Abstract
Chitin exposure in the lung induces eosinophilia and alternative activation of macrophages, and is correlated with allergic airway disease. However, the mechanism underlying chitin-induced polarization of macrophages is poorly understood. Here, we show that chitin induces alternative activation of macrophages in vivo, but does not do so directly in vitro. We further show that airway epithelial cells bind chitin in vitro and produce CCL2 in response to chitin both in vitro and in vivo. Supernatants of chitin exposed epithelial cells promoted alternative activation of macrophages in vitro, whereas antibody neutralization of CCL2 in the supernate abolished the alternative activation of macrophages. CCL2 acted redundantly in vivo, but mice lacking the CCL2 receptor, CCR2, showed impaired alternative activation of macrophages in response to chitin, as measured by arginase I, CCL17 and CCL22 expression. Furthermore, CCR2KO mice exposed to chitin had diminished ROS products in the lung, blunted eosinophil and monocyte recruitment, and impaired eosinophil functions as measured by expression of CCL5, IL13 and CCL11. Thus, airway epithelial cells secrete CCL2 in response to chitin and CCR2 signaling mediates chitin-induced alternative activation of macrophages and allergic inflammation in vivo.
Keywords: CCL2, CCR2, chitin, macrophage, eosinophil, epithelial, airway, lung, asthma, allergy
INTRODUCTION
Exposure to fungal- and insect-related antigens early in life are a significant risk factor in the development of allergy and asthma (1). Besides intrinsic protease activity present in many allergens, the additional presence of pathogen-associated molecular patterns (PAMP) such as lipopolysaccharide and β-glucan contributes to the allergenicity of inhaled environmental allergens (2, 3). Common to the cell wall of fungi and the exoskeleton of insects and crustaceans is the polysaccharide chitin, a linear chain of β-1,4-N-acetylglucosamine sugars. Chitin is among the most abundant polymers on our planet and is recognized by, and elicits responses from, organisms across the kingdoms of life. As an example, N-acetylglucosamine oligosaccharides trigger antifungal immune responses in plants via specific chitin receptors that share homology with mammalian chitnases and chitinase-like proteins (4, 5). In humans, elevated chitin exposure in the workplace and at home is correlated with asthma and other allergic diseases (6–8). Furthermore, murine models have demonstrated that exposure to chitin particles results in innate allergic inflammation characterized by alternatively activated macrophages and eosinophilia (9–11). Thus, chitin represents a potential allergy promoting PAMP with a significant public health impact.
Among airway immune cells, macrophages are “ambidextrous” cells capable of initiating or suppressing inflammatory responses (12). In response to environmental or microbial exposures, macrophages are polarized into phenotypically distinct activation states: classical (M1) or alternative (M2). M2 macrophages antagonize pro-inflammatory Th1 responses and are associated with the development of Th2-associated inflammation central to the pathogenesis of allergy and asthma. Although chitin exposure induces M2 polarization in vivo (9, 10), macrophages exposed to chitin in vitro fail to acquire an M2 phenotype and instead secrete TNF-α (13–16). Thus, the disparity of polarization between macrophages exposed to chitin in vitro and in vivo suggests that an intermediary is required for chitin-induced M2 polarization in vivo.
Airway epithelial cells are the initial point of contact for inhaled allergens and coordinate with pulmonary dendritic cells (DC) to induce Th2 responses central to the pathogenesis of asthma. In a model of house dust mite (HDM) induced asthma, activation of epithelial cells was required for the subsequent development of allergic responses in the airway (2). Here, we investigated the intermediary cells and products that facilitate chitin-induced M2 polarization and allergic airway inflammation. We demonstrate that airway epithelial cells produce CCL2 (monocyte chemotactic protein-1, MCP-1) in response to chitin and that the CCL2 receptor CCR2 is required for chitin-induced M2 polarization and allergic inflammation in vivo.
MATERIAL AND METHODS
Mice
CCR2 knockout (stock# 004999), CCL2 knockout (stock# 004434) mice, aged 5–8 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 wild type mice (strain code 01C55), aged 5–8 weeks, were obtained from NCI (Frederick, MD). For all experiments, five mice were used per group. Mice were housed and cared for according guidelines from the University of Wisconsin Animal Care and Use Committee, who approved this work.
Reagents and cell culture
Chitin purified from crab shells was purchased from Sigma (C9752). AMJ2-C11 murine macrophages (CRL-2456) and LA-4 murine lung epithelial cells (CCL-196) were obtained from ATCC. AMJ2-C11 cells were maintained in RPMI with 10% heat inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin (complete RPMI). LA-4 cells were maintained in F-12 Ham’s media with 15% FBS and 1% penicillin/streptomycin. Anti-CCL2 neutralizing antibody (MAB479) and mouse cytokine antibody array (ARY006) were purchased from R&D Systems.
Chitin purification
Ground chitin particles were dissolved in 12.5M HCl and incubated for 30 minutes at 40°C with frequent agitation. The solution was transferred to a cooled beaker and slowly neutralized with ice cold NaOH. The insoluble fraction was collected and washed in H2O three times followed by a wash in ethanol. The purified chitin particles were then dried in a speedvac before storage at −20°C. Before use, chitin particles were resuspended in endotoxin-free PBS, sonicated, then filtered through a 10μm nylon filter. Protein was less than 2% by mass as determined by BCA assay. Endotoxin levels as measured by Pyrogent Plus assay (Lonza) were less than 0.03EU/mL.
Binding Assays
For chitin binding assays only, chitin particles less than 10μm were labeled with flourescein isothiocyante (FITC) as previously described (17). For all other exposures, unlabeled chitin particles were used. AMJ2-C11 macrophages or LA-4 lung epithelial cells were incubated with chitin particles over varying doses and time intervals. Cell samples were washed three times, fixed with 0.5% paraformaldehyde, and analyzed by fluorescence microscopy. All binding assays were performed at 4°C. Binding index is calculated as number of particles/100 cells.
