Abstract
Forty-five years ago Shik and colleagues were the first to demonstrate that electrical stimulation of the dorsal pontine reticular formation induced fictive locomotion in decerebrate cats. This supraspinal motor site was subsequently termed the “mesencephalic locomotor region (MLR)”. Cholinergic neurons of the pedunculopontine tegmental nucleus (PPT) have been suggested to form, or at least comprise in part, the neuroanatomical basis for the MLR, but direct evidence is lacking. In an effort to clarify the location and activity profiles of pontine reticulospinal neurons supporting locomotor behaviors, we employed in the present study a retrograde tracing method in combination with single unit recordings and antidromic spinal cord stimulation as well as characterized the locomotor- and behavioral state-dependent activities of both reticulospinal and non-reticulospinal neurons. The retrograde labeling and antidromic stimulation responses suggested a candidate group of reticulospinal neurons that were non-cholinergic and located just medial to the PPT cholinergic neurons and ventral to the cuneiform nucleus (CnF). Unit recordings from these reticulopsinal neurons in freely behaving animals revealed that the preponderance of neurons fired in relation to motor behaviors and that some of these neurons were also active during REM sleep. By contrast, non-reticulospinal neurons, which likely included cholinergic neurons, did not exhibit firing activity in relation to motor behaviors. In summary, the present study provides neuroanatomical and electrophysiological evidence that non-cholinergic, pontine reticulospinal neurons may constitute the major component of the long-sought neuroanatomic MLR in mammals.
Keywords: mesencephalic locomotor region, rat, single unit activity, sleep-wake REM sleep
Locomotor activity is a complex process requiring neural circuits in the thoracic and lumbar regions of the spinal cord that endogenously generate periodic motor commands for movement, otherwise known as central pattern generators (CPG), as well as supraspinal modulatory inputs. It has long been appreciated that the pontine reticular formation (PRF) contains supraspinal circuitry that participates in motor control. For example, Shik and colleagues (1966) showed that electrical stimulation confined to the dorsal PRF triggered otherwise immobile decerebrate cats to walk/gallop on a treadmill, so-called “fictive locomotion” (Mori et al., 1978; Shik et al., 1966). This circumscribed area of the PRF was subsequently termed the “mesencephalic locomotor region” (MLR). To date, however, the precise neuroanatomical location, neurochemical phenotype and behavioral state-dependent activity of these pontine reticulospinal neurons remains largely unresolved.
Several studies have suggested, albeit inferentially, that cholinergic PPT neurons located within the lateral pontine tegmentum (LPT) might form, or at least comprise in part, the neuroanatomic basis of the MLR (Bernau et al., 1991; Cabelguen et al., 2003; Noga et al., 1991; Skinner and Garcia-Rill, 1984). For example, PPT cholinergic neurons “fit” the location of the proposed MLR and injections of cholinergic agonist into the rostral ventromedial medulla (RVM), a region that receives PPT cholinergic inputs, induces stepping behaviors (Kinjo et al., 1990). Importantly, however, the long latency (10-15 min) and short duration (5-10 sec) of these agonist-driven motor responses cast doubt on the primacy of this cholinergic system in regulating locomotor behaviors. And, moreover, carbachol injections into the RVM have also been reported to induce REM sleep, including muscle atonia (Lai and Siegel, 1990; Vanni-Mercier et al., 1991).
Interestingly, a band of non-cholinergic cells located in the lateral pontine tegmentum (LPT) that project directly to both the ventral horn of the spinal cord and the ventromedial medulla (VMM) was recently revealed (Sukhotinsky et al., 2005). While this cell group also fits the general location proposed for the MLR, lying roughly medial to the PPT and ventral to the cuneiform nucleus (CnF), the role of these neurons in locomotion remains unknown. Of note, LPT neurons express high levels of the orexin 2 receptor and receive substantial orexinergic input (Lu et al., 2006; Marcus et al., 2001). Disruption of orexin signaling causes narcolepsy and cataplexy (Scammell et al., 2009) whereas injections of an orexin agonist into the LPT region can elicit locomotion in decerebrate cats (Takakusaki et al., 2005) and lesions of the LPT result in cataplexy in rats and humans (Fernandez et al., 1995; Lu et al., 2006; Plazzi et al., 1996). These findings taken together suggest that a population of neurons within the LPT may be critically involved in maintaining postural muscle tone and facilitating locomotion. In the present study, we recorded LPT neuronal activity and correlated the firing activity of these neurons with both the level of motor behavior and behavioral state, including active wake, quiet wake, NREM and REM sleep states. We also investigated, for the first time, the antidromic responses of these reticulospinal neurons to spinal cord stimulation, in freely moving animals and with the goal of correlating the firing activity of these neurons with movement and behavioral state. Our findings indicate that only spinally-projecting, non-cholinergic neurons fire correlatively with motor behaviors and as such likely comprise the MLR and possibly represent a clinically important target for treating a wide spectrum of movement disorders, including the postural and gait disorders of Parkinson's disease (PD) and cataplexy.
EXPERIMENTAL PROCEDURES
Animals
All experiments were performed in pathogen free male Sprague Dawley rats (n=7; 250-300gms, Charles River). The animals were maintained on a 12:12 light-dark cycle with ad libitum access to water and food. Care of the rats in the experiment met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals and all protocols were approved by the BIDMC Institutional Animal Care and Use Committees.
Surgical Procedure
Rats were weighed and anesthetized (Ketamine: 100 mg/kg + Xylazine: 10mg/kg, ip) and fixed in a stereotaxic frame. Body temperature and blood pressure were monitored throughout the procedure. Rats were first implanted with electrodes for sleep-wake (S-W) and neuronal recording. Briefly, once the cranium was exposed the target locations were marked. Two cortical screw electrodes (one frontal cortex and one contralateral parietal cortex) were used to record the EEG, and two flexible multistranded wires were implanted in the nuchal muscles to record muscle activity (EMG). The electrodes were connected to a plug and secured onto the skull using dental cement. In addition, the rats were implanted with a miniature microdrive that was secured onto the skull using dental cement. The construction of the microdrive was a modified version of that used in our previous studies (Thankachan et al., 2001; Thankachan et al., 2009). Briefly, the microdrive consisted of a two 26G internal cannulae driven into a two 22G outer cannulae by a nut-screw-spring assembly, which allowed us to target the LPT bilaterally. The internal cannulae was positioned 1.5mm above the dorsal part of LPT at using the following stereotaxic coordinates: P 8.0mm, L 1.8mm, DV 6.5mm (Paxinos and Watson, 1998). Six 25μ microwires (formvar coated, AM systems, WA) were soldered at one end to an electrode plug and protected by Silastic tubing (Bentec Medical Inc., CA) and cut at the other end to expose the insulation and then inserted through the internal cannulas. The wires extended 1.5mm from the tip of the internal cannulas and could be moved up and down to record single neuronal activity from the LPT/MLR. Finally, a small incision was made over the T1 area of the spinal cord. A bipolar twisted insulated electrode (125μ; Plastics One, Roanoke, VA) was guided through the ventral horn of the spinal cord [(ML= 0.5 mm, DV = 1.6 mm; (Watson et al., 2008)] and held in place with dental cement. The incision area was closed with absorbable sutures. The electrode with the contact pin was passed under the skin and threaded to the skull with dental cement. Following a 2 week post recovery and adaptation period to the recording chamber, neuronal activity and sleep recordings were performed.
Sleep recording
The rat recording protocol is similar to the electrophysiological recordings conducted in cats (Thankachan et al., 2001) and transgenic mice (Thankachan et al., 2009). 2-weeks post-surgery the rats were connected for recording EEG and EMG signals. During the recording periods the rats were singly housed in plexiglass cages with wood shavings and food and water was available ad libitum.
