Abstract
Psychostimulants like cocaine and amphetamine are commonly abused by young adults who often state that they take these drugs to increase social or cognitive performance. The current study tested the hypothesis that individuals at early stages of occasional stimulant use show subtle executive dysfunctions such as verbal fluency deficits. 155 young (age 18-25), non-dependent occasional users of stimulants and 49 stimulant naïve comparison subjects performed the Delis-Kaplan Verbal Fluency test. Correlation and median split analyses were conducted to account for stimulant history and co-drug use. Compared to stimulant naïve subjects, occasional stimulant users generated significantly more responses on an over-learned verbal fluency task (Category Fluency), but at the expense of increased error rates (Set Loss and Repetition Errors). These performance differences were not related to lifetime uses of stimulants or marijuana. Taken together, these results support the hypothesis that individuals who are using stimulants occasionally exhibit subtle executive dysfunctions when required to generate verbal sets under time pressure. In particular, occasional stimulant users apply quickly but inaccurately verbal rules, which may represent a mix of diminished cognitive flexibility along with increased rigidity and impulsivity. This specific executive dysfunction may help to identify individuals at risk for stimulant use or dependence.
Keywords: stimulant, cocaine, amphetamine, executive function, verbal fluency
1. Introduction
Since prices for recreational stimulants like powder cocaine are falling, prescription stimulants – the drugs of choice for the treatment of attention deficit hyperactivity disorder (ADHD, e.g. amphetamines (Adderall®), methylphenidate (Ritalin®)) – and illegal, recreational drugs (cocaine) together contribute to the increasing rates of stimulants being abused, particularly by young adults. US survey data indicate that approximately 98,000 adolescents aged 12-17 would meet criteria for abuse of or dependence on stimulants (Herman-Stahl et al., 2006). The reasons for abusing stimulants are multifactorial. Individuals who use stimulants frequently report that they use prescription stimulants to improve performance on tests and recreational stimulants to feel more comfortable in social situations. While the non-medical use of prescription stimulants is associated with lower grade points (McCabe et al., 2005), it remains unclear whether an objective neurocognitive deficiency precedes stimulant initiation. Longitudinal studies of children at risk of substance use report that deficits in ‘neurobehavioral inhibition’ at age 16 accurately predicted substance use disorders at age 19 with an 85% accuracy (Kirisci et al., 2004a;Tarter et al., 2003) and thus point towards a preexisting neuropsychological characteristic.
Chronic stimulant users are characterized by a deterioration of executive functions (for review see e.g. (Yucel et al., 2007b), a group of higher cognitive abilities of organization and integration. Executive impairments were shown to contribute to the initiation of drug use, for instance by increasing the probability of drug-seeking behaviors (Tarter et al., 2003). Evidence for an effect of prescription stimulants on neurocognitive functioning derives from studies on ADHD. Methylphenidate was shown to improve performance on over-learned tasks (Bedard et al., 2002), while tasks with higher cognitive demands show mixed results (Bedard et al., 2002;Aron et al., 2003;Langleben et al., 2006;Scheres et al., 2003). Most studies on neuropsychological functioning in chronic cocaine users identifies mild (Goldstein et al., 2004;Woicik et al., 2008) to severe neurocognitive deficits (Gillen et al., 1998), particularly in the executive functioning domain, while a lack of impairments of certain neuropsychological functions has also been reported (Hoff et al., 1996). A recent review of neuroimaging results (Li & Sinha, 2008) comes to the conclusion that an altered striatal response combined with a heightened striatal response underlies executive deficits in stimulant addiction.
Dysfunctions of prefrontal brain areas, which are most affected by drug use (Rogers & Robbins, 2001;Goldstein et al., 2004), can be assessed employing verbal fluency tasks. These tests led to inconsistent findings: studies either reported better (O'Malley et al., 1992;Hoff et al., 1996;Rahman & Clarke, 2005) or comparable performance between cocaine (Woicik et al., 2008;Bolla et al., 1999) or crack-cocaine users (Di, V et al., 2002) and non-using subjects or reported poorer performance in cocaine users (Gillen et al., 1998;Bolla et al., 1999;Kalechstein et al., 2003). The investigation of chronic stimulant users faces the challenge to differentiate potentially predisposing neurocognitive processing patterns from cumulative effects of stimulant use. Examining occasional stimulant users – users who recently started using psychostimulants and who have not developed problems with stimulant use – on the other hand opens the unique possibility to determine whether these individuals already show subtle neuropsychological problems, which potentially promoted stimulant use. In the present study we therefore administered a verbal fluency test to a large sample of young, occasional stimulant users and stimulant naïve comparison subjects to investigate what predisposes individuals to use stimulant-type drugs. Studying this population, two confounds have to be addressed. First, the prevalence of ADHD, which itself is associated with impaired cognitive functioning, is markedly higher among individuals with substance use disorders (Arias et al., 2008;Kalbag & Levin, 2005). We addressed this issue by excluding subjects with an ADHD diagnosis. Second, marijuana use is highly prevalent among stimulant users (McCabe et al., 2005). Chronic cannabis abusers have shown impaired performance on a variety of executive function tasks (Yucel et al., 2007a) with deficits being attributed to duration and frequency of cannabis use (Messinis et al., 2006). The potential influence of marijuana on stimulant users' verbal performance was addressed by classifying stimulant users into low and high marijuana users (see data analysis section for details).
