Abstract
Background
We previously demonstrated that stage IIIB/IV non-small cell lung cancer (NSCLC) never smokers lived 50% longer than former/current smokers. This observation persisted after adjusting for age, performance status, and gender. We hypothesized that smoking-dependent differences in the distribution of driver mutations might explain differences in prognosis between these subgroups.
Methods
We reviewed 293 never smokers and 382 former/current smokers with lung adenocarcinoma who underwent testing for EGFR and KRAS mutations and rearrangements in ALK between 2009 and 2010. Clinical outcomes and patient characteristics were collected. Survival probabilities were estimated using the Kaplan-Meier method. Group comparison was performed with log-rank tests and Cox proportional hazards methods.
Results
While the overall incidence of these mutations was nearly identical (55% never smokers vs. 57% current/former smokers, p=0.48), there were significant differences in the distribution of mutations between these groups: EGFR mutations- 37% never smokers vs. 14% former/current smokers (p<0.0001); KRAS mutations- 4% never smokers vs. 43% former/current smokers (p<0.0001); ALK rearrangements- 12% never smokers vs. 2% former/current smokers (p<0.0001). Among never smokers and former/current smokers, prognosis differed significantly by genotype. Patients harboring KRAS mutations demonstrated the poorest survival. Smoking status, however, had no influence on survival within each genotype.
Conclusion
Never smokers and former/current smokers with lung adenocarcinomas are not homogeneous subgroups. Each is made up of individuals whose tumors have a unique distribution of driver mutations which are associated with different prognoses, irrespective of smoking history.
Keywords: non-small cell lung cancer, adenocarcinoma, EGFR, KRAS, ALK, never smoker
Background
We have witnessed a sea change in the management of patients with non-small cell lung cancer (NSCLC) in the past decade. Prior to 2004, biologic diversity was seen largely through the lens of histology, with patients grouped into adenocarcinoma, squamous, and large cell carcinoma subtypes. The predictive and prognostic power of this schema was limited, however. It was not until the initial efficacy studies of the epidermal-growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with advanced NSCLC that evidence for a mechanistic model of disease heterogeneity emerged 1, 2. While the overall response rate to EGFR TKIs in unselected patients was a modest 10%, subgroup analyses showed comparatively higher response rates and survivals in women, never smokers, and Asians 1–3. The subsequent identification of mutations 4–6 in the tyrosine kinase domain of EGFR that predicted for response to EGFR TKIs made clear that the limited efficacy found in these early studies was a by-product of the relatively low frequency of EGFR mutations in NSCLC as a whole. Conversely, those subpopulations with higher response rates were found to have a greater proportion of EGFR mutations, approaching 50% in never smokers with lung adenocarcinoma. In the wake of these findings, genotyping efforts have identified driver mutations in the majority of lung adenocarcinoma specimens.7 Mutations in KRAS account for approximately 25% of cases 8; mutations in EGFR, 15% of cases; rearrangements of ALK, 3–7% of cases 9; and mutations in HER2, BRAF, and PIK3CA, 1–2% of cases.
In addition to smoking-dependent variations in the incidence of EGFR mutations 10, 11, differences in the frequency of KRAS mutations between subgroups have also been described. As few as 8% of never smokers and as high as 57% of former/current smokers with lung adenocarcinoma harbors a KRAS mutation 12. While 3–5% of unselected patients with lung adenocarcinoma have ALK rearrangements, the frequency appears to be much higher in never smokers 13. There is a suggestion that the prognosis associated with these mutations also varies. While treatment with EGFR TKIs has likely altered the prognosis of patients with EGFR mutations 14–16, the prognostic relevance of KRAS mutations is less well characterized. The prognostic significance of ALK rearrangements remains largely unknown.
We recently reported data on the prognostic impact of smoking history in patients with stage IIIB/IV NSCLC 17. Patients who were never smokers were found to have a longer overall survival (OS) when compared to patients who smoked < 15 pack-years and ≥ 15 pack-years. This relationship persisted even after adjusting for differences in age, performance status, and gender on multivariate analysis.
We hypothesized that tumor biology was the principle driver of the differences in survival between these subgroups. We therefore reviewed the mutation status, clinical characteristics, and survival of never smokers and former/current smokers with lung adenocarcinomas at our institution.
