Abstract
The emerging field of micro-technology has opened up new possibilities for exploring cellular chemotaxis in real space and time, and at single cell resolution. Chemotactic cells sense and move in response to chemical gradients and play important roles in a number of physiological and pathological processes, including development, immune responses, and tumor cell invasions. Due to the size proximity of the microfluidic device to cells, microfluidic chemotaxis devices advance the traditional macro-scale chemotaxis assays in two major directions: one is to build well defined and stable chemical gradients at cellular length scales, and the other is to provide a platform for quantifying cellular responses at both cellular and molecular levels using advanced optical imaging systems. Here, we present a critical review on the designing principles, recent development, and potential capabilities of the microfluidic chemotaxis assay for solving problems that are of importance in the biomedical engineering field.
Keywords: Microfluidics, Mammalian cell chemotaxis, Molecular gradients
INTRODUCTION
Chemotaxis is a dynamic process where cells move up a chemoattractant or down a chemorepellent concentration gradient.26,43 Mammalian cell chemotaxis represents an important class of cell–cell communication through chemical signaling and plays instrumental roles in a number of physiological and pathological processes. Development: All mammals begin their life as a single cell, and the cell undergoes subsequent divisions. Daughter cells migrate to designated spatial locations and form functional organs. Immune cell trafficking: Immune cells are the first line of defense when a multi-cellular organism is attacked by an infectious agent or foreign materials.4,21,46 Immune cells circulate throughout the body and are ready to migrate and invade any types of tissues. Immune cell migration is important both in the homeostatic process of tissue maintenance and the fight against pathogens. Tumor cell migration and invasion: Cancer metastasis, where cancer cells leave a primary tumor and establish a secondary tumor in a different organ, is the leading cause of all cancer death.8,78 Despite its clinical importance, the underlying molecular and physical mechanisms with which cancer cells use to migrate and invade (two critical steps of cancer metastasis) are poorly understood.18 The challenges come from the complexity of the cancer microenvironments, as well as cancer cells’ ability to adapt and remodel their microenvironments.17,30,36,85
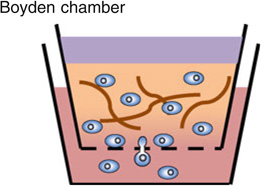
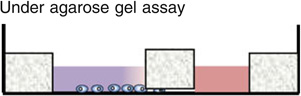
The Boyden chamber has traditionally been the key instrument for mammalian cell chemotaxis,6 although a number of other methods including the Zigmond chamber,90 the Dunn chamber,89 the micropipette-based assay72,86 and the under agarose gel assay have also been used (see figures embedded in Table 1). All of these approaches are based on introducing a chemical gradient to the cells of interest, but are often inadequate in providing a reproducible, controllable, and stable linear gradient. In Table 1, we list the advantages and limitations of each of these chemotaxis assays. One of the most common cell migration assay is the Boyden chamber assay. In this assay, cells of interest are seeded in an insert with a porous membrane at the bottom, and the insert is subsequently placed in a solution containing a chemical attractant. The number of cells transmigrating from within the insert through the membrane and into the chemoattractant solution is used as a measure for chemotactic sensitivity. Although this assay is easy and quick to implement, the limitations are (1) it only gives population based, and end-point results; (2) it does not provide a steady chemical gradient for cells; (3) it does not distinguish chemokinesis vs. chemotaxis. With the rapid advancements in intra-vital imaging,16 it is now clear that cellular microenvironments are dynamic and complex.36,69 Cells are constantly interacting with the extracellular matrix, and their phenotypes change with the evolving microenvironments.30,33,67,85 There is a need for introducing space and time into chemotaxis studies, both in the context of building spatially and temporally controllable chemical gradients, as well as following cellular dynamics in real time and space.
TABLE 1.
Macro-scale assays for studying mammalian cell chemotaxis.
| Advantages | Limitations | |
|---|---|---|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
Microfluidic device overcomes the limitations posed by the macro-scale chemotaxis assays, and it has demonstrated its potential for creating well defined spatial and temporal chemical concentration gradients 1,50–52,55,57,74,84; as well as complex microenvironments such as co-culture, and presence/absence of fluid flows.14,65 Furthermore, its compatibility with an optical microscope has enabled us to study chemotaxis in real time and at single cell level. In this review, we will present both physical and biological considerations for designing microfluidic chemotaxis devices, and their potentials to provide a fundamental understanding of mammalian cell chemotaxis.
MOLECULAR GRADIENTS AND TRANSPORT
Molecular Gradients in Living Systems
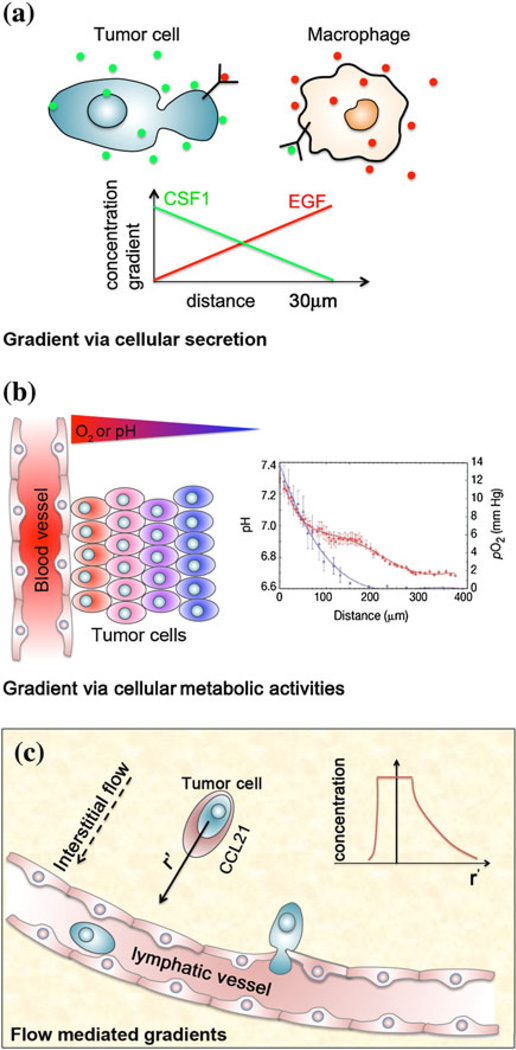
The formation of molecular gradients requires the generation and removal of the molecules in different spatial locations within a cell culture. In vivo, molecules are typically generated through cellular secretion, and are removed through cellular metabolism or protein degradation. These molecules actively bind to or dissociate from the extracellular matrix, and are distributed within the extracellular matrix via the convective-diffusive transport. These activities contribute to the complex molecular gradients within a cell culture. Figure 1 shows three examples of how molecular gradient are formed in living systems.