Recording of single neuronal activity in LPT/MLR
Extracellularly recorded action potentials with amplitude >3:1 signal-to-noise were amplified (10,000x; A-M systems, Everett, WA), filtered (band pass 300 Hz to 10 kHz; A-M systems, Everett, WA), digitized (power1401, CED, UK) and recorded using Spike2 software (CED, UK). The recording procedure was similar to that used previously in cats and mice (Mallick et al., 1998; Thankachan et al., 2001; Thankachan et al., 2009). The data was collected for 8-10 hours each day, so as to acquire neuronal activity during multiple episodes of all stages of the S-W cycle. Separation of action potentials (“spikes”) was performed using a spike sorting analysis (Spike2, CED, UK) procedure.
Stimulation of spinal cord ventral horn area and antidromically activated neurons in LPT/MLR
After recording neurons over multiple episodes of S-W cycles, the spinal cord ventral horn area was stimulated using the pre-implanted twisted bipolar stimulating electrode (150μ, Teflon coated wire). To determine if spinal cord stimulation induced motor activity a brief (60 sec) high frequency (150-350 Hz) stimulation (trains of rectangular cathodal pulses of 150 to 200μA amplitude and 0.5-ms width) was delivered through the stimulating electrode implanted in the spinal cord ventral horn and this was done against the background of quiet wake or sleep with low muscle tone. To determine if the LPT/MLR neurons projected to the spinal cord, low frequency (1Hz) stimulation (10-15 mins, current strength of 10-50μA amplitude and 0.5-ms width) was delivered through the electrodes placed in the spinal cord ventral horn in an attempt to antidromically invade LPT/MLR neurons. The electrodes were implanted unilaterally in the spinal cord, ipsilateral to the neuronal recordings within the LPT/MLR. For antidromic response, all units followed these criteria: a) response at a relatively constant latency (<5 ms); b) an ability to follow high-frequency stimulation (≥333 Hz); c) collision of putative antidromic action potentials with orthodromic action potentials.
Data analysis
Classification of the neurons based on their discharge rate during S-W and during gross locomotor behavior during waking
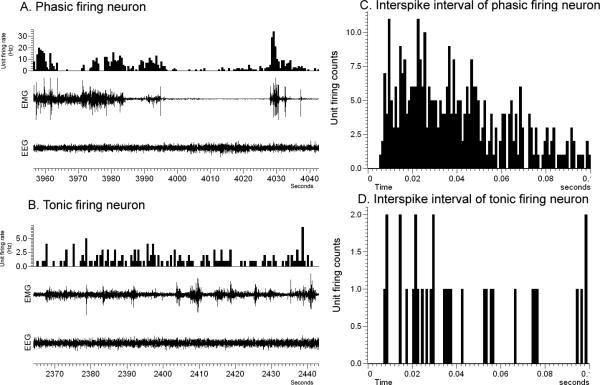
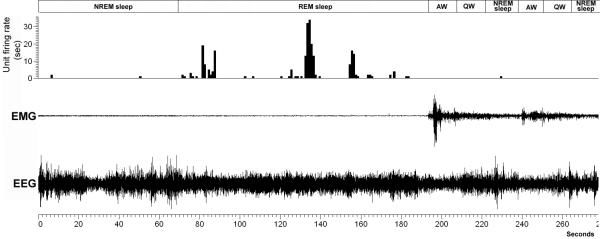
Neuronal activity was measured by analyzing the firing rate of each neuron in a 60 sec epoch during the S-W cycle, including active wake (AW), quiet wake (QW), non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep states. The mean firing rate for each of the S-W and gross locomotor behavior states was calculated from five 60 sec epochs. Based on the mean neuronal discharge activity during AW, QW, NREM and REM, the neurons were subdivided into different groups. The AW/REM active neurons exhibited higher firing rates in AW and REM sleep as compared to NREM (20-90% increase compared to NREM sleep). The slow and fast firing AW/REM active neurons were distinguished based on their highest discharge rate during AW and REM, with a cut off at 2Hz for the former group and >2 Hz for the latter group. The neurons that showed the highest activity during AW state (firing higher than 60-80%) compared to REM and NREM sleep were classified as AW-active neurons. REM active neurons showed 20-90% higher activity during REM sleep as compared to AW and NREM sleep. The slow and fast firing REM active neurons were characterized based on their highest discharge rate during REM state, with a cut off at 2Hz for the former group and >2 Hz for the latter group. The activities of these groups of neuron were further studied in relation to their responses during gross locomotor behaviors. The data expressed as mean firing rate represents the activity of the neuron as action potentials or spikes per second (HZ) ± standard error. The neuronal activity between the S-W states were compared statistically by repeated measures ANOVA (one-way analysis of variance), followed by post hoc comparison using Student-Newman-Keuls test. We further classified neurons into the following categories based on the rate histograms or inter-spike interval (ISI) histograms generated by Spike 2 analysis: 1) Phasically firing neurons: (burst firing) characterized by bursts of activity separated by pauses of discharge or periods of low activity, a sharply positively skewed ISI's distribution and an asymmetrical discharge density histogram (Fig. 1A & 1C); or 2) Tonically firing neurons: those with regular or slight irregular firing frequency and a symmetrical discharge density histogram (Fig. 1B & 1D). Also phasic firing pattern with synchronous higher amplitude EMG (seen only during higher motor behavior) was used to correlate the neuronal firing to motor activity
Figure 1.
A representative figure showing phasic (A) and tonic (B) firing neurons and their inter-spike interval histograms (C & D).
Defining a motor-active or motor-related (motor-responsive) neuron
Nuchal muscle instead of leg muscle electrodes were used for the EMG recordings as the experimental requirement for additional electrodes for spinal cord stimulation interfered surgically with the implantation of leg EEG electrodes. It was however evident when comparing the EMG traces with the time-locked video behavioral recordings that high amplitude changes in nuchal EMG activity occurred in synchrony with motor behaviors (i.e., foraging, grooming, paw licking, moving in the cage, standing etc) during active wake and nuchal EMG activity was corresponding low during quiescent behavioral states (NREM, REM sleep and quiet wake).
The neuronal responses to active motor behavior were determined by correlating the activity of the electromyogram (EMG) recording with the firing pattern of the recorded neuron. A neuron was defined as motor related if its firing rate was highest during an active motor episode (during AW) and as compared to QW and sleep states. The active motor episode was defined by higher EMG amplitude for a minimum of 30 seconds. For each recorded neuron, firing activity was compared between the behavioral states using repeated measures one-way analysis of variance (ANOVA), followed by post hoc comparison using Student-Newman-Keuls test. Individual unit data was processed to generate rate histogram and inter-spike interval histograms (ISIH).
Identification of the recording site & histology
Several post hoc criteria were used to determine the electrode position with respect to cytoarchitectonic features of LPT neuron. After completion of the recordings, the rats were anesthetized [sodium pentobarbital (i.p.)] and an anodal current (1 mA for 30s) was applied to each recording electrode to deposit iron (seen in the Prussian blue stain) and mark the recorded neuron. Anodal current was also passed through the stimulating electrode to generate a small lesion in the spinal cord area. The depth of the marking lesions was then used to confirm the depth of the electrode tip and hence the site at which neuron(s) with spinally-projecting characteristics (antidromically activated by spinal cord stimulation) were recorded. Because marking lesions could only be made after data collection was complete, electrode tip locations for all other neurons recordings obtained on successive days were reconstructed using a depth scale on the microdrive assembly.
Following the passing of current, the animals were transcardially perfused with saline and 4% phosphate-buffered formalin under deep anesthesia. Serial coronal brain sections (40μm) were cut on a freezing microtome, mounted on slides and stained with neutral red. The marked recording sites (blue mark) were then examined using a microscope and plotted on diagram adapted from the stereotaxic map of the rat brain atlas of Paxinos and Watson (1998). All other recording sites were calculated from the marked site. The brain was sectioned and the ferrous deposit (blue mark revealed by Perl's Prussian blue reaction) identified by microscopy.