Methods
Study Design and Patients
Patients with lung adenocarcinomas who were tested for mutations in EGFR (exon 19 deletions, exon 21 L858R substitutions) and KRAS as well as rearrangements in ALK at MSKCC with tissue obtained between May 2009 and May 2010 were reviewed. Testing was performed under a reflex molecular profiling program for patients with lung adenocarcinoma histologies (Lung Cancer Mutation Analysis Program, LC-MAP) without selection by any specific pathologic or clinical feature. Smoking history was determined through the use of a prospectively administered questionnaire. Never smokers were defined as those patients who smoked < 100 cigarettes in their lifetime. Medical records were reviewed to determine gender, ethnicity, age, Karnosfky performance status (KPS), stage, and treatment history. All chart review/tissue collection was approved by the MSKCC Institutional Review Board/Privacy Board. For the purposes of the analysis, patients were grouped into those with early stage disease (stage I-IIIA) and those with advanced stage disease (IIIB/IV and recurrent disease) as per the IASLC 7th edition TNM staging system.
Genotype Analysis
EGFR exon 19 deletions and exon 21 L858R point mutations were detected using a PCR-based assay.18 A 207-bp genomic DNA fragment encompassing exon 19 was amplified using the following primers (FW1: 5’-GCACCATCTCACAATTGCCAGTTA-3’; REV1: 5’-Fam-AAAAGGTGGGCCTGAGGTTCA-3’). A 222-bp genomic DNA fragment spanning exon 21 was amplified using the following primers (FW1: 5’-CCTCACAGCAGGGTCTTCTCTGT-3’; REV1: 5’-Fam-TCAGGAAAATGCTGGCTGACCTA). PCR products were subjected to capillary electrophoresis on an ABI 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA). KRAS exon 2 mutations were identified through direct sequencing 19, and rearrangements in ALK were identified through fluorescence in-situ hybridization (FISH) using a dual-color break-apart probe 20. Positive cases were defined as the presence of a split signal indicating rearrangement of the ALK locus at 2p23 or the presence of a single red signal indicating loss of the 5’ DNA sequence in ≥ 15% of cells. Where tissue was available, PCR for specific EML4-ALK transcript variants was performed to confirm the presence of an EML4-ALK translocation. Based on past data demonstrating the non-overlapping nature of mutations in EGFR and KRAS and rearrangements in ALK 21, 22, only patients who were wild-type (WT) for EGFR and KRAS underwent testing for ALK rearrangements.
Statistical Analysis
Overall survival (OS) for advanced stage patients was measured from the date of diagnosis of stage IIIB/IV or recurrent disease until the date of death. Patients who did not die during the study period were censored at the time of last available follow-up. Survival and follow-up data were obtained through medical records or the Social Security death index and updated as of June 2011. Survival probabilities were calculated using the Kaplan-Meier method. Group comparison was performed with log-rank tests (for univariate analyses) and Cox proportional hazards methods adjusted for age, gender, and KPS (for multivariate analyses). We examined the effect of smoking history on OS within each genotype (EGFR, KRAS, ALK, other/uncharacterized) and reversely, the effect of genotype within each smoking subgroup (never smokers and former/current smokers). Due to the small number of patients in some subgroups defined by the combination of genotype/smoking history, it was not feasible to fit a comprehensive model examining the interaction between the two variables.
Patients became eligible for the study at the time of their molecular diagnosis. In order to account for the potential length-time bias associated with differences between the date of diagnosis of advanced disease and the date of molecular testing, all analyses were performed using left truncation (or delayed entry) techniques. With this method, survival probabilities and hazard ratios are calculated conditional on patients having survived until the date of their molecular diagnosis. Statistical analyses were performed using SAS statistical software (SAS Institute, Inc, Cary, NC) and the ‘survival’ package in R (http://www.r-project.org/).
Results
Patient Characteristics and mutation frequency
Of the 675 patients with lung adenocarcinoma who we analyzed between May 2009 and May 2010, 293 (43%) were never smokers and 382 (57%) were former/current smokers. Patient characteristics are summarized in Table 1. There were no significant differences in gender, age, stage, or KPS between never smokers with EGFR and KRAS mutations. Never smokers harboring an ALK rearrangement were more likely to be men when compared to patients who had EGFR mutations (49% vs. 30%, p=0.02) or KRAS mutations (49% vs. 8%, p=0.02). Never smokers with ALK rearrangements were significantly younger than patients with EGFR mutations (median age 57 vs. 63, p=0.006). Among former/current smokers, no significant differences in clinical characteristics were present between patients with EGFR mutations, KRAS mutations, and ALK rearrangements.
