Abstract
Dendritic cells (DCs) are potent inducers of T cell immunity and autologous DC vaccination holds promise for the treatment of cancers and chronic infectious diseases. In practice, however, therapeutic vaccines of this type have had mixed success.
Here we show that brief exposure to inhibitors of mechanistic Target Of Rapamycin (mTOR) in DCs during the period that they are responding to TLR agonists makes them particularly potent activators of naïve CD8+ T cells, and able to enhance control of B16 melanoma in a therapeutic autologous vaccination model in the mouse. The improved performance of DCs in which mTOR has been inhibited is correlated with an extended lifespan following activation and prolonged, increased expression of costimulatory molecules. Therapeutic autologous vaccination with DCs treated with TLR agonists plus the mTOR inhibitor rapamycin results in improved generation of antigen-specific CD8+ T-cells in vivo and improved anti-tumor immunity compared to that observed with DCs treated with TLR agonists alone. These findings define mTOR as a molecular target for augmenting DC survival and activation and document a novel pharmacologic approach for enhancing the efficacy of therapeutic autologous DC vaccination.
Introduction
Dendritic cells (DCs) are professional antigen presenting cells responsible for initiating adaptive immune responses (1, 2). They can be generated from precursor cells in vitro and are of great interest for their potential use in autologous vaccine therapies for cancer and chronic infectious diseases (3, 4). Autologous DC vaccines have exhibited limited success clinically (3, 4), and the short lifespan of activated DCs is recognized as one obstacle to this promising therapeutic approach (5, 6). While genetic approaches to modulating DC lifespan and function can enhance DC vaccine potency in animal tumor models (5, 7, 8), pharmacological approaches for improving DC immune activity in the context of vaccine therapies are desirable for reasons of clinical feasibility.
One of the central nutrient sensing pathways, controlling a diverse array of cellular responses including cell activation, metabolism, and survival, is governed by mechanistic target of rapamycin (mTOR) (9–12). mTOR is activated by the PI3K/Akt signaling pathway, which is downstream of a number of growth factor receptors as well as TLRs, and we have previously reported that this signaling axis is critically involved in orchestrating the metabolic demands necessary for DC activation (13). Inhibition of mTOR by rapamycin, a macrolide product of the bacterium Streptomyces hygroscopicus, is widely reported to extend the lifespan of eukaryotic cells and organisms (14–16). Based on this evidence, we hypothesized that mTOR could play a regulatory role in controlling DC lifespan and activation following TLR stimulation.
We decided to directly examine the role of mTOR in DCs during the activation process following exposure to TLR agonists. We have found that inhibiting mTOR during activation considerably extends the lifespan of DCs, and this is accompanied by enhanced and prolonged costimulatory molecule expression following activation. These phenotypes effectively increase the window of time during which DCs are able to interact with and stimulate antigen-specific T cell responses. Consistent with their increased lifespan and prolonged activation kinetics, DCs activated in the presence of mTOR inhibitors induce enhanced primary antigen-specific CD8+ T cell responses and stronger and more effective anti-tumor responses in a therapeutic vaccination treatment model. These findings suggest a novel approach for potentiating the efficacy of autologous DC vaccination for the therapeutic treatment of cancers.
Material and Methods
Mice and reagents
C57Bl/6 mice were purchased from The Jackson Laboratory, and re-derived stocks were maintained at the Trudeau Institute under specific pathogen-free conditions under protocols approved by the Institutional Animal Care and Use Committee.
Lipopolysaccharide (LPS; Escherichia coli serotype 0111:B4) was from Sigma-Aldrich and used at 100 ng/ml. Pam2CSK4 (1 µg/mL), R848 (1 µg/mL), and CpG (250 ng/mL), and Rapamycin (100 nM) were purchased from Invivogen. KU 0063794 (100 nM) was purchased from Tocris Biochemicals. All antibodies for FACS analysis were from BD Bioscience except for anti- CD11c and CD40, which were purchased from eBioscience. Ovalbumin (250 µg/mL endotoxin-free egg white) was prepared in lab. Kb-OVA tetramers were produced by the Molecular Biology Core Facility at the Trudeau Institute.
Mouse DC culture, retroviral transduction, purification, and activation
Bone marrow–derived DCs were generated as described (17). Briefly, bone marrow cells were differentiated in the presence of GM-CSF (20 ng/mL) in complete DC media (RPMI containing 10% fetal calf serum, 100 U/mL penicillin/ streptomycin, and 2mM L-glutamine) for 6 days. Retroviral transduction of DCs was accomplished as described previously (18). Sequences for Luciferase or mTOR short hairpin RNAs (shRNAs) were obtained from Open Biosystems and cloned into LMP retroviral vectors. Recombinant retroviruses were obtained after the transfection of 293T packaging cells with the use of Lipofectamine (Invitrogen); retrovirus-containing supernatants were collected 48 hours after transfection and used for spin infection (2500 rpm, 2 hours) of day 2 and 3 bone marrow DC cultures in 6-well plates. After 6 days in culture with GM-CSF, DCs were harvested, and transduction efficiency was assessed by human CD8 expression via FACS; typical transduction rates were around 80–90%. On day 6 of culture, DCs were washed in complete DC media and were pulsed as indicated with media alone, rapamycin, LPS, or rapamycin+LPS. Where applicable, cells were pulsed with 250 µg/mL OVA. For retroviral transduction assays, DCs were purified from cultures using CD11c+ or human CD8+ selection (as indicated) with MACS bead sorting (Miltyeni Biotech) according to the manufacturer’s protocol. For IL-10 receptor blocking studies, anti-IL10 receptor monoclonal antibody 1B1.3A and control antibody (HRPN) were purchased from BioXCell.
Human DC Culture
Human myeloid DCs were isolated from human blood using MACS positive selection beads. In brief, filters from hospital blood donors were generously donated by the CVPH Medical Center in Plattsburgh, NY. Blood cells were obtained by reverse flushing filters in sterile HBSS. PBMCs were obtained by centrifugation of blood samples over Ficoll-Paque Plus (density 1.077 g/mL) (GE Healthcare). Myeloid DCs were enriched using the BDCA-1 Positive Selection DC Islation Kit (Miltenyi Bioscience) per the manufacturer’s instructions. Freshly isolated DC (1×105 cells/well in 200 µl) were cultured in complete RPMI 1640 medium containing 10% FCS, 100 U/mL penicillin/ streptomycin, and supplemented with 20 ng/mL GM-CSF. DCs were stimulated with 1 µg/mL R848 in the presence or absence of rapamycin or KU (both used at 100 nM). At indicated times, DCs were harvested and analyzed by FACS for maturation markers.
Western blotting
Transduced DCs were MACS-purified based on expression of human CD8. Cell lysate preparation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrophoretic transfer, immunoblotting, and development with enhanced chemiluminescence were accomplished as described previously (19).
Cytokine measurements and flow cytometry
DC supernatants were analyzed for IL-12p70, TNFα, and IL-10 by FACS using the Cytometric Bead Array Mouse Inflammation Kit (BD Bioscience) per the manufacturer’s instructions.
Metabolism Assays
Glucose and Lactate levels in the media after indicated stimulation conditions were measured using the Glucose Assay Kit and Lactate Assay Kit from Eton Bioscience, Inc. per the manufacturer’s instructions.
