Abstract
In kidney transplant recipients, cardiovascular disease (CVD) is the leading cause of death. The relationship of kidney function with CVD outcomes in transplant recipients remains uncertain. We performed a post-hoc analysis of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial to assess risk factors for CVD and mortality in kidney transplant recipients. Following adjustment for demographic, clinical and transplant characteristics, and traditional CVD risk factors, proportional hazards models were used to explore the association of estimated GFR with incident CVD and all-cause mortality. In 4016 participants, mean age was 52 years and 20% had prior CVD. Mean eGFR was 49±18 mL/min/1.73m2. In 3,676 participants with complete data, there were 527 CVD events over a median of 3.8 years. Following adjustment, each 5 mL/min/1.73m2 higher eGFR at levels below 45 mL/min/1.73m2 was associated with a 15% lower risk of both CVD [HR = 0.85 (0.80, 0.90)] and death [HR = 0.85 (0.79, 0.90)], while there was no association between eGFR and outcomes at levels above 45 mL/min/1.73m2. In conclusion, in stable kidney transplant recipients, lower eGFR is independently associated with adverse events, suggesting that reduced kidney function itself rather than pre-existing comorbidity may lead to CVD.
Keywords: Kidney Transplant, Glomerular Filtration Rate, Cardiovascular Disease, Chronic Kidney Disease, Epidemiology, Mortality
INTRODUCTION
In kidney transplant recipients, cardiovascular disease (CVD) is the leading cause of mortality, accounting for 30% of deaths among recipients who die with a functioning transplant (1). Reduced kidney function, typically quantified as estimated glomerular filtration rate (eGFR), is an independent risk factor for CVD in the general population, in part, reflecting the close association of CVD risk factors and glomerular filtration rate (GFR). The relationship of GFR to CVD risk in transplant recipients may differ following transplantation, because the level of GFR may no longer reflect lifelong exposure to CVD risk factors (2). Accordingly, better understanding of the epidemiology and risk factors for CVD in this population is critically important to identify higher risk transplant recipients and potentially treat modifiable risk factors.
Several retrospective studies have assessed CVD outcomes in kidney transplant recipients. To date, the association between GFR and subsequent CVD remains uncertain, with most existing data drawn from administrative registries and clinical databases, suggesting an association between kidney function and CVD events in transplant recipients (3–4). Recently, the PORT Study, a retrospective registry of 23,575 incident kidney transplant recipients from 14 transplant centers, demonstrated that traditional CVD risk factors, non-traditional CVD risk factors, and transplant-specific factors predicted future CVD events in this population (3), while, in a subset of the PORT registry, lower estimated GFR (eGFR) 1-year post-transplant was associated with increased mortality (5). Similarly, a retrospective single center study in Belgium evaluated over 2,000 kidney transplant recipients who survived at least 1 year following transplantation and noted that traditional CVD risk factors and transplant-specific risk factors were associated with future CVD events (6). Large prospective studies with rigorous ascertainment of CVD events systematically evaluating the association between kidney function and CVD outcomes in transplant recipients are limited to a post hoc analysis of 1052 participants in the Assessment of Lescol in Renal Transplantation (ALERT) Study that described age, diabetes, electrocardiogram changes and worse allograft function as factors associated with fatal CVD (7–8).
Accordingly, we analyzed data from the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial cohort, a randomized study examining the effect of B-vitamin therapy on CVD and mortality outcomes in 4110 prevalent, stable kidney transplant recipients (9), in order to explore risk factors for CVD and all-cause mortality in kidney transplant recipients, specifically focusing on the relationship between eGFR and outcomes.
METHODS
Study Design
The current report is a post hoc analysis of FAVORIT (clinicaltrials.gov: NCT00064753), a multi-center double-blind randomized controlled clinical trial conducted to determine whether lowering homocysteine levels with vitamin therapy reduced the rate of pooled arteriosclerotic CVD outcomes. FAVORIT was approved by the Institutional Review Boards at the participating institutions (see acknowledgements), and methods are described in detail elsewhere (9–10). FAVORIT randomized 4110 stable kidney transplant recipients from August 2002 through January 2007 to either a standard multivitamin with high doses of folic acid (5 mg), vitamin B6 (pyridoxine; 50 mg) and vitamin B12 (cyanocobalamin; 1 mg) versus a multivitamin containing low doses of vitamin B6 (1.4 mg) and vitamin B12 (2 µg) with no folic acid. Follow-up contacts occurred every six months through January 31, 2010 to obtain study related outcomes through June 24, 2009. As there were no differences between randomized groups in study outcomes, we combined both groups for these analyses.
Study population
Men and women age 35 to 75 years who were at least 6 months post-kidney transplant were screened for eligibility at 30 transplant centers in the United States, Canada and Brazil. Recruitment in Brazil began later in the study than recruitment at United States and Canadian sites. Entry criteria included stable kidney function, initially defined by an estimated creatinine clearance ≥ 30 mL/min in both men and women and redefined after July 7, 2005 in women only as ≥ 25 mL/min, and elevated serum homocysteine level (≥ 11 µmol/L for women; ≥ 12 µmol/L for men).
Serum Creatinine and eGFR
Analyses used serum creatinine data drawn at the baseline study visit and assayed from frozen sera available in 4016 (98.0%) participants following a single thaw. Serum creatinine was measured in 2011 at the FAVORIT central laboratory at the United States Department of Agriculture Jean Mayer Human Nutrition Research Center on Aging at Tufts University using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc, Center Valley, PA) instrument that is calibrated to an isotope dilution mass spectrometry traceable standard. GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, a newer estimating equation with better performance at higher GFR levels (11–12). An eGFR of 60–74 ml/min per 1.73 m2 was selected as the reference group for categorical analyses, since this range is the highest level of measured GFR expected for a single kidney, and higher eGFR values may reflect decreased creatinine generation, which may be associated with increased mortality (13–15).