FIGURE 1.
Molecular gradients in living systems. (a) Molecular gradients via cellular secretion. Illustration of a tumor cell communicating with a macrophage via paracrine signaling. The macrophage secretes EGF, which induces the tumor cell to secrete CSF1. CSF1 is a chemoattractant for macrophage. The diffusive peri-cellular EGF/CSF1 concentration profiles of the macrophage/tumor cell are drawn, respectively. (b) Molecular gradients via cellular metabolic activities. Graph is reproduced from Carmeliet and Jain7 with permission from the Nature Publishing Group. Shown here is a drawing of a portion of the tumor cells adjacent to a blood vessel within a tumor body. An oxygen concentration or pH gradient toward the blood vessel is formed as a result of oxygen consumption by the tumor cells, and the diffusion limited transport of oxygen from the blood vessel to the tumor cells. The graphs show the measured oxygen and pH gradients in a human xenograph. (c) Flow mediated molecular gradient. The graph is reproduced from Shields et al.75 Interstitial flow caused by the pressure difference between blood and lymphatic vessels, and the osmotic pressure points toward the lymphatic vessel. The peri-cellular CCL21 distribution around the tumor cell in the interstitial space is modified by the flow. Here, the insert shows an asymmetric CCL21 concentration profile centered at the center of the tumor cell.
The first example of molecular gradient formation is from cellular secretion and the subsequent diffusion into the surrounding extracellular matrix (see Fig. 1a). In mammalian cell culture, cells communicate via either autocrine or paracrine signaling. Autocrine signaling refers to a cell signaling to itself via self-secreted molecules. Paracrine signaling refers to one cell signaling to another cell of either the same or different cell type53 (see Fig. 1a). In both cases, cells secrete chemokines or growth factors. Tumor cells, for example, are known to secrete an array of chemokines (e.g., IL8, CCL21, SDF-1α) and growth factors (e.g., epidermal growth factor (EGF)) to form a tight control of their microenvironments, and to enhance their ability to migrate to a distant site. For a complete list of chemokines and growth factors secreted by tumor cells, please refer to Roussos et al.69 Immune cells are another class of cell type that utilizes molecular gradients within their surrounding as guidance cues to migrate.39,72 Dendritic cells, for instance, are known to migrate up lymphoidal chemokine, CCL21 and CCL19, gradient toward lymphatic vessels.35 Recently, it has been shown that the autocrine signaling via the vascular endothelial growth factor (VEGF) gradients plays a critical role in network formation of endothelial cells.71 The subject of the chemotaxis-assisted endothelial cell network formation will be discussed in a later section.
The second example of the molecular gradient formation is the oxygen concentration or pH gradient as a result of cellular metabolic activities.7,12,38,81 Tumor cells are known to have a high metabolic rate, and hence a high oxygen consumption rate. Tumor cells initially grow as an avascular mass until the tumor body reaches a critical size, where the cells in the center becomes hypoxic due to the diffusion limited oxygen supply from their surrounding tissues.81 This generates an oxygen concentration gradient with the highest concentration being at the edge of the tumor body. Verbridge et al. described this phenomenon computationally.81 In subsequent events, hypoxic tumor cells secrete pro-angiogenic factors that promote angiogenesis (sprouting of new blood vessels), an important hallmark of tumor progression.28 In a vascularized tumor, blood vessels are oxygen suppliers to the tumor cells. Due to tumor metabolic activities, there is an oxygen and pH gradient adjacent to the blood vessel in the tumor mass as shown in Fig. 1b. It should be noted that the characteristic length scale of the oxygen gradient profile in Fig. 1 is about 100 µm, which is close to the average inter-blood vessel distance observed in vascularized tissue. This length scale, is determined by the Krogh length,29 which is . Here, DO2 is the diffusivity of oxygen in the tissue, CO2 is the oxygen concentration and RO2 is the cellular oxygen consumption rate.
The third example illustrates how fluid flows can modify the shapes of the molecular gradients in living systems (see Fig. 1c). Fluid flows are known to impact cellular behaviors in a significant way; mostly in their role as a mechanical force driver.58,59 It is only recently that the role of fluid flows as a chemical gradient mediator has been revealed and discussed.65,75 Interstitial flow, a slow flow in the order of ~1 µm/s, is ubiquitous in interstitial space (or tissues). It is driven by the hydrostatic pressure difference between the arterial and venous vessels and the osmotic pressure. In Fig. 1c, a tumor cell situated in the interstitial space is subjected to the interstitial flow pointing toward the lymphatic vessel. The tumor cell is secreting CCL21, and forms a peri-cellular CCL21 concentration profile via the molecular diffusion. This concentration profile is symmetric with respect to the tumor cell in the absence of the flow; but skewed toward the flow in the presence of the flow (see the insert in Fig. 1c). Recent work from the Swartz lab using a modified Boyden Chamber assay has demonstrated that the interstitial flow may distort the secreted chemokine concentration profile around the tumor cells, and guide the tumor cells to move along the direction of the flow and toward the lymphatic vessel,75 a phenomenon named as autologous chemotaxis.
Physical Principles in Molecular Transport
Molecular transport typically takes place in two major forms; diffusion based, and convective flow based. In this section, we will discuss the general physical principles governing the molecular transport within living tissues. An emphasis will be given to the relevant time and length scales.
