau C, Fouquet Y, Charlou JL, et al. (2009) Archaeal communities associated with shallow to deep subseafloor sediments of the New Caledonia Basin. Environ Microbiol 11: 2446–2462. [DOI] [PubMed] [Google Scholar]
- 74. Cadillo-Quiroz H, Brauer S, Yashiro E, Sun C, Yavitt J, et al. (2006) Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA. Environ Microbiol 8: 1428–1440. [DOI] [PubMed] [Google Scholar]
- 75. Galand PE, Fritze H, Yrjälä K (2003) Microsite-dependent changes in methanogenic populations in a boreal oligotrophic fen. Environ Microbiol 5: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 76. Webster G, Sass H, Cragg BA, Gorra R, Knab NJ, et al. (2011) Enrichment and cultivation of prokaryotes associated with the sulphate methane transition zone of diffusion controlled sediments of Aarhus Bay, Denmark under heterotrophic conditions. FEMS Microbiol Ecol 77: 248–263. [DOI] [PubMed] [Google Scholar]
- 77. Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, et al. (2006) Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc Natl Acad Sci USA 103: 2815–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Y, Maignien L, Zhao X, Wang F, Boon N (2011) Enrichment of a microbial community performing anaerobic oxidation of methane in a continuous high-pressure bioreactor. BMC Microbiol 11: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beal EJ, House CH, Orphan VJ (2009) Manganese- and Iron-Dependent Marine Methane Oxidation. Science 325: 184–187. [DOI] [PubMed] [Google Scholar]
- 80. Segerer A, Langworthy T, Stetter K (1988) Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from solfatara fields. Syst Appl Microbiol 10: 161–171. [Google Scholar]
- 81. Kemnitz D, Kolb S, Conrad R (2005) Phenotypic characterization of Rice Cluster III archaea without prior isolation by applying quantitative polymerase chain reaction to an enrichment culture. Environ Microbiol 7: 553–565. [DOI] [PubMed] [Google Scholar]
- 82. Smith PF, Langworthy TA, Smith MR (1975) Polypeptide nature of growth requirement in yeast extract for Thermoplasma acidophilum. J Bacteriol 124: 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Takano Y, Chikaraishi Y, Ogawa NO, Nomaki H, Morono Y, et al. (2010) Sedimentary membrane lipids recycled by deep-sea benthic archaea. Nat Geosci 3: 858–861. [Google Scholar]
- 84. Biddle JF, Lipp JS, Lever MA, Lloyd KG, Sørensen KB, et al. (2006) Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc Natl Acad Sci USA 103: 3846–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specificity and binding sites (shading) of primers 490f and 818r targeting MBG-D. These features are shown on a partial alignment of representative archaeal 16S rDNA sequences from positions 490 to 508 and from 818 to 837 (E. coli numbering), respectively.
(DOC)
Hierarchical cluster analysis and ANOSIM statistics on TTGE profiles. (A) Hierarchical cluster analysis, performed from TTGE banding patterns for each sample of the sediment core 1 (Fig. 1A), using the Jaccard coefficient and the UPGMA method. The dashed vertical line indicates the distance that was chosen for cluster separation. (B) ANOSIM statistics for comparisons of communities using TTGE similarity values. Upper, Intermediate and Deeper refers to clusters defined in Figure 2.
(DOC)
Number and affiliation of archaeal 16S rRNA gene sequences identified in clone libraries. Data were obtained on samples from sediment core 1. MBG-D: Marine Benthic Group D; RC-V: Rice Cluster V; DSEG: Deep Sediment Euryarchaeotal Group; Val-III: Valkea-III; MCG: Miscellaneous Crenarchaeotal Group; MGI: Marine Group I; MBG-B, -A: Marine Benthic Group B and A.
(DOC)
Spearman rank correlations between the abundance of prokaryotic groups, depth, OM and OC. Only correlations with p value <0.05 are shown. *p value <0.01. **p value <0.001. OM, Organic matter content, OC, Organic carbon content, Mst: Methanosaetaceae, MM: Methanomicrobiales, Cre: Crenarchaeota, Arc: Archaea, Bac: Bacteria; MBG-D: Marine Benthic Group D.
(DOC)
Occurrence of the main uncultured euryarchaeotal and crenarchaeotal/thaumarchaeotal lineages in freshwater lake sediments. 16S rRNA gene sequences used for this table were previously published. The affiliation was based on published trees and new construction of trees using archaeal sequences from lake sediments retrieved from NCBI. “×” : detected; “−” : undetected; “?” ambiguous affiliation. MBG-D: Marine Benthic Group D, LDS: Lake Dagow Sediments, RCIII: Rice Cluster III, RCV: Rice Cluster V, DSEG: Deep Sediment Euryarchaeotal Group, Val-III: Valkea-III, MCG: Miscellaneous Crenarchaeotal Group, MBG-A: Marine Benthic Group A, MBG-B: Marine Benthic Group B, MGI: Marine Group I.
(DOC)