Abstract
Background
Studies of the effects of alcohol on NMDA receptor function and gene expression have depended on rodent or post-mortem human brain models. Ideally, the effects of alcohol might better be examined in living neural tissue derived from human subjects. In this study, we used new technologies to reprogram human subject-specific tissue into pluripotent cell colonies and generate human neural cultures as a model system to examine the molecular actions of alcohol.
Methods
Induced pluripotent stem (iPS) cells were generated from skin biopsies taken from 7 individuals, 4 alcohol dependent subjects and 3 social drinkers.. We differentiated the iPS cells into neural cultures and characterized them by immunocytochemistry using antibodies for the neuronal marker beta III-tubulin, glial marker s100β, and synaptic marker synpasin1. Electrophysiology was performed to characterize the iPS-derived neurons and measure the effects of acute alcohol exposure on the NMDA receptor response in chronically alcohol exposed and non-exposed neural cultures from one non-alcoholic. Finally, we examined changes in mRNA expression of the NMDA receptor subunit genes GRIN1, GRIN2A, GRIN2B, and GRIN2D after 7 days of alcohol exposure and after 24-hour withdrawal from chronic alcohol exposure.
Results
Immunocytochemistry revealed positive staining for neuronal, glial, and synaptic markers. iPS-derived neurons displayed spontaneous electrical properties and functional ionotropic receptors. Acute alcohol exposure significantly attenuated the NMDA response, an effect that was not observed after 7 days of chronic alcohol exposure. After 7 days of chronic alcohol exposure, there were significant increases in mRNA expression of GRIN1, GRIN2A, and GRIN2D in cultures derived from alcoholic subjects but not in cultures derived from non-alcoholics.
Conclusions
These findings support the potential utility of human iPS-derived neural cultures as in vitro models to examine the molecular actions of alcohol on human neural cells.
Keywords: iPS cell, alcohol, NMDA receptor, electrophysiology, gene expression
Introduction
Alcohol use disorders, including alcohol abuse and dependence, are among the most prevalent mental disorders in the United States, with approximately 8.5% of the US population being affected by them during a one-year period (Grant et al., 2004). Despite the high prevalence of these disorders, the molecular mechanisms underlying many of the behavioral effects of alcohol and those that lead to the development of tolerance and dependence are not well understood (Lewohl et al., 2000, Hanchar et al., 2006).
Ligand-gated ion channels, including the N-methyl-D-aspartate (NMDA) and GABAA receptors are targets for the actions of alcohol (Vengeliene et al., 2008) and these may play a role in the development of alcohol use disorders. In the central nervous system (CNS), NMDA receptors play important roles in synaptic transmission, synaptic plasticity (such as long-term potentiation), and excitotoxicity (Nagy, 2004). The conventional NMDA receptor is a tetrameric structure consisting of two NR1 and two NR2 (NR2A-D) subunits (Bigge, 1999) that surround a cation channel with a high permeability to calcium (Cavara and Hollmann, 2008). Under normal resting conditions the NMDA receptor is blocked by magnesium (Mg2+) ions, which reside inside the channel pore. This blockade is removed upon membrane depolarization in conjunction with the combined binding of the agonist glutamate and the co-agonist glycine. Because these channels are activated only when electrical and chemical signals are present simultaneously, NMDA receptors are thought to act as coincidence detectors (Nagy, 2008b).
Research using both in vivo and in vitro models has established that both acute and chronic exposure to alcohol affects the NMDA receptor. Acutely, alcohol acts as an NMDA antagonist (Tomberg, 2010), leading to significant decreases in the size of NMDA excitatory post-synaptic potentials (EPSPs) (Nie et al., 1994) and inhibition of NMDA-dependent long-term potentiation (LTP) (Puglia and Valenzuela, 2010). Chronic exposure to alcohol leads to an increased density of receptors and facilitation of NMDA receptor functioning, due to receptor subunit phosphorylation and increased expression of various NMDA receptor subunit mRNAs (Nagy, 2008b). Significantly increased expression after chronic alcohol exposure has been observed in NR1 subunit mRNA in the amygdala (Floyd et al., 2003) and protein in the hippocampus (Maler et al., 2005), in NR2A and NR2B subunit mRNA in the cortex and hippocampus (Follesa and Ticku, 1995) and protein in the hippocampus (Maler et al., 2005). After a 48-hour withdrawal period, NR1, NR2A, and NR2B mRNA levels returned to baseline in the hippocampus and cortex (Follesa and Ticku, 1995), and protein levels were returned to baseline in the amygdala (Roberto et al., 2006) and the cortex (Kalluri et al., 1998). The changes observed in NMDA receptor expression and function are believed to contribute to the development of alcohol tolerance and dependence, and the CNS hyperexcitability and excitotoxicity observed during alcohol withdrawal (Nelson et al., 2005, Nagy, 2008a, Nagy, 2008b)
Other studies have compared post-mortem human brain tissue derived from alcoholics and non alcoholics (Lewohl et al., 1997, Lewohl et al., 2000). NMDA receptor NR1, NR2A, and NR2B subunit mRNA expression in both the superior frontal and primary motor cortices was significantly lower in alcoholic individuals with cirrhosis of the liver than either non-alcoholic controls or alcoholics without co-morbid disease (Ridge et al., 2008). Studies using post-mortem human brain tissue bypass the questions of relating animal models to humans. However, using post-mortem brain tissue does not allow for examination of electrophysiological effects of alcohol or controlled exposure to alcohol on gene expression. In addition, factors including gender, brain pH, age of death, ethnicity, history of medication, and post-mortem interval also contribute to variability among post-mortem samples (Hynd et al., 2003, Preece and Cairns, 2003).