Diffusive Molecular Transport
Cells in a 3D cell culture are surrounded by extracellular matrix (ECM), a hydrogel that contains mostly water. When cells secrete chemicals, the secreted molecules diffuse into their surrounding ECM. This process, in its simplest form can be described by the standard diffusion equation:
| (1) |
where C is the chemical concentration and D is the diffusion coefficient of the molecule of interest. Let’s assume that we have a single spherical cell, and it secretes a chemical at a constant rate at the cell surface r = R, where r is the distance to the center of the cell, and R is the radius of the cell. We also assume that the concentration of the chemical at the cell surface is a constant, and zero farther away. The solution of the concentration field using Eq. (1) and the above boundary conditions can be obtained using a Comsol Multiphysics Analysis software (Burlington, MA) and the result is shown in the first column of Fig. 2. The concentration profiles in the bottom panel show that the molecules spread out over time. The characteristic time for a molecule to diffuse through a distance L is L2/2kD, where k is the spatial dimension. If we use cell diameter 20 µm as the length scale, and molecular diffusion coefficient of 100 µm2/s for molecules with a molecular weight of 10 kDa, the characteristic time is ~1 s. It takes a longer time for the large molecule to diffuse as its diffusion coefficient decreases with the increase of the molecular weight. Diffusion coefficient D can be estimated using Einstein-Stokes equation where kB is Boltzmann’s constant, T the temperature, η the solvent viscosity, and rH the hydrodynamic radius of the molecule for diffusion of spherical particles through liquid.64 For diffusion coefficients of molecules with a range of molecular weight (342–1,950,000 Da), please refer to Lebrun and Junter.47
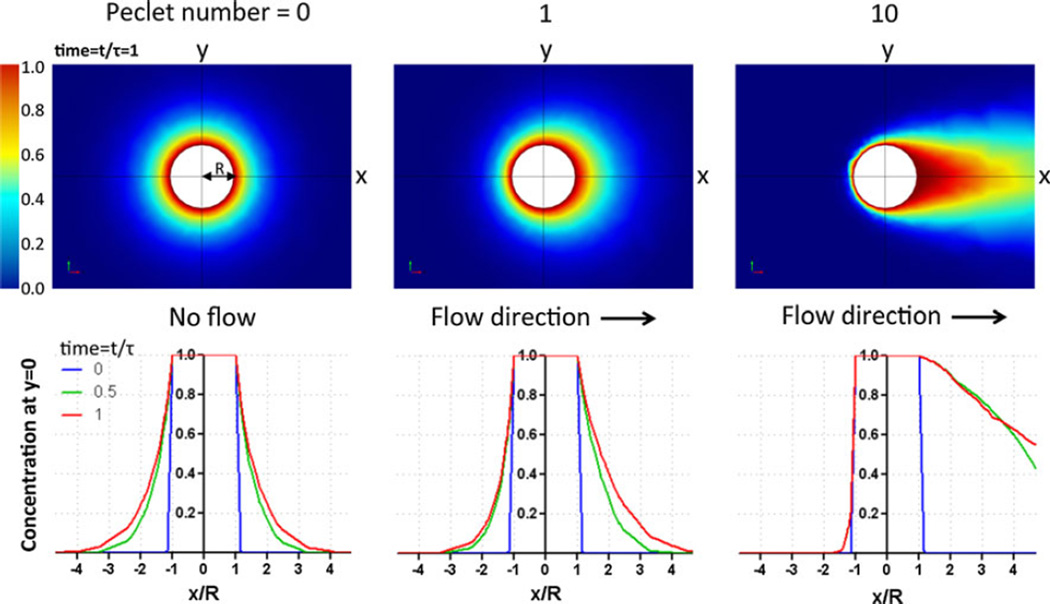
FIGURE 2.
Flow effects on the transport of molecules secreted by a single cell. Computation results of the chemical concentration distribution C of a spherical cell at various flow rates (columnwise, Pe = 0, 1, 10) along +x-axis direction. Graphs are adapted from Helm et al.37 and Shields et al.75 We assume that the cell is spherical with radius R, it is secreting one type of molecules at a constant rate, and the concentration at far site is zero. Top panel: color renditions of the chemical concentration field C in the xy-plane at a time point t/τ = 1. Here the characteristic time τ = R2/D. Bottom panel: Chemical concentration profiles along the x-axis. For each Peclet number, concentration profiles at three time points, 0, 0.5, and 1 are plotted.
Convective vs. Diffusive Molecular Transport
In the presence of fluid flows, molecular transport process can be described by:
| (2) |
where U⃖ is the fluid flow velocity. Here, the first and second terms on the right side represents the diffusive and convective transport, respectively. To evaluate the relative contribution from the convective vs. diffusive transport, one defines a dimensionless number, the Peclet number,
| (3) |
When Pe ≫ 1, molecular transport is dominated by the convective flow; when Pe ≪ 1, transport is dominated by the diffusive process.
Let’s continue with the example of molecular transport around a single spherical cell. In the absence of the flow, the concentration field is symmetric around the cell; but in the presence of the flow, the concentration field loses its symmetry (see Fig. 2).37,65,75,79 One important class of fluid flow that mediates molecular transport within a tissue is the interstitial flow.9,79 Assume that we have an interstitial fluid flow of 5 µm/s, the Peclet number for the flow around a cell is: UL/D ~ 1, sufficient to distort the chemical concentration profile as shown in Fig. 2. Here we use length scale of 20 µm for a cell and a diffusion coefficient of 100 µm2/s. The flow mediated chemical gradients have recently been implicated in autologous chemotaxis of tumor cells toward lymphatic vessels as described in Fig. 1c,27,75 which may subsequently play an important role in cancer metastasis.