Retrograde tracing of LPT/MLR neurons after CTb injections in the spinal cord
Rats (n=4) were injected with 15nl of cholera toxin B subunit (CTb, 1% in saline) in the spinal ventral horn at the C8-T1 level. 2 weeks after the injection the rats were transcardially perfused with saline and 4% phosphate-buffered formalin. Brains were cut at 40μm sections and processed immunohistochemically for CTb and choline acetyltransferase (ChAT). In brief, the sections were incubated in primary antibody (polyclonal goat anti-CTb antibody; 1:10,000; List Biologicals) for 24 hours at room temperature followed by incubation in secondary antibody (biotinylated donkey anti-goat; 1:100 Vector, Burlingame, CA) for 2 hours. CTb was visualized by avidin-biotin peroxidase technique (ABC) in which the sections were incubated in the ABC solution (1:200 in PBS, Vectastain ABC-elite kit, Vector, Burlingame, CA) for 1 hour followed by detection with nickel intensified DAB (diaminobenzedine) reaction for 20 mins. The sections were then processed for ChAT immunohistochemistry. All processed sections were mounted on slides, dehydrated and delipidated and cover slipped with permanent mount solution.
RESULTS
Anatomic location of spinally-projecting supraspinal neurons
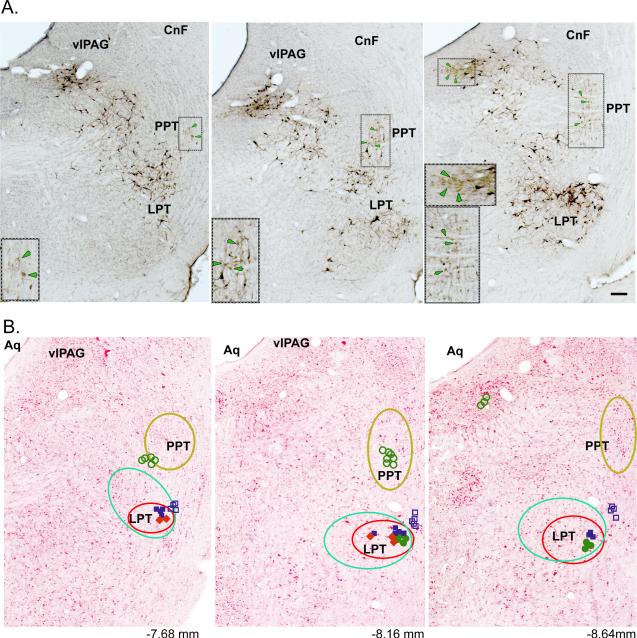
Injection of the retrograde tracer CTb in the spinal cord at the level of C8-T1 (n=4) revealed many retrograde labeled neurons (black color) in the lateral pontine tegmentum (LPT) just below the ventrolateral periaquaductal gray (vlPAG) and medial to the pedunculopontine tegmental nucleus (PPT) cholinergic neurons (light brown color) (Fig. 2A), with a preponderance of these labeled cells on the side ipsilateral to the injection. Consistent with previous studies (Rye et al., 1988; Skinner et al., 1990), double-immunolabeling of CTb and ChAT confirmed that these spinally-projecting neurons were in fact non-cholinergic. Table 1 shows the distribution of both spinally-projecting and ChAT-positive neurons in the LPT and PPT/LDT regions. On the basis of this labeling, we set forth to characterize the motor- and state-dependent firing activities of these non-cholinergic spinal projecting neurons of the LPT, which may in fact comprise the long-sought mesencephalic locomotor region (MLR). To examine the role of these LPT neurons in motor and sleep-wake behaviors, we performed simultaneous recordings of EEG/EMG and single-unit discharges of neurons in the LPT (presumptive MLR) and in surrounding regions, including the PPT, in freely behaving rats. In addition, we analyzed the antidromic responses of all recorded neurons to spinal cord stimulation.
Figure 2.
A. Histological image showing retrogradely labeled CTb cells (CTb injection at the level of C8-T1, the spinal ventral horn) in the lateral pontine tegmentum (LPT) (black color) region and ChAT–positive cells (light brown color & green arrows) just below the ventrolateral periaquaductal gray (vlPAG) and the cuneiform nucleus (CnF) and medial to the pedunculopontine tegmental nucleus (PPT) cholinergic neurons marked by the box. Inset: A larger magnification of the boxed area. B. Schematic images illustrate the reconstruction of the location of the recorded neurons on the background of neutral red stained sections. The placement of the recorded neurons is based on the Prussian blue that marks electrode tip.
Each neuron is represented by a symbol designating their firing pattern and activity across behavioral states (AW and REM sleep). Symbols:  Filled blue (Phasic AW/REM);
Filled blue (Phasic AW/REM);  Open blue (Tonic AW/REM);
Open blue (Tonic AW/REM);  Filled green (Phasic REM);
Filled green (Phasic REM);  Filled red (Phasic AW);
Filled red (Phasic AW);  Open green (Tonic REM); AW- Active wake active; AW/REM- Active wake and REM sleep active, REM-REM sleep active; Phasic – Phasic firing neurons show intermittent higher activity with occasional burst firing (usually preceded or followed by silent or low activity); Tonic – Tonic firing neurons show low or high firing activity but with regular interval. Numbers in B represent approximate AP distance (in mm) from bregma (Paxinos & Watson, 1998). The yellow circled region shows neurons with no relation to motor behavior nor antidromic response to spinal cord stimulation. Spinally-projecting neurons depicted here are CTb labeled cells (green circle) and neurons (red circle) with higher activity during motor behavior with antidromic projection to spinal cord. Bar in A. 50μm.
Open green (Tonic REM); AW- Active wake active; AW/REM- Active wake and REM sleep active, REM-REM sleep active; Phasic – Phasic firing neurons show intermittent higher activity with occasional burst firing (usually preceded or followed by silent or low activity); Tonic – Tonic firing neurons show low or high firing activity but with regular interval. Numbers in B represent approximate AP distance (in mm) from bregma (Paxinos & Watson, 1998). The yellow circled region shows neurons with no relation to motor behavior nor antidromic response to spinal cord stimulation. Spinally-projecting neurons depicted here are CTb labeled cells (green circle) and neurons (red circle) with higher activity during motor behavior with antidromic projection to spinal cord. Bar in A. 50μm.
Table 1.