Administration of Chitin
Mice were anesthetized via an intraperitoneal injection of etomidate. The anesthetized mouse was then suspended from their front incisors and intubated using a BioLite Intubation System (18). Chitin particles were suspended at indicated concentrations in 20μL PBS and administered via the intubation tube into the airway.
Bronchoalveolar Lavage
At varied intervals after chitin administration, mice were anesthetized with isoflurane and euthanized by exsanguination. 1mL of PBS/2mM EDTA was administered via intratracheal catheter to lavage the airway. BALF was placed on ice prior to centrifugation at 300 × g for 5 minutes. Cell-free supernatants were frozen at −20°C for later analysis. The BALF cell pellet was resuspended in PBS/1%BSA for analysis by flow cytometry or lysed for RNA extraction. For isolation of alveolar macrophages, the BALF cell pellet was resuspended in PBS/1%BSA, incubated with anti-mouse CD11c-biotin antibody (BD Bioscience) on ice for 30 minutes, washed, and incubated with streptavidin coated magnetic beads (BD Bioscience). For chemokine secretion experiments, isolated CD11c+ cells were resuspended in RPMI with 10% FBS and 1% penicillin/streptomycin and incubated overnight in 24-well plates at 37°C.
Mouse Airway Epithelial Cell Isolation
Airway epithelial cells were isolated as previously described (19) with slight modifications. The pulmonary vasculature was perfused with 5mL PBS via the right ventricle and Liberase (Roche) was instilled via a catheter placed in the trachea, followed by 1mL of 1% low melt agarose (ISCBioExpress). The animal was placed on ice to harden the agarose prior to removing the lungs, which were then placed in 2mL Liberase solution for 1 hour at room temperature. Lungs were then teased apart with forceps in DMEM with 1% penicillin/streptomycin (Hyclone) and shaken for 10 minutes at 200r/min. The resulting suspension was filtered through a 40μm filter, centrifuged at 250 × g for 10 minutes. After centrifugation, the cells were stained with anti-mouse CD45-biotin antibody (eBioscience) on ice for 30 minutes, washed, and incubated with streptavidin coated magnetic beads (BD Bioscience) for CD45+ cell depletion. The CD45-depleted suspension was stained with anti-mouse CD326-PE antibody (eBioscience) on ice for 20 minutes, washed, and incubated with anti-PE coated magnetic beads (BD Bioscience). The CD326+ cells were resuspended in DMEM with 10% FBS and 1% penicillin/streptomycin and incubated overnight in 6-well plates at 37°C. A portion of the cells was analyzed by flow cytometry to assess purity.
Measurement of chemokines
Following overnight incubation at 37°C, cell-free supernatants were collected and stored at −20°C for later analysis by ELISA. ELISA kits for CCL2, CCL17, CCL22, and Ym1 were obtained from R&D Systems.
Flow Cytometry
Lung cell suspensions were prepared by mincing lungs through a 70μm filter using a 3mL syringe plunger. The resulting homogenate was digested with Liberase/DNAse I and the red blood cells were lysed with ammonium chloride/potassium bicarbonate buffer. All samples were blocked with anti-mouse CD16/32 antibody prior to staining with fluorochrome-conjugated antibodies. Events were gated on FSC/SSC parameters to exclude debris and on live cells based on Violet Fixable Live-Dead stain (Molecular Probes) Fluorochrome-conjugated antibodies used were Mac-3-FITC, Siglec F-PE, CD90.2-PerCP-Cy5.5, CD11b-PECy7, Ly6C-APC, CD11c-Alexa700, Ly6G-APC-Cy7. Antibodies were obtained from BD Biosciences, eBioscience, and Biolegend. Cells were collected for analysis on a BD Biosciences LSRII cytometer and data analyzed with FloJo software (Tree Star).
Real-Time PCR
Total RNA was isolated from lung homogenates or isolated cell populations using a Qiagen RNeasy mini kit and cDNA was prepared using the BioRad iScript cDNA synthesis kit. cDNA was amplified using BioRad SSoFast EvaGreen Supermix in a BioRad MyIQ Real Time PCR detection system. Primers were designed using Primer-BLAST (20). Relative transcript quantity was calculated using the comparative Ct method with β-actin transcript as the control transcript (21).
ROS measurement
The presence of reactive oxygen species (ROS) in cell-free BALF was assessed using 2′-7′-dichlorofluorescin diacetate (DCF), which becomes fluorescent after exposure to peroxidase and hydrogen peroxide (22). 80μL of BALF or PBS was added to 120μL of DCF/HBSS solution in 96-well black opaque plates. After a 20-minute incubation in the dark, fluorescence intensity above an empty control 96-well black plate was measured using a Bio-Rad Versadoc5000MP imaging system.
Statistics
The means of fold change in transcript, chemokine protein content, or numbers of leukocytes were compared to control using an unpaired t-test with a two-tailed p-value < 0.05 considered statistically significant. For experiments where percent reduction vs. wild type mice were reported, a one-sample t-test was used to compare values to a hypothetical value of zero (i.e. no change) with a p-value < 0.05 considered statistically significant. All statistical analysis was performed using Prism software (Graph Pad). In all figures, error bars represent the standard deviation of the data.
