Abstract
The enteric microbiota contributes to gastrointestinal health and its disruption has been associated with many disease states. Some patients consume probiotic products in attempts to manipulate the intestinal microbiota for health benefit. It is important for gastroenterologists to improve their understanding of the mechanisms of probiotics and the evidence that support their use in practice. Clinical trials have assessed the therapeutic effects of probiotics for several disorders, including antibiotic-or Clostridium difficile-associated diarrhea, irritable bowel syndrome, and the inflammatory bowel diseases. Although probiotic research is a rapidly evolving field, there are sufficient data to justify a trial of probiotics for treatment or prevention of some of these conditions. However, the capacity of probiotics to modify disease symptoms is likely to be modest and varies among probiotic strains—not all probiotics are right for all diseases. The current review provides condition-specific rationale for using probiotics as therapy and literature-based recommendations.
Keywords: Clostridium difficile, IBD, IBS, irritable bowel, clinical practice, evidence based practice, Crohn’s disease and colitis, yogurt, pouchitis
INTRODUCTION
For over a hundred years it has been recognized that certain microorganisms may impart health benefits to the host when administered in adequate amounts. These microorganisms, termed probiotics, have recently become a topic of significant focus in basic and clinical investigation. Relevant to the practice of gastroenterology, probiotics are commonly used by patients with gastrointestinal complaints or diseases. Increasingly, probiotics are also being recommended by the clinicians who treat these conditions.1
The goal of this review is to provide clinicians with an overview of the rationale and data which support or refute the role of probiotics for treating commonly encountered gastrointestinal disorders. The information provided is based on review of primary literature from randomized controlled trials (RCTs), meta-analyses, expert consensus panel recommendations and society-based practice recommendations. References are provided for more in depth reading and tables or figures summarize key information.
THE HUMAN MICROBIOME AND PROBIOTIC MECHANISMS
To understand the role that probiotics may have in influencing health, it is important to have an appreciation of the roles of the normal intestinal microbiome (commensal microbiota). The human gastrointestinal tract is host to over 500 bacterial species as well as a less well-described virome. These microbiota form a virtual bioreactor facilitating digestion, nutrient provision and the shaping of our immune system.2 Our intestinal bacteria weigh up to 1 kg and bacterial cells outnumber human cells by 10:1. The bacterial genome may outnumber the human genome by 100:1. Nutritional factors including several B vitamins, vitamin K, folate, and short chain fatty acids are produced by these bacteria. Up to 10% of anindividual’s daily energy needs can be derived from the byproducts of bacterial fermentation. Gastrointestinal microbiota are also critical for normal immune system development.3 The physiologic impact mediated by our resident microbes is substantial enough to have earned the label of “other organ” from some.4
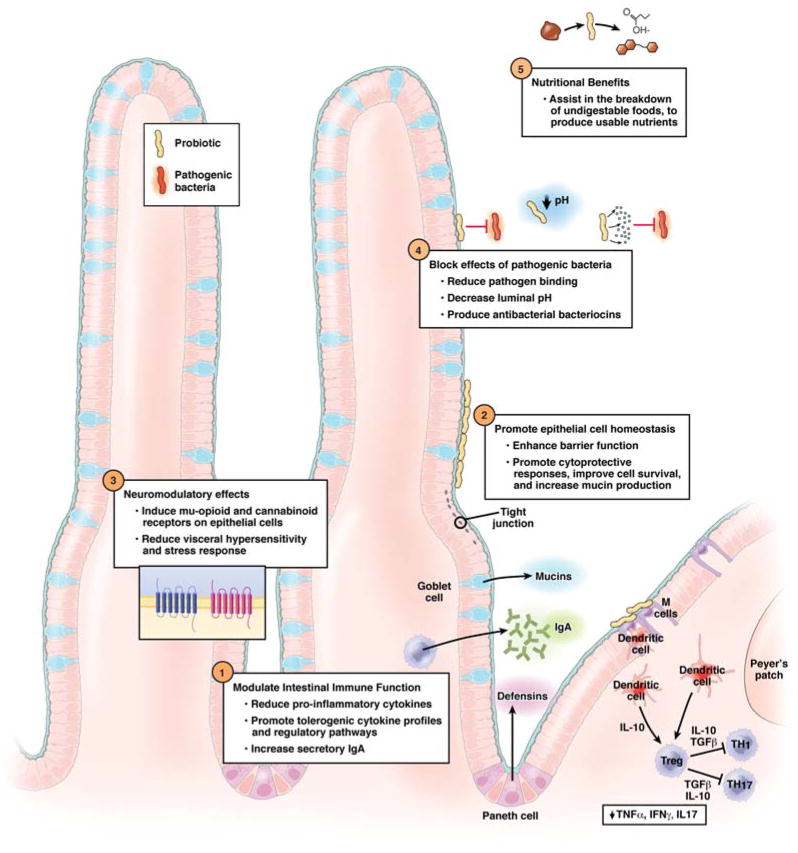
Beyond contributing to or modifying the metabolic and nutritional functions of the commensal microbiota, probiotic bacteria have several putative mechanisms by which they may confer specific beneficial effects. General categories include modulation of immune or sensory-motor function, enhancement of mucosal barrier function and anti-pathogen effects (Figure 1).5–7 Some of these mechanisms have been worked out in animal models and/or in vitro systems only.