Other Baseline Variables
Baseline characteristics at study enrollment included demographics (age, sex, race, country of origin); smoking status (current, former or never); past medical history (baseline CVD, diabetes mellitus); transplant characteristics (living donor kidney, time since transplant); physical examination findings (body mass index (BMI), systolic and diastolic blood pressure); and laboratory variables (serum creatinine, homocysteine, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides). Baseline blood pressure was the average of two measurements and hypertension was defined by systolic blood pressure ≥140 mm Hg, diastolic ≥90 mm Hg or antihypertensive medication use at study enrollment. Diabetes was defined by the use of insulin or oral hypoglycemic medications or patient history. Baseline CVD was defined as prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower extremity arterial revascularization. Race was defined as white, black, or other, with individuals who identified as ‘other’ classified as white for eGFR estimation. The 27 individuals missing data on race were defined as white for GFR estimation. Body mass index was calculated using the formula: weight [kg]/ height [m]2. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald equation at triglyceride levels below 400 and measured in the 234 participants with triglyceride levels above 400 mg/dL (16).
Study Outcomes
The primary CVD outcome is a composite of CVD death, myocardial infarction, resuscitated sudden death, stroke, coronary revascularization, or peripheral, carotid, aortic or renal artery procedures. The first four components of this primary outcome were centrally reviewed and adjudicated by the FAVORIT Clinical Endpoints Committee; the remaining outcomes were identified through medical record abstraction. The Clinical Endpoints Committee also reviewed records for unstable angina cases and urgent coronary revascularization procedures in search of myocardial infarctions that were not identified by the clinical site staff. The secondary outcome is all-cause mortality. To account for semi-competing risks, we also examined a composite outcome of CVD and all-cause mortality. For primary analyses, participants were not censored at the time of return to dialysis or at re-transplantation.
Statistical Analyses
Linear regression for continuous variables and chi-square tests for categorical variables were used to compare baseline data by eGFR group. Kaplan-Meier survival analysis was used to estimate the survival function among study participants by eGFR level. Cox proportional-hazards regression was used to examine the association between baseline covariates and time to study outcomes. Parsimonious models were a priori adjusted for age, sex, race, diabetes, prior CVD, treatment allocation and country of origin, while, reflecting the available data in FAVORIT, extended multivariable models were a priori further adjusted for smoking, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, triglyceride level, transplant vintage, and living versus deceased donor kidney. Non-violation of the proportional hazards assumption was assessed through visual examination of the log-log plot of survival. Several potentially clinically important interaction terms with kidney function were tested, including with prior CVD and with transplant vintage. Two sensitivity analyses were performed. The first examined the relationship of eGFR and other covariates with the composite CVD outcome and the all-cause mortality outcomes, censoring 3-months after return to dialysis. The second included total homocysteine in extended models. All analyses were performed using SAS 9.2. Dr. Carpenter had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
RESULTS
Among 4110 enrolled participants, 94 were excluded for missing baseline creatinine, leaving 4,016 participants described in Table 1. Mean age was 52 (SD, 9) years; 37% were women, 40% diabetic and 20% had a history of CVD. Mean eGFR was 48.9 (SD, 17.7) mL/min/1.73m2 and median time since kidney transplant was 4.0 (25th–75th percentile, 1.7–7.4) years. Individuals with lower eGFR were older, had longer duration since transplant and higher systolic blood pressure, were more often white and women, and were more likely to have a history of CVD. HDL cholesterol was significantly lower and triglycerides significantly higher in participants with lower eGFR (Table 1).
Table 1.