Table 1.
Patient Clinical Characteristics by Genotype and Smoking History
| EGFR mutation | KRAS mutation | ALK rearrangement | Uncharacterized | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never smoker | Former/current smoker | p | Never smoker | Former/current smoker | p | Never smoker | Former/current smoker | p | Never smoker | Former/current smoker | p | |
| Characteristic | ||||||||||||
| Total Patients | 110 (38%) | 54 (14%) | <0.001 | 14 (5%) | 157 (41%) | <0.001 | 35 (12%) | 9 (2%) | <0.001 | 134 (46%) | 162 (43%) | |
| Age | ||||||||||||
| Median | 63 | 68 | 0.38 | 63 | 66 | 0.27 | 57 | 71 | 0.10 | 66 | 66 | 0.24 |
| Range | 39–88 | 45–86 | 31–78 | 40–86 | 32–84 | 42–84 | 39–89 | 33–86 | ||||
| ≥70 | 38 (35%) | 24 (44%) | 3 (21%) | 55 (35%) | 9 (26%) | 5 (56%) | 49 (37%) | 50 (31%) | ||||
| KPS | ||||||||||||
| Median | 90 | 80 | 0.95 | 80 | 80 | 0.71 | 80 | 80 | 0.4 | 90 | 80 | 0.04 |
| Range | 60–90 | 60–90 | 60–90 | 50–90 | 60–90 | 80–90 | 60–90 | 30–90 | ||||
| ≤70 | 11 (16%) | 5 (15%) | 0 | 27 (25%) | 5 (14%) | 0 | 18 (20%) | 31 (27%) | ||||
| Pack-years smoked | ||||||||||||
| Median | 18 | 32 | 15 | 31 | ||||||||
| Range | 1–90 | 1–150 | 1–60 | 1–150 | ||||||||
| Gender | ||||||||||||
| Women (%) | 77 (70%) | 39 (74%) | 0.71 | 11 (92%) | 110 (70%) | 0.18 | 18 (51%) | 4 (44%) | 1.0 | 104 (73%) | 82 (50%) | 0.16 |
| Stage | ||||||||||||
| I–IIIA | 42 (38%) | 20 (37%) | 0.20 | 4 (29%) | 50 (32%) | 0.12 | 5 (14%) | 1 (11%) | 0.42 | 45 (36%) | 48 (30%) | 0.82 |
| IIIB/IV | 68 (62%) | 34 (63%) | 10 (71%) | 107 (68%) | 30 (86%) | 8 (89%) | 89 (64%) | 114 (70%) | ||||
| Ethnicity | ||||||||||||
| White | 87 (79%) | 47 (87%) | 0.54 | 10 (71%) | 144 (92%) | 0.38 | 26 (74%) | 8 (89%) | 0.29 | 116 (87%) | 141 (87%) | 0.89 |
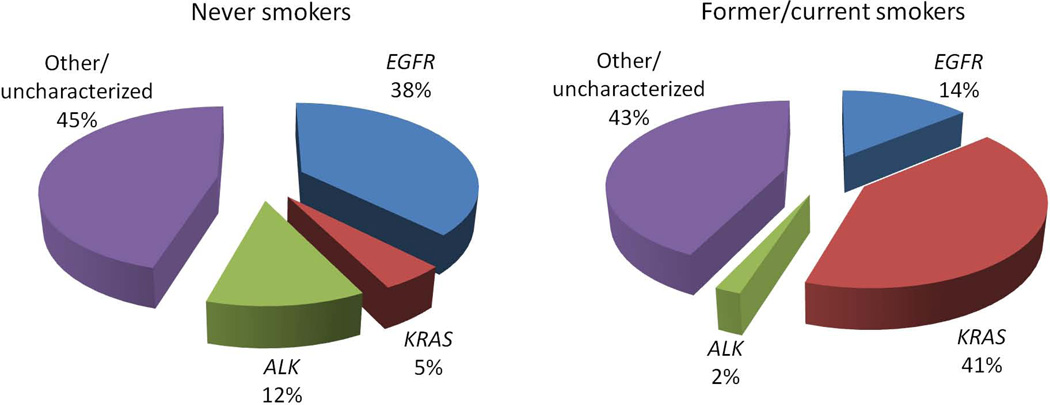
The relative distribution of mutations between never smokers and former/current smokers is shown in Figure 1. The overall incidence of EGFR and KRAS mutations and rearrangements in ALK was virtually identical in never smokers (55%, 95% CI: 48–60%) and former/current smokers (57%, 95% CI: 52–63%) (p=0.43). Never smokers had a significantly higher incidence of EGFR mutations (38% vs. 14%, p<0.0001) and ALK rearrangements (12% vs. 2%, p<0.0001) than did former/current smokers. In contrast, KRAS mutations were more common in former/current smokers than in never smokers (41% vs. 5%, p<0.0001). The majority of patients had an identifiable mutation in one of these genes.