Figure 1.
Mechanisms of action for probiotics in the gastrointestinal tract
Soluble products secreted or shed by probiotics also mediate important physiologic benefits; thus viable bacteria are not necessarily required for all benefits.8, 9 The mechanisms by which probiotics exert benefit varies by specific probiotic strain and likely depends on the clinical indication.10, 11 Therefore, as with antibiotic prescribing, clinical use of probiotics should focus on matching the probiotic strain and dosage to the condition for which it has shown benefit in clinical trials. In the future, greater understanding of probiotic specific mechanisms could allow for precise selection of a particular probiotic strain to target a patient’s specific pathogenic defect and clinical problem.
PROBIOTIC CONCEPTS FOR PRACTICE
What makes a probiotic a probiotic?
Definitions of the terms probiotic, prebiotic and synbiotic are provided in Table 1. This review focuses on probiotics, though some probiotics have been tested as part of a synbiotic product. Lactobacillus and bifidobacterium species are the most commonly used probiotics. However, one of the first probiotics, which is still in use, is the non-pathogenic Escherichia coli Nissle 1917 (ECN). Most probiotics were initially cultured from humans and resemble known commensal gut bacteria. However, the commensal population they resemble typically represents only a fraction of the total luminal bacteria. Saccharomyces boulardii is a probiotic yeast strain with the potential advantage of having resistance to most antibiotics.
Table 1.
Definitions
| Probiotics | Live microorganisms that confer a health benefit on the host when administered in adequate amounts |
| Prebiotic | Dietary substances that nurture specific changes in the composition and/or activity of the gastrointestinal microbiota (favoring beneficial bacteria), thus conferring benefit(s) upon host health |
| Synbiotics | Products that contain both probiotics and prebiotics |
Adapted from Reference 17
According to current definitions, probiotics should survive both gastric acid and bile to reach the small intestine and colon where they exert their effects. Clinical and basic investigations on probiotics have used a multitude of probiotic species, both as single strains and multi-species products. Many of these probiotics are available in a lyophilized (freeze-dried) pill form, though some are available in yogurt or as packets (sachets) which can be mixed into non-carbonated drinks. Whether synergism or antagonism exists between probiotic species when offered together has not been examined in clinical studies, though both scenarios are theoretically possible. Though not exhaustive, Table 2 lists several of the more commonly available probiotic preparations which have shown benefit in human trials. Probiotics are considered dietary supplements; thus, they are not covered by medical insurance and their production is not regulated by the Food and Drug Administration. As such, product quality, purity and viability have been reported to be variable.12 However, several clinically tested probiotic products with quality-controlled production are now marketed by reputable companies.
Table 2.
Common probiotic products specifically tested for gastrointestinal disorders
| Brand Name (Company) | Bacterial Species | Clinical Condition | Effectiveness15,16 | Practice Guidelines17–19 | Bacteria Count/Dosing | Cost/Quantity |
|---|---|---|---|---|---|---|
| Activia (Dannon, White Plains, NY) | B. lactis DN-173 010, (plus yogurt starters L. bulgaricus, L. lactis and Streptococcus thermophilus) | IBS | C | 1b** | 4 oz/cup, 1–4 QD | $10–18/24 count |
|
| ||||||
| Align (Proctor & Gamble, Cincinnati, OH) | Bifidobacterium infantis 35624 | IBS | B | 1b** | 1 billion/1 QD | $29.99/28 count |
|
| ||||||
| BioGaia (Everidis Health Sciences, Saint Louis, MO) | L. reuteri protectis SD2112 (ATCC 55730 or DSM 17928) | Infectious Diarrhea Treatment | A | 1a* | 100 million QD | $29.99 |
| IBS | C | 1b* | ||||
|
| ||||||
| Bio-K+ (Bio-K plus International inc., Laval, QC, Canada) | L. acidophilus CL1285 and L. casei LBC80R | AAD Prevention | NS | 1b** | 50 billion/capsule | $29.99/15 count |
| CDAD Prevention | 1b** | BID | ||||
|
| ||||||
| Culturelle (Valio, Helsinki, Finland/Amerifit Brands, Inc., Cromwell, CT) | L. rhamnosus GG (LGG) (LGG also included in Danimals yogurt, Dannon) | AAD Prevention | A | AAP, 1b*,1b** | 10 billion/1 QD | $18–25/30 count |
| Infectious Diarrhea Treatment | A | AAP, 1a*, 2b** | ||||
| Infectious Diarrhea Prevention | B | 1b*, 1b** | ||||