The findings of executive dysfunctions in chronic stimulant users and atrisk populations invite the hypothesis that occasional stimulant users would show verbal fluency impairments, potentially preceding and predisposing to stimulant initiation. However, stimulants enhance cognitive performance in stimulant naïve subjects (Fillmore et al., 2005;de et al., 2002;de et al., 2000) and stimulant users (Garavan et al., 2008;Fillmore et al., 2006), and are being consumed specifically to improve performance. Assuming that an executive deficit existed prior to stimulant initiation, occasional stimulant users might now perform similarly to comparison subjects due to this stimulant-inherent enhancement of cognitive functioning. Thus, only subtle impairments were anticipated in this cohort of occasional users, most likely to be more apparent on non-habitual rather than over-learned verbal fluency tasks as these require more cognitive resources.
2. Materials and Methods
2.1. Sample
The study protocol was approved by local the Human Subjects Review Board (UCSD) and was carried out in accordance with the Declaration of Helsinki. Stimulant using subjects were recruited via recruitment flyers mailed to >7000 students at local universities, via internet ads (e.g. Craigslist), and using local university newspapers. 1025 stimulant users underwent intensive phone screens. 155 non-dependent stimulant using subjects and 49 stimulant naïve comparison subjects were included into the study. All individuals were informed that this study was aimed to examine behavior and brain functioning of people who use stimulants occasionally and all subjects gave written informed consent. Subjects were between the ages of 18 and 25. The inclusion criteria for healthy comparison subjects were (1) no lifetime use of stimulants; (2) no lifetime history of substance or alcohol related problems. Occasional stimulant users were defined as having (1) at least three distinct off-prescription uses of cocaine or prescription stimulants (amphetamines and/or methylphenidate) in the past six months; (2) no evidence for lifetime stimulant dependence; (3) never sought treatment of substance or alcohol related problems. Stimulant users and comparison subjects were assessed by experienced interviewers using the SSAGA (Semi Structured Assessment for the Genetics of Alcoholism, (Bucholz et al., 1994)) and diagnoses were based on consensus meetings with a clinician specialized in psychiatric, particularly substance use disorders (MPP) and trained study personnel. The following were exclusion criteria for both groups (1) evidence for Attention Deficit Hyperactivity Disorder (ADHD) as assessed with an extended section of the SSAGA; (2) use of stimulants for medical reasons, e.g. ADHD; (3) lifetime use of MDMA > 20 times (mean number of lifetime uses stimulant users: 3.22±4.99, comparison subjects: 0.06±0.32); (4) evidence for current (and past 6 months) of the following Axis I diagnoses: Panic Disorder, Social Phobia, Post-traumatic stress disorder, Major Depressive Disorder; (5) evidence for lifetime Bipolar disorder, Schizophrenia or other cognitive disorders, OCD; (6) evidence for Antisocial Personality Disorder; (7) any current positive urine toxicology test (exception: marijuana) and (8) head injuries or loss of consciousness for longer than 5 minutes.
2.2. Task Description
As part of a neuropsychological test battery further comprising the California Verbal Learning Test (CVLT II, Reske et al., 2010) and three other tests of the Delis-Kaplan Executive Function System (Delis et al. 2001, Trail Making Test, Card Sorting Test, Color-Word Interference Test, (Reske et al., in preparation)), the D-KEFS Verbal Fluency Test was carried out to assess verbal fluency capacities in occasional stimulant users. Participants were advised to refrain from excessive alcohol use the night prior to neuropsychological testing and, in case of stimulant users, to refrain from stimulant use for at least three days prior to testing.
The verbal fluency test comprises three testing conditions: Letter Fluency, Category Fluency and Category Switching and measures the participants' ability to generate words fluently in an effortful, phonemic format (Letter Fluency), from over-learned semantic concepts (Category Fluency), or while shifting between over-learned concepts (Category Switching). For the Letter Fluency test, subjects are required to quickly generate words beginning with a particular letter (F, A, S) while being instructed that names of places, people or numbers (e.g. four) and the same word with different endings (e.g. take, takes, taking) are counted as errors. In comparison to low-demanding category fluency tasks, where words from a semantic category shall be produced, letter fluency tasks present a novel task that cannot be performed habitually. For the Category Fluency subtest, subjects are asked to generate words belonging to a designated semantic category (animals, boy's names). The Category Switching test requires the examinee to generate words alternating between two over-learned semantic categories (fruits, furniture). Subtests are restricted to 60 seconds each and subjects are asked to produce as many words as possible without repeating words.
2.3. Data Analysis
Performance data were entered into the program for the analysis of D-KEFS data (Delis et al., 2001), which also scores the data. Measures used are raw total scores. The following measures were considered:
Letter Fluency. Number of correct responses generated within each 60-second trial.
Category Fluency. Number of correct responses within each 60-second trial.