RESULTS
Interaction of chitin with macrophages in vitro and in vivo
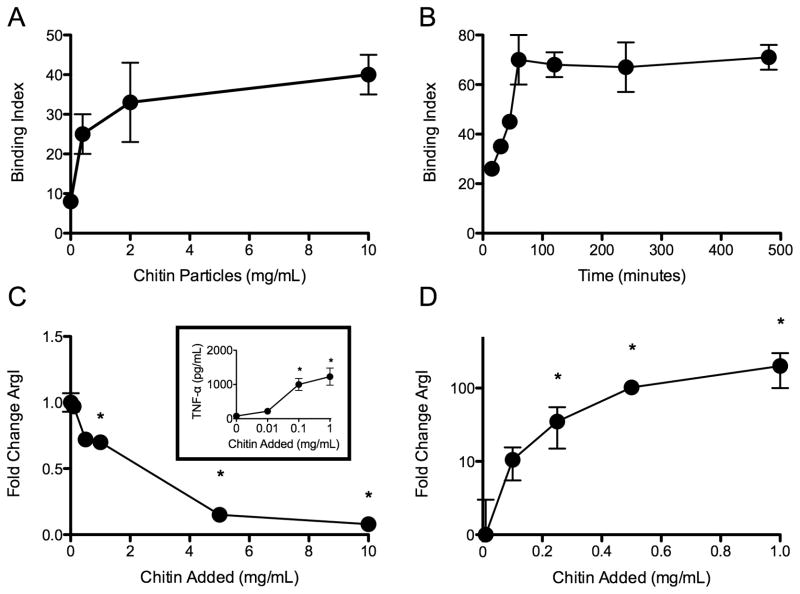
To understand the direct effect of chitin exposure on macrophages, we investigated the binding of chitin particles to macrophages and the activation profile of the cells after they were exposed to chitin particles in vitro. AMJ2-C11 macrophages bound FITC-labeled chitin particles in a dose- and time-dependent, saturable manner (Fig 1A, 1B). Because FITC may alter surface binding properties of particles, we confirmed our findings with unlabeled chitin particles that were stained after binding with the chitin selective dye Uvitex 2B. To assess the activation state of macrophages exposed to chitin, we measured expression of arginase I (ArgI), a canonical product of the M2 phenotype (23, 24). Chitin particles failed to induce ArgI expression in AMJ2-C11 macrophages, but did activate the cells and induce production of TNF-α, a marker of classical activation in vitro (Fig 1C). Chitin also failed to induce ArgI expression in alveolar macrophages in vitro (data not shown). Because the context of chitin exposure likely affects the activation state of macrophages, we also evaluated the activation of macrophages in vivo. Accordingly, ArgI expression was strongly induced in the lung following intratracheal exposure to chitin particles (Fig 1D). Thus, chitin promotes disparate macrophage activation states in vitro and in vivo and the cells exhibit an M2 phenotype only in vivo.
Figure 1. Macrophages bind chitin and become M2 polarized in vivo but not in vitro.
(A) Dose-dependent, and (B) time-dependent binding of chitin particles by AMJ2-C11 macrophages. Binding was performed at 4°C in vitro (n=3). Chitin-induced ArgI expression (C) and release of TNF-α [inset] in vitro (n=3), and (D) in vivo (n=3). *P<0.05 vs. no chitin control.
Chitin binds to airway epithelial cells and elicits CCL2 in vitro
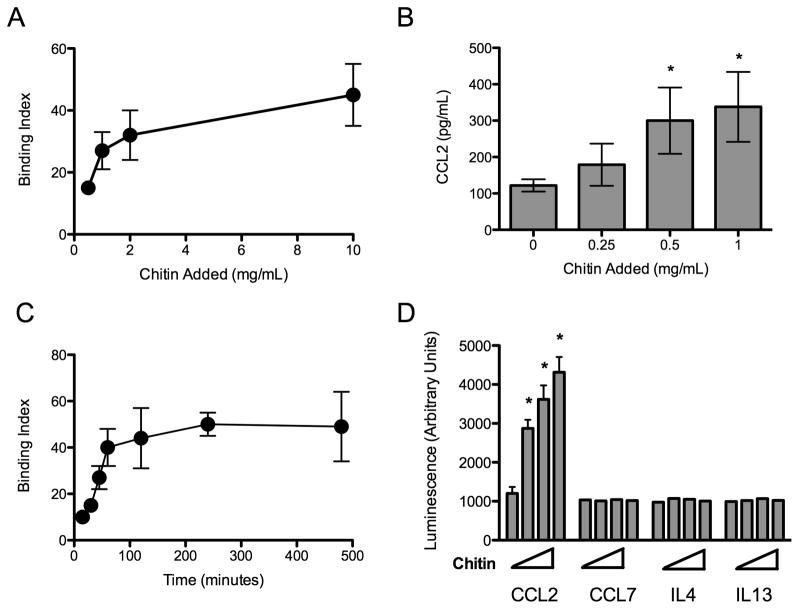
Due to the apparent dichotomy of macrophage activation observed above, we hypothesized that chitin was alternatively activating macrophages indirectly in vivo. Epithelial cells form a barrier to particles and high molecular weight molecules in the airway and are activated by chitin-containing allergens such as house dust mite (2, 3). To test whether chitin interacts with airway epithelial cells, we exposed LA-4 murine epithelial cells to FITC-labeled chitin particles in vitro. LA-4 cells bound FITC-labeled chitin particles in a dose- and time-dependent, saturable manner (Fig 2A, 2B). Unlabeled chitin particles bound to LA-4 epithelial cells in a similar fashion. To determine if LA-4 cells were activated by exposure to chitin particles, we used a proteomic array to investigate chitin-induced release of cytokine and chemokine into the cell supernate. Exposure to chitin particles induced a dose-dependent increase in CCL2 secretion, but did not induce any of the other 39 targets in the array (Fig 2C). Notably, chitin exposure to LA-4 epithelial cells failed to induce IL-4 or IL-13 production, cytokines that promote the alternative activation of macrophages. In addition, CCL7, which shares a receptor with CCL2, was also not produced by chitin-exposed epithelial cells in vitro. We confirmed the dose-dependent increase in CCL2 production by chitin-exposed epithelial cells by measuring CCL2 in epithelial cell supernatants in amounts ranging up to 300 pg/ml (Fig 2D).