Figure 1.
Relative frequency of driver mutations in never smokers and former/current smokers with lung adenocarcinoma. Other/uncharacterized denotes patients without a mutation in EGFR or KRAS or rearrangement in ALK. Differences in the frequency of each mutation between never smokers and former/current smokers were significant in all cases (p<0.0001 for all genotypes).
There were no significant differences in the frequencies of EGFR exon 19 deletions vs. L858R substitutions in never smokers (56% vs. 43%, p=0.22) or former/current smokers (44% vs. 56%, p=0.50). G12D transition mutations (43%) were the single most common KRAS variant in never smokers. G12C and G12V transversion mutations were most common in former/current smokers (64%). Rearrangements in ALK were detected predominantly through the presence of a split FISH signal in both groups.
Treatment history
The median number of treatments given to patients within each genotype is listed in Table 2, and did not vary by smoking status. Evaluation of treatment response was not possible given the absence of uniform pre- and post-treatment imaging. Most patients with an EGFR mutation, regardless of smoking history, were treated with an EGFR TKI (never smoker vs. former/current smokers: 88% vs. 76%, p=0.57). Treatment with an EGFR TKI was also given to 7–27% of patients without a documented EGFR mutation, the frequency of which was highest in patients with ALK rearrangements. There was no significance difference between never smokers and former/current smokers in the proportion of patients with KRAS mutations or ALK rearrangements who were given an EGFR TKI. Forty percent of never smokers and former/current smokers with an ALK rearrangement had been treated with Xalkori (crizotinib) as part of a clinical trial, with a median duration of therapy of 7 months in each group.
Table 2.
Treatment by Genotype, Advanced Stage Patients
| EGFR mutation | KRAS mutation | ALK rearrangement | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Never smoker |
Former/current smoker |
p | Never smoker |
Former/current smoker |
p | Never smoker |
Former/current smoker |
p | |
| Characteristic | N (%) | N (%) | N (%) | N(%) | N (%) | N (%) | |||
| Number of regimens | |||||||||
| Median | 2 | 2 | 1 | 1 | 2 | 2 | |||
| Range | 0–6 | 1–5 | 0–4 | 0–6 | 0–5 | 1–6 | |||
| Treatment with EGFR TKI | |||||||||
| Yes | 60 (88%) | 25 (76%) | 0.57 | 1 (10%) | 8 (7%) | 0.95 | 8 (27%) | 1 (13%) | 0.29 |
| No | 3 (4%) | 3 (9%) | 9 (90%) | 77 (72%) | 22 (73%) | 7 (87%) | |||
| Unknown | 5 (7%) | 5 (15%) | 0 | 22 (21%) | 0 | 0 | |||
| Treatment with crizotinib* | |||||||||
| Yes | 12 (40%) | 3 (40%) | |||||||
| Median duration (months) | 7 | 7 | |||||||
| Mean duration (months) | 7 | 5 | |||||||
| Duration range (months) | 0.4–18.8 | 0.5–8.8 | |||||||
Patients were not treated beyond disease progression.