Category Switching. Combined number of correct items given, independent of the switching accuracy.
Set Loss Errors total. Set Loss Errors score violations defined according to specified criteria for the respective conditions, for example, for the Letter Fluency task, words starting with a letter other than the designated letter or grammatical variants of words (e.g. fast, faster, fastest; sing, sang; non-words), for the Category Fluency task words that do not belong to the specified category (e.g. fur when “animals” are to be named), grammatical variants (cat, cats), words that are ordinate (e.g. animal) or superordinate (e.g. living things) and, for the Category Switching task, words that do not belong to either target category (e.g. coffee when asked to switch between Fruits and Furniture) or grammatical variants. The cohort consisted of high functioning, young students, who committed a relatively low number of Set Loss Errors per condition. Due to the fact that errors in different conditions are assumed to be based on the similar neuropsychological processes, i.e. the attenuated ability to apply defined rules, Set Loss Errors are summed across all six test trials (three letter fluency conditions, two category conditions and one switching condition).
Repetition Errors total. Repetition Errors refer to any response that is repeated within the 60 seconds of a given trial. Analogous to the rationale employed for Set Loss Errors, Repetition Errors are summed across all six trials of the verbal fluency test.
Performance data were analyzed using SPSS 16.0 (www.spss.com). Kolmogorov-Smirnov tests were carried out to test whether performance data followed a normal distribution. While raw scores for Letter Fluency, Category Fluency and Category Switching met normal distribution criteria, Set Loss and Repetition Errors did not (p's<0.001). Levene's tests further revealed that groups' variances were not homogeneous for Repetition Errors (p<0.02). Non-parametric Kruskal-Wallis tests were thus used instead of parametric analyses of variance to test for group differences. Non-parametric post hoc Mann-Whitney tests were applied for subsequent pairwise comparisons between below and above median THC stimulant users and comparison subjects.
The underlying idea of our analysis approach was, first, to identify differences in verbal fluency performance between stimulant users and stimulant naïve comparison subjects while acknowledging one of the most striking potentially conflicting factors: marijuana (Δ9 tetrahydrocannabinol, THC) use. The number of distinct lifetime marijuana uses, as assessed with the SSAGA and Timeline Follow-Back methods, differed between stimulant users and comparison subjects (p<0.001, see Tab. 1). In stimulant users, THC use preceded stimulant use by 2.59±2.01 (M±SD) years and lifetime stimulant use was significantly correlated with lifetime marijuana co-use (Pearson's correlation coefficient: 0.239, p=0.003). To account for a potential effect of lifetime THC use on verbal fluency performance, we divided the group of stimulant users into below and above median THC users based on their lifetime marijuana use (median of InTHC: In(1 + lifetime marijuana uses)). 77 stimulant users (Below Median THC Stimulant Users, 34 females) reported a lifetime marijuana use below the stimulant users' median of 5.889 (InTHC) and were compared to 78 stimulant users classified as Above Median THC Stimulant Users (27 females) and 49 comparison subjects (see Tab. 1). A secondary analysis compared marijuana users with THC naïve participants (stimulant users and comparison subjects, respectively) to further explore if marijuana impacted verbal fluency performance (analysis of covariance, ANCOVA, controlling for lifetime stimulant use).
Table 1.
Sociodemographics, drug use and personality/clinical measures in 155 non-dependent stimulant users and 49 comparison subjects and in subgroups of stimulant users with below and above median marijuana (THC) use.
| Stimulant Users, n=155 |
Below Median THC Stimulant Users, n=77 |
Above Median THC Stimulant Users, n=78 |
Comparison Subjects, n=49 |
|||||
|---|---|---|---|---|---|---|---|---|
| m | sd | m | sd | m | sd | m | sd | |
| Sociodemographics | ||||||||
| Age | 20.80 | 1.52 | 20.66 | 1.56 | 20.94 | 1.480 | 21.22 | 2.21 |
| Education | 14.44 | 1.2 | 14.35 | 1.28 | 14.53 | 1.21 | 14.68 | 1.37 |
| Females (%) | 39% | 44% | 35% | 55% | ||||
| Race/Ethnicity | ||||||||
| Caucasian (n) | 129 | 63 | 66 | 35 | ||||
| Afro-American (n) | 2 | 1 | 1 | 0 | ||||
| Asian (n) | 14 | 10 | 4 | 12 | ||||
| Other/Mixed (n) | 10 | 3 | 7 | 2 | ||||
| Verbal IQ (WTAR) | 108.36 | 7.21 | 107.45 | 7.68 | 109.23 | 6.67 | 109.65 | 6.88 |
| Impulsivity (BIS) | 66.29 | 11.142 | 65.21 | 10.243 | 67.36 | 11.932 | 61.57 | 6.933 |
|
Drug Use (# lifetime) |
||||||||
|
Prescription Stimulants |
31.04 | 74.16 | 20.55 | 38.37 | 41.40 | 96.59 | N/A | |
|
Recreational Stimulants |
29.94 | 77.59 | 23.49 | 92.59 | 36.31 | 59.13 | N/A | |
| THC | 859.34 | 1355.73 | 118.92 | 110.93 | 1590.27 | 1604.50 | 30.31 | 100.293 |