Figure 2. Airway epithelial cells bind chitin and produce CCL2 following chitin exposure.
(A) Dose-dependent and (B) time-dependent binding of chitin particles to LA-4 epithelial cells. Binding was performed at 4°C in vitro (n=3). (C) Chitin induced CCL2, CCL7, IL-4, and IL-13 production (n=3). Chitin added in amounts of 0, 0.25, 0.5, and 1.0 mg/mL is represented by the crescendo. (D) CCL2 production by LA-4 cells 16 hours following chitin exposure (n=3). *P<0.05 vs. no chitin control.
Chitin exposed airway epithelial cells alternatively activate macrophages in a CCL2-dependent manner in vitro
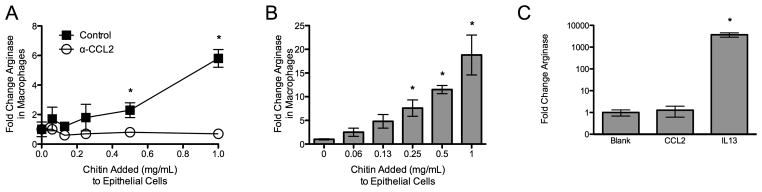
To further investigate whether chitin-exposed epithelial cells could promote the alternative activation of macrophages, we incubated AMJ2-C11 macrophages in supernatants from chitin-exposed LA-4 cells. Supernatants from chitin-exposed epithelial cells resulted in a chitin dose-dependent increase in ArgI expression in the macrophages (Fig 3A). Given our observation that chitin induces CCL2 secretion from epithelial cells in vitro, and prior work that demonstrated an impairment in alternative activation of macrophages in CCL2-deficient animals (25, 26), we investigated whether CCL2 in epithelial cell supernatant is required for alternative activation of macrophages in vitro. We used a CCL2-neutralizing antibody to block the product in chitin-exposed epithelial cell supernatants added to the macrophages. In the presence of neutralizing CCL2 antibody, chitin-exposed epithelial cell supernatant lost the ability to induce ArgI expression in macrophages (Fig 3B). However, unlike recombinant IL-13, recombinant CCL2 alone was not sufficient to induce ArgI expression in macrophages (Fig 3C). Therefore, CCL2 is necessary but not sufficient to mediate ArgI expression in macrophages exposed to chitin-stimulated epithelial cells in vitro.
Figure 3. Epithelial cell CCL2-dependent M2 polarization in vitro.
(A) ArgI expression in macrophages cultured in chitin-exposed epithelial cell supernatant for 16 hours (n=5, *P<0.05 vs. no chitin added). (B) Experiment in panel B done in the presence of 100μg/mL CCL2 neutralizing or isotype control antibody (n=3, *P<0.05). (C) Effect of recombinant CCL2 or IL13 (50ng/mL) on AMJ2-C11 macrophage ArgI expression in vitro (n=3, *P<0.05 vs. PBS).
Chitin induces CCL2 from airway epithelial cells in vivo
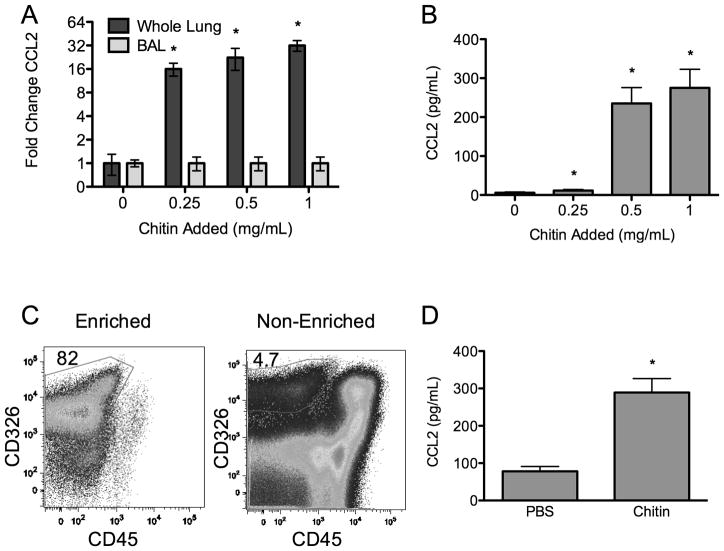
Next, we investigated whether CCL2 is produced by lung epithelial cells after exposure to chitin particles in vivo. Following chitin administration into the airway, we found a dose-dependent increase in CCL2 expression in whole lung homogenates (Fig 4A). While CCL2 protein in cell-free BALF was increased in a dose-dependent fashion by chitin exposure (Fig 4B), we found no increase in CCL2 in the BALF cell pellet (Fig 4A), suggesting a lung parenchymal source of CCL2. To investigate whether lung epithelial cells produce CCL2 in response to chitin exposure, we isolated these cells from chitin-and PBS control-exposed lungs. We achieved a nearly 20-fold enrichment of epithelial cells from whole lung homogenates by depleting hematopoietic cells (CD45+) and selecting for cells expressing the epithelial marker CD326 (EpCAM) (27) (Fig 4C). Following overnight culture of cells ex vivo, the CD45+ cell populations from chitin-exposed mice did not increase CCL2 production. In contrast, CD326+ enriched epithelial cells from chitin-exposed mice secreted significantly more CCL2 than PBS control-exposed mice (Fig 4D). Hence, CD326+ epithelial cells represent a source of CCL2 in vivo following chitin exposure in the airway.
Figure 4. Chitin induces epithelial cell CCL2 secretion in vivo.