In vitro T-cell responses
For staggered DC activation experiments, DCs were pulsed with indicated treatments for 24 hours and then the media changed to normal growing media (no TLR agonist or mTOR inhibitor). After 24 hour activation, DC was changed daily prior to T cell co-culture to prolong survival of activated DCs. DCs were co-cultured for 4 days at a 1:5 ratio with CFSE-labeled CD8+ selected OT-I splenocytes (MACS) on day 2, 3, or 4 following initial DC activation.
Tumor Challenge Experiments
For all tumor studies, mice were challenged with 1×105 Ova-expressing B16 melanoma cells intradermally on the peritoneal surface. For therapeutic vaccine studies, mice were challenged with tumor on Day 0 and then received autologous DC transfer subcutaneously in the left footpad on Day 3 after tumor challenge. Mice were monitored for tumor growth periodically, and tumor sizes were measured with digital calipers (Fisher Scientific). At time of harvest, mice were sacrificed and tumor was excised with final tumor volumes and weights measured. For analysis of tumor infiltrating cells, tumors were mechanically dissociated in HBSS with a 1 mL syringe stopper and passed through 70 µm cell strainers to obtain single cell suspensions of tumor content.
Statistics
For analysis of CFSE-labeled DCs in the draining LN, 2-way ANOVA was performed on log-transformed data. For analysis of tetramer positive cells in the blood of mice, 2-way ANOVA was performed on data that were transformed by √(percent +0.5) as appropriate for percentage data with very low values (20). Tumor volumes were analyzed using Student’s t-test on log-transformed values. Tumor weights were first classified by whether or not they exceeded the lower limit of detection for the assay (10 mg) then analyzed using Fisher’s exact test.
Declaration of Ethical Compliance with Standards for Study of Human Subjects
Human DCs and monocytes were collected from white blood cells destined to be discarded after being removed by filtration from blood collected for other purposes. The white blood cells were provided to us as samples that were identified by numbers only.
Results
mTOR regulates lifespan and activation of DCs following stimulation by TLR agonists
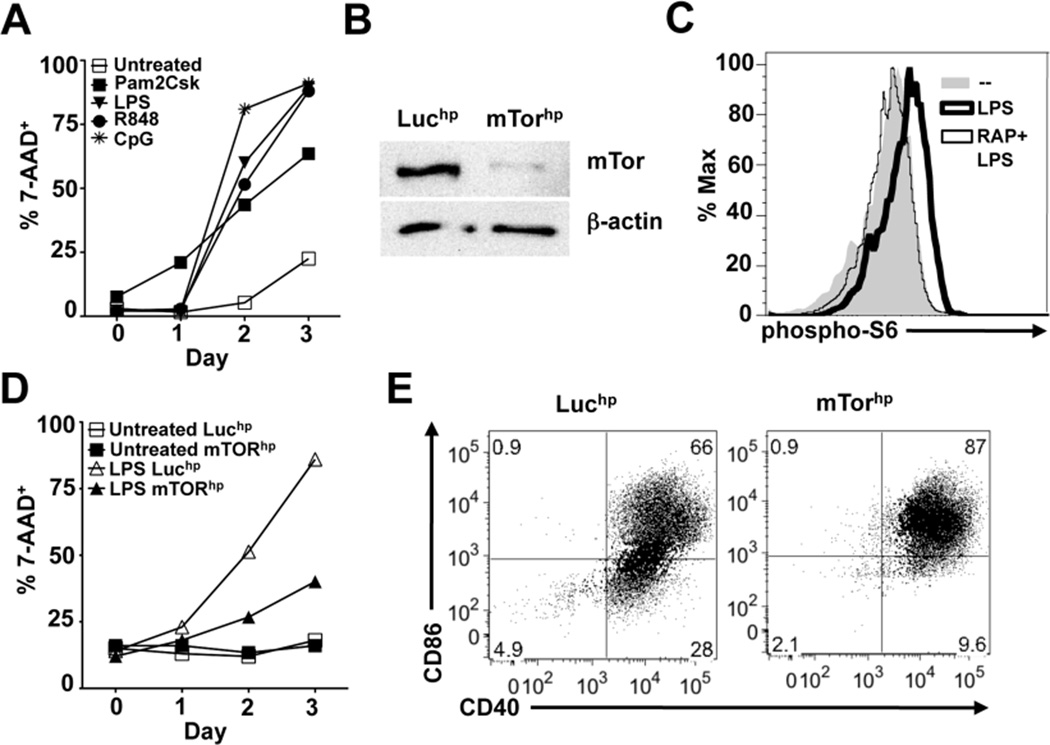
DC lifespan is dramatically reduced following activation by TLR agonists (Figure 1A) (21, 22). We directly assessed the role of mTOR in DC activation and longevity using retroviral transduction of shRNAs targeting mTOR or luciferase (mTORhp and Luchp respectively) into bone marrow cells being cultured in GM-CSF (13). Transduced DCs emerged equally well from Luchp (control) and mTORhp cultures (Supplemental Figure 1A), and mTOR protein expression levels were substantially reduced in DCs transduced with mTORhp (Figure 1B). Retroviral transduction alone did not induce a mature phenotype in unstimulated DCs (Supplemental Figure 1B). mTOR signaling, measured as phosphorylation of one of its targets, S6 kinase, is activated by stimulation with LPS and inhibited by rapamycin (Figure 1C) (23). We therefore asked whether mTOR knockdown would affect the lifespan, or ability to become activated, of DCs responding to the TLR4 agonist LPS. We observed that mTOR deficiency attenuated cell death induced by LPS activation (Figure 1D), indicating that TLR-driven mTOR signaling is an important determinant of DC lifespan following activation. Furthermore, mTOR knockdown resulted in higher percentages of LPS-stimulated DCs becoming CD40+ CD86+ (Figure 1E), demonstrating that TLR-driven mTOR signaling negatively regulates costimulatory molecule expression following LPS activation. This was not a reflection of CD40+ CD86- cells being less viable than CD40+ CD86+ cells, since we were unable to detect differences in 7-AAD staining in these two populations (Supplemental Figure 1C).
Figure 1.
Inhibition of mTOR expression prolongs DC lifespan and promotes expression of costimulatory molecules CD40 and CD86. (A) DCs were pulsed with Pam2CSK4, LPS, R848, or CpG for 6 hours and then washed and cultured in complete medium. Cell viability was monitored daily by FACS analysis of 7-AAD staining of CD11c+ cells. (B) Western blot for mTOR and β-actin protein in Luciferase hpRNA (Luchp) or mTOR hpRNA (mTORhp) -transduced DCs. (C) DCs were stimulated with media alone, LPS, or rapamycin + LPS for 30 minutes. Cells were subsequently fixed and stained for phosphorylated S6 protein as a molecular readout for mTOR activation and analyzed by FACS. (D) Luchp and mTORhp-transduced DCs were left untreated or stimulated with LPS and monitored daily for cell viability by analysis of 7-AAD staining of CD11c+ cells. (E) Luchp or mTORhp DCs were cultured with or without LPS for 24 hours and analyzed by FACS for CD40 and CD86 expression. All graphs in this figure represent mean values of replicate wells; all experiments were performed at least twice with similar results.