Four additional potentially influencing aspects were investigated next: First, the effect of cumulative stimulant use on neurocognitive performance is still a matter of debate. While impairments were shown to be apparent in chronic users, higher levels of lifetime stimulant use were shown to be largely uncorrelated with neuropsychological test scores. Moreover, a few significant correlations suggested either a better functioning with more stimulant use (Toomey et al., 2003) or a cognitive decline with cocaine abuse (Ardila et al., 1991;Bolla et al., 1999). We examined the effect of cumulative lifetime stimulant use on verbal fluency performance by directly comparing stimulant users with below and above median lifetime stimulant use (median of InSTM: In(1 + lifetime marijuana uses)) with comparison subjects: According to their lifetime stimulant use, stimulant users were divided into below (n=77) and above median (n=78) users (median InSTIM=3.2581). For these analyses, lifetime marijuana use was included as a covariate (ANCOVAs covarying for InTHC). Second, we analyzed the effect of the preferred stimulant type by comparing stimulant users with a preference for prescription stimulants (prescription amphetamines and/or methylphenidate, total of n=55, comprising n=35 users describing lifetime consumption of prescription stimulants only and n=20 users characterized by a predominant use of prescription stimulants (≥ 80% of total stimulant use)) or cocaine (total of n=43, with n=13 users with cocaine use only and n=30 with predominant cocaine use (≥ 80% of total stimulant use)) with users without a clear preference (uses of prescription stimulants and cocaine between 20 and 80% of total use, n=57) and comparison subjects. Specifically, analyses of covariance (ANCOVA) were applied controlling for lifetime marijuana use (InTHC). Third, the effect of subsyndromal ADHD on verbal fluency performance was analyzed. Specifically, participants (stimulant users and comparison subjects) with and without ADHD symptoms during ages 6 through 13 as assessed with the SSAGA (maximum of n=10 attention deficit symptoms and n=11 hyperactivity symptoms) were compared. Fourth, based on an increased “female vulnerability” for cocaine related problems (Rahman & Clarke, 2005) and hints at gender-specific verbal performance among cocaine users (Rahman & Clarke, 2005), we compared performance in male and female participants. In particular, verbal fluency performance was assessed integrating gender as a second fixed factor into the univariate analysis as well as in male and female stimulant users and comparison subjects separately covarying for substance use.
3. Results
155 stimulant users (94 males, 61 females) and 49 healthy comparison subjects (22 males, 27 females) did not differ on age (see Tab. 1, t202=1.254, p=0.215), education (t200=1.142, p=0.255), or gender distribution (χ2=3.764, p=0.052). Moreover, groups did not differ on Verbal IQ (t194=1.048, p=0.296, n.s.) as estimated using the Wechsler Test for Adult Reading (WTAR), but stimulant users were characterized by higher impulsivity ratings as assessed with the Barratt Impulsivity Scale (BIS, t202=2.795, p=0.001, see Tab. 1)
133 stimulant users and 23 comparison subjects reported to have tried any form of tobacco (lifetime) and 106 stimulant users and 12 comparison subjects reported to smoke currently, where stimulant users reported to smoke a mean of 6.23±4.82 cigarettes per day on 6.05±1.76 days per week (information on weekly use available for 64 stimulant users; the exact weekly nicotine consumption was available for 4 of the 12 smoking comparison subjects only, which prevented statistical comparisons across groups) drink on an average of 3.23 (±1.59) days per week while comparison subjects drank on 1.62±0.78 days per week (t187=6.131, p<0.001). In a typical week, stimulant users reported to drink an average of 20.17 (±14.37) standard drinks per week (comparison subjects: 4.77 (±3.39) drinks, t189=6.797, p<0.001).
3.1. Comparison of Stimulant Users and Comparison Subjects
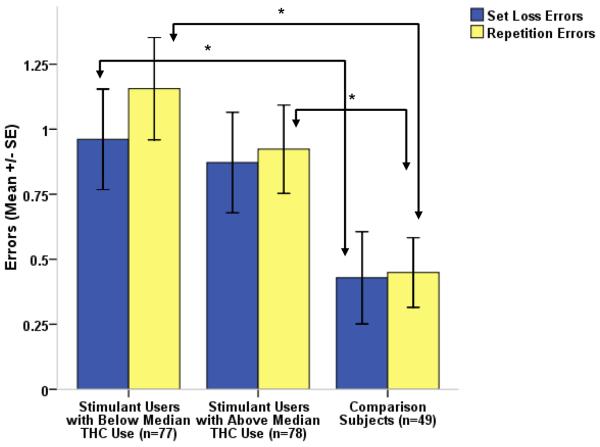
Groups (stimulant users with below and above median THC use and comparison subjects) differed on Category Fluency performance (Kruskal-Wallis χ2= 6.68, p=0.035), Set Loss Errors total (summed up across all six test conditions, χ2= 7.213, p=0.027) and Repetition Errors total (summed up across all six test conditions, χ2=8.59, p=0.014). Post hoc Mann-Whitney tests revealed that, on requests to generate verbal sets under time pressure, stimulant users sacrificed accuracy for speed speed-accuracy trade-off): Specifically, stimulant users produced significantly more responses than comparison subjects on the over-learned Category Fluency task (below median THC users: p=0.015, above median THC users: p=0.030; for means refer to Tab. 2) but generated significantly more Set Loss total (below median THC: p=0.007, above median THC: p=0.057, n.s.) and Repetition Errors total than comparison subjects (p=0.004 and p=0.016, see Fig. 1).