One way potentially to bypass the difficulties that arise with the use of animal and post-mortem human brain models is to derive pluripotent cells from samples taken from living participants, and then differentiate these pluripotent cells into the tissue of interest. Takakashi and Yamanaka reported that it was possible to reprogram mouse or human fibroblasts into a pluripotent state by virally introducing 4 factors into the cell culture, generating induced pluripotent stem (iPS) cells (Takahashi et al., 2007, Takahashi and Yamanaka, 2006). Subsequently, other groups have used protocols developed for human embryonic stem (hES) cells to differentiate iPS cells into neural cultures containing neurons and glia (Hu et al., 2010, Johnson et al., 2007). iPS cell derivation and neural differentiation provides a minimally invasive method to obtain and examine subject-specific neural tissue.
The ability to generate neurons from participant fibroblasts has made it possible to examine and study living neurons from individual subjects with different diseases (Chamberlain et al., 2008). Current research has focused on the use of iPS cell technologies to examine mechanisms of neurodegenerative disease such as amyotrophic lateral sclerosis, spinal muscular atrophy, and Parkinson’s disease (Yamanaka, 2009), and genetic, neurodevelopmental diseases including Angelman syndrome and Prader-Willi syndrome (Chamberlain et al., 2010). To our knowledge, there are no reports of research using human iPS-derived neural cells as a model for the molecular actions of alcohol and the development of alcohol use disorders.
Here, we present results focusing on the NMDA receptor to explore the potential use of human iPS-derived neural cells to examine the biological and molecular effects of alcohol. Electrophysiological recordings revealed that iPS-derived neurons in our cultures were able to generate action potentials, exhibited spontaneous synaptic activity, and expressed functional GABAA, AMPA, and NMDA receptors. Chronic effects of alcohol exposure were also detectable using electrophysiological and gene expression analysis.
Materials and Methods
iPS Cell Derivation and Culture
Fibroblasts cell lines were generated from skin punch biopsies taken from participants enrolled in clinical studies of the effects of alcohol in social drinkers and a treatment trial for problem drinkers at the University of Connecticut Health Center (UCHC). Participants signed a consent form that was approved by the UCHC institutional review board and a punch biopsy sample was taken from the inner, upper arm. Biopsy samples were finely minced and cultured in Dulbecco’s Modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS, Invitrogen) to establish primary fibroblast cultures. Fibroblast lines used in the current report were generated from 2 women and 5 men. Four of the participants met criteria for current DSM-IV alcohol dependence, including tolerance to alcohol, while three participants were classified as non-alcohol dependent social drinkers. Cell lines derived from non-alcohol dependent social drinkers are labeled 1, 2, and 3, while alcohol-dependent participants are labeled 4, 5, 6, and 7. The two cell lines derived from women are numbers 4 and 6.
Human fibroblast cells were reprogrammed by the UCHC Stem Cell Core using retrovirus constructs expressing 5 reprogramming factors (OCT4, SOX2, KLF4 c-MYC, and LIN28). iPS cell colonies were identified based on characteristic morphology approximately 2–4 weeks after viral transduction. Selected colonies were expanded as separate clones and the expression of pluripotency markers SSEA-3/4 and NANOG was verified by the UCHC Stem Cell Core using immunocytochemistry.
iPS cells were cultured and expanded by growth on a layer of irradiated mouse embryonic fibroblasts (MEFs) using human embryonic stem (hES) cell media containing Dulbecco’s Modified Eagle Medium with F12 (DMEM/F12, 1:1 ratio) (Invitrogen) supplemented with 20% Knockout Serum Replacer (Invitrogen), 1× non-essential amino acids (Invitrogen), 1mM L-glutamine solution (Invitrogen), 0.1mM β-mercaptoethanol (MP Biomedicals, Solon, OH, USA), and 4ng/mL basic fibroblast growth factor (bFGF) (Millipore, Billerica, CA, USA). Colonies were observed on a daily basis, and any colonies exhibiting spontaneous differentiation were removed. iPS media was replaced on a daily basis. iPS cells were passed every 7 days onto irradiated MEFs using a 1mg/mL dispase (Invitrogen) in DMEM/F12.