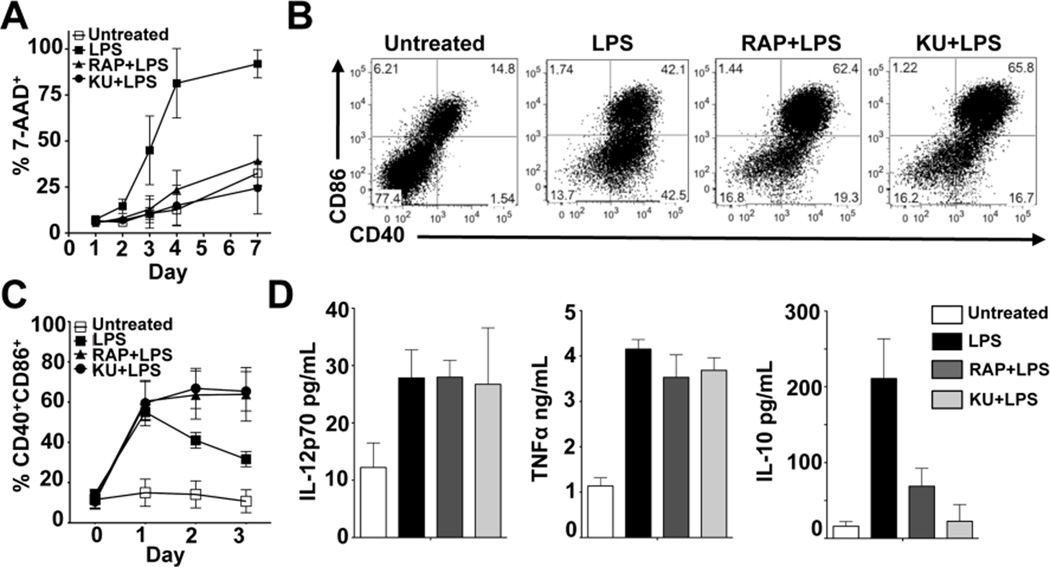
We next tested whether treatment of DCs with rapamycin, a clinically-utilized inhibitor of mTOR signaling (24), or the synthetic ATP-competitive inhibitor of mTOR, KU 0063794 (KU), could recapitulate the phenotypes observed in DCs transduced with mTORhp. Consistent with the outcome of the mTORhp experiments, mTOR inhibitors prolonged DC lifespan following activation with LPS (Figure 2A) or with agonists for TLR2, TLR7/8, and TLR9 (data not shown). Rapamycin and KU treatment alone did not impact the viability of unstimulated DCs (data not shown). Additionally, mTOR inhibition by rapamycin or KU during LPS stimulation resulted in an increase in the percentage of DCs expressing CD40 and CD86 (Figure 2B), and prolonged the expression of these costimulatory molecules in response to LPS (Figure 2C). mTOR inhibitors were observed to similarly stabilize the expression of CD80, MHC-I, and MHC-II on LPS-activated DCs (data not shown).
Figure 2.
Pharmacological inhibition of mTOR augments DC lifespan and costimulatory molecule expression. (A) DCs were stimulated with LPS in the presence or absence of RAP or KU. Cells were monitored daily for cell viability as described for Figure 1. Data are presented as mean +/− SD of 4 independent experiments. (B) DCs were unstimulated or stimulated with LPS, RAP + LPS, or KU+LPS and analyzed by FACS 24 hours later for CD40 and CD86 expression. Data are representative of more than 4 independent experiments. (C) DCs were treated as in (B) and analyzed daily by FACS for CD40 and CD86 expression gated on live CD11c+ cells. Data are presented as mean +/− SD of 4 independent experiments. (D) DCs were treated as indicated and supernatants collected 24 hours later for analysis by Cytometric Bead Array for IL-12p70, TNFα, and IL-10. Data are presented as mean +/− SD of 2 independent experiments.
mTOR functions through two, functionally distinct, signaling complexes: mTORC1, which is sensitive to direct disruption by rapamycin treatment, and mTORC2, which is insensitive to the direct inhibitory effects of rapamycin but can be regulated by mTORC1 activity in some contexts (25, 26). mTORC1 signaling regulates protein translation through its interaction with p70S6 kinase and 4E-BP1 while mTORC2 signaling is thought to be involved in regulating cytoskeleton dynamics and negatively regulating Akt signaling via phosphorylation of Ser473 (25, 27, 28). We observed that activation induced Akt Ser473 phosphorylation was inhibited by both rapamycin and KU (Supplemental Figure 1D). Therefore our experiments are unable to distinguish between the role of mTORC1 versus mTORC2 since both of the inhibitors that we utilized reduce mTORC2-mediated Akt phosphorylation.
Consistent with previous reports, mTOR inhibitors did not negatively affect the production of the pro-inflammatory cytokines IL-12p70 and TNFα by LPS-activated DCs, but did inhibit IL-10 production (Figure 2D) (29, 30). We found that DCs pulsed with ovalbumin in the presence of LPS and rapamycin were highly competent to process and present antigen to CD8+ and CD4+ T cells in vitro (Supplemental Figures 1E and 1F). Thus, mTOR inhibition in DCs during TLR stimulation protects them from activation-associated cell death, and allows them to retain an activated phenotype for prolonged periods without compromising proinflammatory cytokine production or their ability to stimulate T cells in vitro. These effects could not be explained by the observed reduction in IL-10 production, since inhibition of IL-10 signaling using anti-IL-10R antibody did not recapitulate the effects of mTOR inhibition on either lifespan or the duration of costimulatory molecule expression (Supplemental Figures 2A and 2B).
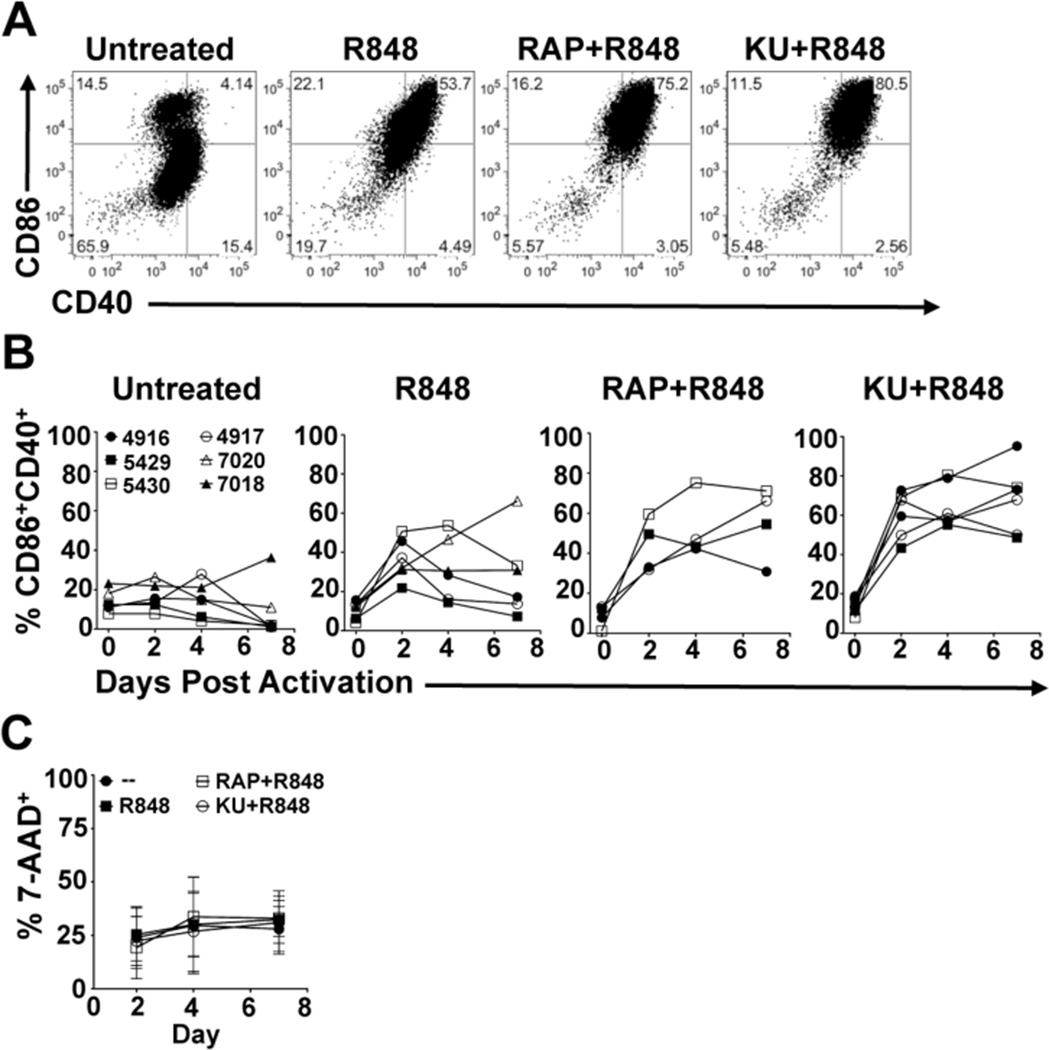
A recent report indicated that, consistent with our findings in mouse BMDCs, rapamycin is able to augment costimulatory molecule expression in human myeloid DCs (29). We examined this directly and found that treatment with rapamycin or KU allowed enhanced costimulatory molecule expression in response to stimulation with the TLR agonist R848 in human myeloid DCs (Figure 3A). Moreover, enhanced expression of CD40 and CD86 was prolonged as in the mouse DCs when mTOR was inhibited (Figure 3B). Unlike our results with murine BMDCs, we did not observe the same activation-associated cell death in human myeloid DCs as a result of TLR stimulation (Figure 3C). However, these findings demonstrate that the enhancing effects of mTOR inhibition on the stability of costimulatory molecule expression observed in mouse DCs is recapitulated in human myeloid DCs, and that this phenotype is not intrinsically dependent on prolonging post-activation survival in these cells.