Table 2.
Comparison of verbal fluency performance of stimulant users with below (n=77) and above (n=78) median marijuana use and 49 comparison subjects (non-parametric Kruskal-Wallis tests). Post hoc Mann-Whitney tests reveal that both subgroups of stimulant users outperform comparison subjects on the Category Fluency task, an over-learned verbal capacity while performing more errors than comparison subjects.
| Below Median THC Stimulant Users, n=77 |
Above Median THC Stimulant Users, n=78 |
Comparison Subjects, n=49 |
Kruskal-Wallis | ||||
|---|---|---|---|---|---|---|---|
| m | sd | m | sd | m | sd | p | |
| Letter Fluency | 43.35 | 10.796 | 46.28 | 12.124 | 43.59 | 8.974 | 0.342 |
| Category Fluency | 48.44 | 9.875 | 47.27 | 7.702 | 43.29 | 8.884 | 0.035 |
| Category Switching | 14.68 | 3.156 | 15.30 | 2.720 | 14.65 | 2.712 | 0.419 |
|
| |||||||
| Set Loss Errors | 0.96 | 1.697 | 0.87 | 1.708 | 0.43 | 1.242 | 0.027 |
| Repetition Errors | 1.16 | 1.725 | 0.92 | 1.501 | 0.45 | 0.937 | 0.014 |
Figure 1.
While outperforming comparison subjects on the over-learned capacity to recall words from a semantic group (Category Fluency), stimulant users produced more Set Loss and Repetition Errors than comparison subjects, reflecting a tendency to disrespect rules which is known to be associated with frontal lobe impairments.
On the other hand, stimulant users with below and above median marijuana use, i.e. those who had relatively few versus those that had many uses of marijuana, did not differ significantly on any of these variables (pCategory Fluency=0.777, pSet Loss Errors total=0.352, pRepetition Errors total=0.570). Further, we tested for linear relationships between recency of marijuana use (measured in days as assessed with the Timeline Follow-Back method) and verbal fluency performance in stimulant users. For the variables differing between stimulant users and comparison subjects, correlations were insignificant (Category Fluency: p=0.415; Set Loss Errors total: p=0.647; Repetition Errors total: p=0.844, n.s.), strengthening that marijuana co-use did not affect verbal fluency performance in stimulant users.
A secondary comparison of marijuana users (n=177, lifetime use: 787.62±1310.506) and marijuana-naïve participants (n=33) to further elucidate the potential effect of marijuana (ANCOVAs covarying for lnSTIM) also revealed no effect of marijuana on any of the verbal fluency measures (all p's > 0.5, n.s.).
3.2. Effect of Lifetime Stimulant Use
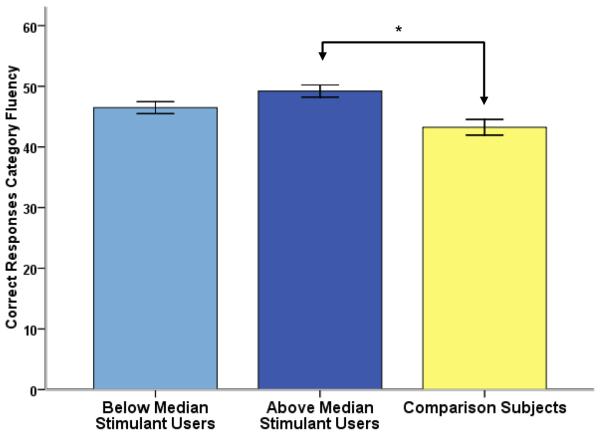
Next, the group of stimulant users was divided into users with below (n=77, 31 females; age: 20.58±1.39, education: 14.34±1.29) and above (n=78, 30 females; age: 21.01±1.62, education: 14.54±1.19) median stimulant use. Age and education did not differ between subgroups of users (age: t153=1.764, p=0.08; education: t153=1.005, p=0.316). Below and above median stimulant users were compared with comparison subjects in ANCOVAs including lnTHC as a covariate. While subgroup neither differed for Letter Fluency (F2,200=1.421, p=0.244, n.s.), Category Switching (F2,200=0.057, p=0.945, n.s.), Set Loss total (F2,200=0.946, p=0.39) nor Repetition Errors total (F2,200=2.738, p=0.067), a significant group difference for Category Fluency performance (F2,200=6.661, p=0.002, see Fig. 2) was observed. Post hoc Bonferroni tests revealed that individuals with above median stimulant use relative to comparison subjects showed better performance during Category Fluency (p=0.001). In comparison, this effect was less pronounced for individuals with below median stimulant use (p=0.052, n.s.). However, there were no significant correlations between lifetime stimulant use (lnSTIM) and performance on any verbal fluency performance measures within the stimulant user group. Taken together, these analyses reveal that, in occasional, non-dependent users of psychostimulants, relatively frequent rather than infrequent use (lnSTIM = E4.2662 vs. 2.4553) is associated with increased over-learned verbal fluency performance. No evidence, however, was found for a linear increase of verbal fluency performance with lifetime use.