Molecular Transport Within a Mammalian Cell Culture
To model the molecular transport within a 2D or 3D cell culture, several factors need to take into account considering that cells are active participants of the transport process: (1) Cells are constantly secreting chemokines or growth factors, and forming diffusive peri-cellular gradients (see Fig. 1a). (2) When exposed to a chemical gradient, each cell responds by moving up/down the gradient.
To model the molecular concentration gradient formation within a cell culture, we assume that cells follow a continuous cell density distribution n, and secrete one type of chemokine. The concentration field of the chemokine C can then be described by:
| (4) |
where α (mol/s) is the rate of chemokine release, and τh (s) is the half-life time of the protein degradation.
Modeling Chemotaxis-Assisted Vascular Morphogenesis
We present an example on how chemotaxis via autocrine signaling can be used to explain successfully the early stages of vascular network formation using an integrated experimental and theoretical modeling approach.5,31,71
In experiments, Human Umbilical Vein Endothelial Cells (HUVECs) are randomly seeded on a Matrigel coated surface, cellular motility and chemotaxis are observed using a video-microscopy. In theoretical modeling, the cell density is assumed to follow a continuous spatial distribution n, and the cell matter is modeled as a fluid subjected to a specific force field depending on the concentration gradient of a chemoattractant, vascular endothelial growth factor (VGEF-A). Here,
| (5) |
where β ((N/kg)/(mol/m3)) is the chemotactic index that measures the strength of the chemotactic response, β∇C is the chemotactic force per unit mass, and v˖ is the cell velocity field. The top equation is the conservation of cell number, and the lower one describes the force balance.
The dimensional analysis from the Eqs. (4) and (5) gives rise to a length scale, , which is the characteristic length of the endothelial network; and a time scale is the cellular close-packing density) which describes the time it takes for the endothelial cell network formation to occur. Using the half life of VGEF-A of ~64 min, one obtains the length scale of ~100 µm, which is in good agreement with the experimental observation.71 The time scale, however, has not yet been verified in experiments, as the measurement of parameter β remains to be a challenge.40
The proposed model further validate the prediction that cellular chemotaxis in VGEF-A gradients play critical roles in network formation. By switching off the chemoattractant mechanism, i.e., β = 0 in Eq. (5), the simulation presents a clear cut of inhibition of network formation. This phenomenon is reproduced in experiments by placing a saturated amount of VGEF-A into the cell culture.
MICROFLUIDICS FOR MAMMALIAN CELL CHEMOTAXIS
Microfluidic technology presents us a unique opportunity to build physiologically relevant, 3D, and well defined microenvironments for mammalian cells. The size proximity of the device to the cells enables the observation of cell responses at a single cell level and in real time. In this session, we will present general physical and engineering considerations for the design and construction of microfluidic devices for studies of the mammalian cell chemotaxis. For a comprehensive list of microfluidic chemotaxis devices that are in the literature, please see excellent recent review articles cited here3,44,49 and references therein.
Diffusive vs. Convective Flow-Based Microfluidic Gradient Generator
There are two major classes of microfluidic gradient generators, one is the convective flow-based device where molecules are mainly transported by the convective flow41,54; the other is the diffusive based device where the molecules are mainly transported by the molecular diffusion.1,10 Both types of devices are able to generate well defined gradients.
Convective Flow-Based Microfluidic Chemical Concentration Gradient Generator
This was first introduced by the Whitesides’ lab,41 where fluid flows of high and low chemical concentration were brought into contact through a series of serpentine microfluidic channels, and form a chemical concentration gradient when exiting to a cell chamber (see Fig. 3a). This device has been widely used to create molecular gradients in the bulk fluid of the cell chamber or on the surface of a substrate. Applications of this device include studies of immune, and tumor cell chemotaxis.39,42,57,68,84 The advantage of the convective flow-based chemotaxis device is the short gradient establishing time, which can be as short as a few milliseconds. The disadvantage often comes from the unwanted fluid flows in the cell chamber. This flow can potentially cause three problems: the first is the shear stress exerting on the cells from the fluid flow, which is known to be able to alter cellular behaviors59; the second is that the flow washes away the auto/paracrine signals secreted by the cells, which are often necessary for cell survival or migration74,83; and the third is that it remodels the biomatrix in the case of a 3D cell culture.48,59 In addition, when patterning molecular gradients on a surface using flow-based microfluidic generation, one needs to pay attention to the problem of Taylor dispersion within a microfluidic channel, where the solutes are transported down the channel at different rates across the channels.77 This may lead to a difference in the surface and bulk gradients.
FIGURE 3.
Microfluidic chemotaxis devices. Shown here are four representative microfluidic chemotaxis devices that illustrate the principles of molecular gradient generation via convective flow and molecular diffusion. (a) A convective flow based microfluidic device made of PDMS (reproduced from Kim et al.44 with the permission of the Royal Society of Chemistry). Fluid flow with different chemical concentrations (symbolized by the colors) are introduced to the inlets of the device. These flows merge and mix through a series of serpentine channel arrangements, and form a gradient when exiting to the cell chamber. Note that the fluid flow is in direct contact with the cells in the cell chamber. (b) A diffusion based microfluidic device (reproduced from Abhyankar et al.1 with the permission of the Royal Society of Chemistry). Chemicals and buffers are added to the source and sink, or the inlet/outlet of the channel. Cells are added through the cell addition port. A chemical gradient is generated through diffusion along the center channel. Note that cells do no have direct contact with the flow. (c) A convective-diffusive hybrid device (reproduced from Shamloo et al.74 with the permission of the Royal Society of Chemistry). Here, the chemical/buffer flow through source/sink channels, respectively. The sink/source channels are separated from the cell chamber by the microcapillaries with the cross section area of 20 µm × 20 µm. The capillaries allows for quick transport of the molecules from sink/source channels to the cell chamber, but prevent the flows from the direct contact with the cells in the cell chamber. The gradient establishment time is controlled by cell chamber width squared divided by 2D, where D is the diffusion coefficient of the molecules. (d) A hydrogel based microfluidic chemotaxis device with gradient buffering capability (reproduced from Haessler et al.35 with the permission from the National Academy of Sciences). Three parallel channels are patterned in a 1 mm thick agarose gel membrane. Chemical/buffer flow through the source/sink channel, and a linear concentration gradient is formed in the center channel via molecular diffusion through the agarose ridges. (i–iv) The steps for the pre-establishment of a gradient in the agarose gel. The gradient establishment time is controlled by the cell chamber height squared divided by 2D. Because the chamber width is typically larger than channel height, this design has the advantage of fast gradient establishment time.