Baseline characteristics for the FAVORIT cohort, stratified by eGFR category.
| eGFR mL/min/1.73m2 | |||||||
|---|---|---|---|---|---|---|---|
| <30 (N = 515) |
30–45 (N = 1354) |
45–60 (N = 1209) |
60–75 (N = 614) |
>=75 (N = 324) |
Overall (N = 4016) |
p-value | |
| Age (SD), yr | 52.8 ± 9.1 | 52.4 ± 9.4 | 51.8 ± 9.4 | 51.4 ± 9.8 | 49.9 ± 9.3 | 51.9 ± 9.4 | <0.0001 |
| Women, n (%) | 237 (46) | 573 (39) | 446 (35) | 205 (33) | 74 (28) | 1496 (37) | <0.0001 |
|
Race, n (%) White |
400 (78) | 1038 (77) | 931 (77) | 436 (72) | 210 (65) | 3015 (76) | <0.0001 |
| Black | 75 (15) | 222 (17) | 198 (16) | 122 (20) | 89 (28) | 706 (18) | |
| Other | 37 (7) | 84 (6) | 73 (6) | 51 (8) | 23 (7) | 268 (5) | |
|
Treatment Group, n (%) High Dose Vitamin |
259 (50) | 689 (51) | 600 (50) | 309 (50) | 152 (47) | 2009 (50) | 0.78 |
| Low Dose Vitamin | 256 (50) | 665 (49) | 609 (50) | 305 (50) | 172 (53) | 2007 (50) | |
|
Location, n (%) United States |
375 (73) | 1012 (75) | 865 (72) | 427 (70) | 245 (76) | 2924 (73) | <0.0001 |
| Canada | 89 (17) | 170 (13) | 127 (11) | 65 (11) | 29 (9) | 480 (12) | |
| Brazil | 51 (10) | 172 (13) | 217 (18) | 122 (20) | 50 (15) | 612 (15) | |
| Median Graft Vintage (25th–75th), yr | 5.2 (1.9–9.5) | 4.3 (1.9–8.1) | 3.7 (1.6–7.0) | 3.4 (1.7–6.5) | 3.1 (1.3–6.1) | 4.0 (1.7–7.4) | <0.0001 |
| Living Donor Kidney, n (%) | 178 (35) | 556 (41) | 563 (47) | 263 (43) | 112 (35) | 1672 (41) | <0.0001 |
| History of CVD, n (%) | 125 (24) | 280 (21) | 222 (18) | 112 (18) | 58 (18) | 797 (20) | 0.03 |
| History of Diabetes, n (%) | 204 (40) | 544 (40) | 485 (40) | 252 (41) | 136 (42) | 1621 (40) | 0.96 |
|
Smoking, n (%) Current |
62 (12) | 168 (13) | 113 (9) | 66 (11) | 35 (11) | 444 (11) | 0.11 |
| Former | 216 (42) | 491 (37) | 490 (41) | 252 (41) | 129 (40) | 1578 (40) | |
| Never | 233 (46) | 679 (51) | 593 (50) | 291 (48) | 155 (49) | 1951 (49) | |
| Prevalent Hypertension, n (%) | 493 (96) | 1267 (94) | 1100 (91) | 553 (90) | 283 (88) | 3696 (92) | <0.0001 |
| Systolic Blood Pressure (SD), mm Hg | 138.2 ± 21.0 | 136.2 ± 19.6 | 136.1 ± 19.8 | 135.5 ± 18.9 | 133.0 ± 18.8 | 136.0 ± 19.7 | 0.0009 |
| Diastolic Blood Pressure (SD), mm Hg | 77.6 ± 12.6 | 78.1 ± 12.2 | 79.0 ± 12.4 | 79.0 ± 11.6 | 78.8 ± 11.5 | 78.5 ± 12.2 | 0.02 |
| Body Mass Index (SD), kg/m2 | 30.6 ± 6.6 | 29.3 ± 6.1 | 29.0 ± 6.3 | 28.4 ± 5.9 | 28.4 ± 6.5 | 29.2 ± 6.2 | <0.0001 |
| Total Cholesterol (SD), mg/dL | 189.4 ± 51.1 | 184.7 ± 43.9 | 184.8 ± 43.8 | 183.6 ± 40.1 | 177.9 ± 39.4 | 184.6 ± 44.0 | 0.002 |
| HDL Cholesterol (SD), mg/dL | 44.6 ± 14.8 | 45.6 ± 13.7 | 46.5 ± 13.8 | 48.0 ± 14.4 | 46.7 ± 12.8 | 46.2 ± 13.9 | 0.0001 |
| LDL Cholesterol (SD), mg/dL | 102.7 ± 39.3 | 100.7 ± 35.0 | 101.5 ± 33.8 | 101.2 ± 31.3 | 97.6 ± 31.3 | 101.0 ± 34.4 | 0.17 |
| Triglycerides (SD), mg/dL | 240 ± 372 | 204 ± 149 | 192 ± 128 | 178 ± 116 | 175 ± 111 | 199 ± 183 | <0.0001 |
| Total Homocysteine (SD), µmol/L | 20.5 ± 8.5 | 17.8 ± 6.1 | 16.3 ± 5.8 | 15.5 ± 5.3 | 15.3 ± 4.3 | 17.1 ± 6.3 | <0.0001 |
| Serum Creatinine (SD), mg/dL | 2.6 ± 0.6 | 1.9 ± 0.3 | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.7 ± 0.6 | <0.0001 |
| eGFR (SD), mL/min/1.73m2 | 24.9 ± 4.1 | 37.8 ± 4.2 | 52.0 ± 4.3 | 66.5 ± 4.2 | 88.0 ± 10.7 | 48.9 ± 17.7 | <0.0001 |
Values are means unless otherwise indicated. SD, standard deviation. History of cardiovascular disease (CVD) defined by myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and lower extremity arterial revascularization.
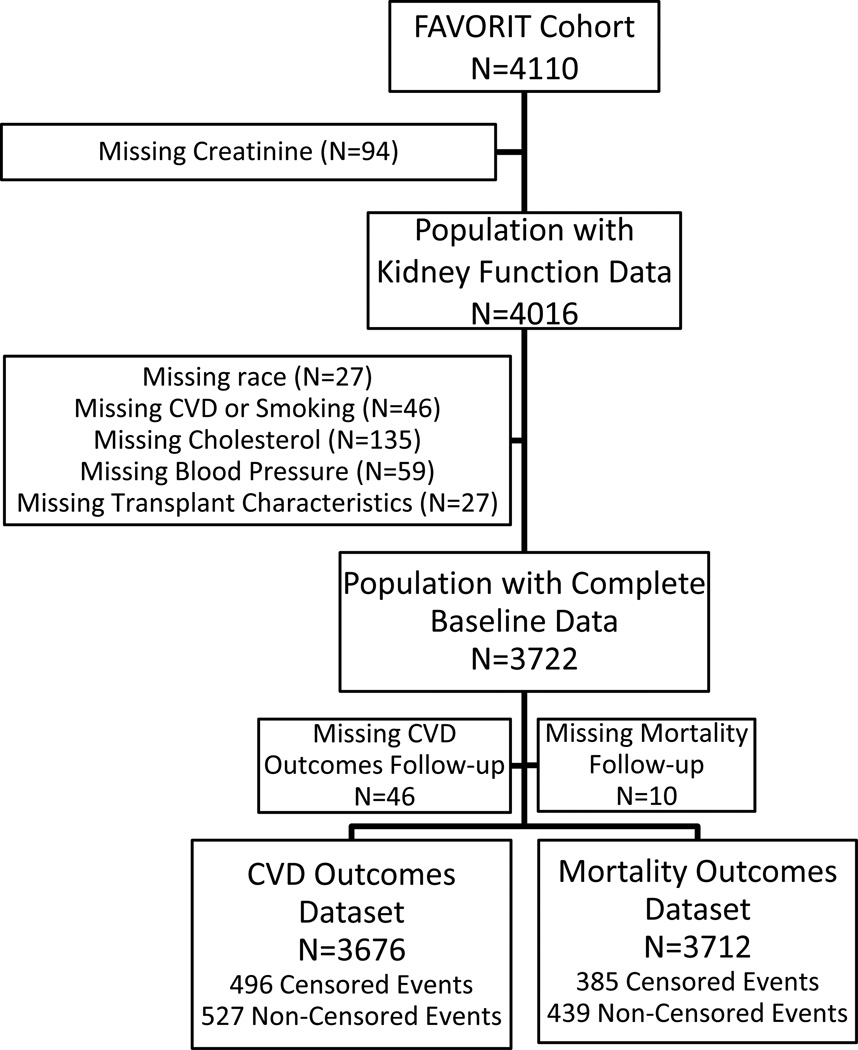
The final population with complete data for CVD analyses included 3,676 (91.5%) participants; of the initial 4,016 participants with eGFR results, 27 were excluded for missing race, 46 for loss to follow-up, 46 for missing baseline clinical data (history of CVD or smoking), 135 for missing cholesterol data, 57 for missing blood pressure or BMI, and 27 for missing data on transplant characteristics (Figure 1). Participants excluded for missing data were similar to included participants with the exception of significantly fewer participants from Brazil being excluded (data not shown). Adjusted mortality analyses included 3,714 participants as an additional 36 participants had data on vital status at study completion. Administrative censoring occurred on June 24, 2009, at which time 2,788 participants remained enrolled in the trial, 493 participants were deceased, 822 had incomplete follow-up, and 7 participants had no follow-up.
Figure 1.