Figure 3.
Pharmacological inhibition of mTOR augments the duration of costimulatory molecule expression in human myeloid DCs. (A) Human myeloid DCs were either unstimulated or stimulated with R848 in the presence or absence of rapamycin (RAP) or KU. CD40 and CD86 expression was analyzed by FACS. Day 4 costimulatory molecule expression from a representatitve donor (5430) is depicted. (B) DCs were treated as in (A) and analyzed daily by FACS for CD40 and CD86 expression. (C) DCs were treated as in (A) and analyzed for viability at indicated times by FACS analysis of 7-AAD staining. For each treatment, data from 4–6 individual donors is depicted.
mTOR promotes commitment to glycolytic metabolism following exposure to LPS in in mouse but not human DCs
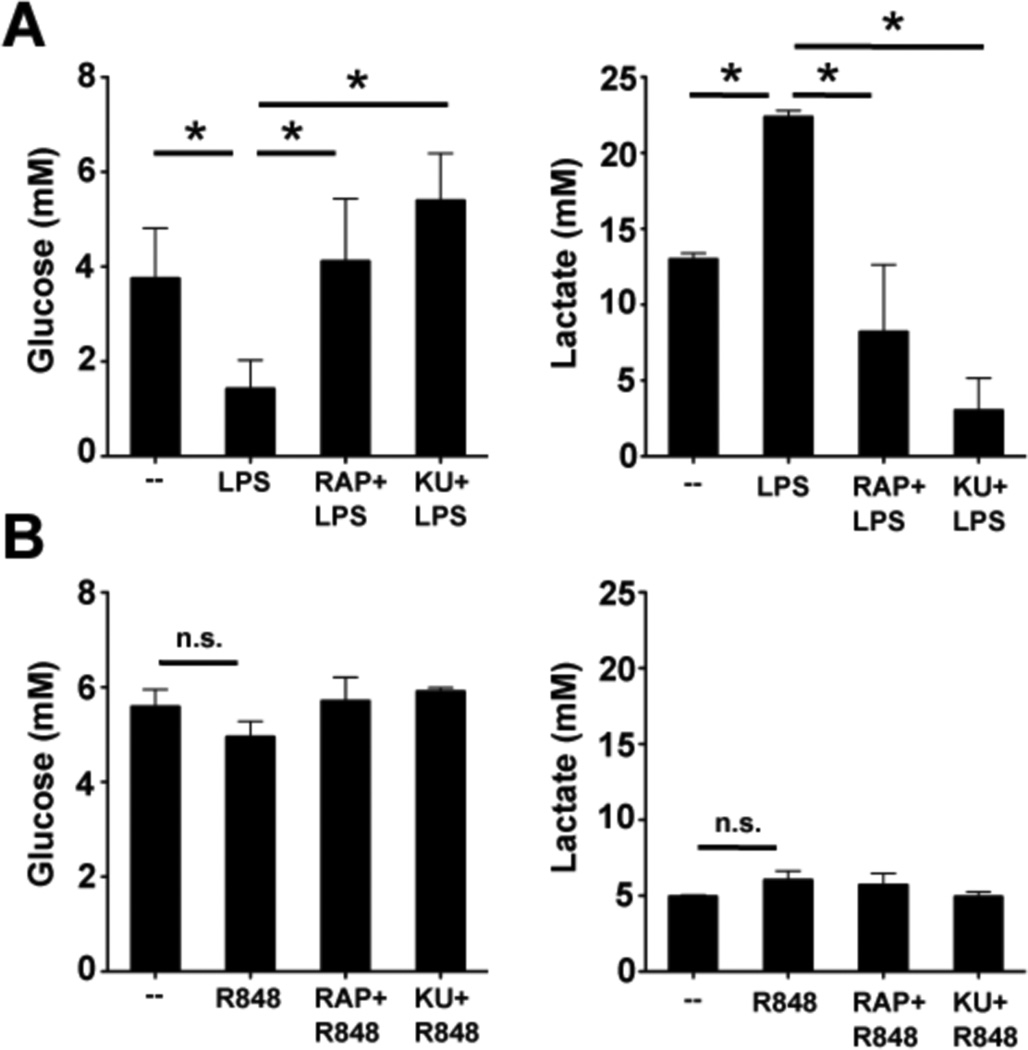
We recently showed that the metabolism of mouse DCs switches away from oxidative phosphorylation towards aerobic glycolysis following activation by TLR agonists (13). We found that the death of DCs following stimulation with TLR agonists is in part due to the fact that they are glucose-dependent and able to rapidly exhaust available glucose, and perhaps other nutrients, in tissue culture medium (13). Consequently, daily feeding with glucose in vitro is able to extend the lifespan of activated DCs (13). Because high levels of glucose consumption generally correlate with shorter lifespan, and mTOR is documented to control the induction of glycolytic metabolism (31, 32), we reasoned that mTOR inhibitors might extend the lifespan of activated DCs by limiting their dependence on glucose. Consistent with this, we found that LPS-induced increases in glucose consumption and the production of lactate (the end product of glycolysis) 48 hours after activation were profoundly diminished by mTOR inhibitors (Figure 4A). In contrast, neither the use of glucose, nor the production of lactate by human myeloid DCs were affected by exposure to TLR agonists (Figure 4B), and mTOR inhibitors had no measurable effects on either of these metabolic parameters. These findings may help explain the differences in survival of activated mouse vs. human DCs in our system (Figure 2A vs. Figure 3C).