Diffusion-Based Microfluidic Chemotaxis Device
This device, on the other hand, uses the mechanism of molecular diffusion to establish a gradient,1 in which fluid flows do not come in direct contact with the cells. Figure 3b shows a typical diffusion based gradient generator. Chemical/buffer are placed in the inlet/outlet of the channel, and the cells are seeded within the channel. A membrane is placed at the inlet of the channel to prevent the convective flow from the cell chamber. Chemicals are introduced to the channel entirely through molecular diffusion. The advantage of the diffusion based microfluidic device is that the cells are free of fluid flow induced shear stresses. The disadvantage is that the gradient establishment time is long. This time scale depends on the geometry of the device as well as the size of the molecules. In the case of Fig. 3b, the time for a gradient to reach a steady state is approximately L2/2D. For a channel length of 2 mm, the gradient generation time is roughly 5.6 h for the 10 kDa molecule with the diffusion coefficient of 100 µm2/s. This long diffusion time also causes the diffusion based device to be more susceptible to any disturbance than the flow-based device, as the former takes a longer time to recover. Diffusion based device is generally good for slow moving cells such as endothelial cells, but not for fast moving cells such as immune cells.
A Hybrid Microfluidic Chemotaxis Device
A number of improvements have been made to overcome the limitation of the long establishment time of the diffusion based microfluidic device.10,22,70,74 The key feature is to shorten the distance between the sink and source regions through the clever spatial arrangement of the microfabricated channels. Figure 3c is a hybrid device, where it uses both convective and diffusive transport to establish a gradient in the cell chamber with no direct shear stresses imposing on the cells.73,74 Chemical/buffer flow through the two side channels, and form a chemical gradient in the cell chamber. The key innovation here is the use of the microcapillaries that connect the side channels with the cell chamber. These small channels have an cross section area of 20 µm × 20 µm; they are small enough to successfully isolate the cell chamber from the convective flows in the side channels, but large enough to efficiently bring the chemicals into/away from the cell chamber. The gradient establishment time here is controlled by the width of the cell chamber. In the case of chamber width of 400 µm, the establishment time is ~13 min; or for chamber width of 1 mm, the establishment time is 83 min. This device has been successfully used in revealing roles of VEGF gradients in endothelial cell chemotaxis as well as the vascular vessel formation.73,74
A Hydrogel-Based Microfluidic Chemotaxis Device
An alternative way to improve the gradient establishment time for the diffusion based microfluidic device is to use hydrogel as the base material for the device. Figure 3d demonstrates the gradient buffering capability of the agarose gel based device. In Fig. 3d, three parallel channels are patterned in a 1 mm thick agarose gel membrane. The chemical/buffer flow through the two side channels, and cells are seeded in the center channel. Here, the agarose gel walls allow the chemical to diffuse from/to the side to/from the cell chamber, and at the same time provide a barrier for the cells from the convective flow. The advantage of this device is its ability to pre-establish the gradient in the agarose gel surrounding the cell chamber, and then introduce the cells into the center channel at a later time. Under this scenario, the gradient establishment time is decreased approximately from L2/4D to h2/4D, where L is the distance from the sink to source channel and h the channel height. For a typical 3D chemotaxis experiments, the minimum channel height is 250 µm. This leads to a gradient establishment of ~2.6 min.34 This device has been successfully used to study immune cell chemotaxis.34,35
Designing Principles That are Specific to Mammalian Cell Chemotaxis
Soft vs. Hard Materials for Microfluidics
Polydimethylsiloxane (PDMS), an elastomer upon cross linking is the material introduced about three decades ago to fabricate micro/nano-meter size devices through a replica molding process, also known as soft lithography.87 PDMS has several salient physical and chemical properties that are ideal for being the base material for microfluidic devices: (1) PDMS retains its shape when cross linked which makes it ideal for micro-molding; (2) It is transparent which allows for real time microscopic observation through the device; (3) It has a low surface energy, which makes it easy for surface chemistry modification. PDMS based microfluidic devices have been widely used for various applications in biology, including molecular gradient patterning,76 bacteria54 and mammalian cell chemotaxis.42,52,74
Soft hydrogels have been introduced as the fabrication materials for microfluidics as their applications extend from studies of macromolecules to cells.10,11,13,19,32,60,88 The inclusion of the living cells to microfluidic devices adds one more layer of complexity, in that the cells need oxygen and nutrient supplies, and metabolic waste removal.66,83 To build a microenvironment that is physiologically realistic for cells, soft materials such agarose gel,10 alginate,11,45 gelatin60 and collagen88 have been introduced as the base materials for microfluidic devices. These soft hydrogels have advantages over PDMS in that they allow nutrients and chemicals necessary for cell survival to diffuse through, and have the mechanical properties that are tunable and close to those of the cells. As a result, hydrogel gel based devices typically afford a simpler structural design, and does not require the extra plumbing for providing cells with nutrients as in the case of PDMS devices. With the rapid advancements in biomaterials, hydrogel based microfluidic device has the potential to truly recreate an in vivo like microenvironments for mammalian cells in the near future.