Flowchart of FAVORIT cohort members. Censored analyses indicate individuals for whom event ascertainment ceased at 3 months post-dialysis initiation.
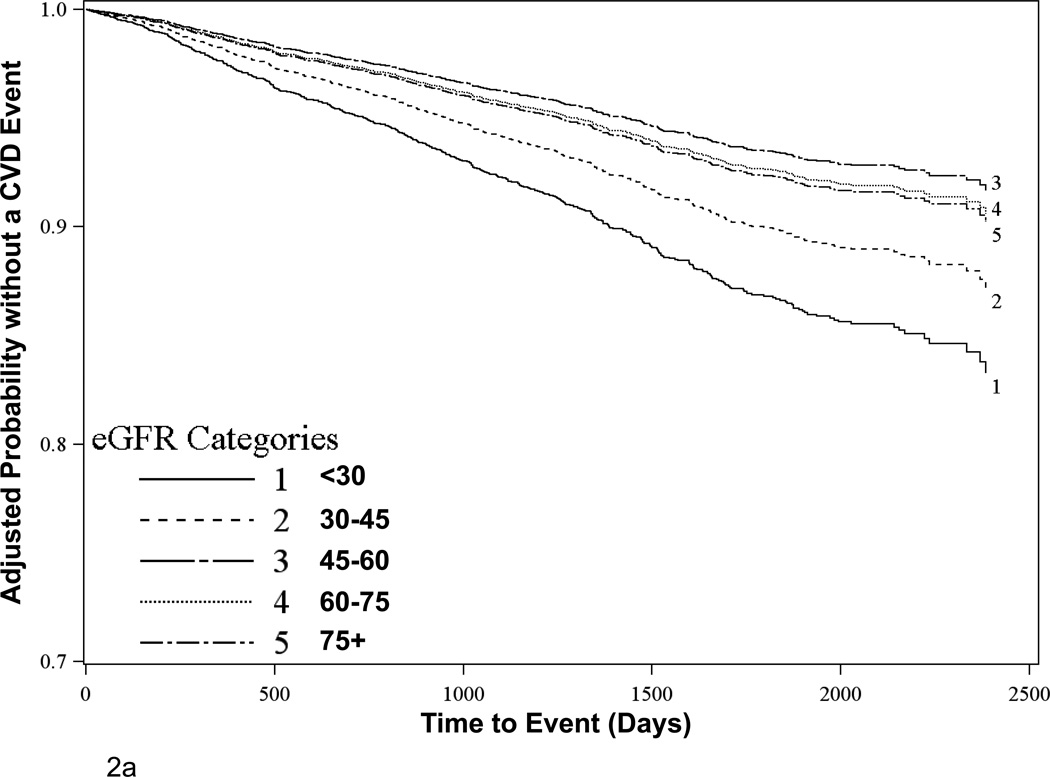
There were 584 CVD events and 493 deaths, with 37 CVD events and 62 deaths occurring more than 3 months after dialysis-dependent recurrent kidney failure. Among the 3,676 participants with complete data, there were 527 CVD events over mean follow-up time of 3.8 (SD 1.6) years (comprising the primary composite endpoint), with a higher proportion of events occurring at lower eGFR levels. When eGFR was examined as a categorical variable in clinically relevant groups, risk increased only with progressively lower eGFR levels below 45 mL/min per 1.73m2 (Table 2, Figure 2a). Accordingly, in parsimonious Cox proportional hazards models adjusting for age, sex, race, treatment assignment, country, baseline CVD and diabetes, each 5 mL/min per 1.73m2 higher eGFR for eGFR levels below 45 mL/min per 1.73m2 was associated with a 17% lower risk of a CVD event [Hazard Ratio (HR) = 0.83 (0.78, 0.88), p<0.0001] while there was no relationship appreciated for eGFR levels above 45 [HR = 1.01 (0.97, 1.05), p=0.74]; this effect was unchanged in extended multivariable models [HR = 0.85 (0.80, 0.90), p<0.0001 for each 5 mL/min per 1.73m2 rise at eGFR below 45 mL/min per 1.73m2 and HR = 1.01 (0.96, 1.05), p=0.74 for each 5 mL/min per 1.73m2 rise at eGFR above 45 mL/min per 1.73m2]. Other factors significantly associated with greater CVD risk in transplant recipients included several traditional CVD risk factors, such as older age, prior CVD, diabetes, current smoking, and higher systolic blood pressure. Other traditional CVD risk factors, including sex, LDL cholesterol and triglycerides were non-significant, while HDL cholesterol was of borderline statistical significance and lower BMI and lower diastolic blood pressure were associated with increased CVD risk. The impact of eGFR on CVD outcomes was comparable to that of systolic blood and age. Interactions for eGFR with prior CVD and with transplant vintage were non-significant (p=0.37 and 0.83, respectively). The breakdown of events comprising the CVD outcome is shown in Table 3 along with event-specific risk by baseline eGFR.
Table 2.