(A) Chitin induced CCL2 transcript expression in whole lung homogenate and BAL and (B) CCL2 protein level in BAL (n=4, *P<0.05). (C) To verify an epithelial source of CCL2, CD326+ cells were isolated from chitin- or PBS-exposed lungs. The purity of cell separation was assessed by flow cytometry. (D) Chitin-exposed CD326+ cells cultured overnight ex vivo following isolation secrete produce CCL2 (n=3, *P<0.05 vs. PBS).
Chitin induced alternative activation of macrophages is CCR2 dependent in vivo
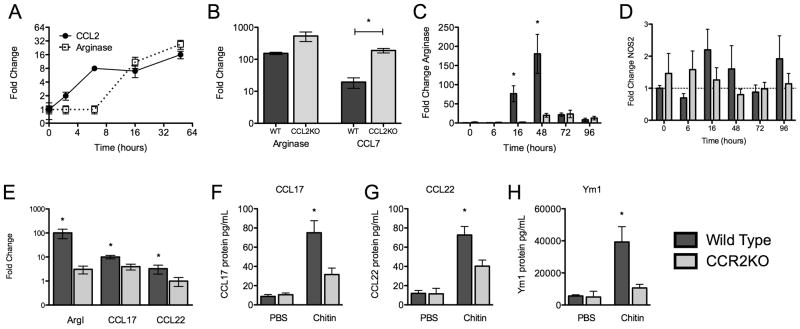
To see if CCL2 plays a role in chitin-induced alternative activation of macrophages in vivo, we analyzed the expression kinetics of CCL2 and the alternative activation marker ArgI in the lung. CCL2 is expressed as early as 2 hours following exposure to chitin while ArgI is not expressed until 16 hours (Fig 5A), temporally supporting a role for CCL2 in the induction of ArgI. To determine whether CCL2 is necessary for ArgI expression in the lung, we administered chitin to the airways of CCL2KO mice. We found no impairment in Arg I expression. However, we observed that CCL2KO mice had a significant compensatory increase in CCL7 expression in response to chitin (Fig 5B).
Figure 5. Chitin induces CCR2-dependent M2 polarization in vivo.
(A) Kinetics of ArgI and CCL2 expression following chitin exposure in vivo (n=3). (B) ArgI and CCL7 expression in wild type and CCL2KO mice (n=2, *P<0.05). (C) Chitin induction of ArgI expression in whole lung in wild-type and CCR2KO mice (n=3; *P<0.05, WT vs. CCR2KO). (D) Chitin induction of NOS2 expression in whole lung in wild-type and CCR2KO mice (n=3; *P<0.05, WT vs. CCR2KO). (E) Chitin-induction of ArgI, CCL17 and CCL22 expression in CD11c+ cells from BALF of wild-type and CCR2KO mice (n=2; *P<0.05, WT vs. CCR2KO). (F–H) Chitin induced CCL17, CCL22, and Ym1 protein from CD11c+ cells (n=2; *P<0.05, WT vs. CCR2KO).
Because CCL7 signals through the same receptor as CCL2 – that is, CCR2 (28, 29) – and contributes to CCR2-mediated activity in parallel with CCL2 (30), we investigated whether CCR2 is required for chitin-induced ArgI expression in vivo. Whole lung homogenates showed significantly delayed and reduced ArgI expression after chitin exposure in CCR2KO mice compared to wild-type mice (Fig 5C). To address whether the defect in M2 polarization in chitin-exposed CCR2KO mice was due to an increase in M1 polarization, we measured NOS2 expression in whole lung homogenates. Both wild-type and CCR2KO mice failed to demonstrate an increase in the M1 polarization marker NOS2 following exposure to chitin (Fig 5D). To determine if the CCR2-dependent reduction in ArgI expression occurred in alveolar macrophages, and therefore reflected an impaired M2 phenotype, we isolated and analyzed CD11c+ cells from BAL. Isolated CD11c+ cells were >95% CD11blo/Mac-3+, indicating their identity as macrophages rather than dendritic cells. CD11c+ cells from CCR2KO mice had significantly lower levels of ArgI expression than did wild-type mice (Fig 5E). To substantiate a defect in M2 polarization in CCR2KO mice, we also measured the expression of other M2 signature chemokines in isolated CD11c+ cells. Both CCL17 and CCL22 were expressed at significantly lower levels in CD11c+ cells from CCR2KO mice compared to WT mice (Fig 5E). Similarly, CD11c+ cells isolated from the BALF of chitin-exposed CCR2KO mice secreted less CCL17, CCL22, and Ym1 following overnight incubation ex vivo than did CD11c+ cells isolated from the BAL of chitin-exposed wild type mice (Figure 5F–H). Therefore, CCR2 signaling is required for chitin-induced M2 polarization in the lung.
Chitin exposure elicits neutrophilic and eosinophilic inflammation and recruits monocytes in a CCR2-dependent manner
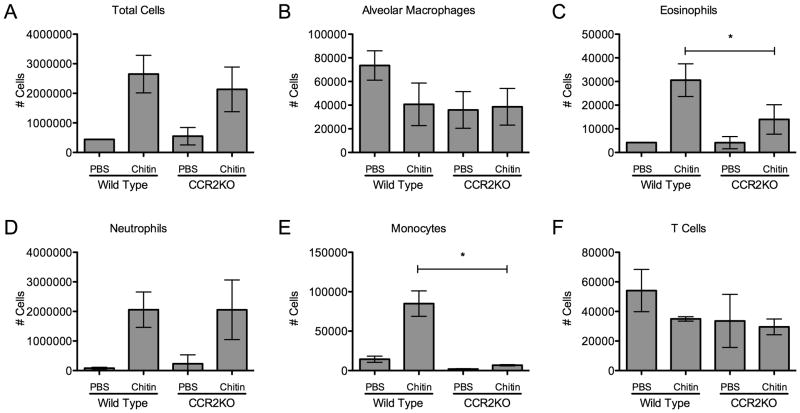
To characterize and compare the allergic inflammatory response in the lungs of wild-type and CCR2KO mice, we quantified leukocyte subsets in whole lung homogenates following chitin exposure. Overall cell numbers, influx of CD11c+/Mac3+/CD11blo macrophages, Ly6Ghi neutrophils, and Thy-1+ T-cells were all unaffected in CCR2KO mice (Fig 6A,B,D, and F). However, recruitment of CD11b+/Ly6Chi monocytes was dependent on CCR2 in response to chitin exposure (Fig 6E). Furthermore, chitin-induced recruitment of SiglecF+/CD11c− eosinophils was significantly reduced in CCR2KO mice (Fig 6C). Thus, CCR2 was linked functionally to the recruitment of eosinophils into the lung following exposure to chitin.