Figure 4.
mTOR inhibition affects activation-induced metabolic changes in mouse but not human DCs. (A) Mouse BMDCs were either left unstimulated, or treated with LPS in the presence or absence of rapamycin or KU as indicated and supernatants collected 48 hours later for analysis of glucose concentration (left) and lactate concentration (right). (B) Human myeloid DCs were either left unstimulated, or treated with R848 in the presence or absence of rapamycin or KU as indicated and supernatants collected 48 hours later for analysis of glucose concentration (left) and lactate concentration (right). Asterisks indicate statistically significant differences between groups (p<0.05). For all graphs data represent the mean +/− SD of data from at least 3 individual mice or donors.
mTOR is a negative regulator of the ability of DCs to activate T cells
The increased duration of costimulatory molecule expression in DCs activated in the presence of mTOR inhibitors indicated that cells treated in this way might be able to continue to activate T cells at times when DCs activated in the absence of mTOR inhibitors are no longer able to do so. To directly test this, we stimulated DCs with LPS and OVA in the presence or absence of mTOR inhibitors, and initiated co-cultures with CD8+ OVA-specific OT-I cells on days 2, 3, or 4 after activation; DCs were thoroughly washed to remove drugs, antigen and TLR agonists prior to addition to these co-cultures and equal numbers of DCs were added to each co-culture condition. To focus this analysis on the survival-independent effects of mTOR inhibition, we performed daily media changes of DCs prior to co-culture with T cells, which minimized cell death (Supplemental Figure 2C); this is consistent with previous work from our laboratory demonstrating that media supplementation can extend the lifespan of LPS-activated DCs (13). As anticipated, the ability of control DCs activated with LPS and OVA to stimulate T cells deteriorated over time following activation (Figure 5A). In contrast, DCs activated in the presence of mTOR inhibitors, which were as capable as control DCs of stimulating T cell proliferation at day 2, retained their ability to stimulate T cells even at day 4 after DC activation (Figure 5A). Thus, the ability of DCs to remain activated and capable of stimulating T cells following exposure to antigen and TLR-agonists is markedly enhanced by the inhibition of mTOR.
Figure 5.
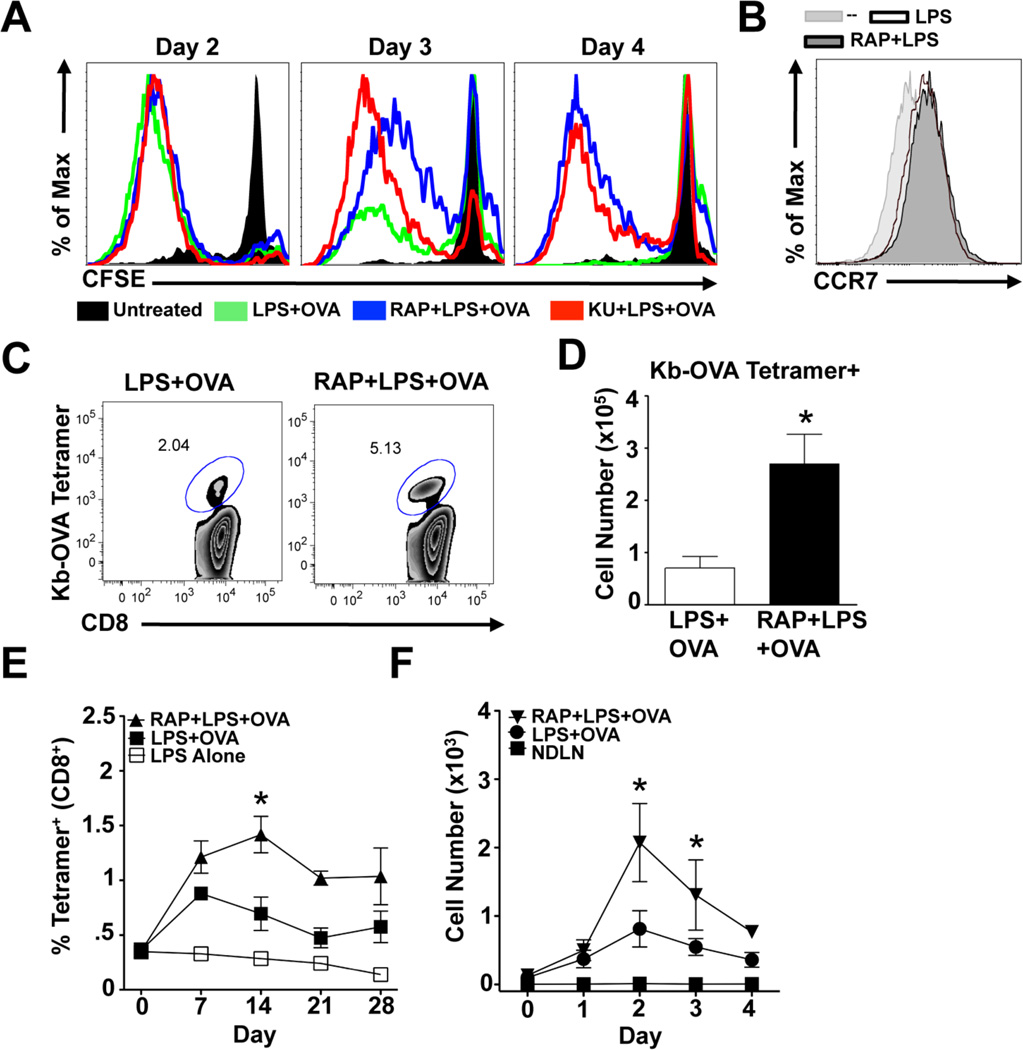
mTOR inhibition in DCs improves their ability to stimulate CD8 T cell responses. (A) DCs were treated as indicated for 24 hours after which cells were washed and replaced with normal media. 2, 3, or 4 days after activation, DCs were co-cultured at a 1:5 ratio with CFSE-labeled OT-I CD8+ T cells for 4 days. T-cell proliferation was determined by CFSE dilution within CD8+ cell population. Data are representative of 3 independent experiments. (B) DCs were treated as indicated for 24 hours and stained for CCR7 expression. Data is representative of two independent experiments. (C) 10 mice per group were immunized subcutaneously with DCs stimulated in vitro for 6 hours with LPS, LPS plus OVA, or rapamycin (RAP) plus LPS plus OVA. 7 days later, draining (popliteal) LNs were harvested and frequencies of Kb-OVA tetramer+ CD8+ cells were determined. FACS plots represents concatenated data from all 10 individual mice per group. Data are representative of more than 3 individual experiments. (D) Total numbers of tetramer+ cells from (C) were calculated. (E) Mice were immunized as in (C) and bled weekly for one month thereafter. The frequencies of Kb-OVA tetramer+, CD44+, CD8+ cells at different times after immunization are shown. Asterisk indicates statistically significant differences between mice immunized with RAP-treated DC and normally activated DCs (p<0.05). (E) CFSE-labeled DCs were treated as indicated and injected subcutaneously into mice. On indicated days, the total number of CFSE+ CD11c+ DCs within draining or non-draining LNs (NDLN) were calculated and are displayed (n = 3–5 per group per day). Asterisks indicate statistically significant differences between RAP-treated and control DC groups (p<0.05). Data are representative of 3 individual experiments.