| CDAD Prevention | B/C | |||||
| CDAD Prevention of Recurrence | B/C | |||||
| Crohn’s Disease | C | |||||
| IBS | B/C (children) | 1a*,1b**+ | ||||
|
| ||||||
| Danactive (Dannon. White Plains, NY) | Lactobacillus casei DN-114001 | AAD Prevention | A | 1b** | 3.1 oz/cup | $5.00/8 count |
| Infectious Diarrhea Prevention | 1b* | 10 billion/cup | ||||
| CDAD Prevention | 1b** | |||||
|
| ||||||
| Florastor (Biocodex, Inc., Creswell, OR) | Saccharomyces Boulardii | AAD Prevention | A | AAP, 1a*, 1b** | 250 mg/1 BID | $19.99/20 count |
| Infectious Diarrhea Treatment | A | 1a*,1b** | ||||
| Infectious Diarrhea Prevention | B | |||||
| CDAD Prevention | B/C | |||||
| CDAD Prevention of Recurrence | B/C | 1b** | ||||
| Crohns | C | |||||
|
| ||||||
| Mutaflor (Ardeypharm, Herdecke, Germany) | E.coli Nissle 1917 (ECN) | UC Induction | B | 100 mg/capsule/BID | $62–$81/60 | |
| UC Maintenance | A | 1b**, BSG “A” | Canada# | |||
|
| ||||||
| VSL*3 (Sigma-Tau Pharmaceuticals, Inc., Towson, MD) | Combination Probiotic Product (Streptococcus thermophilus, B. breve, B. longum, B infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbreuckii/bulgaricus | IBS | B/C | 122.5 billion/capsule | $86/30 Sachets | |
| UC Induction | B | 1b** | 450 billion/sachet | $52/60 count | ||
| UC Maintenance | A | IBS: ½–1 sachet/day | ||||
| Pouchitis: Prevention and Maintaining Remission | A | 1b**, BSG “B” | Pouchitis: 2–4 sachets/day UC: 1–8 sachets/day |
|||
Effectiveness based on expert panel recommendations where: A=strong, positive, well-conducted, controlled studies in the primary literature, B=some positive, controlled studies but presence of some negative studies or inadequate amount of work to establish the certainty; C=some positive studies but clearly inadequate amount of work to establish the certainty. Practice Guidelines include those of 1) World Gastroenterology Organisation’s global guidelines evidence level assignment17 based on the Centre for Evidence Based Medicine system20:
pediatrics,
adults,
as part of a multispecies probiotic product 2)American Academy of Pediatrics recommended (AAP)18 3) British Society of Gastroenterology (BSG) guidelines Grade of Evidence19 Grade A indicates consistent results among RCTs, Grade B indicates consistent cohort studies or smaller RCTs.
E. coli Nissle is currently removed from the U.S. market respecting the FDA’s decision on the classification of the product as a “biologic” instead of its former status as a “medical food.”
Does any yogurt work just like a probiotic?
Lactic acid producing bacteria have been used for centuries in food fermentation. Many yogurts contain live-active lactobacillus cultures and are considered functional food products; however, most are not considered probiotics per se. This term is reserved for products with an adequate number of microorganisms at time of consumption specifically shown to confer health benefits in controlled human trials. Yogurts fortified with an adequate number of viable bacteria shown to exert benefit in controlled trials are classified as probiotics. Given this information, and the knowledge that probiotic benefits appear species specific, expected clinical endpoints may not be achieved by generically recommending yogurt to patients in whom a purported probiotic benefit is desired. It should be noted, however, that yogurt consumption has other benefits including improved lactose tolerance and the provision of protein, vitamin D and calcium.
How long does one have to take a probiotic?
As viable microorganisms, probiotics can survive in the human gut and impact microbes which colonize the gut. Probiotics are often detectable in the stool by culture or gene-based assays during periods of consumption. However, many probiotic strains do not colonize the gut and are no longer recoverable in stool 1–4 weeks after stopping consumption.13 For example, McNulty and colleagues recently evaluated a fermented milk product with probiotic strains matching the commercially available Activia (Dannon, White Plains, NY). The investigators showed that the probiotic product did not change the gut’s overall bacterial composition, but instead altered gene expression patterns relevant to carbohydrate metabolism in the host’s resident gut microbes.14 These changes in the human fecal “metatranscriptome” were transient, confined only to the time of the probiotic consumption. Thus, if sustained benefit from a probiotic is desired, continued consumption is likely required.
Where can probiotics fit into a therapeutic algorithm?