Figure 2.
To investigate the effect of lifetime stimulant use on verbal fluency performance, stimulant users were divided into below and above median users (medianlnSTIM = 3.2581). Stimulant users with higher lifetime stimulant use significantly outperformed comparison subjects on the category fluency task (p=0.001).
3.3. Effect of Preferred Type of Stimulant Used
Differential effects of cocaine and prescription stimulants (amphetamines and/or methylphenidate) were investigated comparing the subgroups of stimulant users preferring cocaine (n=43, 19 females; age: 21.14±1.73 years, education: 14.40±1.27 years), prescription stimulants (n=55, 24 females; age: 20.51±1.28, education: 14.27±1.25) or reporting no preference for either stimulant type (n=57, 18 females; age: 20.82±1.55, education: 14.63±1.21) with comparison subjects. Age and education did not differ between subgroups of users (age: F2,152=2.112, p=0.125, education: F2,152=1.205, p=0.303).
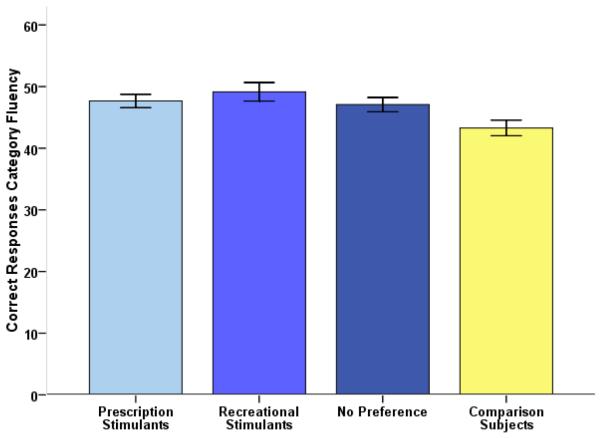
For the Category Fluency task, stimulant users with a preference for cocaine significantly outperformed comparison subjects (analysis of covariance controlling for the effect of lifetime THC use: p=0.014, see Fig. 3, effect size Cohen's d=0.62) while only a strong trend was found for users preferring prescription stimulants (p=0.052, n.s., d=0.52). The amount of Set Loss and Repetition Errors did not differ between subgroups of stimulant users with preferences for different types of stimulants and comparison subjects (Set Loss Errors total: p=0.493, Repetition Errors total: p=0.107).
Figure 3.
Evidence for an effect of stimulant type on verbal performance: Stimulant users with a clear preference for recreational stimulants outperform comparison subjects while only a strong trend (p=0.052) was observed for prescription stimulant users.
3.4. No Effect of Subsyndromal Attention Deficits or Hyperactivity
To investigate whether subsyndromal ADHD symptoms affected verbal fluency performance, we compared 114 participants who did not fulfill any attention deficit or hyperactivity symptoms (maximum total: 21 ADHD symptoms) during ages 6 through 13 as assessed with an extended section of the SSAGA with 88 subjects who reported a mean of 3.93±3.710 symptoms age 6-13. Controlling for lifetime stimulant use, no significant differences on verbal fluency performance or rates of Set Loss or Repetition Errors were found between subjects with and without ADHD symptoms (ANCOVAs with lnSTIM, all p's > 0.071). Taking together our exclusion criterion of ADHD diagnosis and this lack of effect of subsyndromal ADHD symptoms, we can conclude that verbal fluency differences between stimulant users and comparison subjects were not due to increased attention deficits or hyperactivity.
3.5. Gender Differences
Male and female stimulant users did not differ on lifetime stimulant use (p=0.659, n.s.) and estimated verbal IQs of male and female stimulant users and gender-matched comparison subjects were comparable (p=0.853 and 0.238, n.s.). Including gender as an additional fixed factor into the model, no significant differences were observed in verbal fluency performance between occasional stimulant users and comparison subjects (ANCOVAs covarying for lnSTIM and lnTHC; Letter Fluency: p=0.328, Category Fluency: p=0.151; Category Switch: 0.992; Set Loss Errors total: 0.172, Repetition Errors total: p=0.087).
The analysis of verbal fluency data for both genders separately, however, revealed that the differences identified in the combined male and female sample (faster category fluency performance but increased error rates in stimulant users) remained significant for males only but not for females. Specifically, male stimulant users significantly outperformed male comparison subjects on the Category Fluency task (48.63±8.729 vs. 43.27±10.119 correct responses, p=0.020) and showed a strong trend to a worse performance on the Letter Fluency task (p=0.059, n.s.) and to generate more Repetition Errors than comparison subjects (p=0.097, n.s., ANCOVAs covarying for lifetime marijuana use). Male stimulant users and comparison subjects did not differ on Set Loss Errors (p=0.32, n.s.). Female stimulant users and Dcomparison subjects on the other hand did not differ on any of the verbal fluency measures. Given the relatively smaller samples and, therefore, the reduced power to detect significant differences, one should be careful in interpreting these results.