Multivariable models for CVD, all-cause mortality and a composite of CVD and all-cause mortality
| Cardiovascular Disease | All-Cause Mortality | Composite | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95 % CI) | P-value | Hazard Ratio (95 % CI) | P-value | Hazard Ratio (95 % CI) | P-value | ||
| eGFR | <30 | 1.85 (1.35–2.54) | <0.0001 | 2.05 (1.45–2.90) | <0.0001 | 1.69 (1.27–2.42) | |
| 30–44 | 1.39 (1.05–1.84) | 1.34 (0.98–1.84) | 1.36 (1.06–1.74) | ||||
| 45–59 | 0.88 (0.65–1.20) | 1.02 (0.73–1.43) | 0.99 (0.76–1.29) | <0.0001 | |||
| 60–74 | reference | reference | reference | ||||
| 75+ | 1.04 (0.69–1.57) | 1.07 (0.68–1.69) | 1.04 (0.73–1.50) | ||||
| Age | 1.13 (1.08–1.19) | <0.0001 | 1.22 (1.16–1.29) | <0.0001 | 1.19 (1.14–1.25) | <0.0001 | |
| Male Sex | 1.16 (0.95–1.41) | 0.16 | 0.93 (0.75–1.15) | 0.50 | 1.04 (0.88–1.23) | 0.66 | |
| Race | White | reference | 0.25 | reference | 0.93 | reference | |
| Black | 0.81 (0.64–1.04) | 1.05 (0.81–1.36) | 0.86 (0.69–1.07) | 0.11 | |||
| Other/Mixed | 0.92 (0.62–1.35) | 0.99 (0.66–1.50) | 1.25 (0.92–1.71) | ||||
| Country | USA | reference | 0.22 | reference | 0.10 | reference | |
| Canada | 0.81 (0.60–1.10) | 0.75 (0.53–1.05) | 0.79 (0.61–1.02) | 0.16 | |||
| Brazil | 0.80 (0.56–1.14) | 1.25 (0.85–1.84) | 0.88 (0.65–1.18) | ||||
| Low-dose Vitamin | 0.96 (0.81–1.15) | 0.68 | 0.99 (0.82–1.20) | 0.92 | 1.04 (0.90–1.21) | 0.59 | |
| CVD | 2.06 (1.71–2.48) | <0.0001 | 1.48 (1.20–1.82) | 0.0002 | 1.78 (1.51–2.10) | <0.0001 | |
| Diabetes | 2.30 (1.90–2.80) | <0.0001 | 1.88 (1.53–2.31) | <0.0001 | 2.10 (1.78–2.48) | <0.0001 | |
| Smoking | Never | reference | reference | reference | |||
| Former | 1.10 (0.91–1.33) | 0.07 | 1.26 (1.03–1.56) | 0.0004 | 1.19 (1.01–1.41) | 0.002 | |
| Current | 1.38 (1.05–1.82) | 1.79 (1.33–2.41) | 1.54 (1.21–1.97) | ||||
| LDL Cholesterol | 1.01 (0.98–1.04) | 0.41 | 1.00 (0.97–1.03) | 0.87 | 1.02 (0.99–1.04) | 0.15 | |
| HDL Cholesterol | 0.93 (0.87–1.01) | 0.07 | 0.95 (0.88–1.03) | 0.21 | 0.90 (0.84–0.96) | 0.001 | |
| Triglycerides | 1.00 (0.98–1.02) | 0.99 | 1.01 (1.00–1.02) | 0.23 | 1.00 (0.99–1.02) | 0.61 | |
| Systolic BP | 1.17 (1.11–1.23) | <0.0001 | 1.11 (1.05–1.17) | 0.0002 | 1.13 (1.08–1.18) | <0.0001 | |
| Diastolic BP | 0.89 (0.81–0.98) | 0.02 | 0.90 (0.81–1.00) | 0.05 | 0.89 (0.82–0.97) | 0.008 | |
| Body Mass Index | 0.91 (0.84–0.98) | 0.02 | 0.94 (0.86–1.02) | 0.11 | 0.89 (0.83–0.95) | 0.0005 | |
| Transplant Vintage | 1.02 (1.00–1.03) | 0.10 | 1.03 (1.01–1.04) | 0.01 | 1.03 (1.01–1.04) | 0.0007 | |
| Living Donor | 0.84 (0.70–1.01) | 0.07 | 0.64 (0.52–0.79) | <0.0001 | 0.83 (0.70–0.98) | 0.02 | |
Analyses not censored at return to dialysis. Hazard ratio (HR) for age per 5 years, LDL and HDL cholesterol per 10 mg/dL increase, triglycerides per 50 mg/dL increase, systolic and diastolic blood pressure per 10 mm Hg increase, BMI per 5 kg/m2 increase, vintage per year increase.
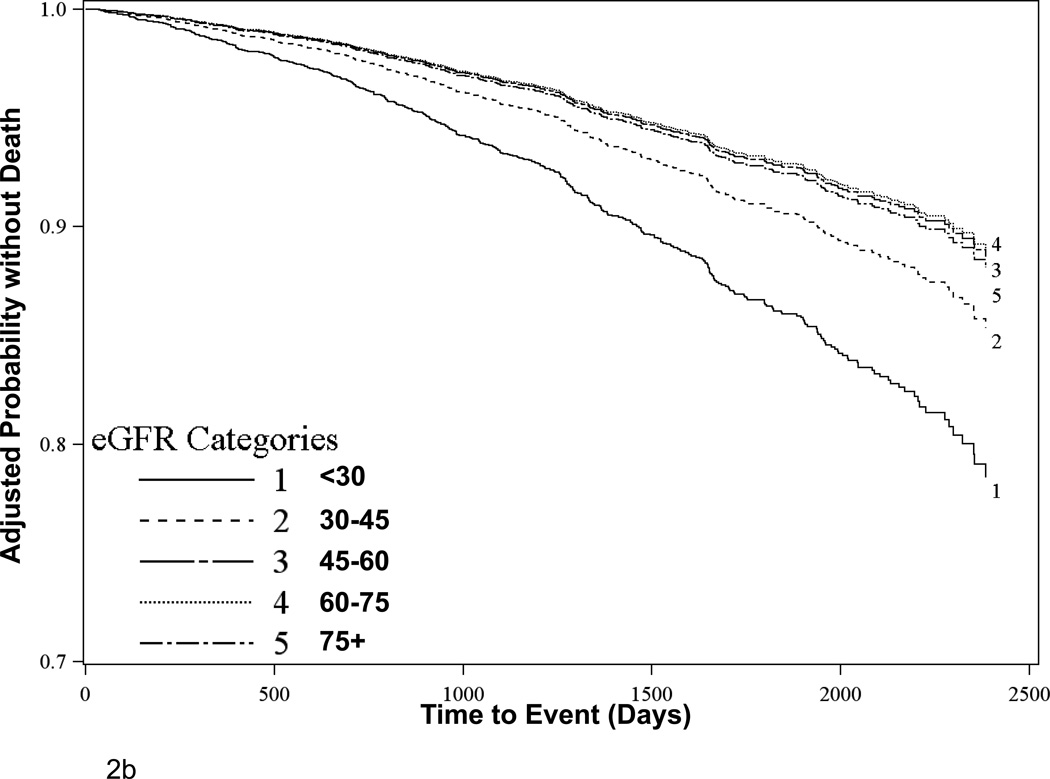
Figure 2.
Adjusted survival estimates for (A) Primary CVD outcomes and (B) All-cause mortality outcomes stratified by baseline eGFR. Figures depict the product-limit estimates of the survivor function at mean levels for continuous covariates and for the reference group as displayed in Table 2 for categorical variables. Analyses exclude participants with missing covariate data.
Table 3.