Data for probiotic use in several GI disorders is reviewed in the following section. For antibiotic associated diarrhea and viral gastroenteritis supporting data are strong and probiotics are among the only treatment modalities available. However, the duration of symptoms in these conditions is typically short regardless of probiotic use. In ulcerative colitis, pouchitis and irritable bowel syndrome adequate data exists for clinicians to consider recommending a therapeutic trial of specific probiotic strains or preparations in selected patients. In these conditions probiotics are usually administered as adjunctive therapy, rather than primary or first-line therapy. The decision to recommend probiotic therapy ultimately depends on the clinical scenario, patient interest and clinician preference. In hepatic encephalopathy, Crohn’s disease and Clostridium difficile-associated diarrhea (CDAD), conventional medical therapies remain the gold standard. Practice relevant probiotic concepts are summarized in Table 3.
Table 3.
Practical considerations relevant to probiotics in practice
|
PROBIOTIC THERAPY FOR GASTROINTESTINAL CONDITIONS
Acute Onset Infectious Diarrhea
Several randomized controlled trials (RCTs) have evaluated the use of probiotics in acute infectious diarrhea. The data are largely from pediatric studies where both prevention and treatment were examined. Trials were conducted across the world with durations of up to 1 year. In the pediatric population, rotavirus has been the most common cause of infectious diarrhea. Data suggests that the benefit of probiotics in preventing acute infectious diarrhea is modest.18, 21 Lactobacillus rhamnosus GG (LGG), L. reuteri and L. casei all have shown benefit, with an approximate NNT of 7 children to prevent 1 case of rotavirus in the child care center setting.22–24 With the currently available rotavirus vaccine in consideration, the American Academy of Pediatrics states that probiotics for preventing acute infectious diarrhea are not universally endorsed, but acknowledges that they may have a role in special circumstances.18 According to the US Center for Disease Control, data is not sufficient to support the use of probiotics such as LGG to prevent traveler’s diarrhea of bacterial origin.
The data supporting treatment of acute infectious diarrhea with probiotics are stronger. LGG is the most effective probiotic reported on to date, reducing both severity and duration of diarrhea by ~1 day.25, 26 The American Academy of Pediatrics supports the recommendation of LGG early in the course of acute infectious diarrhea to reduce symptom duration.18
Antibiotic Associated Diarrhea
Antibiotic use is common in children, and diarrhea develops in ~20% of those taking antibiotics. Prevention of non-C.difficile-related antibiotic associated diarrhea (AAD) with probiotics has been assessed in RCTs. A 2011 Cochrane Review evaluating >3400 patients from 16 studies concluded that the overall evidence suggests a protective effect of probiotics in preventing AAD.27 Studies using LGG and S. boulardii produced the most convincing results.28 The NNT to prevent one case of AAD was ~7 in the Cochrane Review. The American Academy of Pediatrics supports the recommendation of probiotics for prevention of, but not treatment of, AAD.18
In the adult population probiotics also appear effective in limiting AAD. A meta-analysis evaluating studies on various probiotics and antibiotic regimens published between 1977–2005 found that both LGG and S. Boulardii offered a reduction in risk of AAD development (combined RR 0.31 and 0.37 respectively).29 Two recent placebo-controlled RCTs evaluated combination probiotic products for the prevention of antibiotic associated diarrhea as their primary endpoint. Hickson et al used the probiotic mixture currently marketed as DanActive (Dannon, White Plains, NY) in the United States and found that it significantly reduced AAD (12% vs. 34%) in an older cohort of hospitalized patients.30 A second study evaluated a combination probiotic containing both L. casei and L. acidophilus (Bio-K+, Bio-K Plus International, Quebec, Canada) in 255 patients. Patients given the higher dose of probiotic concurrent with antibiotics (and for 5 days afterward) had fewer occurrences of AAD (15.5 vs. 44.1%).31 As a secondary endpoint, both of these studies also showed a reduction in development of C. difficile-associated diarrhea (discussed below).
Clostridium Difficile Associated Diarrhea
C.difficile-associated diarrhea (CDAD) is a common nosocomial and community-based medical condition. While typically linked to antibiotic induced disturbance of the intestinal microbiota, CDAD is now increasingly identified in patients without recent antibiotic exposure.32 Antibiotic therapy with metronidazole, oral vancomycin and now fidaxomicin makeup the current treatment paradigm.33 Recurrence of CDAD remains a clinical problem. In 1994 a trial reported that S. boulardii (500mg bid) offered for 4 weeks after antibiotic therapy reduced overall CDAD recurrence rates.34 However, the finding was only significant for those with a history of recurrent CDAD. A follow up study, designed to be confirmatory, did not find S. boulardii to significantly reduce CDAD recurrence after standard therapy.35 Though a favorable trend was found in patients treated with high-dose vancomycin (2 g/day) in the latter study, the clinical significance of this is less clear. Lactobacillus probiotics have been tested as single species and as combination probiotic products for preventing CDAD recurrence. While some results have been promising, most studies are underpowered, have methodological flaws, or have not been reproduced.36
Probiotic-based primary prevention still may be an approach to the current scourge of C. difficile. The two recent probiotic trials discussed above in the AAD section suggest that this may be feasible. The Hickson study reported that DanActive supplementation in older hospitalized adults reduced AAD, but also CDAD (0% vs. 17% placebo).30 The study evaluating the combination probiotic Bio-K+ also showed a reduction in CDAD in the treated cohort (1.2% vs. 23.8% placebo).31 The high incidence rate of C.difficile positivity in the placebo groups (17% and 23.8%) is a criticism for both of these studies. Nonetheless, if confirmatory studies show similar results, these intriguing findings may lead to a paradigm shift in managing older adults requiring antibiotic therapy.