DCs in which mTOR is inhibited have an enhanced capacity to induce therapeutic CD8 T cell responses in vivo
The ability of mTOR inhibition to prolong DC activation and T-cell stimulatory capacity following maturation in vitro suggested that inhibition of DC mTOR signaling might enhance the ability of these cells to induce T cell responses in vivo. To test this possibility, we stimulated DCs with LPS, LPS plus OVA, or rapamycin plus LPS plus OVA for 6 hours, then washed them extensively and injected them subcutaneously into mice. Endogenous OVA-specific CD8+ T-cell responses in draining LNs were monitored by tetramer staining 7 days following DC transfer. Rapamycin-treated DCs were not impaired in their ability to migrate to LNs draining sites of injection (data not shown and discussed below), consistent with the fact that they increased expression of CCR7 in response to LPS equivalently to DCs that were stimulated with LPS in the absence of rapamycin (Figure 5B). The addition of rapamycin to DCs during the time that they were pulsed with LPS plus OVA enabled them to induce stronger immune responses as measured by LN expansion (Supplemental Figure 3A), and the frequency (Figure 5C) and number (Figure 5D) of Kb-OVA tetramer positive CD8+ T cells in reactive LNs. We also detected antigen-specific CD8+ T cells in blood and spleen of immunized mice, and these cells were present in higher frequencies in the mice that received rapamycin-treated DCs (Supplemental Figures 3B and 3C). We observed that frequencies of circulating antigen-specific CD8+ T cells increased between days 7 and 14 post-immunization with rapamycin-treated DCs, whereas contraction of the antigen-specific population occurred during this period in mice immunized with DCs that had not been treated with rapamycin (Figure 5E). Additionally, we observed higher numbers of injected rapamycin-treated DCs than control DCs in reactive LNs draining sites of immunization on days 3 and 4 after DC transfer (Figure 5F), suggesting that the in vitro survival advantage conferred by mTOR inhibition (Figure 2A) may also be at play in vivo. Based on in vitro data (Figure 5A), we would expect the rapamycin-treated DCs persisting at days 3 and 4 to be able to continue to activate T cells in the in vivo setting.
Our principal focus was on the ability of mTOR inhibition to augment DC activity during the primary T cell response following autologous DC transfer. However, we were also interested in testing whether vaccination with DCs activated in the presence of mTOR inhibitors could induce the development of a population of antigen-specific memory CD8+ T cells. Immunization with DCs pulsed with LPS plus OVA or with rapamycin plus LPS plus OVA resulted in the establishment of robust memory CD8+ T cell populations that, 4 – 5 weeks after priming, were highly capable of responding to challenge infection with OVA-expressing Listeria monocytogenes (Supplemental Figure 4A), or of mediating protection against challenge with OVA-expressing B16 melanoma cells (Supplemental Figure 4B and 4C). Taken together, our data demonstrate that DCs activated in the presence of rapamycin are capable of inducing enhanced CD8 T cell primary expansion and contraction during the primary phase of the response, and the establishment of a population of memory CD8+ T cells that can be recalled by re-exposure to antigen.
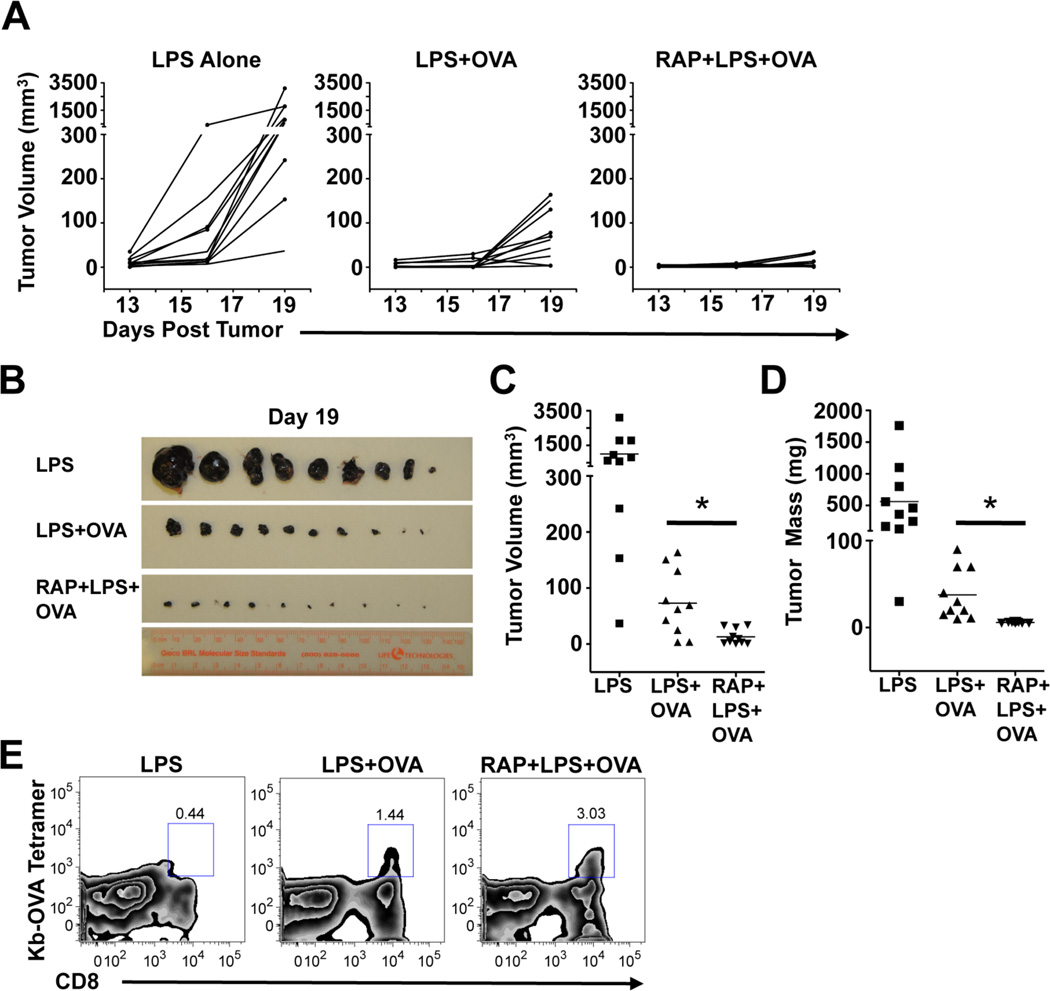
Therapeutic autologous DC vaccination has significant promise for cancer treatment (33). We reasoned that the advantages conferred to DCs by mTOR inhibition might enhance their ability to induce therapeutic anti-tumor immunity. To test this hypothesis, we intradermally inoculated mice with OVA-expressing B16 melanoma cells. Three days later, we vaccinated tumor recipients with DCs that had been pulsed in vitro for 6 hours with LPS, with LPS plus OVA, or with rapamycin plus LPS plus OVA. Mice that received the rapamycin-treated DCs following tumor inoculation had increased frequencies of OVA-specific CD8+ T cells in the blood 1 week after DC vaccination compared to mice that received DCs that had not been treated with rapamycin (data not shown), indicating that the in vivo benefit conferred by rapamycin-treated DCs is not sensitive to systemic tumor-mediated immune suppression. Consistent with a protective role for CD8+ T cells in this system, the kinetics of tumor growth were substantially delayed in mice vaccinated with DCs stimulated with LPS plus OVA (Figure 6A). Most importantly, there was a highly significant reduction in tumor burden, obvious macroscopically (Figure 6B), and as measured by tumor volume (Figure 6C) and mass (Figure 6D) at the time of sacrifice in mice that received the rapamycin-treated DCs. As anticipated, regardless of whether they were treated with rapamycin or not, DCs pulsed with LPS plus OVA conferred significant therapeutic advantage over the control treatment of DCs that had been pulsed with LPS without antigen. The improved therapeutic anti-tumor effect of immunization with rapamycin-treated DCs was associated with a doubling in the frequency of antigen-specific CD8+ tumor-infiltrating lymphocytes observed in single cell suspensions of harvested tumors (Figure 6E). These data provide evidence that mTOR-inhibition in DCs during in vitro stimulation with antigen and TLR-agonists prior to transfer into a recipient can significantly improve the subsequent in vivo therapeutic potential of these cells
Figure 6.