Distribution of pontine spinal projecting (CTb) and cholinergic (ChAT) neurons (cell count/section)
| Ipsi-LPT | Contra-LPT | Ipsi-PPT | Contra-PPT | Ipsi-LDT | Contra-LDT | |
|---|---|---|---|---|---|---|
| CTb | 55.2 ± 4.5 | 35.3 ± 4.6 | 3.6 ± 1.5 | 1.3 ± 0.6 | 6.6 ± 1.5 | 3.0 ± 1.0 |
| ChAT | 7.6 ± 2.5 | 2.3 ± 2.0 | 49.6 ± 2.5 | 50.3 ± 1.5 | 56.0 ± 5.3 | 51.6 ± 5.5 |
| CTb+ChAT | 0 | 0 | 0 | 0 | 0 | 0 |
Ipsi- = ipsi-lateral to the injection side of CTb
Contra- = contra-lateral to the injection side of CTb
CTb = CTb labeled neurons
ChAT= ChAT labeled neurons
CTb + ChAT = Neurons with dual-label of CTb and ChAT
Spinal cord stimulation and the antidromic responses of LPT/MLR neurons in freely behaving rats
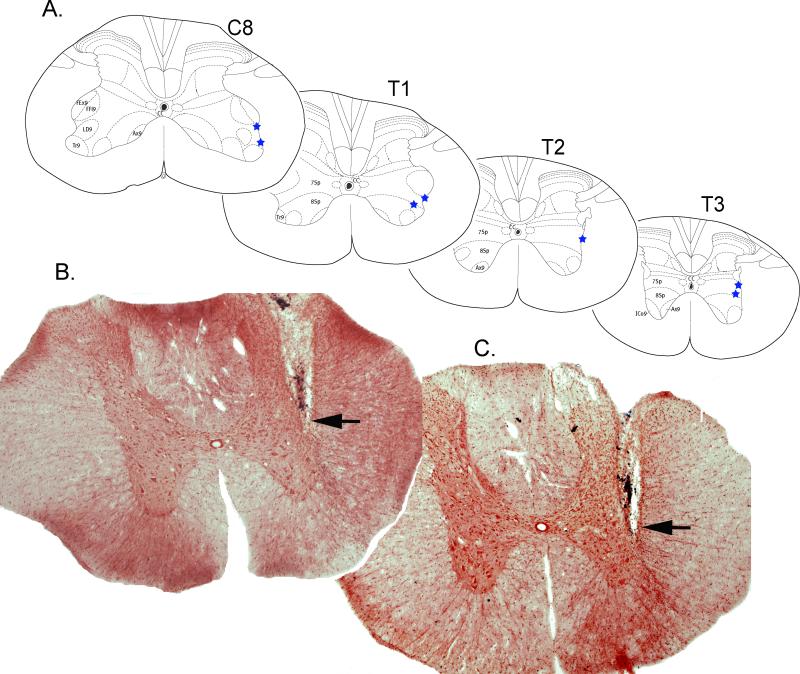
We first applied a brief (60 sec) high frequency electrical stimulus (100-350Hz, 0.5ms width, 50-200uA) through the electrode implanted at the C8-T1 level of the spinal ventral horn and this resulted in motor activation (body or leg movements) and EEG arousal (active wake). Both overt motor behaviors and arousal were observed in the video recordings time-locked to the stimulus and suggests that the C8-T1 level in the spinal cord is an important area for driving motor activity. Histological transversal sections from two rat spinal cord are shown in Fig. 3B and clearly indicate the location of the stimulating electrode track at the level of the thoracic segment of the spinal cord. The schematic diagram in figure 3A illustrates the position of the electrode tip in the spinal cord in seven rats. We next evaluated the antidromic responses of MLR neurons to low frequency (1Hz, with current strength of 10 to 50μA amplitude and 0.5-ms width) stimulation of the spinal cord. In this case, low frequency stimulation strength was used so that the stimulation did not produce motor activation or arousal (active wake) but was still sufficient to activate an antidromic response in spinally-projecting neurons in the LPT/MLR.
Figure 3.
A. Schematic images illustrate the reconstruction of the location of the stimulating electrode in the neutral red stained spinal cord sections. The placement of the tip of the electrode at the level approx. the T1 level, in all 7 (marked by filled blue star) rats. Axial muscles (Ax9); forearm ext (FEx9); forearm flex (FFI9); intercostals (ICo9); lamina 7 (7sp); lamina 8 (8sp); latiss dorsi (LD9); triceps (Tr9). B. & C. Representative histological images from rat (n=2) spinal cord sections showing the stimulating electrode (arrow).
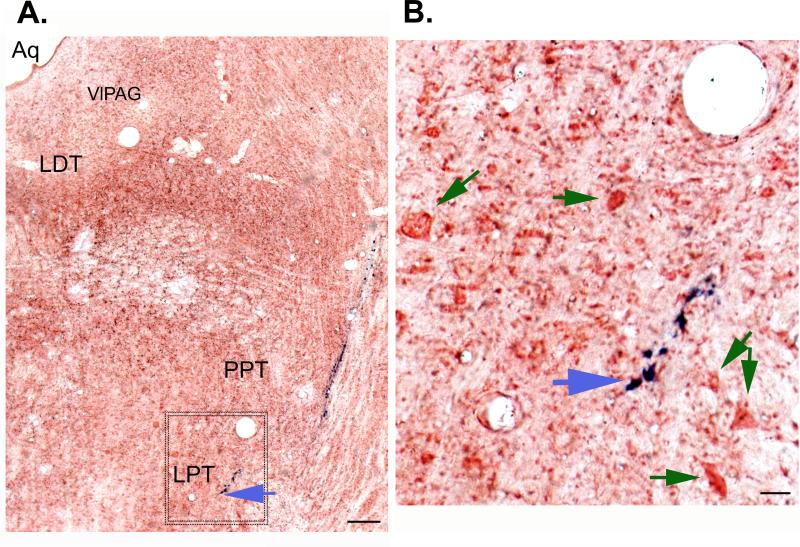
Of the 94 recorded neurons in the MLR and surrounding region, only 54 neurons could be studied for antidromic response to spinal cord stimulation and these neurons were verified histologically using a blue marker dye (Fig. 2B). In the remaining 40 neurons we could record their activity in relation to the behavior and locomotor state, but in these, either their antidromic response could not be studied/ verified (electrode implants in spinal cord inaccurate) or their sites of recording could not be identified by histology. For these reasons, these 40 neurons were excluded from the analysis. A histological section showing the Prussian blue mark (which reveals the final position of the recording electrode tip and the location of the recorded LPT neuron) is shown in Figure 4.
Figure 4.
A. Histological image showing the track of the recording electrode targeted to the lateral pontine tegmentum (LPT) where a phasic active wake (AW) spinally-projecting neuron was recorded. The blue mark (ferrous deposits) track (blue arrow) is from the recording electrode and is the result of the Pearl's Prussian-blue reaction seen against the background of the neutral red stain. B. Image shows an enlarged view of the area shown (box) in the figure 2A. Recording were done from the cells (green arrows) close to the electrode track. Bar in A. 50μm; B. 10 μm.
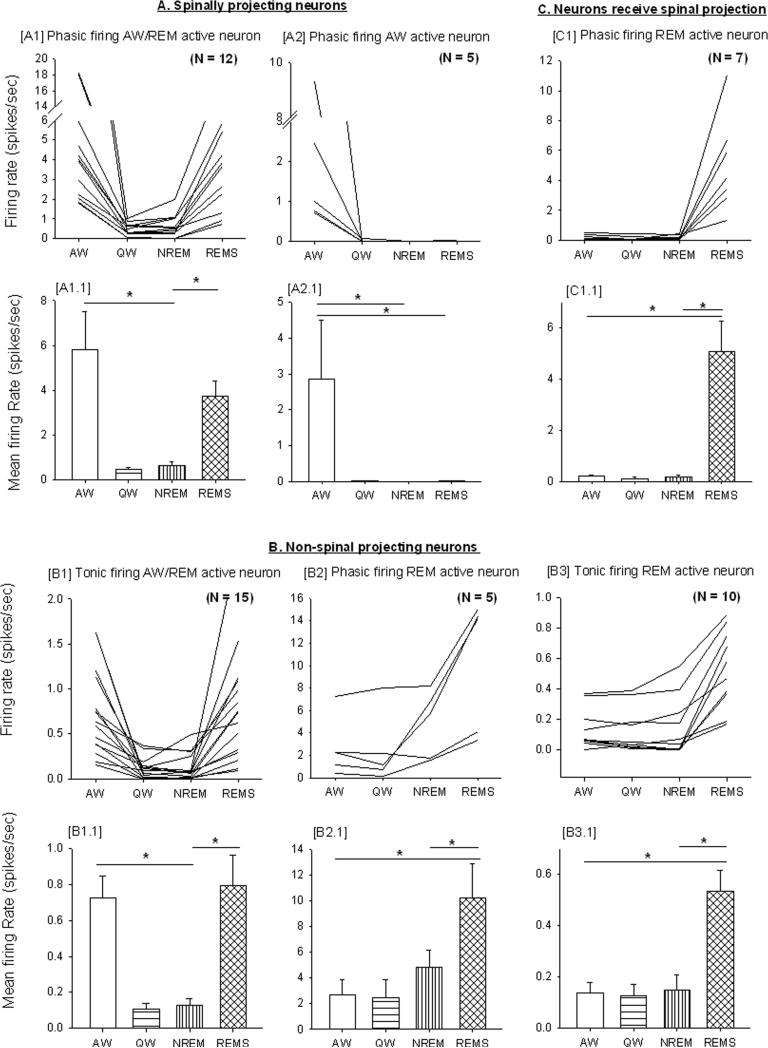
The 54 neurons were classified into three groups (Fig. 5 & Table 2) based on their antidromic response to spinal cord stimulation: 1) neurons that were antidromically activated in response to spinal cord stimulation; 2) neurons that did not show an antidromic response; and 3) neurons that showed a orthodromic activation (i.e., presumably receiving spinal projections) to spinal cord stimulation. Furthermore, and based on their firing pattern, the neurons were sub-divided into phasic and the tonic firing neurons: phasic firing neurons showed intermittent increases in activity with occasional bursting whereas tonic firing neurons showed sustained firing activity.