3D vs. 2D Microfluidic Chemotactic Device
A 2D microfluidic chemotaxis device refers to the device where cells are plated on a planar substrate (e.g., a glass substrate), and the chemical gradients are generated across the substrate.42 Figure 3a is a typical 2D microfluidic device. 2D microfluidic devices are ideal for microscopic imaging, in particular when molecular events need to be observed at the same time with the cellular behavior. Cares must be taken with the surface treatments of the substrate. For the majority of cell types, the substrate needs to be coated with adhesive molecules, such as fibronectin or collagen to allow for the cell adhesion, an essential component of cell migration. Work from the Lauffenburger’s group has demonstrated that the cell motility dependence on adhesion molecule concentration is biphasic when fibroblasts are plated on a fibronectin coated surface. The adhesion molecule concentration at which cells have the maximum motility depends on ligand levels, integrin levels, and the integrin-ligand association constant.61
A 3D microfluidic device refers to a device where the chemical gradients are imposed on a cell-embedded extracellular matrix. It is now well accepted that cells behave very differently in a 3D environment than they do on a 2D substrate.20,33,46 In 3D, cells bind to ligands in the extracellular matrix around all their surfaces, and are mechanically supported by the mesh fiber network in 3D. On a 2D substrate, cells bind to the substrate only on their basal sides, and are mechanically supported on the basal side. Of particular relevance to the chemotaxis studies is the cell migration. For the same type of cells, the mechanism with which cells use to migrate on a 2D substrate or within a 3D biomatrix can be significantly different. For example, adhesion molecules plated on the substrate, which are necessary for 2D cell migration, are not always required for 3D cell migration. Recent work from Lammermann et al.46 shows that immune cells migrating through a 3D collagen matrix are integrin independent, while the integrin binding is essential for their 2D migration on a collagen coated surface.
Due to its physiological relevance, the trend of microfluidic chemotaxis devices has gone toward 3D.2,34,35,70,73,80,82 Almost all the diffusion based microfluidic chemotaxis devices have been transformed from 2D to 3D through the introduction of cell-embedded ECM into the cell chamber (see Figs. 3c, 3d). Fluid flows are not only able to alter cellular behavior,59 but they are also able to degrade or remodel biomatrix.48 It is thus not wise to have fluid flows in direct contact with the biomatrix. Convective flow based microfluidic chemotaxis devices are thus not suitable for 3D chemotaxis studies.34,74 A second designing consideration for the 3D chemotaxis devices is the choice of biomatrix. The biomatrix needs to be compatible with the cells and also can be easily introduced into the microfluidic device. To our knowledge, collagen, and collagen mixed with Matrigel have been the most common biomaterials for 3D microfluidic chemotaxis devices due to their excellent biocompatibility with cells; in that they provide the binding sites for cells to attach to, and the mechanical support for cells to migrate through.2,19,34,35,65,70,74 In addition, collagen allows for studies of cell invasion, where cells can directly invade into the collagen.82 It should be mentioned that the field of synthetic biomaterials has been developing at a rapid pace, where the gel can be fine tuned both mechanically and chemically, which can potentially be useful for microfluidic chemotaxis studies.25
CHALLENGES
A number of major advancements have to be made before microfluidic chemotaxis devices can be routinely used in academic labs or industry. First, current microfluidic devices are far from recapitulating in vivo like microenvironments. The strength of microfluidic devices is their capability of accurately defining biophysical and biochemical parameters within the cellular microenvironments, however, the blueprints for the in vivo like microenvironments have been slow to come. For many biological problems, little or no quantitative information exists with regard to ligand-receptor binding rate, biomatrix stiffness, oxygen tension, and fluid flow rate. The good news is that these numbers are made available at a rapid pace due to the recent focus on quantitative biology.62 Second, the use of microfluidic chemotaxis devices is still largely limited to engineering labs. These devices usually need special engineering skills, as well as tools that are not normally available in a biology lab, such as a plasma cleaner. In comparison to the Boyden chamber assay, microfluidic chemotaxis devices are more difficult to use, and have a steeper learning curve. The difficulties come mostly from the poor interfaces between the microfluidic channels and the fluid pumping systems, and the complexities associated with the imaging and the data analysis. On-chip pumping or the use of a docking systems is promising24 but still in a development stage. Third, imaging in 3D has limited the wide applications of the 3D microfluidic chemotaxis devices. A confocal microscopy has been the workhorse for 3D imaging, however, the cost of the confocal microscope has made it nearly impossible for individual investigators to own the equipment in their labs. This made it difficult for long-term live mammalian cell imaging, which is essential for most chemotaxis studies. In addition, the large data sets (often up to 200 Gb for one experimental run) demand a new way of data management. At the moment, most of the data acquisition is still limited to 2D in 3D chemotaxis studies,34,35 losing much of the information that the experiments offer. This problem can be alleviated as computer and imaging technology advance. Also noted is the availability of 3D imaging analysis softwares such as Imaris (Bitplane AG, Zurich, Switzerland) and ImageJ (NIH, USA), which made 3D data analysis possible in many biology labs.
OUTLOOKS AND PERSPECTIVES
Microfluidic chemotaxis devices have the potential to generate a wealth of quantitative information, and lay the foundation for the theoretical modeling of mammalian cell chemotaxis. Looking ahead, there are several problems that need our immediate attentions.
Multi-Scale Mammalian Cell Chemotaxis
One critical understanding of chemotaxis is to correlate molecular-level with cellular-, or organismic-level events in real time and space. Microfluidic technology, coupled with modern molecular biology tools such as lenti-virus and antibodies, presents us a unique opportunity for imaging the molecular events together with the cellular dynamics. Such quantitative information will be essential to understand the molecular machineries responsible for mammalian cell chemotaxis.