iPS Cell Neural Differentiation
iPS cells were differentiated into neural cells using an established protocol (#SOP-CH-207 Rev A) developed by the WiCell Institute (www.wicell.org, Madison, WI, USA) for the neural differentiation of hES cells. Confluent iPS colonies were harvested from 10 cM plates using 1mg/mL dispase in DMEM/F12. iPS cells were cultured in suspension for 4 days in 75mL low-adhesion flasks using hES cell media without the addition of bFGF to allow for the formation of embryoid bodies (EBs). Half of the media was changed daily. After 4 days, EBs were collected, centrifuged, and washed in phosphate buffered saline (PBS) and placed into new 75mL low adhesion flasks in a neural induction media [DMEM/F12 supplemented with 1× N2 supplement (Invitrogen), 1× non-essential amino acids and 2µg/mL heparin (Sigma-Aldrich, St. Louis, MO, USA)] to allow for the formation of neural epithelial (NE) cells. Half of the media was changed every other day. After 2–4 days in suspension, NE cells were collected and plated onto laminin (20µg/mL in DMEM/F12) coated 6-well cell culture plates to allow the NE cells to adhere to the surface. Cells were cultured in neural induction media, and half of the media was changed every other day. NE cells plated on laminin were cultured for 10–14 days. During this time, formation of neural tube-like rosettes was observed in the culture. NE cells were collected by mechanical detachment, centrifuged, and re-suspended in neural induction media and incubated in 75mL low adhesion flasks for 7–14 days with half of the media changed every other day. NE cells were then collected, pelleted, and resuspended in neural differentiation media [Neurobasal media (Invitrogen) supplemented with 1× non-essential amino acids, 4× B27 Supplement (Invitrogen), 1µM dibutyryl-cAMP (Enzo Life Sciences, Farmingdale, NY, USA), and 200µM ascorbic acid (Sigma-Aldrich)]. NE cells were then mechanically dissociated by pipetting and plated onto 13mm glass coverslips (Fisher Scientific, Pittsburg, PA, USA) pre-coated with polyornithine (0.1mg/mL in water) and Matrigel (0.67mg/mL in DMEM/F12) (BD Biosciences, Bedford, MA, USA) coated at a density of 100,000 cells per coverslip. Cells were allowed to adhere to coverslips for 24 hours, and then cultured in neural differentiation media supplemented with 10ng/mL brain derived neurotrophic factor (BDNF) (Peprotech, Rocky Hill, NJ, USA), 10ng/mL glial derived neurotrophic factor (GDNF) (Peprotech), 10ng/mL insulin-like growth factor (IGF) (Peprotech), and 1µg/mL laminin (Sigma-Aldrich) to allow for the differentiation of neural cells. Half of the media was changed 3 times per week, and neural cells were cultured for 12 weeks or more.
Chronic alcohol treatment
Cultures were fed daily for 7 days with neural differentiation media containing 50 mM ethanol (0.23 mg/dl). Evaporation of alcohol following daily media changes in unsealed culture plates is expected to produce a gradual reduction of alcohol each day. Evaporation of alcohol was examined in sister culture dishes not containing neural cells by measurement of media alcohol concentrations at 2, 4, 6, 8, 10 and 24 hours post-feeding using an AM1 Alcohol Analyzer (Analox Instruments Ltd, The Vale, London, UK). Alcohol concentration was gradually reduced with a ~19-hour half-life, such that by the end of 24 hours the average alcohol concentration was 18.1±2.0 mM (0.07 mg/dl). This daily replenishment of alcohol followed by gradual loss to evaporation in unsealed culture dishes provides a pattern of exposure more similar to that in human daily heavy drinkers than would continuous exposure to 50 mM ethanol in a sealed culture system.
Immunocytochemistry
Neural cultures were fixed with 4% paraformaldehyde in PBS (Invitrogen) for 45 min and stained with mouse monoclonal antibody to beta III-tubulin (Millipore, 1:500), and either rabbit polyclonal antibody to s100β (Abcam, Cambridge, MA, USA 1:250) or synapsin1 (Millipore, 1:500). All primary antibodies were diluted in 0.1% bovine serum albumin in PBS. Appropriate donkey anti-mouse and donkey anti-rabbit alexa fluor 488 and 594 (Invitrogen, 1:600) secondary antibodies were used for visualization.