4. Discussion
In this study, the neuropsychological functioning of a large cohort of individuals who experimented with stimulants (predominantly prescription stimulants or cocaine) was examined and yielded two main results: First, individuals who occasionally use stimulants but do not fulfill the criteria for stimulant dependence performed better on some verbal tasks compared to stimulant naïve individuals. This improvement was limited to verbal tasks which rely heavily on over-learned, automated processing strategies (Category Fluency). Second, this seemingly superior verbal performance in stimulant using subjects was accompanied by increased error rates, i.e. occasional stimulant users generated significantly more Set Loss and Repetition Errors than comparison subjects. In contrast, stimulant using individuals did not differ from comparison subjects on verbal fluency tasks that rely on high cognitive load and non-habitual processing such as Letter Fluency or Category Switching.
The combination of superior performance on overlearned verbal behavior and increased errors by occasional stimulant users may be a sign of greater verbal production in the context of cognitive rigidity and diminished flexibility. Specifically, whereas stimulant users perform better than high functioning comparison subjects on an overlearned task, they are not able to apply the appropriate rules. In comparison, when facing new, cognitively challenging demands stimulant users cannot adequately stick to rules but instead vacillate between impulsive and rigid performance.
Comparing the performance profiles of individuals with preferences for different types of stimulants revealed that recreational but not prescription stimulants were associated with better automated and over-learned verbal fluency performance. One potential explanation for this result is the possibility that individuals who are prone to use cocaine in social contexts may be characterized as being relatively weaker on cognitively challenging task, which require a transfer of knowledge to novel situations and tasks and not only a transcript of memorized facts and strategies.
The results of increased rigidity and diminished flexibility on verbal fluency tasks in occasional stimulant users can contribute to some degree to the question whether executive deficiencies are a pre-existing condition for the propensity to use stimulants. If these executive dysfunctions preceded initiation of stimulant use, they may have served as the incentive to use stimulants in the first place, might represent a vulnerability marker for stimulant use and problems and could enable researchers and in the end clinicians to identify not only subjects at high risk for stimulant related problems but also to develop training strategies to overcome objective or self experienced deficiencies. Alternatively, the verbal fluency performance profile of increased performance on over-learned tasks combined with increased error rates could have been a consequence of stimulant use. However, this latter possibility would be more plausible if there was a dose-relationship to the observed differences, i.e. if individuals with greater numbers of stimulant use would exhibit more pronounced impairments. In contrast, our data clearly contradict a linear correlation between cumulative lifetime stimulant use and verbal fluency performance. Studies on verbal fluency capacities in stimulant dependent or chronic users show mixed results and cannot be drawn on as support. Here, the effect of better performance was solely more pronounced in occasional stimulant users with relatively more lifetime uses of stimulants and in male users. Our findings of altered performance on verbal fluency in high functioning occasional users with very limited exposure to stimulants along with a lack of relationship between performance and lifetime stimulant use are consistent with the view of a pre-morbid, pre-existing executive trait characteristic (Kirisci et al., 2004b;Tarter et al., 2003;Aytaclar et al., 1999) that may have led to stimulant initiation and clearly invite the hypothesis that impairments in executive functioning are a trait characteristic that precedes and promotes stimulant use. Though unlikely given the above, we cannot entirely rule out that the limited variability in age and amount or duration of use may have covered dose – performance relationships.
Though only investigated in a secondary analysis, our study provides evidence for gender-associated effects on neuropsychological performance in stimulant users. Specifically, we show that the specific pattern of verbal fluency performance of increased performance on over-learned tasks along with heightened cognitive rigidity and diminished flexibility was driven by male stimulant users. Gender-specific effects have recently also been described in neuroimaging studies investigating diverse executive functioning domains in stimulant dependent males and females, such as response inhibition (e.g. (Li et al., 2005;Li et al., 2006;Li & Sinha, 2008), in parts in absence of behavioral differences, further supporting the notion to consider effects of gender when investigating executive functioning in stimulant users.
One limiting factor of our study is that the design was not completely prospective; we included stimulant using individuals with very limited exposure to stimulants lacking clinically significant stimulant-related problems. As all other cross sectional studies, we therefore cannot directly account for pre-existing neurocognitive characteristics. The ideal but barely practical study design would include adolescents into a longitudinal approach prior to any drug use. We addressed this methodological challenge by investigating stimulant users with only minimal use and comparing users with relatively low and high lifetime uses, and, lastly, by correlation analyses with cumulative stimulant use. Importantly, our results cannot be attributed to attention deficits or hyperactivity, a common characteristic among stimulant users. We specifically excluded stimulant using and comparison subjects with a lifetime history (diagnosis or treatment) of ADHD and conduct disorder, both of which known to contribute to the propensity to substance use (Arias et al., 2008;Kalbag & Levin, 2005;Marshal & Molina, 2006) and were able to show that even subsyndromal attention deficits and hyperactivity did not account for our results. Moreover, we accounted for another striking characteristic of stimulant use: Occasional stimulant users usually exhibit high lifetime uses of marijuana (McCabe et al., 2005), which itself affects executive functioning (Yucel et al., 2007b). Our statistical approach to choose subgroup analyses comparing stimulant users with low and high lifetime marijuana use rather than eliminating the impact of marijuana (e.g. through covariance analyses) strongly acknowledged the co-existing nature of stimulant and marijuana use. The present results reveal that marijuana did not affect verbal fluency performance in occasional stimulant users.