Role of Mechanical Environment in Chemotaxis
It is now accepted that the mechanical environment plays important roles in cellular behaviors, including proliferation, adhesion, differentiation and migration. 23,63 In particular, cell locomotion and adhesion are known to be regulated by the substrate stiffness.63 It is thus critical to take into account the interplay between the mechanical and chemical environments of the cells when conducting chemotaxis experiments.40
Theoretical Modeling
The complexity of mammalian cell chemotaxis does not exclude it from following underlying biophysical or biochemical laws. Recent immune cell chemotaxis experiments using microfluidic devices revealed that the chemotactic sensitivity of the immune cells follows the ligand-receptor binding kinetics.35,39 However, questions such as whether there is an adaptation system for mammalian chemotaxis, or how the mechanical signaling would be integrated into the chemical signaling pathway remain to be answered. In bacterial chemotaxis, it is known that bacterium has a robust adaptation system through the modification of methylation sites on the trans-membrane chemo-receptor to achieve a high chemotactic sensitivity in a wide range of chemical concentrations.56 It is likely that mammalian cells also possess an adaptation system, possibly through the receptor internalization.15
Looking ahead, microfluidic technology can be an enabling technology for the successful modeling of the complex mammalian cell chemotaxis events. Using the well defined chemical gradients provided by the microfluidic devices, it is possible to obtain key parameters, such as chemotactic index, that is essential for the theoretical modeling. In addition, microfluidic devices will allow us to obtain cellular chemotactic dynamics both in space and time, and at single cell resolution. This quantitative information will be critical for the understanding of the physical and chemical mechanisms underlying mammalian cell chemotaxis.
ACKNOWLEDGMENTS
MW thanks insightful discussions with Abraham Stroock and Melody Swartz. Both authors thank the anonymous reviewers for their careful readings and very useful suggestions. The work was supported by the National Cancer Institute through award number R21CA138366, and through the Cornell Center on the Microenvironment & Metastasis with Award Number U54CA143876, and the Cornell Nanobiotechnology Center.
REFERENCES
- 1.Abhyankar VV, et al. Characterization of a membrane-based gradient generator for use in cell-signaling studies. Lab Chip. 2006;6(3):389–393. doi: 10.1039/b514133h. [DOI] [PubMed] [Google Scholar]
- 2.Abhyankar VV, et al. A platform for assessing chemotactic migration within a spatiotemporally defined 3D microenvironment. Lab Chip. 2008;8(9):1507–1515. doi: 10.1039/b803533d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed T, Shimizu TS, Stocker R. Microfluidics for bacterial chemotaxis. Integr. Biol. 2010;2(11–12):604–629. doi: 10.1039/c0ib00049c. [DOI] [PubMed] [Google Scholar]
- 4.Alberts B, et al. Molecular Biology of the Cell. 5th ed. New York, NY: Garland Science, Taylor and Francis Froup, L.L.C.; 2007. [Google Scholar]
- 5.Ambrosi D, Gamba A, Serini G. Cell directional and chemotaxis in vascular morphogenesis. Bull. Math. Biol. 2004;66(6):1851–1873. doi: 10.1016/j.bulm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 9.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA. 1989;86(14):5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng S-Y, et al. A hydrogel-based microfluidic device for the studies of directed cell migration. Lab Chip. 2007;7:763–769. doi: 10.1039/b618463d. [DOI] [PubMed] [Google Scholar]
- 11.Choi NW, et al. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007;6(11):908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 12.Choi NW, et al. Phosphorescent nanoparticles for quantitative measurements of oxygen profiles in vitro and in vivo. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.11.048. doi: 10.1016/j.biomaterials.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006;71(3):185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Chung S, et al. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9(2):269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 15.Clague MJ, Urbe S, de Lartigue J. Phosphoinositides and the endocytic pathway. Exp. Cell Res. 2009;315(9):1627–1631. doi: 10.1016/j.yexcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 2003;3(12):921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 17.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu. Rev. Cell Dev. Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 19.Cross VL, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cukierman E, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 21.De Bruyn PP. The amoeboid movement of the mammalian leukocyte in tissue culture. Anat. Rec. 1946;95:177–191. doi: 10.1002/ar.1090950209. [DOI] [PubMed] [Google Scholar]
- 22.Diao J, Young L, Kim S, Fogarty EA, Heilman SM, Zhou P, Shuler ML, Wu M, DeLisa MP. A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab Chip. 2006;6:381–388. doi: 10.1039/b511958h. [DOI] [PubMed] [Google Scholar]
- 23.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 24.Domansky K, et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10(1):51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrbar M, et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 2011;100(2):284–293. doi: 10.1016/j.bpj.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenbach M. Chemotaxis. London: Imperial College Press; 2004. [Google Scholar]
- 27.Fleury ME, Boardman KC, Swartz MA. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 2006;91(1):113–121. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29(6):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 29.Fournier RL. Basic Transport Phenomena in Biomedical Engineering. 1st ed. London: Taylor & Francis; 1999. [Google Scholar]
- 30.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 2010;188(1):11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamba A, et al. Percolation, morphogenesis, and Burgers dynamics in blood vessels formation. Phys. Rev. Lett. 2003;90(11) doi: 10.1103/PhysRevLett.90.118101. 118101. [DOI] [PubMed] [Google Scholar]
- 32.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7(6):720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 33.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Natl Rev. Mol. Cell Biol. 2006;7(3):211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 34.Haessler U, et al. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed. Microdevices. 2009;11(4):827–835. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 35.Haessler U, et al. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc. Natl Acad. Sci. USA. 2011;108(14):5614–5619. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Helm CLE, et al. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl Acad. Sci. USA. 2005;102(44):15779–15784. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helmlinger G, et al. Interstitial pH and pO(2) gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3(2):177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 39.Herzmark P, et al. Bound attractant at the leading vs. the trailing edge determines chemotactic prowess. Proc. Natl Acad. Sci. USA. 2007;104(33):13349–13354. doi: 10.1073/pnas.0705889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jannat RA, Dembo M, Hammer DA. Traction forces of neutrophils migrating on compliant substrates. Biophys. J. 2011;101(3):575–584. doi: 10.1016/j.bpj.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon NL, et al. Generation of solution and surface gradients using microfluidic systems. Langmuir. 2000;16(22):8311–8316. [Google Scholar]