Figure 5. Neuronal firing characteristics of LPT neurons.
Individual [upper trace; spikes/second)] and group rate [lower trace; mean firing rate / second)] across sleep-wake states [active wake (AW), quiet wake (QW), non-REM (NREM) sleep and REM sleep]. These neurons were classified into three groups based on their antidromic response to spinal cord stimulation and further sub-divided based upon their firing pattern (tonic/phasic) and rate during period of high cortical EEG activity (active wake and REM sleep). * indicates a significant difference (p<0.05) between behavioral states.
Table 2.
Classification of neurons recorded in the LPT area based on their antidromic response to spinal cord stimulation, firing patterns (tonic/phasic) and rate during active wake and REM sleep.
| Relation to locomotor behavior & behavioral states (N=54) | Relation to spinal cord (projection) stimulation | ||
|---|---|---|---|
| Antidromic activation (Spinally projecting) (N=17) | No antidromic response (Non-spinally projecting) (N=30) | Delayed response (Not spinally projecting but possibly receiving spinal projection) (N=7) | |
| locomotor-related | Phasic AW (5) Phasic AW/REM (12) |
- | - |
| locomotor-unrelated | - | Tonic AW/REM (15) Phasic REM (5) Tonic REM (10) |
Phasic REM (7) |
Neurons: AW- Active wake active; AW/REM- Active wake and REM sleep active, REM- REM sleep active
Firing pattern: Phasic - intermittent higher activity with occasional burst firing (usually precedes or followed by silent or low activity). Tonic - low or high but with sustained regular intervals. (N): number of neurons recorded
Spinally-projecting neurons
Seventeen (n=17) recorded neurons showed antidromic activation with a short latency (2-4ms) (Fig. 5A & Table 2). These neurons also faithfully followed and showed reproducible responses to higher frequency (300Hz) stimulation trains. With regard to behavioral state (AW, QW, NREM sleep and REM sleep), these neurons exhibited the highest discharge rate during active wake (motor behavior) and their phasic firing pattern (burst activity) was correlated to higher motor activity (higher amplitude in EMG recording) seen during various animal behavior like eating, foraging, grooming, paw licking, moving in the cage, standing etc. Of these spinally-projecting neurons, we observed AW/REM active (n=12; 71%) (Fig. 5A1) or AW-active (n=5; 29%) (Fig. 5A2) neurons. The mean firing rate of phasic firing AW/REM active neurons was 5.82±1.70 and 3.75±0.66 during AW and REM sleep, respectively, and this was significantly higher as compared to QW (0.46±0.08; q=6.68, p<0.05 & q=4.10, p<0.05) and NREM sleep (0.64±0.16; q=6.46, p<0.05 & q=3.87, p<0.05) (Fig. 5A1.1). AW-active neurons had a mean firing rate of 2.81±1.65 during AW that was significantly higher than during QW (0.02±0.00; q=4.24, p<0.05), NREM sleep (0.002±0.00; q=4.67, p<0.05) or REM sleep (0.03±0.00; q=4.42, p<0.05) (Fig. 5A2.1). Overall, these neurons fired much faster than tonic non-spinally-projecting AW/REM active neurons (Fig. 5B1). We also conducted collision tests in some (N=4/17) neurons and were successful in blocking the antidromic responses of the neurons with a shorter spike-to-stimulus interval, further confirming that these neurons project directly and monosynaptically to the spinal cord.
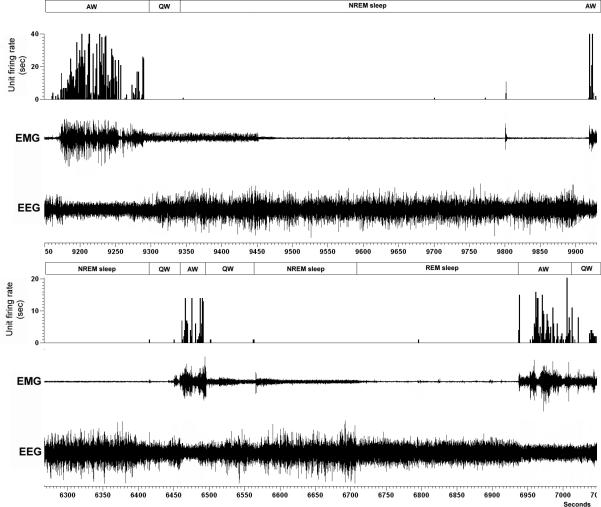
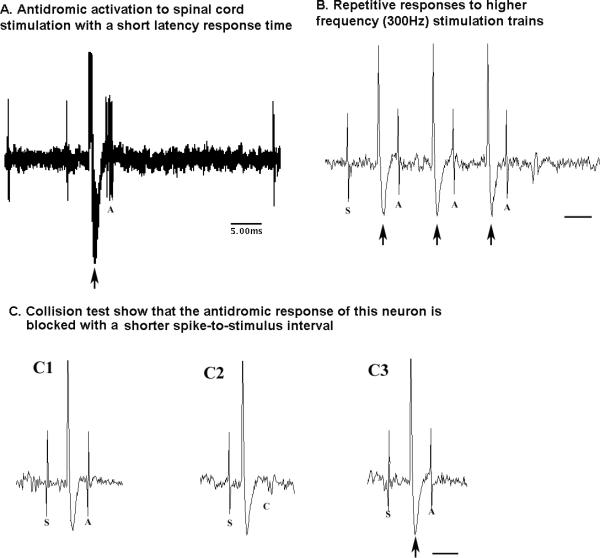
An example of a phasic firing AW active neuron is shown in figure 6. This neuron exhibited burst firing (phasic) activity that was associated with high motor tone in antigravity muscles and which was irrespective of the nature of the motor behavior (i.e., during foraging, grooming, paw licking, moving in the cage, standing etc). In addition, this neuron was antidromically activated by spinal cord stimulation, exhibited a shorter latency response time of 4 ms (Fig. 7A) and showed repetitive responses to higher frequency (300Hz) stimulation trains (Fig. 7B). A collision test showed that the antidromic response (Fig. 7C1 & 7C3) of this neuron could be successfully blocked (Fig. 7C2) with a shorter spike-to-stimulus interval, further confirming that these neurons project monosynaptically to the spinal cord.
Figure 6. An example of a phasic firing AW active neuron.
The neuron exhibits a higher firing rate during motor activity (higher EMG amplitude) in AW state as compared to QW, NREM sleep and REM sleep. This neuron was within the MLR region as verified by the electrode track.
Figure 7. AW active neuron and their response to spinal cord stimulation.
A, shows antidromic activation with a short latency response time of 3.5 ms. (10 overlap tracing);
B, shows repetitive responses to higher frequency (300Hz) stimulation trains;
C, collision test showing that the antidromic response of this neuron is blocked with a shorter spike-to-stimulus interval.