While controversy exists, current society guidelines and expert opinion panels state that existing data are not sufficient to justify recommending available probiotics for preventing primary or recurrent CDAD.15, 36, 37
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is characterized by symptoms of abdominal pain and altered bowel habits which occur over at least three months. This common disorder is managed with varying clinical styles as no dominant therapeutic strategy has emerged.38 The pathophysiology of IBS remains unknown, but several lines of evidence link symptomatic expression of this disorder with the intestinal microbiota. IBS patients may have subtle differences in their luminal and mucosal-associated intestinal microbiota compared to controls.39, 40 New onset IBS symptoms can develop in up to one-third of individuals after recovery from a self-limited episode of infectious gastroenteritis.41, 42 Small-bowel bacterial overgrowth has been reported in a proportion of IBS patients, and antibiotics offer relief of IBS symptoms in some individuals.43, 44 So, while controversy exists, bacteria likely contribute to at least some symptoms of IBS.45
Several clinical trials have investigated the potential for probiotics as therapy in IBS. These trials are the subject of several single topic reviews.16, 46, 47 Systematic summarization of these results is complicated by the inclusion of several probiotic strains/species, single or combination preparations, dosing regimens and unique study designs. Several studies included endpoints which were not clinically applicable or demonstrated improvement over baseline, but not compared to placebo.16 Most studies were short term only; data on long-term efficacy are still lacking.
A meta-analysis of 3 RCTs suggests that LGG moderately improves pain symptoms in children with IBS (NNT=4).48 Traditional IBS treatment endpoints have not been adequately met in studies of other single strain lactobacillus species in adults.16 A Bifidobacterium infantis strain (B.infantis 35624, Align, Proctor and Gamble, Cincinnati, OH) was evaluated in two clinical trials. One study found significant reductions in pain, bloating, bowel movement difficulty and composite symptom score versus placebo and a lactobacillus species.49 In a larger follow-up study, reduction in pain and global relief of IBS symptoms were significantly greater in the B.infantis treated group compared to placebo.50
General recommendations from the American College of Gastroenterology as well as expert consensus panels from both the United States and in Europe are similar.15, 17, 38 There is reasonable rationale for why probiotics may work as treatment for IBS. There are at least some positive controlled studies showing that probiotic supplementation reduces IBS symptoms in some patients. The evidence of benefit is not sufficiently strong to support the general recommendation of probiotics for IBS; however, the benefit appears greatest for bifidobacteria species and certain combinations of probiotics which include bifidobacteria species rather than single species lactobacillus probiotics.
With probiotics, patients might experience a global improvement in symptomatology rather than specific improvement in bowel function. Since treatment options for IBS remain limited in both number and efficacy, a therapeutic trial of probiotics is reasonable for patients interested in this approach.
Inflammatory Bowel Diseases
Evidence points to the intestinal microbiome being a key player in the development and perpetuation of the inflammatory bowel diseases.51 Defects in the innate immune response to commensal intestinal bacteria resulting in an exaggerated adaptive immune response to these organisms are implicated in the pathogenesis of Crohn’s disease (CD).52 Several key CD risk genes have functions related to bacterial killing, and antibiotics have therapeutic efficacy in CD and pouchitis.53, 54 Compared to CD, a central role for gut bacteria is less strongly implicated in the pathogenesis of ulcerative colitis.55 However, the evidence supporting probiotics in patient management is better for UC and pouchitis than for CD.
Several limitations exist with trials which have evaluated probiotic therapy in the inflammatory bowel diseases. These include small cohort sizes, use of different probiotic doses and strains, varied treatment durations and differences in concurrent conventional treatments. Regardless, patients with IBD often take or consider taking probiotics and appreciate their clinician having knowledge of the topic.