Figure 6. Chitin-induced eosinophil and monocyte recruitment is CCR2 dependent.
Cell numbers in leukocyte subsets in whole lung homogenate 48 hours after chitin exposure in wildtype and CCR2KO mice (n=3, *P<0.05 WT vs. control). (A) Total live cells. (B) CD11c+/CD11blo/Mac3+ Alveolar Macrophages. (C) Siglec F+/CD11c−/CD11b+ Eosinophils. (D) Ly6G+/SSChi/CD11b+ Neutrophils. (E) CD11b+/Ly6Chi/Ly6G− monocytes. (F) Thy1+ T cells.
Eosinophils have an activation defect in chitin-exposed CCR2 deficient mice
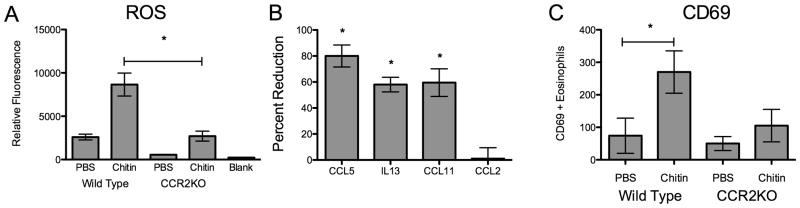
Because we found alterations in the numbers of eosinophils and other innate immune cell recruited to the lung, we investigated whether eosinophil function and other inflammatory parameters in CCR2KO mice were otherwise altered in their response to chitin exposure. Exposure to allergens increases the production of ROS in the airway (31, 32), however the role of CCR2 in allergen or chitin-induced ROS production remains undefined. BAL obtained from chitin-exposed mice converted significantly more of the redox-sensitive H2DCF dye to fluorescent DCF in WT mice as compared to CCR2KO mice (Fig 7A). To further investigate eosinophil function during innate allergic inflammation in chitin-exposed mice, we sorted these cells by FACS and analyzed the expression of products associated with eosinophil activation (33). Sorted SiglecF+/CD11c− eosinophils from whole lung homogenates of chitin-exposed animals showed that CCR2KO mice had 60-to-80% less expression of CCL5, IL13, and CCL11 than similarly exposed WT mice (Fig 7B), and also had reduced surface expression of the activation marker CD69 (Fig 7C). Thus, CCR2 is required for recruitment, activation and function of allergic innate immune cells such as eosinophils after chitin exposure in vivo.
Figure 7. Chitin induced ROS production eosinophil activation is CCR2 dependent.
(A) ROS levels in BALF from wildtype and CCR2KO animals 48 hours after chitin exposure (n=3, *P<0.05 WT vs CCR2KO). Siglec F+/CD11c- lung eosinophils were sorted by FACS from CCR2KO mice vs. wildtype mice 48 hours after chitin exposure. (B) Percent reduction of eosinophil CCL5, IL13, and CCL11 expression in CCR2KO vs. WT mice(n=3, *P<0.05). (C) Number of CD69+ eosinophils isolated from mouse lungs 48 hours following exposure. (n=3, *P<0.05 vs. PBS).
DISCUSSION
Our work provides new insight into the cellular mechanisms that drive M2 polarization during innate allergic inflammation in response to chitin. Our study suggests that macrophages are not M2 polarized directly on exposure to chitin in the lung. Instead, we demonstrate that chitin stimulates CCL2 production from airway epithelial cells, and we establish a pivotal role for CCR2 in chitin-induced macrophage polarization and innate allergic inflammation in the airway. Isolated CD326+ cells from chitin-exposed lungs and LA-4 epithelial cells exposed to chitin in vitro produced elevated levels of CCL2. Compared to WT mice, CCR2KO mice exposed to chitin demonstrated reduced expression of M2 markers (ArgI, CCL17 and CCL22), eosinophil recruitment and eosinophil activation in the lung. Thus, we propose that the respiratory epithelium modulates M2 polarization of macrophages upon chitin exposure.
Airway epithelial cells are among the earliest cells exposed to inhaled substances and actively collaborate with immune cells to mount innate and adaptive responses. Because chitin is associated with many inhaled allergens, we hypothesize that chitin recognition by airway epithelial cells may promote an epithelial pro-allergy program to otherwise innocuous agents via secretion of CCL2. Multiple lines of evidence underscore the association of CCL2 with asthma and allergic airway disease. Elevated CCL2 levels are present in BALF obtained from individuals with asthma (34) and CCL2 is elicited upon airway allergen challenge in humans (35). In animal models, OVA/alum sensitization and challenge provokes increased CCL2 expression (36). Similarly, chitin-associated cockroach (37) or Aspergillus fumigatus antigens (38) also provoke CCL2 production in animal asthma models. Our findings suggest that epithelial cells may be an important source of CCL2 during exposure to chitin-containing allergens, thereby promoting M2 polarization.