Large arrow: stimulation artifact, A: antidromic cell response; S: spontaneous cell firing; C: collision test blocking antidromic cell response. Bar in B and C, 5ms
Non-spinally projecting neurons
30 neurons showed no antidromic response and 7 neurons showed orthodromic responses (Fig. 5B & Table 2). Based on their firing pattern during sleep-wake behavior, we classified the 30 non-antidromic response neurons into three groups; tonic AW/REM active (n=15) (Fig. 5B1), phasic REM-active (n=5) (Fig. 5B2) and tonic REM-active (n=10) (Fig. 5B3). The tonic AW/REM active neurons showed a mean firing rate of 0.73±0.12 and 0.79±0.17 during AW and REM sleep, respectively and this was significantly higher than during QW (0.11±0.03; q=7.48, p<0.05 & q=9.85, p<0.05) or NREM sleep (0.13±0.04; q=6.20, p<0.05 & q=7.48, p<0.05) (Fig. 5B1.1). The mean firing rate of phasic firing REM active neuron was 10.23±2.66 during REM sleep and this was significantly higher than during AW (2.68 ± 1.19; q=4.47, p<0.05), QW (2.45±1.43; q=4.50, p<0.05) or NREM sleep (4.81±1.34; q=4.42, p<0.05) (Fig. 5B2.1). Similarly, tonic REM active neurons also showed a mean firing rate during REM sleep (0.53±0.08) that was higher than during QW (0.12±0.05; q=6.32, p<0.05), NREM sleep (0.15±0.06; q=5.02, p<0.05) or AW (0.14 ± 0.04; q=8.72, p<0.05) (Fig. 5B3.1).
The tonic firing neurons (AW/REM active and tonic REM active) fired slower than phasic spinally-projecting (Fig. 5A) and REM-active neurons (Fig. 5B2 & 5C). Consistent with the absence of antidromic response(s), none of these neurons showed any correlation in firing activity with motor behaviors, with some remaining silent (REM active neurons) and other showing no increase (tonic firing AW/REM active neurons) in firing during active motor behavior during active wake. An example of a tonic firing AW/REM active neuron is shown in figure 8. This neuron exhibited a higher firing rate in AW (higher EMG amplitude) that was irrespective of the type of motor behavior or during the REM sleep state (loss of motor activity) compared to QW and NREM sleep.
Figure 8. An example of a tonic firing AW and REM active neuron.
The neuron exhibits a higher firing rate irrespective of motor behavior in AW (higher EMG amplitude) or the REM sleep state (loss of motor activity) compared to QW and NREM sleep. This neuron was within the PPT region as verified by the electrode track.
The tonic firing neurons also showed sustained higher neuronal discharge during active wake and this was not correlated with motor behaviors and was irrespective of firing during NREM sleep or REM sleep states. Neurons with similar firing patterns have been reported in the dorsal pontine area (El Mansari M. et al., 1989; Siegel et al., 1981; Steriade et al., 1990; Thankachan et al., 2001; Thankachan et al., 2009). Whether some of these neurons are cholinergic remains to be determined. It has been postulated that phasic- and tonic- REM active neurons are differentially involved in regulating the phasic (PGO) and tonic (EEG and atonia) features of REM sleep (Sakai and Jouvet, 1980; Sakai and Koyama, 1996).
Neurons showing a orthodromic activation (i.e., presumably receiving spinal projections) to spinal cord stimulation
7 phasic REM-active neurons showed orthodromic responses (>15ms) to 1.0 Hz spinal cord stimulation and no response to repetitive high frequency (300 Hz) stimulation (Fig. 5C & Table 2). These REM-ON neurons (Fig. 5C1) showed a phasic firing pattern with burst discharge during REM sleep and low/silent activity during other behavioral states (AW, QW and NREM sleep). The mean firing rate during REM sleep was 5.06±1.21 and this was significantly higher than during QW (0.11±0.06; q= 5.56, p<0.05), NREM (0.20±0.06; q=4.53, p<0.05) or AW (0.20±0.07; q=5.88, p<0.05) (Fig. 5C1.1)
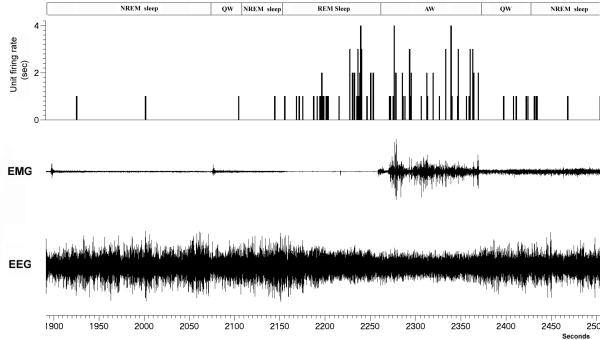
An example of a phasic (burst firing) REM-ON neuron is shown in figure 9. These neurons exhibited a broad spike shape (Fig. 10A) similar to that reported for REM-ON putative neurons in the PPT/LDT area by others (Koyama et al., 1994), These neuron also showed a longer response time of 18ms (Fig. 10B) to 1 Hz spinal stimulation. These neurons did not however follow the repetitive high frequency (300Hz) spinal stimulation train (Fig. 10C) suggesting that they do not project directly to the spinal cord but instead may be activated by an orthodromic response to spinal cord stimulation.
Figure 9. A REM-ON neuron in the PPT region.
This neuron showed burst firing (phasic activity) during REM sleep but was nearly silent during AW, QW and NREM sleep.
Figure 10. REM-ON phasic firing neuron in the PPT region showing orthodromic response to spinal cord stimulation.
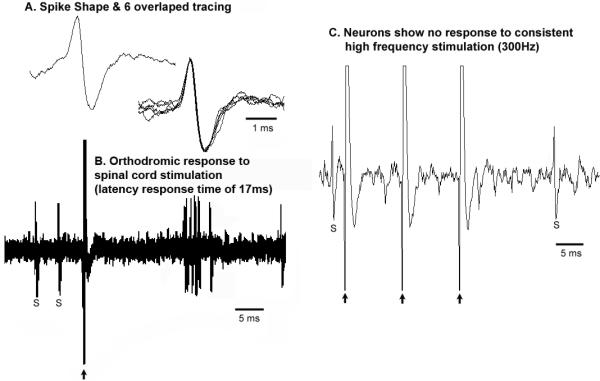
A, “Broad” spike shape (presumably a cholinergic neuron);
B, orthodromic response to spinal cord stimulation (latency response time of 17ms);
C, no response to trains of high frequency stimulation (300Hz).
Large arrow: stimulation artifact, S: spontaneous cell firing
The prominent presence of REM active neurons (=10 tonic + 12 phasic neurons / total 37 non-MLR neurons) indicates that the LPT contains tonic and phasic control network for REM sleep.
DISCUSSION
It has long been known that neurons of the dorsal pons can participate in motor control, although the precise neuroanatomic and neurochemical identity of this supraspinal motor network, the so-called MLR, has remained unresolved (Garcia-Rill et al., 1985; Mori et al., 1978; Shik et al., 1966). In the present study, we employed a retrograde tracing method to identify candidate pontine MLR neurons as well as unit recordings to characterize the locomotor- and state-dependent activities of presumptive non-cholinergic MLR and cholinergic PPT neurons. To this end, we characterized neurons based on their activity during active motor behaviors (which included: eating, foraging, grooming, paw licking, moving in the cage, and rearing) and during NREM sleep and REM sleep states. Our retrograde CTb-based labeling (Fig. 2A) revealed a group of non-cholinergic neurons, lying just medial to the PPT, which we predicted form the neuroanatomic basis of the MLR. In recording from these presumptive MLR neurons, we found that the preponderance of these neurons fired at a high phasic rate (>2 Hz) in relation to motor behaviors and that some of these neurons were also active during REM sleep (but less active than during active-wake) whereas non-reticulospinal neurons that likely include PPT cholinergic neurons fired in a slower tonic mode during active-wake and without relation to motor behaviors or otherwise fired at a high rate only during REM sleep.