Crohn’s Disease
Probiotic use in the management of Crohn’s disease is not supported by currently available RCT data. Trials have found LGG and other lactobacilli not superior toplacebo as an additive to standard care for inducing or maintaining remission in CD or for preventing post operative relapse.56–58 There is also no solid data to support the use of ECN or S. boulardii in CD.59
Ulcerative Colitis
Several published RCTs have shown benefit of probiotics in the management of ulcerative colitis (UC). These studies have examined induction of remission and maintenance of remission typically by comparing the probiotic to oral mesalamine or adding the probiotic to standard therapy. ECN at 200 mg/day was similar in efficacy to 1500 mg of mesalamine for maintaining UC in remission.60
High dose VSL#3 (3.6 trillion cfu/day) has shown therapeutic efficacy in two RCTs evaluating patients with mild-to-moderately active UC. When offered to UC patients having a flare while on a 5-ASA or an immunomodulator, the probiotic cohort demonstrated improved symptom-based disease activity indices and rectal bleeding, but not endoscopic scores, compared to the placebo group.61 A study conducted in India included 144 adults with relapsing UC and showed the VSL#3 group to have significantly higher remission rates (42.9% vs. 15.9%) and endoscopic healing (32% vs. 14.7%).62 Most patients in both groups remained on a stable dose of mesalamine therapy. A high dropout-rate in both groups (29% VSL3, 59% placebo) was a limitation of the latter study. VSL#3 was also shown to improve rates of induction and maintenance of remission in children with UC (n=29 total).63 Recent Cochrane reviews conclude that there is insufficient data to demonstrate that probiotics have efficacy in maintaining remission in UC; however, they have not recently addressed induction of remission in UC.64 Single strain lactobacillus and bifidobacterium (infantis 35624, Align) probiotics did not show efficacy for maintaining UC remission in clinical trials.65, 66
In summary, the overall evidence suggests that ECN and VSL#3 have modest efficacy, similar to and perhaps complementary to mesalamine, in inducing and maintaining remission for mild-to-moderately active UC.
Pouchitis
Chronic or recurrent pouchitis is an important complication occurring in ~10–20% of UC patients after ileal anal pouch formation surgery. VSL#3 was shown beneficial in prophylaxis against pouchitis onset after surgical take-down 67 and in maintaining clinical remission after antibiotic induction.68, 69 These trials were conducted in Europe and included ~20 patients per group. A practice based report from the Cleveland Clinic found only 19% (6 of 31) of patients who were started on VSL#3 after treatment with antibiotics to still be taking the probiotic at 8 months.70 A single study from the Netherlands found that compared to a historical cohort, patients taking LGG had a delayed onset of pouchitis at 3 years (7% vs. 29%).71
Clinical expert-generated guidelines concur that probiotics (VSL#3) can be effective for preventing recurrence of pouchitis.15, 72, 73
Complications of Chronic Liver Disease/Hepatic Encephalopathy
Luminal microbiota play an important role in the pathogenesis of both spontaneous bacterial peritonitis and hepatic encephalopathy. Ammonia produced by gut bacteria is believed to play a key role in hepatic encephalopathy. Antibiotics are employed in clinical practice to reduce severity or frequency of both these chronic liver disease complications. Lactulose, a mainstay of therapy, is a prebiotic for lactobacilli which can limit bacterial ureases.
The role for probiotics in these disorders is an area of ongoing investigation.74 Treatment of hepatic encephalopathy with lactobacillus acidophilus was studied as early as 1965.75 More recently, Liu and colleagues offered a probiotic and prebiotic mixture to 97 patients with minimal hepatic encephalopathy and observed a reduction in ammonia levels and improvement in encephalopathy.76 Another group found that a yogurt-based probiotic supplement significantly improved quantitative measurements of minimal hepatic encephalopathy in nonalcoholic cirrhotics.77 The latter group is now completing a more comprehensive trial using the probiotic LGG in a similar patient cohort.78
Society guidelines and expert consensus panels do not currently support a recommendation of probiotic use for any chronic liver disease associated condition.