Consistent with a role for airway epithelial cell CCL2 in allergic responses, chitin exposure promotes M2 polarization in vivo via an indirect mechanism (10) that is dependent on CCR2. We propose that airway epithelial cell-derived CCL2 is a key chemokine in promoting M2 polarization and innate allergic inflammation in response to chitin. Indeed, CCL2 expression precedes ArgI expression in chitin-exposed lungs. Following chitin exposure, antibody neutralization of CCL2 in chitin-exposed epithelial cell supernatants also inhibits M2 polarization in vitro. Airway epithelial cells are an important source of CCL2 in viral infections associated with asthma exacerbations (39, 40). Our findings that CCL2 promotes M2 polarization in a setting of chitin-induced allergic inflammation are consistent with previous reports demonstrating a role for CCL2 in tumor- (41) and thermal injury- (25) associated M2 polarization.
We observed that epithelial cell CCL2 is necessary for M2 polarization in response to chitin in vitro, however CCL2 was dispensable for M2 polarization in vivo. The upregulation of the CCR2 ligand, CCL7, in CCL2-deficient mice following chitin exposure may obscure the necessary role of CCL2 in chitin-induced M2 polarization we observed in vitro. To address this issue, we investigated the host response to chitin in a CCR2-deficient mouse. In the absence of CCR2, chitin exposure failed to elicit alternatively activated macrophages in the lung. Our results reflect the established central role of CCR2 in mediating the effects of both CCL2 and CCL7 in vivo. For example, in a model of infection with the fungal pathogen Histoplasma capsulatum, CCR2KO mice demonstrated increased susceptibility to the fungus and an increased fungal burden in the lung (42). Neutralization of either CCL2 or CCL7 was not sufficient to increase the fungal burden, however neutralization of both chemokines did. Similarly, CCR2KO mice are more susceptible to Listeria monocytogenes infection whereas CCL2KO or CCL7KO mice demonstrate an intermediate susceptibility phenotype (30). Moreover, M2 polarization canonically depends on IL4Rα and its ligands IL4 and IL13. While neither CCL7 nor IL4 or IL13 were detected following epithelial cell exposure to chitin in vitro, all three products are present in the lung following chitin exposure in vivo. Our work does not preclude a role for IL-4 in chitin induced innate allergic inflammation. However, future studies should address whether the CCL2-CCR2 axis and IL-4Rα signalling represent linear, convergent, or parallel signaling pathways in chitin-initiated M2 polarization.
CCR2KO mice also demonstrate reduced eosinophil recruitment and activation in response to chitin, further suggesting a role for CCR2 in eosinophilic inflammation. Murine eosinophils contain CCR2 mRNA (43) and may express low levels of CCR2 protein on their cell surface (44). However, this is unlikely to explain the reduction in eosinophil recruitment in CCR2KO mice as eosinophils do not migrate in response to CCL2 (45) and migrate toward CCL7 via a CCR3-dependent mechanism (46). Chitin induced eosinophil recruitment is abrogated in mice lacking the receptor for LTB4 (9), suggesting a link between eosinophil recruitment and leukotriene signaling following chitin exposure. Because chitin fails to activate eosinophils directly (47), the defect in activation and recruitment in CCR2KO mice must be linked to upstream factors. M2 macrophages elicited by chitin exposure are potent sources of LTB4 (9). Thus, the reduction in chitin induced eosinophil recruitment in CCR2KO mice may be due to the reduction in M2 polarization.
CCR2 is required for egress (48) and homing (49) of monocytes to sites of inflammation. We show a near-complete reduction of Ly6Chi monocytes recruited to the lung following chitin exposure in CCR2KO mice. Ly6Chi monocytes may differentiate into CD11b+ inflammatory DCs, which accumulate in the allergic airway and are critical mediators of Th2 adaptive immune response in the airway (50). Recently, CCR2+Ly6Chi monocytes were reported to recruit eosinophils to colonic mucosa via CCL11 in an experimental colitis model (51). The partnering of Ly6Chi monocytes with M2 macrophages to recruit and/or activate eosinophils could represent an intriguing interaction to therapeutically target in order to reduce eosinophilic inflammation during asthma and allergy.
In conclusion, we show that airway epithelial cells secrete CCL2 and that CCR2 signaling is required for events leading to M2 polarization and recruitment and activation of eosinophils upon chitin exposure. The findings clarify the mechanisms that drive alternative activation of macrophages in response to chitin, and identify possible targets of therapeutic intervention in the setting of chitin induced innate allergic inflammation.
Acknowledgments
Supported by funds from the American Asthma Foundation (BK) and from the National Institutes of Health grants # T32ES007015 and F30ES019048 (RR).
We thank Marlene Klaila and Titilayo Omobesi for excellent animal care, Dr. Tristan Brandhorst for assistance in preparing and analyzing chitin used in these studies and Dr. Rebecca Brockman-Schneider and Dr. James Gern for advice and assistance with epithelial cell experiments.