Events comprising the cardiovascular disease composite outcome and risk associated with eGFR strata for these events in the subset with complete data
| eGFR mL/min/1.73m2 | ||||||
|---|---|---|---|---|---|---|
| <30 (N = 468) |
30–45 (N = 1229) |
45–60 (N = 1113) |
60–75 (N = 571) |
>=75 (N = 295) |
Total (N = 3676) |
|
| Myocardial Infarction |
N=30 1.85 (1.00,3.41) |
N=49 1.22 (0.70,2.14) |
N=29 0.86 (0.47,1.57) |
N=17 reference |
N=5 0.54 (0.20,1.48) |
N=130 |
| Cardiovascular Disease Death |
N=26 4.54 (1.94,10.6) |
N=32 2.00 (0.88,4.54) |
N=19 1.36 (0.57,3.23) |
N=7 reference |
N=8 2.13 (0.77,5.91) |
N=92 |
| Cardiovascular Procedures |
N=39 1.33 (0.82,2.14) |
N=95 1.24 (0.83,1.86) |
N=58 0.86 (0.56,1.32) |
N=32 reference |
N=20 1.19 (0.68,2.09) |
N=244 |
| Stroke | N=10 not calculated |
N=24 not calculated |
N=8 calculated |
N=9 not calculated |
N=1 not calculated |
N=52 |
| Resuscitated Sudden Death |
N=1 not calculated |
N=5 not calculated |
N=2 not calculated |
N=0 calculated |
N=1 not calculated |
N=9 |
| Total Events | N=106 1.85 (1.35,2.54) |
N=205 1.39 (1.05,1.84) |
N=116 0.88 (0.65,1.20) |
N=65 reference |
N=35 1.04 (0.69,1.57) |
N=527 |
Hazard ratios and 95% confidence intervals are adjusted for age, sex, race, diabetes, prior CVD, treatment allocation, country of origin, smoking, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, triglyceride level, transplant vintage, and living versus deceased donor kidney. Hazard ratios are not reported for outcomes where there are fewer than 5 events in each stratum.
Among 3,712 participants with complete data for vital status, there were 439 deaths over mean follow-up time of 4.0 (SD 1.5) years, with a higher proportion of events occurring at lower eGFR levels (Figure 1). When eGFR was examined as a categorical variable, the lowest risk group had eGFR between 60 and 74 mL/min per 1.73 m2, although this was relationship was nearly identical to that associated with an eGFR of 45–59 or above 75 mL/min per 1.73 m2 (Table 2, Figure 2b). Factors associated with all-cause mortality were similar to those associated with a CVD event (Table 2). In parsimonious Cox proportional hazards models adjusting for age, sex, race, treatment assignment, country, baseline CVD and diabetes, each 5 mL/min per 1.73m2 higher eGFR below 45 mL/min per 1.73m2 was associated with a 16% lower risk of death [Hazard Ratio (HR) = 0.84 (0.79, 0.89), p<0.0001] while there was no relationship appreciated for eGFR levels above 45 [HR = 0.99 (0.95, 1.03), p=0.61]; this effect was unchanged in extended multivariable models [HR = 0.85 (0.79, 0.90), p<0.0001 for each 5 mL/min per 1.73m2 rise at eGFR below 45 mL/min per 1.73m2 and HR = 1.00 (0.95, 1.04), p=0.86 for each 5 mL/min per 1.73m2 rise at eGFR above 45 mL/min per 1.73m2]. Interactions for eGFR with prior CVD and with transplant vintage were non-significant (p=0.53 and 0.41, respectively). Analyses examining a composite outcome of CVD and all-cause mortality were similar (Table 2).
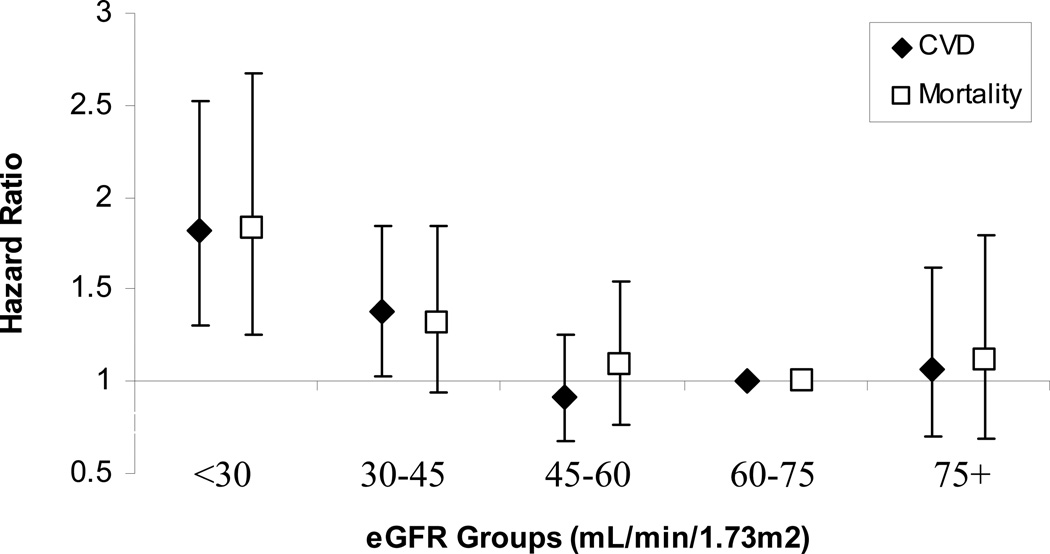
Sensitivity analyses revealed similar results. In extended multivariable models censoring 3-months after recurrent kidney failure, each 5 mL/min per 1.73 m2 higher eGFR up to an eGFR of 45 mL/min per 1.73m2 was associated with 14% lower CVD event risk [HR = 0.86 (0.81, 0.92), p<0.0001] and 12% lower mortality risk [HR = 0.88 (0.82, 0.95), p=0.0006], with no significant relationship at eGFR levels above 45 mL/min per 1.73m2. Models evaluating censored outcomes stratified by clinically relevant eGFR group also remained similar (Figure 3). Homocysteine level was not significant in CVD models, but higher homocysteine level was associated with all-cause mortality [HR = 1.02 (1.01, 1.04, p<0.0001) for each µmol/L increase]; inclusion of homocysteine level in models did not substantially impact the relationship between eGFR and outcomes (data not shown).