SAFETY OF PROBIOTICS
For most populations, probiotic consumption is considered safe and complications rare. A review on the safety of probiotics by Snydman points out that although case reports of bacteremia and endocarditis (LGG) as well as cases of fungemia (S. boulardii) exist, epidemiologic evidence suggests that there is no overall increase in population-risk based on usage data.79 This position is substantiated by a recent US government commissioned review panel report.80 As a caveat however, a high profile multicenter placebo controlled Dutch RCT examining probiotic supplementation in severe acute pancreatitis found a higher incidence of mesenteric ischemia and death in the treatment group.81 This is the only trial to date to infer such a relationship, but supports the concept that probiotics should be avoided in critically ill patients. Indwelling central vein catheters and perhaps cardiac valvular disease may be relative contraindications.82
WHAT LIES AHEAD FOR PROBIOTICS IN DIGESTIVE DISEASES
Inspired investigators and technical advances in genomics are facilitating in-depth scientific investigation of the human microbiome and the functional capacities of probiotics. These advances are sure to bring paradigmatic changes to our fundamental understanding of how microbiota influence health and how they can be manipulated to combat disease and improve quality of life. Future indications and therapeutic directions for probiotics may include conditions as diverse as mood disorders, obesity, autism and diabetes. Recent clinical trials and translational studies suggest that lactobacillus probiotics may offer epithelial cytoprotection to limit symptoms of radiation enteritis, a dose limiting side effect for patients receiving abdominal radiotherapy for malignancy.8, 83, 84 Promise is held for confirmative testing of helminth-based therapy and “turbo-probiotics” designed to secrete human cytokines. Gene based bacterial profiling studies from disease affected humans have identified what may be novel “probiotics” such as Faecalibacterium prausnitizii and Clostridium species IV and XIVa. Finally, the identification, purification and repackaging of probiotic-derived soluble factors possessing proven capacity to modify biologic function may allow us to harness the power of probiotics while averting the potential risks associated with live bacteria. Some suggest that as these advances progress to the clinic we will shift from the term probiotic into the new world of pharmabiotics.85
CONCLUSIONS
Evidence supports a role for considering the recommendation of conventional probiotics for some clinical conditions. Probiotic strain selection should focus on quality tested products with clinically demonstrated benefit for the given disorder. Patients and physicians should expect modest effects and consider using probiotics as a supplement to, rather than a replacement for, conventional therapy. Though challenges exist, ongoing investigations offer great promise for the future. Perhaps one day clinicians will have the opportunity to use directed selection of a probiotic or probiotic derived product to specifically address a patient’s unique disease causing physiologic or genetic defect.
Acknowledgments
Grant Support: MAC has funding from NIH grant DK089016
Work partially supported by NIH grant DK089016. I thank my colleagues at Washington University for their critical assessment of this review and for the knowledge shared by participants at the 2011 FASEB conference on Probiotics.
Abbreviations
- CD
Crohn’s disease
- UC
ulcerative colitis
- IBS
irritable bowel syndrome
- RCT
randomized controlled trial
- AAD
antibiotic associated diarrhea
- CDAD
Clostridium difficile-associated diarrhea
- ECN
E.coli Nissle 1917
- LGG
Lactobacillus rhamnosus GG
Footnotes
Author Involvement: MAC drafted the manuscript in entirety
Disclosures: MAC received support for laboratory research from VSL Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams MD, Ha CY, Ciorba MA. Probiotics as therapy in gastroenterology: a study of physician opinions and recommendations. J Clin Gastroenterol. 2010;44:631–636. doi: 10.1097/MCG.0b013e3181d47f5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 4.Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 5.Ng SC, Hart AL, Kamm MA, et al. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 6.Madsen KL. Interactions between microbes and the gut epithelium. J Clin Gastroenterol. 2011;45(Suppl):S111–114. doi: 10.1097/MCG.0b013e3182274249. [DOI] [PubMed] [Google Scholar]
- 7.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 8.Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2011 doi: 10.1136/gutjnl-2011-300367. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan F, Cao H, Cover TL, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanahan F. Probiotics in perspective. Gastroenterology. 2010;139:1808–1812. doi: 10.1053/j.gastro.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Mileti E, Matteoli G, Iliev ID, et al. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS ONE. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman S, Spicer D. Safety and Reliability of Lactobacillus Supplements in Seattle, Washington. The Internet Journal of Alternative Medicine. 2003:1. [Google Scholar]
- 13.Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol. 2011;45(Suppl):S115–119. doi: 10.1097/MCG.0b013e318227414a. [DOI] [PubMed] [Google Scholar]
- 14.McNulty NP, Yatsunenko T, Hsiao A, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floch MH, Walker WA, Madsen K, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;45(Suppl):S168–171. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 16.Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2011;45(Suppl):S145–148. doi: 10.1097/MCG.0b013e31822d32d3. [DOI] [PubMed] [Google Scholar]
- 17.Guarner F, Khan AG, Garisch J, et al. Probiotics and prebiotics: World Gastroenterology Organisation Global Guidelines. 2011 Available at: http://www.worldgastroenterology.org/probiotics-prebiotics.html.
- 18.Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–1231. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 19.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 20.Centre for Evidence Based Medicine Levels of Evidence. 2009 www.cebm.net. Available at: www.cebm.net.