Rapamycin enhances the ability of DCs to induce therapeutic anti-tumor immunity. (A) Mice inoculated with tumors on Day 0 were each immunized once subcutaneously with 5 × 105 DCs treated as indicated on Day 3 and then monitored every three days for tumor growth. Tumor volumes were measured at each time point. The kinetics of tumor growth for each individual mouse in the experiment (n = 10 per group) is plotted. 19 days after immunization, mice were sacrificed and tumors were excised, photographed (B) and tumor volume (C) and mass (D) were calculated. Data from all individual mice in the experiment are shown, and mean values illustrated by horizontal bars in (C) and (D). Asterisks show statistically significant differences (p<0.05). (E) Tumor single-cell suspensions were analyzed by FACS and the frequencies of Kb-OVA tetramer+ CD8+ T cells within the CD45+ gates are shown. Data are concatenated from all tumors for each mouse group. All tumor experiments were repeated at least three times with similar results.
Discussion
Here we describe mTOR inhibition during DC activation as a novel strategy to improve autologous DC vaccination in a murine melanoma tumor model. We demonstrate that pharmacological inhibition of mTOR prolongs the lifespan of TLR-activated mouse DCs and extends the time period over which they exhibit an activated phenotype. The combined effect of these two processes results in a dramatic difference in outcome. By 2 – 4 days following activation in the absence of mTOR inhibitors, most DCs are dead, and those that remain alive are no longer expressing high levels of costimulatory molecules. In contrast, by 2 – 4 days following activation in the presence of mTOR inhibitors, most of the DCs within the starting population are alive and continuing to express high levels of costimulatory molecules. Not surprisingly in light of these findings, DCs activated in the presence of mTOR inhibitors induce larger CD8 T cell responses both in the local lymphoid compartments and systemically when adoptively transferred into naïve host animals. This enhanced CD8 T cell response induced by DCs in which mTOR is inhibited correlates with a more pronounced protective effect against an aggressive melanoma in a therapeutic vaccination model. Strikingly, these beneficial effects of mTOR inhibition on DCs are induced by a brief 6-hour exposure to mTOR inhibitors at the time of the addition of TLR agonists to these cells. The fact that short-term in vitro pharmacological intervention can have beneficial effects on the kinetics human DC activation status suggests that it may be technically feasible to translate this approach for enhancing autologous DC vaccination into a clinical setting.
We observed identical effects on DC lifespan, costimulatory molecule expression, and cytokine production with either rapamycin- or KU -treatment. However, because both rapamycin and KU treatment showed comparable inhibitory effects on the phosphorylation of Akt Ser473, a downstream target of mTORC2 activity, we can not distinguish from these data which mTOR signaling complex is the predominate effector complex regulating DC activation and lifespan in our system. Future studies genetically targeting the role of mTORC1 vs. mTORC2 in DC activation and survival will be an interesting avenue for further investigation.
Elucidating the role of mTOR during DC activation in response to TLR agonists is complicated by its established role as central mediator of signaling initiated by growth factor receptors, including that for GM-CSF, an important DC differentiation and survival factor (25, 34). Work in human monocyte-derived DCs has demonstrated that mTOR inhibition can disrupt GM-CSF signaling in these cells, inhibiting differentiation and leading to apoptosis (34). Consistent with this, the inclusion of pharmacological inhibitors of mTOR in mouse bone marrow DC cultures did negatively affect the final yield of DCs (data not shown). In contrast, the suppression of mTOR expression by retrovirally introduced mTOR-targeting shRNA did not prevent GM-CSF-driven differentiation of mouse DCs from bone marrow, possibly because the hairpin is not expressed until after the DC precursors have finished proliferating in response to GM-CSF (35). Taken together, these data indicate that mTOR signaling may be important for different functions at different times over the lifespan of a DC. The fact that mTOR inhibition actually promoted increased DC survival following activation by TLR agonists emphasizes the emerging concept that the effects of mTOR regulation of DC metabolism and growth factor signaling may be highly context specific. Understanding the nuances of the functional role of mTOR in DCs has potential to provide important insights about the underlying cell biology controlling activation and survival signals in these cells.
One of the challenges raised by the findings reported here showing adjuvant-like effects of inhibiting mTOR in DCs is to reconcile our data with the expansive literature on the use of mTOR inhibitors to promote immune tolerance. Inhibition of mTOR by rapamycin is reported to support the induction of tolerogenic DCs (36, 37) and to inhibit both Flt3 ligand and GM-CSF-driven DC differentiation in vitro (29, 34, 38, 39). In contrast, our data strongly support an emerging view that, in certain contexts, mTOR inhibitors administered simultaneously with TLR stimulation can enhance the activation of DCs (29, 30). The underlying explanation for this apparent disagreement may lie in the details of how experiments are performed and in the nature of the DCs being tested. Based on the role of mTOR in signaling by a number of growth factors and cytokines (12, 25, 34), we speculate that specific culture conditions during in vitro generation of DCs may determine the effect of mTOR inhibition on these cells. For example, Flt3 ligand-differentiated DCs may respond differently to mTOR inhibitors during TLR activation than GM-CSF-differentiated DCs. We speculate that by restricting mTOR inhibition specifically to the initial phases of TLR activation, we can modulate DC activity without inducing the deleterious effects that may be associated with disrupted growth factor signaling in some DC subtypes.
We previously proposed that the prolonged increase in aerobic glycolysis in activated DCs serves to rapidly generate ATP, while conserving the metabolic intermediates such as amino acids and fatty acids that we predicted would be important for the complete enactment of the activation process (13). Nevertheless, we observed that mTOR-inhibition in the context of TLR activation potently inhibits metabolic commitment to glycolysis, but does not affect TLR-mediated activation, insofar as antigen processing and presentation, IL-12 and TNFα production, costimulatory molecule expression, cellular migration, and DC:T cell interactions are concerned. Thus it is clear that TLR-agonist mediated DC activation does not require commitment to aerobic glycolysis when mTOR is inhibited. Indeed, our findings indicate that sustained glycolysis subsequent to TLR activation is associated with rapid cell death and that restricting glucose consumption by mTOR inhibition prolongs the lifespan of these cells without compromising their ability to stimulate T cells in vitro or in vivo. A strong association between glucose consumption and cell death is widely reported in the literature and restricting glucose usage and caloric intake leads to increased cellular and organismal longevity (10, 14, 15, 40–42). Interestingly, the strong metabolic shift to aerobic glycolysis upon LPS stimulation was not observed in human myeloid DCs, which may be an important factor in the different survival kinetics of activated human and mouse DCs in our systems. However, mTOR inhibition augmented and prolonged costimulatory molecule expression in both human and mouse DCs, indicating that mTOR may be regulating DC survival and costimulatory molecule expression by two different mechanisms. The underlying mechanism by which inhibition of mTOR allows DCs to enact their activation program in the absence of a switch to glycolysis as well as the apparent differences in metabolic regulation of DCs between human and mouse are current focuses of research in our laboratory.