Figure 3.
Hazard of CVD and mortality outcomes in extended models censoring 3-months after recurrent kidney failure.
DISCUSSION
In stable kidney transplant recipients, lower eGFR is strongly and independently associated with both CVD events and all-cause mortality, specifically at levels below 45 mL/min per 1.73m2. Similar to the general population and the PORT registry, those transplant recipients with the highest eGFR values (≥75 mL/min per 1.73m2) did not have a lower risk of death than individuals with much lower eGFR results. These findings expand considerably on two recent studies (3, 7) by exploring the relationship between eGFR, including CKD stage, and outcomes in a large, generalizable transplant cohort with rigorous ascertainment of CVD events and calibrated serum creatinine values. Critically, the current study identifies transplant patients with lower eGFR as being at increased risk of major outcomes in addition to progression to kidney failure, suggesting that transplant recipients, particularly those with lower eGFR, may be a high-yield population for research into strategies for CVD risk reduction.
The presence of an association between low eGFR and cardiovascular disease in transplant recipients suggests that comorbid conditions associated with low GFR itself rather than concurrent comorbidities that result in both low GFR as well as systemic cardiovascular disease may be impacting cardiovascular risk. In the general population, reduced eGFR is independently associated with CVD events and mortality (17–20). It is likely that this relationship reflects several processes, including a greater duration and severity of traditional CV risk factors in individuals with reduced GFR, the effects of non-traditional CV risk factors that accompany reduced GFR (21–22), and perhaps effects of reduced GFR itself. Finding a similar relationship in kidney transplant recipients as in the general population suggests a possible direct effect of reduced GFR, since the transplanted kidney is likely to not have had a long exposure to traditional and non-traditional CVD risk factors. These findings are particularly important as many kidney transplant recipients receive less aggressive CVD risk factor management than would be expected given their underlying comorbid burden (23).
In 2002, the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) proposed a CKD classification system, which stratified chronic kidney disease into stages based on eGFR levels (24). This has been widely adopted, with CKD stage 3 to be subdivided into stage 3a (eGFR 45–59 mL/min per 1.73 m2) and stage 3b (eGFR 30–44 mL/min per 1.73 m2) for better risk assessment (25). To date, this classification system is not adequately studied in transplant recipients (26–27). The current study adds to the literature on the utility of the CKD classification system, which now divides stage 3 into two categories, by showing progressively increased risk of both CVD and mortality outcomes in univariate and multivariable adjusted analyses at eGFR levels below 45 mL/min per 1.73 m2 and a flat risk relationship at eGFR levels above 45 mL/min per 1.73 m2; this considerably expands on the findings in the ALERT Study, where risk was quantified based on each 1 mg/dL increment of serum creatinine (7, 28). This relationship in FAVORIT is supported by findings for all-cause mortality from the PORT registry study (5), but conflicts with a single-center study of 431 transplant recipients where there was no significant relationship between CKD stage and mortality (29). Importantly, this latter study did not subdivide CKD stage 3 to appreciate the gradated risk that is seen within this stage and had few patients with CKD stage 2 or CKD stage 4 (29).
The current study has multiple strengths. These are most notable for the large size of the FAVORIT cohort as well as the extensive ascertainment of CVD risk factors, the systematic prospective ascertainment of CVD events, and the measurement of serum creatinine at a single laboratory. Additionally, kidney transplant recipients are among the healthiest of kidney failure patients, with most having undergone pre-transplant CVD screening, although the value of this screening remains uncertain (30–31). There are also several weaknesses. First, we lack data on albuminuria; however, albuminuria may be a less important risk factor in transplant recipients than in individuals in the general population, reflecting that 1) in some transplant recipients albuminuria may be from the native kidneys rather than the transplant, and 2) in individuals without a kidney transplant, albuminuria likely is a kidney marker of lifelong vascular burden (18, 32–35). Whether the level of albuminuria in a transplant kidney reflects current systemic vascular disease burden requires further study. Second, eGFR was estimated based on a single assessment of serum creatinine; however, we were able to confirm the results presented in this manuscript by performing similar analyses using creatinine values assayed at the time of screening for FAVORIT eligibility. Additionally, despite the potential inaccuracies with a single ascertainment, which would bias to a null finding, the relationship between eGFR and CVD and mortality outcomes remained highly significant in the FAVORIT cohort. Third, we are relying on data from a randomized clinical trial analyzed as a cohort. However, the clinical trial was negative, finding no effect of the vitamin intervention on outcomes (36). Additionally, we adjust for intervention assignment in all analyses.
In conclusion, in a large, generalizable prevalent kidney transplant population, we have identified a substantial and graded association between eGFR and subsequent CVD and mortality outcomes. Further research is essential to evaluate strategies to modify this risk.
ACKNOWLEDGMENTS
We would like to acknowledge the tremendous contributions of the participants in the study and the contributions of the doctors, nurses, and administrative staff in hospitals and clinical centers in Brazil, Canada, and the United States who assisted with trial conduct.
FAVORIT is supported by cooperative agreement U01 DK61700 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Dr. Kusek, an employee of the NIDDK, was involved in the study design, interpretation, and writing of the report. Support also provided by the Office of Dietary Supplements, NIH. PamLab LLC of Covington, LA provided the high- and low-dose multivitamins. DEW was supported by K23DK071636 and a Carl Gottschalk Career Development Award from the American Society of Nephrology.