5. Summary and Conclusion
Psychostimulants like methylphenidate and dextroamphetamine boost over-learned verbal fluency performance in non-dependent, occasional stimulant users. However, stimulant users are characterized by an increased cognitive rigidity and diminished flexibility, cannot refrain from breaking rules and generate more set loss and repetition errors. The absence of a relationship of cumulative stimulant use to this speed-accuracy trade-off suggests that executive impairments precede and potentially promote stimulant initiation. Our results are consistent with the notion that executive dysfunctions serve as a vulnerability marker or predictor for stimulant use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int.J.Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Gelernter J, Chan G, Weiss RD, Brady KT, Farrer L, Kranzler HR. Correlates of co-occurring ADHD in drug-dependent subjects: prevalence and features of substance dependence and psychiatric disorders. Addict.Behav. 2008;33:1199–1207. doi: 10.1016/j.addbeh.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J.Am.Acad.Child Adolesc.Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. J.Child Adolesc.Psychopharmacol. 2002;12:301–309. doi: 10.1089/104454602762599844. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J.Neuropsychiatry Clin.Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J.Stud.Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- de WH, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in Ehumans. Behav.Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- de WH, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Di S,V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend. 2005;77:151–159. doi: 10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos.Trans.R.Soc.Lond B Biol.Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen RW, Kranzler HR, Bauer LO, Burleson JA, Samarel D, Morrison DJ. Neuropsychologic findings in cocaine-dependent outpatients. Prog.Neuropsychopharmacol.Biol.Psychiatry. 1998;22:1061–1076. doi: 10.1016/s0278-5846(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Herman-Stahl MA, Krebs CP, Kroutil LA, Heller DC. Risk and protective factors for nonmedical use of prescription stimulants and methamphetamine among adolescents. J.Adolesc.Health. 2006;39:374–380. doi: 10.1016/j.jadohealth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Kalbag AS, Levin FR. Adult ADHD and substance abuse: diagnostic and treatment issues. Subst.Use.Misuse. 2005;40:1955–1958. doi: 10.1080/10826080500294858. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J.Neuropsychiatry Clin.Neurosci. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Reynolds M, Habeych M. Relation between cognitive distortions and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: a prospective study. Drug Alcohol Depend. 2004b;76:125–133. doi: 10.1016/j.drugalcdep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Reynolds M, Habeych M. Relation between cognitive distortions and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: a prospective study. Drug Alcohol Depend. 2004a;76:125–133. doi: 10.1016/j.drugalcdep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Monterosso J, Elman I, Ash B, Krikorian G, Austin G. Effect of methylphenidate on Stroop Color-Word task performance in children with attention deficit hyperactivity disorder. Psychiatry Res. 2006;141:315–320. doi: 10.1016/j.psychres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol.Psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci.Biobehav.Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP, Molina BS. Antisocial behaviors moderate the deviant peer pathway to substance use in children with ADHD. J.Clin.Child Adolesc.Psychol. 2006;35:216–226. doi: 10.1207/s15374424jccp3502_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am.J.Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Clarke CD. Sex differences in neurocognitive functioning among abstinent recreational cocaine users. Psychopharmacology (Berl) 2005;181:374–380. doi: 10.1007/s00213-005-2257-8. [DOI] [PubMed] [Google Scholar]
- Reske M, Delis DC, Wittmann M, Berger F, Paulus MP. Executive Dysfunctions Develop With Increasing Stimulant Use – Evidence From A Non-Dependent Cohort. In: in preparation. [Google Scholar]
- Reske M, Eidt CA, Delis DC, Paulus MP. Non-Dependent Stimulant Users of Cocaine and Prescription Amphetamines Show Verbal Learning and Memory Impairments. Biol.Psych. 2010 June 3rd; doi: 10.1016/j.biopsych.2010.04.021. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr.Opin.Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J.Abnorm.Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, Faraone SV, Tsuang MT. A twin study of the neuropsychological consequences of stimulant abuse. Arch.Gen.Psychiatry. 2003;60:303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, ia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The Neuropsychology of Cocaine Addiction: Recent Cocaine Use Masks Impairment. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Solowij N, Brewer WJ. Understanding drug addiction: a neuropsychological perspective. Aust.N.Z.J.Psychiatry. 2007a;41:957–968. doi: 10.1080/00048670701689444. [DOI] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Solowij N, Brewer WJ. Understanding drug addiction: a neuropsychological perspective. Aust.N.Z.J.Psychiatry. 2007b;41:957–968. doi: 10.1080/00048670701689444. [DOI] [PubMed] [Google Scholar]