Survival
Of the 440 patients with advanced stage disease included in this analysis, 228 died during the analysis period. The median overall survival of advanced stage never smokers vs. former/current smokers was 20 months (95% CI: 16–27) vs. 12 months (95% CI: 11–13). This difference was significant on univariate (p<0.001) and multivariate analyses, adjusting for age, gender, and KPS (adjusted HR = 1.9, 95% CI: 1.4–2.5; p<0.001). The median overall survival for all patients with EGFR mutations, KRAS mutations, and ALK rearrangements was 23 months (95% CI: 19–36), 11 months (95% CI: 8–13), and 35 months (95% CI: 20-NR), respectively. There were significant survival differences between patients with KRAS mutations and EGFR mutations (adjusted HR = 2.3, 95% CI 1.5–3.6; p<0.001) and patients with KRAS mutations and ALK rearrangements (adjusted HR = 2.9, 95% CI 1.4–5.8; p<0.001). There was no significant difference in survival between patients with ALK rearrangements and EGFR mutations (adjusted HR = 0.8, 95% CI 0.4–1.6; p=0.42).
These differences persisted within the never smoker and former/current smoker subgroups. Among advanced stage never smokers, patients with KRAS mutations had a higher risk of death compared to patients with EGFR mutations (adjusted HR = 2.7, 95% CI 1.1–6.7; p=0.04) and patients with ALK rearrangements (adjusted HR = 4.6, 95% CI 1.5–14.1; p=0.008). There was no significant difference in survival between never smokers with ALK rearrangements vs. EGFR mutations (adjusted HR = 0.6, 95% CI 0.2–1.4, p=0.23).
Among smokers, patients with KRAS mutations had significantly worse survival relative to patients with EGFR mutations (adjusted HR = 3.3, 95% CI 1.6–5.7, p<0.0001). Meaningful survival comparisons with smokers harboring ALK rearrangements were not possible given the small numbers detected, though there were similar trends in survival as with never smokers and the overall cohort. A planned analysis of disease-free survival among early stage patients could not be performed given the absence of events to date.
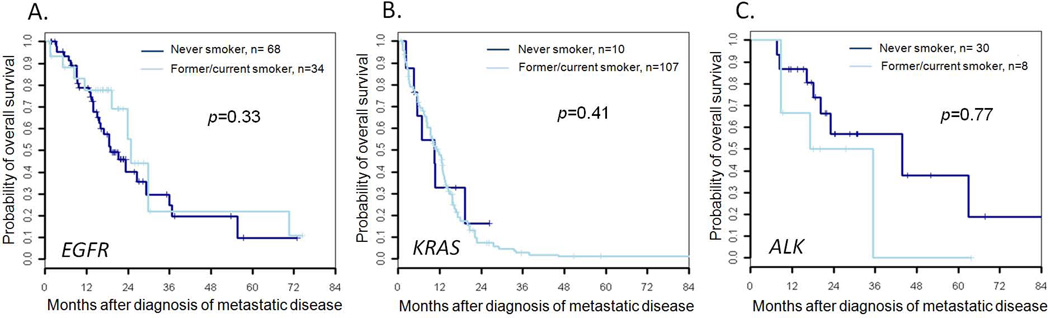
Despite these differences in genotype-specific survival, there were no differences in survival between never smokers and former/current smokers who harbored a given mutation. The median overall survival of never smokers vs. former/current smokers with EGFR mutations was 25 months (95% CI: 19-NR) vs. 19 months (95% CI: 16–37) (p=0.33). The median overall survival of never smokers vs. former/current smokers with KRAS mutations was 11 months (95% CI: 8–13) vs. 10 months (95% CI: 6-NR) (p=0.77). The median overall survival of never smokers vs. former/current smokers with ALK rearrangements was 26 months (95% CI: 9-NR) vs. 44 months (95% CI: 20-NR) (p=0.41). The associated Kaplan-Meier survival curves are found in Figures 2A–C.
Figure 2.
A–C. Overall survival in advanced stage (IIIB/IV) lung adenocarcinoma never smokers and former/current smokers harboring (A) EGFR mutations; (B) KRAS mutations; and (C) ALK rearrangements.
Multivariate analysis adjusting for age, gender, and KPS similarly found no significant differences between former/current smokers and never smokers with EGFR mutations (adjusted HR = 0.9, 95% CI 0.4–2.0, p=0.80) and KRAS mutations (adjusted HR = 1.1, 95% CI 0.5–2.5, p=0.80). Multivariate analysis for patients with ALK rearrangements could not be performed due to small sample sizes in both groups.