Abbreviations
- ArgI
Arginase I
- BALF
Bronchoalveolar lavage fluid
- CCL
CC Chemokine ligand
- CCR
CC Chemokine receptor
- DCF
2′-7′-dichlorofluorescin diacetate
- H2DCF
Reduced 2′-7′-dichlorofluorescin diacetate
- M2
Alternatively activated macrophage
- DC
Dendritic cell
- WT
Wild type
References
- 1.Ahluwalia SK, Matsui EC. The indoor environment and its effects on childhood asthma. Curr Opin Allergy Clin Immunol. 2011;11:137–143. doi: 10.1097/ACI.0b013e3283445921. [DOI] [PubMed] [Google Scholar]
- 2.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–37760. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 5.Ohnuma T, Onaga S, Murata K, Taira T, Katoh E. LysM domains from Pteris ryukyuensis chitinase-A: a stability study and characterization of the chitin-binding site. J Biol Chem. 2008;283:5178–5187. doi: 10.1074/jbc.M707156200. [DOI] [PubMed] [Google Scholar]
- 6.Howse D, Gautrin D, Neis B, Cartier A, Horth-Susin L, Jong M, Swanson MC. Gender and snow crab occupational asthma in Newfoundland and Labrador, Canada. Environ Res. 2006;101:163–174. doi: 10.1016/j.envres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua AA. Fungal exposure modulates the effect of polymorphisms of chitinases on emergency department visits and hospitalizations. Am J Respir Crit Care Med. 2010;182:884–889. doi: 10.1164/rccm.201003-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 11.Kogiso M, Nishiyama A, Shinohara T, Nakamura M, Mizoguchi E, Misawa Y, Guinet E, Nouri-Shirazi M, Dorey CK, Henriksen RA, Shibata Y. Chitin particles induce size-dependent but carbohydrate-independent innate eosinophilia. J Leukoc Biol. 2011;90:167–176. doi: 10.1189/jlb.1110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol. 2004;31:3–7. doi: 10.1165/rcmb.f279. [DOI] [PubMed] [Google Scholar]
- 13.Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, Levitz SM. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182:3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Tsuji S, Yamashita M, Henriksen RA, Myrvik QN, Shibata Y. Phagocytosis of N-acetyl-D-glucosamine particles, a Th1 adjuvant, by RAW 264.7 cells results in MAPK activation and TNF-alpha, but not IL-10, production. Cell Immunol. 2006;239:103–112. doi: 10.1016/j.cellimm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Shinohara T, Pantuso T, Tsuji S, Yamashita M, Shinohara S, Myrvik QN, Henriksen RA, Shibata Y. Depletion of cellular cholesterol enhances macrophage MAPK activation by chitin microparticles but not by heat-killed Mycobacterium bovis BCG. Am J Physiol Cell Physiol. 2008;295:C341–9. doi: 10.1152/ajpcell.00446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamacher J, Arras M, Bootz F, Weiss M, Schramm R, Moehrlen U. Microscopic wire guide-based orotracheal mouse intubation: description, evaluation and comparison with transillumination. Lab Anim. 2008;42:222–230. doi: 10.1258/la.2007.006068. [DOI] [PubMed] [Google Scholar]
- 19.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 20.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 21.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 22.Keston AS, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigematsu K, Asai A, Kobayashi M, Herndon DN, Suzuki F. Enterococcus faecalis translocation in mice with severe burn injury: a pathogenic role of CCL2 and alternatively activated macrophages (M2aMphi and M2cMphi) J Leukoc Biol. 2009;86:999–1005. doi: 10.1189/jlb.0409235. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. CCL2, a product of mice early after systemic inflammatory response syndrome (SIRS), induces alternatively activated macrophages capable of impairing antibacterial resistance of SIRS mice. J Leukoc Biol. 2004;76:368–373. doi: 10.1189/jlb.1203645. [DOI] [PubMed] [Google Scholar]
- 27.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szymczak WA, Deepe GSJ. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol. 2009;183:1964–1974. doi: 10.4049/jimmunol.0901316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye YL, Wu HT, Lin CF, Hsieh CY, Wang JY, Liu FH, Ma CT, Bei CH, Cheng YL, Chen CC, Chiang BL, Tsao CW. Dermatophagoides pteronyssinus 2 regulates nerve growth factor release to induce airway inflammation via a reactive oxygen species-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2011;300:L216–24. doi: 10.1152/ajplung.00165.2010. [DOI] [PubMed] [Google Scholar]
- 33.Rose CEJ, Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010;7:361–374. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 35.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–1383. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez-A C, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell EM, I, Charo F, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/−mice: the role of mast cells. J Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- 38.Blease K, Mehrad B, Lukacs NW, Kunkel SL, Standiford TJ, Hogaboam CM. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia. J Immunol. 2001;166:1832–1842. doi: 10.4049/jimmunol.166.3.1832. [DOI] [PubMed] [Google Scholar]
- 39.Renois F, Jacques J, Talmud D, Deslee G, Leveque N, Andreoletti L. Respiratory echovirus 30 and coxsackievirus B5 can induce production of RANTES, MCP-1 and IL-8 by human bronchial epithelial cells. Virus Res. 2010;152:41–49. doi: 10.1016/j.virusres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deepe GSJ, Wuthrich M, Klein BS. Progress in vaccination for histoplasmosis and blastomycosis: coping with cellular immunity. Med Mycol. 2005;43:381–389. doi: 10.1080/13693780500245875. [DOI] [PubMed] [Google Scholar]
- 43.Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 44.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira SH, Lira S, Martinez-A C, Wiekowski M, Sullivan L, Lukacs NW. Increased responsiveness of murine eosinophils to MIP-1beta (CCL4) and TCA-3 (CCL1) is mediated by their specific receptors, CCR5 and CCR8. J Leukoc Biol. 2002;71:1019–1025. [PubMed] [Google Scholar]
- 46.Chung IY, Kim YH, Choi MK, Noh YJ, Park CS, Kwon DY, Lee DY, Lee YS, Chang HS, Kim KS. Eotaxin and monocyte chemotactic protein-3 use different modes of action. Biochem Biophys Res Commun. 2004;314:646–653. doi: 10.1016/j.bbrc.2003.12.134. [DOI] [PubMed] [Google Scholar]
- 47.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robays LJ, Maes T, Lebecque S, Lira SA, Kuziel WA, Brusselle GG, Joos GF, Vermaelen KV. Chemokine receptor CCR2 but not CCR5 or CCR6 mediates the increase in pulmonary dendritic cells during allergic airway inflammation. J Immunol. 2007;178:5305–5311. doi: 10.4049/jimmunol.178.8.5305. [DOI] [PubMed] [Google Scholar]
- 51.Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, Hogan SP. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993–6003. doi: 10.4049/jimmunol.1003844. [DOI] [PMC free article] [PubMed] [Google Scholar]