Abbreviations
- FAVORIT
Folic Acid for Vascular Outcome Reduction in Transplantation
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- BMI
body mass index
- HR
hazard ratio
- SD
standard deviation
Footnotes
Data included in this manuscript were presented in poster form at the American Heart Association Cardiovascular Disease Epidemiology and Prevention 2011 Scientific Sessions on March 25, 2011 in Atlanta, GA.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation
FAVORIT INVESTIGATORS
Deborah Adey, MD (University of Vermont); Edward Alfrey, MD (Southern Illinois University); Paul Bolin, Jr., MD (East Carolina University); Andrew Bostom, MD (Rhode Island Hospital); Daniel C. Brennan, MD, FACP (Washington University-St. Louis); Barbara Bresnahan, MD (Medical College of Wisconsin); Edward Cole, MD (University of Toronto); David Conti, MD (Albany Medical Center); Fernando Cosio, MD (Mayo Clinic); Gabriel Danovitch, MD (University of California-Los Angeles); Alfredo Fabrega, MD (Banner Good Samaritan Transplant Services); Lorenzo Gallon, MD (Northwestern University); Andrew House, MD (London Health Sciences Center); Lawrence Hunsicker, MD (University of Iowa); Bertram Kasiske, MD (Hennepin County Medical Center); Clifton Kew, MD (University of Alabama-Birmingham); Matthew Koch, MD (private practice); M.S. Anil Kumar, MD (Reata Pharmaceuticals); Mariana Markell, MD (SUNY Health Science Center); Arthur Matas, MD (University of Minnesota); Douglas Norman, MD (Oregon Health Sciences University); Akinlolu Ojo, MD (University of Michigan); Alvaro Pacheco-Silva, MD, PhD (Universidade Federal de Sao Paulo); Alice Peng, MD (Cedars-Sinai Health System); Todd Pesavento, MD (Ohio State University); John Pirsch, MD (University of Wisconsin-Madison); Ajay Singh, MD (Brigham and Women’s Hospital); Stephen Smith, MD (Duke University); John Vella, MD (Maine Medical Center); Matthew Weir, MD (University of Maryland); Muhammad Yaqub, MD (Indiana University).
Contributor Information
Daniel E Weiner, Tufts Medical Center, Boston, MA.
Myra A Carpenter, University of North Carolina, Chapel Hill, NC.
Andrew S Levey, Tufts Medical Center, Boston, MA.
Anastasia Ivanova, University of North Carolina, Chapel Hill, NC.
Edward H Cole, University of Toronto, Ontario, Canada.
Lawrence Hunsicker, University of Iowa College of Medicine, Iowa City, IA.
Bertram L Kasiske, Hennepin County Medical Center and the University of Minnesota, Minneapolis, MN.
S Joseph Kim, University of Toronto, Ontario, Canada.
John W Kusek, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Andrew G Bostom, Rhode Island Hospital, Providence, RI.
REFERENCES
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8. doi: 10.1053/j.ajkd.2010.10.007. e1-526. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11(9):1735–1743. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 3.Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10(2):338–353. doi: 10.1111/j.1600-6143.2009.02949.x. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Israni AK, Snyder JJ, Skeans MA. The Relationship Between Kidney Function and Long-term Graft Survival After Kidney Transplant. Am J Kidney Dis. 2011;57(3):466–475. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Vanrenterghem YF, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85(2):209–216. doi: 10.1097/TP.0b013e318160254f. [DOI] [PubMed] [Google Scholar]
- 7.Jardine AG, Fellstrom B, Logan JO, Cole E, Nyberg G, Gronhagen-Riska C, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46(3):529–536. doi: 10.1053/j.ajkd.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Soveri I, Holdaas H, Jardine A, Gimpelewicz C, Staffler B, Fellstrom B. Renal transplant dysfunction--importance quantified in comparison with traditional risk factors for cardiovascular disease and mortality. Nephrol Dial Transplant. 2006;21(8):2282–2289. doi: 10.1093/ndt/gfl095. [DOI] [PubMed] [Google Scholar]
- 9.Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009;53(1):121–128. doi: 10.1053/j.ajkd.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152(3):448. doi: 10.1016/j.ahj.2006.03.004. e1-7. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikkalingaiah KB, Grant ND, Mangold TM, Cooke CR, Wall BM. Performance of simplified modification of diet in renal disease and Cockcroft-Gault equations in patients with chronic spinal cord injury and chronic kidney disease. Am J Med Sci. 2010;339(2):108–116. doi: 10.1097/MAJ.0b013e3181c62279. [DOI] [PubMed] [Google Scholar]
- 14.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114(15):1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48(3):392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 21.Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, et al. Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int. 2008;73(5):615–621. doi: 10.1038/sj.ki.5002716. [DOI] [PubMed] [Google Scholar]
- 22.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51(2):212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilmore HL, Skeans MA, Snyder JJ, Israni AK, Kasiske BL. Cardiovascular disease medications after renal transplantation: results from the patient outcomes in renal transplantation study. Transplantation. 2011;91(5):542–551. doi: 10.1097/TP.0b013e31820437bd. [DOI] [PubMed] [Google Scholar]
- 24.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 25.KDIGO Guideline for CKD Classification and Management. [cited 2011 March 16];Guideline 1. Definition and Stages of Chronic Kidney Disease. Available from: http://www.kdigo.org/clinical_practice_guidelines/CKD.php. [Google Scholar]
- 26.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53(6):915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Fellstrom B, Jardine AG, Soveri I, Cole E, Neumayer HH, Maes B, et al. Renal dysfunction is a strong and independent risk factor for mortality and cardiovascular complications in renal transplantation. Am J Transplant. 2005;5(8):1986–1991. doi: 10.1111/j.1600-6143.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 29.Kukla A, Adulla M, Pascual J, Samaniego M, Nanovic L, Becker BN, et al. CKD stage-to-stage progression in native and transplant kidney disease. Nephrol Dial Transplant. 2008;23(2):693–700. doi: 10.1093/ndt/gfm590. [DOI] [PubMed] [Google Scholar]
- 30.Kasiske BL, Israni AK, Snyder JJ, Camarena A. COST Investigators. Design Considerations and Feasibility for a Clinical Trial to Examine Coronary Screening Before Kidney Transplantation (COST) Am J Kidney Dis. 2011;57(6):908–916. doi: 10.1053/j.ajkd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lentine KL, Hurst FP, Jindal RM, Villines TC, Kunz JS, Yuan CM, et al. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis. 2010;55(1):152–167. doi: 10.1053/j.ajkd.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguilar MI, O'Meara ES, Seliger S, Longstreth WT, Jr, Hart RG, Pergola PE, et al. Albuminuria and the risk of incident stroke and stroke types in older adults. Neurology. 2010;75(15):1343–1350. doi: 10.1212/WNL.0b013e3181f73638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3(6):595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Seaghdha CM, Hwang SJ, Upadhyay A, Meigs JB, Fox CS. Predictors of incident albuminuria in the Framingham Offspring cohort. Am J Kidney Dis. 2010;56(5):852–860. doi: 10.1053/j.ajkd.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, et al. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56(5):861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the folic Acid for vascular outcome reduction in transplantation trial. Circulation. 2011;123(16):1763–1770. doi: 10.1161/CIRCULATIONAHA.110.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]