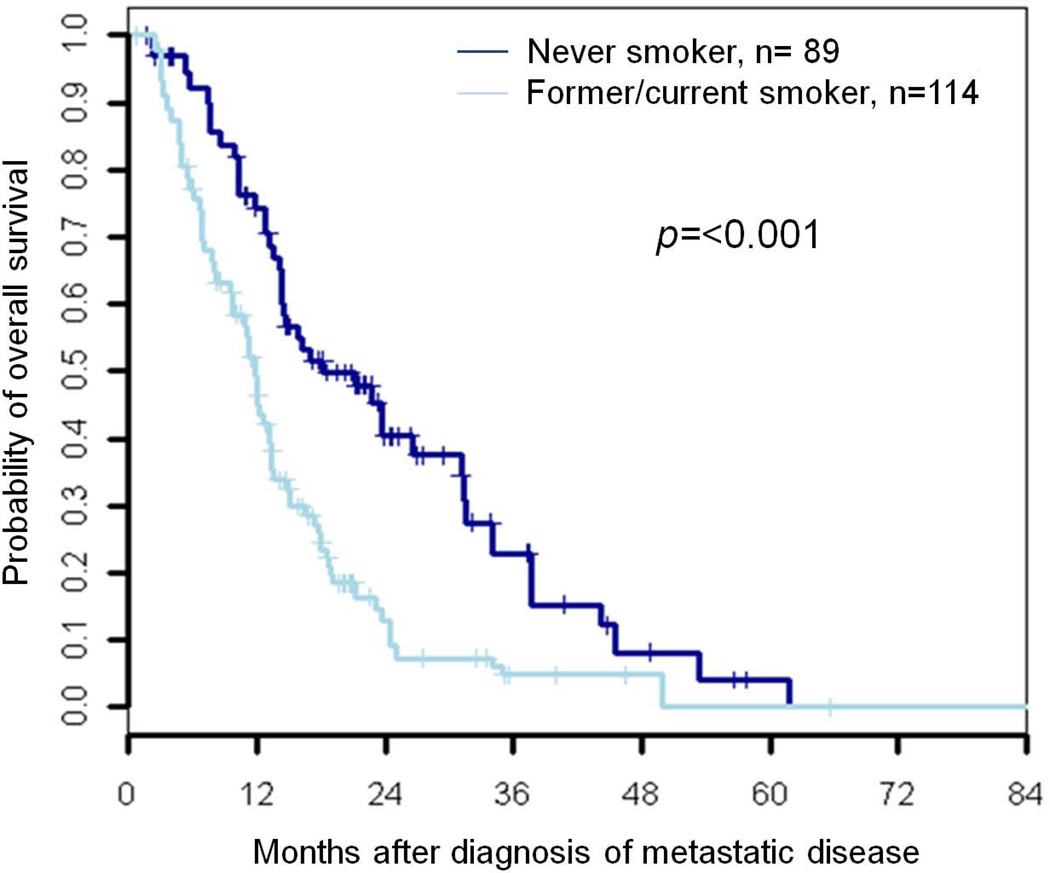
While mutations in EGFR, KRAS, and rearrangements in ALK comprise the majority of driver mutations in both never smokers and former/current smokers, some 40% of patients in each group lack these genetic aberrations. These other/uncharacterized patients were the only subgroup to exhibit a significant difference in overall survival when grouped by smoking status (median OS 18 months vs. 12 months; unadjusted HR = 2.0, 95% CI 1.33–2.98, p<0.001) (Figure 3). Similar findings were present on multivariate analysis (adjusted HR 1.8, 95% CI 1.2–2.7; p=0.006).
Figure 3.
Overall survival in advanced stage (IIIB/IV) lung adenocarcinoma never smokers and former/current smokers with other/uncharacterized genotype.
Discussion
In an attempt to explain the survival advantage that never smokers exhibit over smokers with lung adenocarcinomas, we hypothesized that a unique distribution of driver mutations in never smokers, enriched for those mutations with better prognoses, leads to an improved outcome when compared to smokers.
Our data demonstrate that while the overall incidence of driver mutations is identical, the distribution of mutations in patients with lung adenocarcinomas differs significantly based on smoking status. Never smokers had a significantly higher proportion of EGFR mutations and ALK rearrangements, totaling 50%. Conversely, former/current smokers had a significantly higher proportion of KRAS mutations, totaling 41%.