- 21.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;45(Suppl):S149–153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 22.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 23.Pedone CA, Arnaud CC, Postaire ER, et al. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract. 2000;54:568–571. [PubMed] [Google Scholar]
- 24.Szajewska H, Kotowska M, Mrukowicz JZ, et al. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 25.Szymanski H, Pejcz J, Jawien M, et al. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains--a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–253. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 26.Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010:CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;11:CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367–372. doi: 10.1016/j.jpeds.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 29.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 30.Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636–1641. doi: 10.1038/ajg.2010.11. [DOI] [PubMed] [Google Scholar]
- 32.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 33.Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1–9. doi: 10.1097/MOG.0b013e32834bc9a9. [DOI] [PubMed] [Google Scholar]
- 34.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 35.Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 36.Na X, Kelly C. Probiotics in clostridium difficile Infection. J Clin Gastroenterol. 2011;45(Suppl):S154–158. doi: 10.1097/MCG.0b013e31822ec787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 38.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 39.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JK, Thabane M, Garg AX, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. quiz 660. [DOI] [PubMed] [Google Scholar]
- 42.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 43.Ford AC, Spiegel BM, Talley NJ, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel BM. Questioning the bacterial overgrowth hypothesis of irritable bowel syndrome: an epidemiologic and evolutionary perspective. Clin Gastroenterol Hepatol. 2011;9:461–469. doi: 10.1016/j.cgh.2011.02.030. quiz e459. [DOI] [PubMed] [Google Scholar]
- 46.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 47.Brenner DM, Moeller MJ, Chey WD, et al. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–1049. doi: 10.1038/ajg.2009.25. quiz 1050. [DOI] [PubMed] [Google Scholar]
- 48.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302–1310. doi: 10.1111/j.1365-2036.2011.04665.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 50.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 51.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 56.Schultz M, Timmer A, Herfarth HH, et al. Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol. 2004;4:5. doi: 10.1186/1471-230X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousvaros A, Guandalini S, Baldassano RN, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–839. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 58.Prantera C, Scribano ML, Falasco G, et al. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–409. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45(Suppl):S139–144. doi: 10.1097/MCG.0b013e31822103f7. [DOI] [PubMed] [Google Scholar]
- 60.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209. 1209, e1201. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Miele E, Pascarella F, Giannetti E, et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 64.Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;12:CD007443. doi: 10.1002/14651858.CD007443.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Fujimori S, Gudis K, Mitsui K, et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25:520–525. doi: 10.1016/j.nut.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 66.Shanahan F, Guaraner F, von Wright A, et al. A one year, double-blind, placebo controlled trial of a Lactobacillus or a Bidfidobacterium probiotic for maintenance of steroid-induced remission of ulcerative colitis. Gastroenterology. 2006;130:A-44, 249. [Google Scholar]
- 67.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 68.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 70.Shen B, Brzezinski A, Fazio VW, et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721–728. doi: 10.1111/j.1365-2036.2005.02642.x. [DOI] [PubMed] [Google Scholar]
- 71.Gosselink MP, Schouten WR, van Lieshout LM, et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876–884. doi: 10.1007/s10350-004-0525-z. [DOI] [PubMed] [Google Scholar]
- 72.Pardi DS, D’Haens G, Shen B, et al. Clinical guidelines for the management of pouchitis. Inflamm Bowel Dis. 2009;15:1424–1431. doi: 10.1002/ibd.21039. [DOI] [PubMed] [Google Scholar]
- 73.Holubar SD, Cima RR, Sandborn WJ, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. :CD001176. doi: 10.1002/14651858.CD001176.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Lata J, Jurankova J, Kopacova M, et al. Probiotics in hepatology. World J Gastroenterol. 17:2890–2896. doi: 10.3748/wjg.v17.i24.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macbeth WA, Kass EH, McDermott WV., Jr Treatment of Hepatic Encephalopathy by Alteration of Intestinal Flora with Lactobacillus Acidophilus. Lancet. 1965;1:399–403. doi: 10.1016/s0140-6736(65)90002-4. [DOI] [PubMed] [Google Scholar]
- 76.Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 77.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 78.Bajaj JS. [Accessed 12/11/2011];2011 clinicaltrials.gov/ct2/show/NCT00992290. Available.
- 79.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(Suppl 2):S104–111. doi: 10.1086/523331. discussion S144–151. [DOI] [PubMed] [Google Scholar]
- 80.Hempel S, Newberry S, Ruelaz A, et al. Services USDoHaH. Safety of Probiotics to Reduce Risk and Prevent or Treat Disease. 2011. [PMC free article] [PubMed] [Google Scholar]
- 81.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 82.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264. doi: 10.1093/ajcn/83.6.1256. quiz 1446–1257. [DOI] [PubMed] [Google Scholar]
- 83.Ciorba MA, Stenson WF. Probiotic therapy in radiation-induced intestinal injury and repair. Ann N Y Acad Sci. 2009;1165:190–194. doi: 10.1111/j.1749-6632.2009.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol. 2010;26:88–94. doi: 10.1097/MOG.0b013e3283361927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shanahan F, Collins SM. Pharmabiotic manipulation of the microbiota in gastrointestinal disorders, from rationale to reality. Gastroenterol Clin North Am. 2010;39:721–726. doi: 10.1016/j.gtc.2010.08.006. [DOI] [PubMed] [Google Scholar]