- 42.Jeon NL, et al. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002;20(8):826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- 43.Kay RR, et al. Changing directions in the study of chemotaxis. Nat. Rev. Mol. Cell Biol. 2008;9(6):455–463. doi: 10.1038/nrm2419. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Kim HJ, Jeon NL. Biological applications of microfluidic gradient devices. Integr. Biol. 2010;2(11–12):584–603. doi: 10.1039/c0ib00055h. [DOI] [PubMed] [Google Scholar]
- 45.Kunze A, et al. Micropatterning neural cell cultures in 3D with a multi-layered scaffold. Biomaterials. 2011;32(8):2088–2098. doi: 10.1016/j.biomaterials.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 46.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 47.Lebrun L, Junter GA. Diffusion of sucrose and dextran through agar gel membranes. Enzyme Microb. Technol. 1993;15(12):1057–1062. doi: 10.1016/0141-0229(93)90054-6. [DOI] [PubMed] [Google Scholar]
- 48.Lee P, et al. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed. Microdevices. 2006;8(1):35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- 49.Lin F. A microfluidics-based method for analyzing leukocyte migration to chemoattractant gradients. In: Handel TM, Hamel DJ, editors. Methods in Enzymology, Vol. 461: Chemokines, Part B. New York: Academic Press; 2009. pp. 333–347. [DOI] [PubMed] [Google Scholar]
- 50.Lin F, Butcher EC. T cell chemotaxis in a simple microfluidic device. Lab Chip. 2006;6(11):1462–1469. doi: 10.1039/b607071j. [DOI] [PubMed] [Google Scholar]
- 51.Lin F, et al. Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem. Biophys. Res. Commun. 2004;319(2):576–581. doi: 10.1016/j.bbrc.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 52.Lin F, et al. Neutrophil migration in opposing chemoattractant gradients using microfluidic chemotaxis devices. Ann. Biomed. Eng. 2005;33(4):475–482. doi: 10.1007/s10439-005-2503-6. [DOI] [PubMed] [Google Scholar]
- 53.Maheshwari G, Wiley HS, Lauffenburger DA. Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J. Cell Biol. 2001;155(7):1123–1128. doi: 10.1083/jcb.200109060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao HB, Cremer PS, Manson MD. A sensitive, versatile microfluidic assay for bacterial chemotaxis. Proc. Natl Acad. Sci. USA. 2003;100(9):5449–5454. doi: 10.1073/pnas.0931258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meier B, et al. Chemotactic cell trapping in controlled alternating gradient fields. Proc. Natl Acad. Sci. USA. 2011;108(28):11417–11422. doi: 10.1073/pnas.1014853108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mello BA, Tu Y. Perfect and near-perfect adaptation in a model of bacterial chemotaxis. Biophys. J. 2003;84(5):2943–2956. doi: 10.1016/S0006-3495(03)70021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosadegh B, et al. Epidermal growth factor promotes breast cancer cell chemotaxis in CXCL12 gradients. Biotechnol. Bioeng. 2008;100(6):1205–1213. doi: 10.1002/bit.21851. [DOI] [PubMed] [Google Scholar]
- 58.Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res. 2004;68(3):258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 2005;118(20):4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 60.Paguirigan AL, Beebe DJ. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nat. Protoc. 2007;2(7):1782–1788. doi: 10.1038/nprot.2007.256. [DOI] [PubMed] [Google Scholar]
- 61.Palecek SP, et al. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385(6616):537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann. Biomed. Eng. 2005;33(11):1–22. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 63.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pluen A, et al. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys. J. 1999;77(1):542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl Acad. Sci. USA. 2011;108(27):11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Regehr KJ, et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009;9(15):2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renkawitz J, Sixt M. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 2010;11(10):744–750. doi: 10.1038/embor.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ricart BG, et al. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J. Immunol. 2011;186(1):53–61. doi: 10.4049/jimmunol.1002358. [DOI] [PubMed] [Google Scholar]
- 69.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat. Rev. Cancer. 2011;11(8):573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saadi W, et al. Generation of stable concentration gradients in 2D and 3D environments using a microfluidic ladder chamber. Biomed. Microdevices. 2007;9(5):627–635. doi: 10.1007/s10544-007-9051-9. [DOI] [PubMed] [Google Scholar]
- 71.Serini G, et al. Modeling the early stages of vascular network assembly. EMBO J. 2003;22(8):1771–1779. doi: 10.1093/emboj/cdg176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Servant G, et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287(5455):1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shamloo A, Heilshorn SC. Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab Chip. 2010;10(22):3061–3068. doi: 10.1039/c005069e. [DOI] [PubMed] [Google Scholar]
- 74.Shamloo A, et al. Endothelial cell polarization and chemotaxis in a microfluidic device. Lab Chip. 2008;8(8):1292–1299. doi: 10.1039/b719788h. [DOI] [PubMed] [Google Scholar]
- 75.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11(6):526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Song L, et al. Dicyostelium discoideum chemotaxis: threshold for directed motion. Eur. J. Cell Biol. 2006;85(9–10):981–989. doi: 10.1016/j.ejcb.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Squires TM, Quake SR. Microfluidics: fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005;77(3):977–1026. [Google Scholar]
- 78.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 79.Swartz MA. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001;50(1–2):3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 80.Tayalia P, Mazur E, Mooney DJ. Controlled architectural and chemotactic studies of 3D cell migration. Biomaterials. 2011;32(10):2634–2641. doi: 10.1016/j.biomaterials.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verbridge SS, et al. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng. Part A. 2010;16:2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vickerman V, et al. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8(9):1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker GM, Zeringue HC, Beebe DJ. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4(2):91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 84.Wang SJ, et al. Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis. Exp. Cell Res. 2004;300(1):180–189. doi: 10.1016/j.yexcr.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 85.Wolf K, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003;160(2):267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong K, et al. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl Acad. Sci. USA. 2006;103(10):3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia YN, Whitesides GM. Soft lithography. Annu. Rev. Mater. Sci. 1998;28:153–184. [Google Scholar]
- 88.Zheng Y, et al. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials. 2011;32(23):5391–5401. doi: 10.1016/j.biomaterials.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Zicha D, Dunn GA, Brown AF. A new direct-viewing chemotaxis chamber. J. Cell Sci. 1991;99(Pt 4):769–775. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- 90.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 1977;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]