Importantly, we found no significant differences in the overall survival of never smokers and former/current smokers who bear identical genotypes. This observation is, to our knowledge, the first of its kind, suggesting that former/current smokers, independent of genotype, do not have a poorer prognosis compared to never smokers. While our analysis was relatively large, the low incidence of ALK rearrangements was a limiting factor in the survival analysis of these patients. While there was a numerical difference in survival in never smokers and former/current smokers with ALK rearrangements, the difference was not significant. Ultimately, a larger sampling of patients will be needed to clarify our observation.
We note that former/current smokers with EGFR mutations and ALK rearrangements in our analysis were not light tobacco consumers, with median pack years smoked of 18 and 15, respectively. These data are particularly compelling when placed beside those from the recent whole genome sequencing of a smoking-related lung adenocarcinoma tumor, suggesting that the high somatic mutation rate caused by smoking may not substantially alter the course of tumors driven by certain oncogenes, such as EGFR and KRAS.23
It is important to note that this study was not sufficiently equipped to resolve the impact of targeted therapy versus standard chemotherapy in these subpopulations. Data suggest that both EGFR TKI and ALK-directed therapies alter the clinical courses of patients harboring sensitizing EGFR mutations and ALK rearrangements relative to chemotherapy. 14, 24 This study does not address the untreated natural histories of the genotypes tested. The prognoses of each mutation subtype were undoubtedly affected by the proportion who received an appropriate targeted therapy: this has bearing on the current study only insofar as disparities in the delivery of a targeted therapy might significantly differ between never smokers and smokers. This was not the case. Indeed, as new and improved treatments emerge for patients with specific genotypes, we anticipate that the prognostic differences that currently exist between smokers and never smokers will likely change. The promise of this is perhaps greatest for patients whose tumors harbor KRAS mutations, where an effective directed-therapy has not yet been found.
Forty percent of patients with lung adenocarcinomas do not harbor mutations in EGFR or KRAS or rearrangements in ALK. Our data intimate at the presence of an as yet unknown oncogenic driver event or series of events that is differentially present among never smokers and former/current smokers in this subgroup of patients. To date, mutations in BRAF (3%, predominantly in former/current smokers)25, HER2 (4.8% overall, up to 8% in never smokers)26, PIK3CA (2%)27, and AKT1 (1%) have been identified in addition to those tested in this analysis. The prognoses associated with these mutations are, however, unclear.
While we report herein that all EGFR and KRAS mutations were mutually exclusive, the data presented in this paper cannot comment rigorously on the mutual exclusivity of ALK rearrangements and mutations in EGFR and KRAS based on the mutational testing protocol we used. Once either an EGFR or a KRAS mutation was detected, ALK testing was not pursued routinely. As a result, all patients with ALK rearrangements were EGFR and KRAS WT; however, we did not test all patients with EGFR and KRAS mutations for the coincident presence of ALK rearrangements. Our routine testing procedures were based on past series and our own internal series (manuscript in preparation) that have demonstrated mutual exclusivity of these oncogenic events 21, 22. Recent data from the Lung Cancer Mutation Consortium demonstrated overlapping EGFR and KRAS mutations in 8% (3/38) of tumor samples that harbored rearrangements in ALK.28 We found the frequency of co-incident EGFR or KRAS mutations in the 44 ALK rearranged cases in our series to be 0% (95% CI: 0–10%).
From a practical standpoint, while we advocate stratifying patients by genotype in clinical studies of targeted therapies, we recognize that this is sometimes not possible, particularly when a predictive biomarker has not been well characterized. Furthermore, despite state-of-the-art molecular testing, a known mutation will not be identified in many patients. Our data suggest that stratification of patients by smoking history should be performed in all trials where genotype is not a pre-specified eligibility criterion, given the significant prognostic difference that we observed. Varying proportions of never smokers and former/current smokers within treatment arms may confound survival results, and should be accounted for at study inception, rather than post-hoc.
Finally, we note that although co-morbidity, either in range or severity, was not captured by us as part of this study, its influence on survival apart from performance status was previously shown by Toh et. al. to be non-significant on both univariate and multivariate analysis.29
In conclusion, our data demonstrate that never smokers and former/current smokers with lung adenocarcinomas are not homogeneous subgroups. Each group is made up of individuals with a set of disparate mutations that, in sum, generates an overall prognosis. Never smokers carry a higher proportion of EGFR mutations, but this should not lead to reflexive treatment of never smokers with an EGFR TKI. Conversely, EGFR mutations do occur in former/current smokers, who exhibit a similar survival outcome as never smokers when treated with an EGFR TKI. All patients with lung adenocarcinoma, regardless of smoking history, should undergo testing for EGFR mutations and rearrangements in ALK in an effort to match patients with an appropriate targeted therapy. Patients who are wild-type for mutations in EGFR and KRAS and who do not exhibit ALK rearrangements should have their tumors are prioritized for future molecular profiling efforts. Clinical trials of unselected lung adenocarcinoma patients and trials of patients who do not harbor mutations in EGFR/KRAS or rearrangements in ALK should be stratified by smoking history.