Insofar as the authors are aware, the present study is the first to characterize the unit activity of dorsal pontine reticulospinal neurons in a freely behaving mammal. Our neuronal recordings have demonstrated that the firing activities of reticulospinal neurons are both locomotor- and behavioral state-dependent. More specifically, these neurons exhibited burst firing (phasic) activity that was correlated with high motor tone in antigravity muscles and which was irrespective of the nature of the motor behavior (i.e., during foraging, grooming, paw licking, moving in the cage, standing etc). Interestingly, the majority of these reticulospinal neurons (12/17) also showed higher firing activity during REM sleep. A similar pattern of phasic firing neurons from this general region has been reported from recordings in cats, monkey, rats and mice (Dormont et al., 1998; Matsumura et al., 1997; Siegel et al., 1981; Steriade et al., 1990; Thankachan et al., 2009; Weinberger et al., 2008); importantly, however, none of these previous studies correlated the phasic firing activity with motor behavior. In addition, no attempt has been made to distinguish between PPT cholinergic neurons and reticulospinal neurons, let alone their precise physiological role and function. This is an important point given that the phasic firing neurons may be related to the phasic components of active wake, such as motor activity itself. Our recording results clearly show a population of reticulospinal neurons lying medial to the PPT (based on post hoc histological analysis) whose firing rate is associated with motor behaviors and whose discharge rate is >2 Hz, both of which are strong indicators that these neurons are involved in the regulation of motor behaviors and therefore may represent the substrate of the MLR.
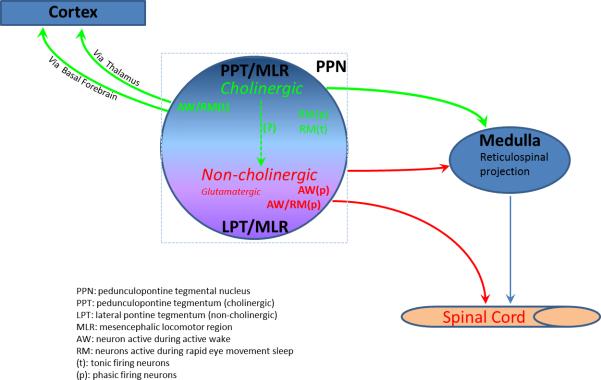
In a recently performed study in humans, neurons within the PPT were reported to be movement responsive, i.e., these neurons showed burst firing only during movement of arms or limb (Weinberger et al., 2008). Although these neurons were assumed to be cholinergic, our data would suggest these neurons are likely non-cholinergic as non-reticulospinal neurons do not show movement-associated firing pattern. As previously noted the role of cholinergic neurons in locomotor control is not clear but tracing studies have been unable to establish the existence of direct cholinergic projections from the PPT to the spinal cord, at least in mammals (Rye et al., 1988; Skinner et al., 1990). Since the MLR was not originally defined on the basis of specific neuronal subtypes, whether or not PPT cholinergic neurons constitute in part the neuroanatomic MLR remains unanswered. It is for example possible that PPT cholinergic neurons could control motor behavior via projections to the RVM or neighboring pontine reticulospinal neurons. Nevertheless, there are several lines of evidence that contradict a significant role for PPT cholinergic neurons in motor control. First, although cholinergic agonists in the RVM can induce stepping behaviors, the latency (10-15 min) and duration (5-10 sec) of these agonist-driven responses are long and short, respectively (Kinjo et al., 1990). Second, PPT cholinergic inputs to the RVM are relatively sparse (Rye et al., 1988; Rye et al., 1987; Skagerberg and Bjorklund, 1985). Third, RVM reticulospinal neurons are primarily GABAegric and project to both the dorsal and ventral horns (Hossaini et al., 2012; Morgan et al., 2008; Skagerberg and Bjorklund, 1985). Fourth, stimulation of the RVM appears to inhibit motor activity, which is consistent with a GABAergic projection (Hajnik et al., 2000). Fifth, in the present study, none of the non-reticulospinal neurons, including some of which are likely cholinergic neurons, showed any correlative firing patterns with motor behaviors. Finally, ibotenic acid lesions of the PPT do not produce cataplexy while lesions of non-cholinergic neurons by orexin-saporin in the LPT do (Fuller et al., 2011; Lu et al., 2006). Thus while more evidence is needed to definitely demonstrate that PPT cholinergic neurons are either a part of or otherwise modulate the activity of MLR neurons, we cannot at this time rule out the possibility that cholinergic PPT neurons and non-cholinergic reticulospinal neurons work together to control different aspects of motor behavior (see figure 11).
Figure 11. Pontine neural circuitry regulating motor behaviors and arousal.
PPT cholinergic neurons regulate arousal via ascending projections to the thalamus and basal forebrain and motor function via descending projections to the RVM (green color). The reticulospinal neurons (red color) regulate motor behavior via a direct projection to the spinal cord (red color).
One particularly intriguing and novel observation from the present study is that the majority of spinally-projecting neurons (n=12/17) show phasic activity during both AW and REM sleep, i.e., AW/REM active. This firing pattern has not been reported in earlier studies and may have important clinical implications (Dormont et al., 1998; Matsumura et al., 1997; Takakusaki et al., 1997; Weinberger et al., 2008). For example, during AW these neurons presumably contribute to the activation of spinal cord circuitry for motor behaviors, but during REM sleep this activating influence is inhibited by the sublaterodorsal tegmental nucleus (SLD) (Lu et al., 2006). If this were to be the case, understanding this circuit arrangement may have important implications for understanding the pathogenesis of REM sleep behavioral disorder and would merit further investigation.
The majority of non-spinally projecting neurons (25/37) are tonic firing neurons. As compared with the presumptive MLR neurons, these non-spinally-projecting neurons exhibited slow tonic firing (<2 Hz) during AW/REM sleep (N=15) or were only active during REM sleep (REM active/ REM-on) (N=10). As perhaps expected, the firing patterns of these non-spinally-projecting neurons showed no correlation with changes in motor behaviors, which included foraging, grooming, paw licking, moving in the cage, and standing. Post hoc histological verification showed these neurons were recorded within the region of the PPT (Fig. 2B). Similar firing patterns have been previously reported in this region in cat, rats and mice (El Mansari M. et al., 1989; Steriade et al., 1990; Thankachan et al., 2001; Thankachan et al., 2009) and likely represent thalamic projection neurons (Steriade and McCarely, 2005). Other studies, but from in vitro preparations, have shown that cholinergic neurons in the PPT/LDT have broad and narrow spike patterns that vary in firing rates and patterns. In our in vivo work, four of the tonic REM-active cells recorded from the LDT region had a broad spike pattern which was highly reminiscent of the pattern previously described for REM-ON putative cholinergic neurons in the LDT (Koyama et al., 1994). If these REM-ON neurons are indeed cholinergic, they are likely to be more critically involved in the regulation of REM sleep timing and not REM-related motor activity. Although both cholinergic and glutamatergic neurons in the PPT/LDT region project to the thalamus (Motts and Schofield, 2010), in the present study we were unable to definitely determine the neurochemical phenotype of these REM-active cells.
The remaining of the recorded non-spinally projecting neurons (12/37) exhibited a phenotype of phasic firing activity during REM sleep. These REM-active neurons also exhibited a very fast firing rate (>2Hz) and intermittent busting firing pattern with a broad spike shape. Some of these phasic REM-active neurons showed a delay response to spinal stimulation (>15ms). This is an interesting neuroanatomic feature of these neurons that has not been previously described. Since these neurons showed longer response times to spinal cord stimulation (orthodromic response) but no response to repetitive high frequency pulse, they probably do not project to spinal cord, but instead may receive input from the spinal cord. Future studies will be necessary to characterize the role of these REM-active neurons in REM sleep regulation, in particular in phasic REM phenomenon, such as rapid eye and tongue movements.