One of the intriguing findings of our study is the disparity in sensitivity of IL-10 versus IL-12p70/TNFα production to mTOR inhibition. The mTOR substrate 4EBP1 drives cap-dependent translation (43–46), so despite the fact that similar observations have been reported previously regarding the specific inhibition of IL-10 production in DCs by mTOR ablation (29, 30), it was surprising to us that whilst TLR-agonist induced IL-10 production was inhibited by rapamycin, the production of IL-12p70 and TNFa remained unaffected. This finding raises the possibility that translation of IL-12p70 and TNFa transcripts can be initiated by cap-independent processes (47). In light of our observations on the effects of mTOR on the surface expression of costimulatory molecules, we speculate that normal cellular dynamics like vesicular trafficking may also have slowed in rapamycin-treated cells. This is consistent with the fact that mTOR controls the expression of genes involved in cholesterol and fatty acid synthesis (32).
There is a ongoing interest in biomedical research in harnessing the immune-stimulatory properties of DCs for immune intervention against tumors. The Food and Drug Administration recently approved Provenge, the first autologous cellular immunotherapy for use in cancer patients (48), which marks an important hallmark in advancing the use of cellular immunotherapy strategies in the clinic. Furthermore, DC vaccination strategies have shown significant promise in early-phase clinical trials in human melanoma patients (49). Our studies using a mouse model of melanoma outline a potential new strategy for augmenting DC activation in the context of therapeutic autologous vaccination. Because brief exposure to mTOR inhibitors in vitro enhances the activation phenotype in both mouse and human DCs, we believe that our findings may be of significant relevance to ongoing clinical research aimed at improving the potency of DC therapeutic vaccines.
Our data define mTOR signaling as a new molecular target for augmenting DC survival and activation. We show that mTOR inhibition extends the lifespan of DCs, and enhances the expression of key costimulatory molecules involved in the initiation of adaptive immune responses. Ongoing studies are aimed at further understanding the consequences of mTOR inhibition in the context of the beneficial effects on DCs reported here. The fact that mTOR inhibitors, including rapamycin, are approved for use in human patients, and that our approach requires that DCs be exposed to rapamycin only in vitro prior to transfer into recipients, increases the feasibility of potentially translating this simple strategy to clinical settings. In summary, our studies demonstrate a novel pharmacological approach for temporally extending DC lifespan, prolonging DC activation, and improving the outcome of autologous DC vaccination for experimentally induced cancer in mice.
Supplementary Material
Acknowledgements
The authors thank Brent Berwin and Dick Dutton for tumor cells, Larry Johnson for help with statistical analysis, and the CVPH Medical Center for donation of blood filters.
Footnotes
This work was supported by NIH grant AI053825 (E.J.P.), NIH institutional post-doctoral training award AI049823 (E.A.), and by the Trudeau Institute.
References
- 1.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 3.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 4.Palucka K, Ueno H, Zurawski G, Fay J, Banchereau J. Building on dendritic cell subsets to improve cancer vaccines. Curr Opin Immunol. 2010;22:258–263. doi: 10.1016/j.coi.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, Kang TH, Noh KH, Bae HC, Kim SH, Yoo YD, Seong SY, Kim TW. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunol Lett. 2009;122:58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Kang TH, Noh KH, Kim SH, Lee YH, Kim KW, Bae HC, Ahn YH, Choi EY, Kim JS, Lee KM, Kim TW. Enhancement of DC vaccine potency by activating the PI3K/AKT pathway with a small interfering RNA targeting PTEN. Immunol Lett. 2010;134:47–54. doi: 10.1016/j.imlet.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Kang TH, Bae HC, Kim SH, Seo SH, Son SW, Choi EY, Seong SY, Kim TW. Modification of dendritic cells with interferon-gamma-inducible protein-10 gene to enhance vaccine potency. J Gene Med. 2009;11:889–898. doi: 10.1002/jgm.1371. [DOI] [PubMed] [Google Scholar]
- 8.Okada N, Mori N, Koretomo R, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther. 2005;12:129–139. doi: 10.1038/sj.gt.3302358. [DOI] [PubMed] [Google Scholar]
- 9.Gulati P, Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans. 2007;35:236–238. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 10.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer AJ, Codogno P. Nutrient sensing: TOR's Ragtime. Nat Cell Biol. 2008;10:881–883. doi: 10.1038/ncb0808-881. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RG, Bui T, White C, Madesh M, Krawczyk CM, Lindsten T, Hawkins BJ, Kubek S, Frauwirth KA, Wang YL, Conway SJ, Roderick HL, Bootman MD, Shen H, Foskett JK, Thompson CB. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity. 2007;27:268–280. doi: 10.1016/j.immuni.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel DRG, Torrie JH. Principles and procedures of statistics, with special reference to the biological sciences. New York: McGraw-Hill; 1960. [Google Scholar]
- 21.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 22.Matsue H, Edelbaum D, Hartmann AC, Morita A, Bergstresser PR, Yagita H, Okumura K, Takashima A. Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 23.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 24.McMahon G, Weir MR, Li XC, Mandelbrot DA. The Evolving Role of mTOR Inhibition in Transplantation Tolerance. J Am Soc Nephrol. 2011;22:408–415. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 28.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csibi A, Blenis J. Appetite for destruction: the inhibition of glycolysis as a therapy for tuberous sclerosis complex-related tumors. BMC Biol. 2011;9:69. doi: 10.1186/1741-7007-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 34.Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101:1439–1445. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol. 2008;180:7931–7937. doi: 10.4049/jimmunol.180.12.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 37.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, Liew FY, Geller DA, Thomson AW. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 39.Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Liu L, Tollefsbol TO. Glucose restriction can extend normal cell lifespan and impair precancerous cell growth through epigenetic control of hTERT and p16 expression. FASEB J. 2010;24:1442–1453. doi: 10.1096/fj.09-149328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Tollefsbol TO. p16(INK4a) suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanisms. PLoS One. 2011;6:e17421. doi: 10.1371/journal.pone.0017421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, Platanias LC. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem. 2008;283:8601–8610. doi: 10.1074/jbc.M707934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar V, Pandey P, Sabatini D, Kumar M, Majumder PK, Bharti A, Carmichael G, Kufe D, Kharbanda S. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 47.Shatsky IN, Dmitriev SE, Terenin IM, Andreev DE. Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol Cells. 2010;30:285–293. doi: 10.1007/s10059-010-0149-1. [DOI] [PubMed] [Google Scholar]
- 48.Drake CG. Update on prostate cancer vaccines. Cancer J. 2011;17:294–299. doi: 10.1097/PPO.0b013e3182325e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridolfi L, Petrini M, Fiammenghi L, Maria Granato A, Ancarani V, Pancisi E, Brolli C, Selva M, Scarpi E, Valmorri L, Nicoletti SV, Guidoboni M, Riccobon A, Ridolfi R. Dendritic cell-based vaccine in advanced melanoma: update of clinical outcome. Melanoma Res. 2011 doi: 10.1097/CMR.0b013e32834b58fa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.