Acknowledgement
Supported by NCI P01-CA129243
Footnotes
Disclosures: Mark G. Kris- Pfizer (Consultant), Boehringer-Ingelheim (Consultant), Roche China (Consultant); Vincent A. Miller- Clovis (Consultant), Astellis (Consultant), Genentech (Consultant), Arqule (Consultant), Boehringer-Ingelheim (Consultant).
References
- 1.Kris MG, Natale RB, Herbst RS, et al. Efficacy of Gefitinib, an Inhibitor of the Epidermal Growth Factor Receptor Tyrosine Kinase, in Symptomatic Patients With Non-Small Cell Lung Cancer: A Randomized Trial. JAMA. 2003;290(16):2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in Previously Treated Non-Small-Cell Lung Cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) The Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kris M, Lau CY, Ang D, et al. Initial results of LC-MAP: An institutional program to routinely profile tumor specimens for the presence of mutations in targetable pathways in all patients with lung adenocarcinoma. J Clin Oncol. 2010;28(15s) abstr 7009. [Google Scholar]
- 8.Riely GJ, Marks J, Pao W. KRAS Mutations in Non-Small Cell Lung Cancer. Proc Am Thorac Soc. 2009;6(2):201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 9.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular Predictors of Outcome With Gefitinib in a Phase III Placebo-Controlled Study in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2006;24(31):5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 12.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and Distinctive Spectrum of KRAS Mutations in Never Smokers with Lung Adenocarcinoma. Clinical Cancer Research. 2008;14(18):5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. New England Journal of Medicine. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. The Lancet Oncology. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 17.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2010;116(3):670–675. doi: 10.1002/cncr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Q, Pao W, Ladanyi M. Rapid Polymerase Chain Reaction-Based Detection of Epidermal Growth Factor Receptor Gene Mutations in Lung Adenocarcinomas. J Mol Diagn. 2005;7(3):396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pao W, Wang TY, Riely GJ, et al. KRAS Mutations and Primary Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib. PLoS Med. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham D, Kris MG, Riely GJ, et al. Use of Cigarette-Smoking History to Estimate the Likelihood of Mutations in Epidermal Growth Factor Receptor Gene Exons 19 and 21 in Lung Adenocarcinomas. J Clin Oncol. 2006;24(11):1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 21.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK Fusion is Linked to Histological Characteristics in a Subset of Lung Cancers. J Thorac Oncol. 2008;3(1):13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer with <i>EML4 –<i>ALK Fusion Gene. Annals of Surgical Oncology. 2010;17(3):889–897. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee W, Jiang Z, Liu J, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465(7297):473–477. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 24.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The Lancet Oncology. 2011;12(11):1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik PK, Arcila ME, Fara M, et al. Clinical Characteristics of Patients With Lung Adenocarcinomas Harboring BRAF Mutations. Journal of Clinical Oncology. 2011;29(15):2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arcila M, Chaft J, Nafa K, Kris M, Zakowski M, Ladanyi M. Molecular and clinicopathologic characteristics of HER2-mutant lung adenocarcinoma (ADC) J Clin Oncol. 2011;29(suppl) abstr 10596. [Google Scholar]
- 27.Pietanza M, Arcila M, Chaft J, et al. Characteristics of patients with non-small cell lung cancer harboring mutations in PIK3CA. J Clin Oncol. 2011;29(suppl) abstr 7587. [Google Scholar]
- 28.Kris M. The Lung Cancer Mutation Consortium. IASLC 12th Annual Targeted Therapies of the Treatment of Lung Cancer Meeting; Santa Monica, CA. 2012. [Google Scholar]
- 29.Toh C-K, Gao F, Lim W-T, et al. Never-Smokers With Lung Cancer: Epidemiologic Evidence of a Distinct Disease Entity. Journal of Clinical Oncology. 2006;24(15):2245–2251. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]