In summary, this single unit recording study has for the first time electrophysiologically and anatomically delineated a delimited node of dorsal pontine reticulospinal neurons that are linked to motor activity per se and whose locomotor- and state-dependent firing properties make them likely candidate neurons to form the neuroanatomic basis of the MLR. Clarifying the precise location and phenotype of these spinally-projecting neurons has long been a scientific and clinical goal as the potential implications for the treatment of motor disorders is significant. The in vivo evidence provided herein that the presumptive MLR neurons are distinct from cholinergic PPT neurons is a feature of potential major importance. For example, Karachi and colleagues (2010) have suggested that the PPT cholinergic neurons might play an important role in the control of gait and posture and possibly be a pharmacological target for gait disorder in Parkinson's disease (Karachi et al., 2010). Moreover, the PPT has been the principal target for deep brain stimulation (DBS) in the treatment of motor disorders in PD (Amara et al., 2011; Plaha and Gill, 2005; Stefani et al., 2007) and so understanding the in vivo role of PPT neurons in motor control is of clear clinical relevance. Interestingly, recent studies have shown that PPT-based DBS for the treatment of gait and posture disorder (Alam et al., 2011) also increases REM sleep (Lim et al., 2009). This side-effect of an increase in REM sleep is not surprising given that REM-active/REM-ON and putatively cholinergic neurons have been recorded in the PPT, including in the present study. On the basis of the recordings performed in the present study, we propose that the current clinical application of DBS may not be optimized with respect to efficacy given the evidence that non-cholinergic MLR neurons and not neighboring cholinergic PPT neurons appear to be the most directly linked to movement. Moreover, different stimulation paradigms may be warranted given the neuronal phenotype of these presumptive MLR neurons; this however will require further investigation.
Locomotor activity is modulated by supraspinal inputs
Stimulation of pontine reticular formation triggers locomotion in decerebrate animals
This region of the pons has been termed the “mesencephalic locomotor region” (MLR)
In this paper we characterize the neuroanatomic and electrophysiologic MLR
The MLR may be a clinically important target for treating movement disorders
ACKNOWLEDGEMENTS
We thank Quan Ha for technical assistance. This work was supported by National Institutes of Health Grants NS062727, NS061841, NS073613 and NS051609.
ABBREVIATIONS
- AW
Active wake
- ANOVA
one-way analysis of variance
- CTb
cholera toxin B subunit
- ChAT
choline acetyltransferase
- CnF
cuneiform nucleus
- DBS
deep brain stimulation
- EEG
electroencephalogram
- EMG
electromyogram
- LPT
lateral pontine tegmentum
- MLR
mesencephalic locomotor region
- (NREM) sleep
non-rapid eye movement
- PD
Parkinson's disease
- PPT
pedunculopontine tegmental nucleus
- PRF
pontine reticular formation
- PGO
ponto-geniculo-occipital wave
- QW
quiet wake
- (REM) sleep
rapid eye movement
- RVM
rostral ventromedial medulla
- SLD
sublaterodorsal tegmental nucleus
- VMM
ventromedial medulla
- vlPAG
ventrolateral periaquaductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain. 2011;134:11–23. doi: 10.1093/brain/awq322. [DOI] [PubMed] [Google Scholar]
- Amara AW, Watts RL, Walker HC. The effects of deep brain stimulation on sleep in Parkinson's disease. Ther Adv Neurol Disord. 2011;4:15–24. doi: 10.1177/1756285610392446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernau NA, Puzdrowski RL, Leonard RB. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 1991;557:83–94. doi: 10.1016/0006-8993(91)90119-g. [DOI] [PubMed] [Google Scholar]
- Cabelguen JM, Bourcier-Lucas C, Dubuc R. Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J Neurosci. 2003;23:2434–2439. doi: 10.1523/JNEUROSCI.23-06-02434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormont JF, Conde H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res. 1998;121:401–410. doi: 10.1007/s002210050474. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Fernandez JM, Sadaba F, Villaverde FJ, Alvaro LC, Cortina C. Cataplexy associated with midbrain lesion. Neurology. 1995;45:393–394. doi: 10.1212/wnl.45.2.393-a. [DOI] [PubMed] [Google Scholar]
- Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossaini M, Goos JA, Kohli SK, Holstege JC. Distribution of glycine/GABA neurons in the ventromedial medulla with descending spinal projections and evidence for an ascending glycine/GABA projection. PLoS One. 2012;7:e35293. doi: 10.1371/journal.pone.0035293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehericy S, Hirsch EC, Francois C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull. 1990;24:509–516. doi: 10.1016/0361-9230(90)90104-8. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Jodo E, Kayama Y. Sensory responsiveness of “broad-spike” neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–1031. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Cardiovascular and muscle tone changes produced by microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medial medulla. Brain Res. 1990;514:27–36. doi: 10.1016/0006-8993(90)90432-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, Lang AE, Wennberg RA, Murray BJ. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol. 2009;66:110–114. doi: 10.1002/ana.21631. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Thankachan S, Islam F. Differential responses of brain stem neurons during spontaneous and stimulation-induced desynchronization of the cortical eeg in freely moving cats. Sleep Res Online. 1998;1:132–146. [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res. 1997;28:155–165. doi: 10.1016/s0168-0102(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140:376–386. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M. Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol. 1978;41:1580–1591. doi: 10.1152/jn.1978.41.6.1580. [DOI] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Cholinergic and non-cholinergic projections from the pedunculopontine and laterodorsal tegmental nuclei to the medial geniculate body in Guinea pigs. Front Neuroanat. 2010;4:137. doi: 10.3389/fnana.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci. 1991;11:1691–1700. doi: 10.1523/JNEUROSCI.11-06-01691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Montagna P, Provini F, Bizzi A, Cohen M, Lugaresi E. Pontine lesions in idiopathic narcolepsy. Neurology. 1996;46:1250–1254. doi: 10.1212/wnl.46.5.1250. [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Sakai K, Jouvet M. Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus. Brain Res. 1980;194:500–505. doi: 10.1016/0006-8993(80)91231-7. [DOI] [PubMed] [Google Scholar]
- Sakai K, Koyama Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport. 1996;7:2449–2453. doi: 10.1097/00001756-199611040-00009. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Wheeler RL, McGinty DJ, Harper RM. Discharge pattern of reticular formation unit pairs in waking and REM sleep. Exp Neurol. 1981;74:875–891. doi: 10.1016/0014-4886(81)90260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Kinjo N, Henderson V, Garcia-Rill E. Locomotor projections from the pedunculopontine nucleus to the spinal cord. Neuroreport. 1990;1:183–186. doi: 10.1097/00001756-199011000-00001. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brainstem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarely RW. Brainstem control of wakefulness and sleep. Plenum; New York: 2005. [Google Scholar]
- Sukhotinsky I, Hopkins DA, Lu J, Saper CB, Devor M. Movement suppression during anesthesia: neural projections from the mesopontine tegmentum to areas involved in motor control. J Comp Neurol. 2005;489:425–448. doi: 10.1002/cne.20636. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shiroyama T, Kitai ST. Two types of cholinergic neurons in the rat tegmental pedunculopontine nucleus: electrophysiological and morphological characterization. Neuroscience. 1997;79:1089–1109. doi: 10.1016/s0306-4522(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, Koyama Y. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–1020. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankachan S, Islam F, Mallick BN. Role of wake inducing brain stem area on rapid eye movement sleep regulation in freely moving cats. Brain Res Bull. 2001;55:43–49. doi: 10.1016/s0361-9230(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Thankachan S, Kaur S, Shiromani PJ. Activity of pontine neurons during sleep and cataplexy in hypocretin knock-out mice. J Neurosci. 2009;29:1580–1585. doi: 10.1523/JNEUROSCI.5151-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Carbachol microinjections in the mediodorsal pontine tegmentum are unable to induce paradoxical sleep after caudal pontine and prebulbar transections in the cat. Neurosci Lett. 1991;130:41–45. doi: 10.1016/0304-3940(91)90222-f. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G. The spinal cord: a Christopher and Dana Reeve Foundation text and atlas. Elsevier/ Academic Press; Amsterdam; Boston: 2008. 2009. [Google Scholar]
- Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res. 2008;188:165–174. doi: 10.1007/s00221-008-1349-1. [DOI] [PubMed] [Google Scholar]