Electrophysiology
Electrophysiological recordings were obtained from neurons from subject 1 (a non-alcoholic) using previously described techniques (Lemtiri-Chlieh and Levine, 2010). Neurons were selected for recording based on neural morphology (neurite projections and pyramidal-shaped soma). Artificial cerebrospinal fluid (aCSF) containing 125mM NaCl, 2.5mM KCl, 1.25mM NaH2PO4, 1mM MgCl2-6H2O, 25mM NaHCO3, 2mM CaCl2, and 25mM dextrose was perfused into the recording chamber at 1mL/min at room temperature. To examine NMDA responses to locally applied glutamate, the bath solution contained the AMPA receptor antagonist DNQX (10µM) under Mg2+ free conditions to relieve the voltage-dependent Mg2+ block of NMDA receptors.
To examine the generation of action potentials, spontaneous synaptic activity, and AMPA and NMDA responses, an internal recording solution was used containing 4mM KCl, 125mM K-Gluconate, 10mM HEPES, 10mM phosphocreatine, 1mM EGTA, 0.2mM CaCl2, 4mM Na2-ATP, and 0.3mM Na-GTP (pH 7.3). To examine GABA responses, the pipette solution contained 130mM KCl, 10mM HEPES, 10mM phosphocreatine, 1mM EGTA, 0.1mM CaCl2, 1.5mM MgCl2, 4mM Na2-ATP, and 0.3mM Na-GTP (pH 7.3), which allowed GABA-induced synaptic currents to be recorded at normal resting membrane potential. Whole cell current clamp recordings were used to examine the ability of cells to generate action potentials and to observe AMPA responses to 50µM glutamate (in the presence of Mg) and NMDA responses to a combination of 50µM glutamate and 10µM glycine (in the presence of DNQX and the absence of Mg2+). Whole cell voltage clamp recordings were used to examine spontaneous synaptic activity in the neural culture as well as to observe responses to 50µM GABA. To elicit AMPA, NMDA, and GABA responses, a micropipette containing the appropriate solution was placed adjacent to the recorded cell. The solution was puffed onto the cell using 5psi of positive pressure.
To examine the effects of acute alcohol exposure on the NMDA response in both control and chronically ethanol-treated neural cultures, a micropipette filled with 50µM glutamate and 10µM glycine was placed next to a patched neuron. Whole cell recordings were done in current clamp mode. aCSF used contained 10µM DNQX and did not contain Mg2+. The glutamate/glycine solution was puffed onto the cell every 30 sec and the responses were recorded. A baseline recording was taken for 5 min, and then a Mg2+ free aCSF supplemented with 50mM alcohol was perfused into the recording chamber. aCSF containing alcohol was perfused onto the cell for 5 min, with the glutamate/glycine puff occurring every 30 sec. Peak amplitude of the responses in the final 2 min of the baseline period and the final 2 min of the acute alcohol exposure period were calculated using the Axon program. Alcohol effects were analyzed via paired t-test using Graphpad Prism software.
To observe the effects of acute alcohol exposure on GABAA and AMPA responses, the same paradigm was used as described above, except the solution being puffed onto the cell was either 50 µM GABA or 50 µM glutamate to elicit GABAA or AMPA responses, respectively. AMPA responses were observed using an external solution containing 1mM Mg2+ without the addition of DNQX.
Effects of Alcohol on NMDA Subunit mRNA Expression
To examine the effects of chronic alcohol exposure on NMDA subunit mRNA expression, iPS-derived neural cells from 7 subjects (4 alcoholics and 3 non-alcoholics) were cultured for 15–18 weeks and subjected to three treatment conditions; a 7-day sham treatment, a 7-day alcohol treatment, and a 7-day alcohol treatment with a 24-hr withdrawal period (n=4–7 coverslips per subject per condition). Neural differentiation media with or without 50 mM alcohol was replaced daily. In the condition examining withdrawal, after 7 days of alcohol treatment, alcohol-containing media was replaced with normal neural differentiation media for 24 hours.
RNA was extracted from neural cultures using TRIzol Reagent (Invitrogen) following the manufacturer’s directions and cDNA was synthesized from 2µg RNA from all conditions using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA).
Neural cell culture cDNA was analyzed by real time polymerase chain reaction (RT-PCR) using an Applied Biosystems 7500 instrument and TaqMAN Assay on Demand (Applied Biosystems) probes and primers for: GRIN1 (Hs00609557_m1), GRIN2A (Hs00168219_m1), GRIN2B (Hs00168230_m1), and GRIN2D (Hs00181352_m1). Expression of these genes was normalized relative to the expression of the housekeeping gene GUSB (Applied Biosystems, 4326320E) co-amplified with target genes. PCR cycles were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. A standard curve consisting of a 4-level 2-fold serial dilution series of pooled cDNA taken from all samples in the experiment was used to determine relative mRNA expression levels. Expression after 7 days of alcohol exposure and 7 days of alcohol exposure plus a 24-hour withdrawal period was normalized to each subject’s sham condition. Data were analyzed for statistical significance with mixed model repeated measures test using SPSS software.
Results
Human iPS Cell Neural Differentiation
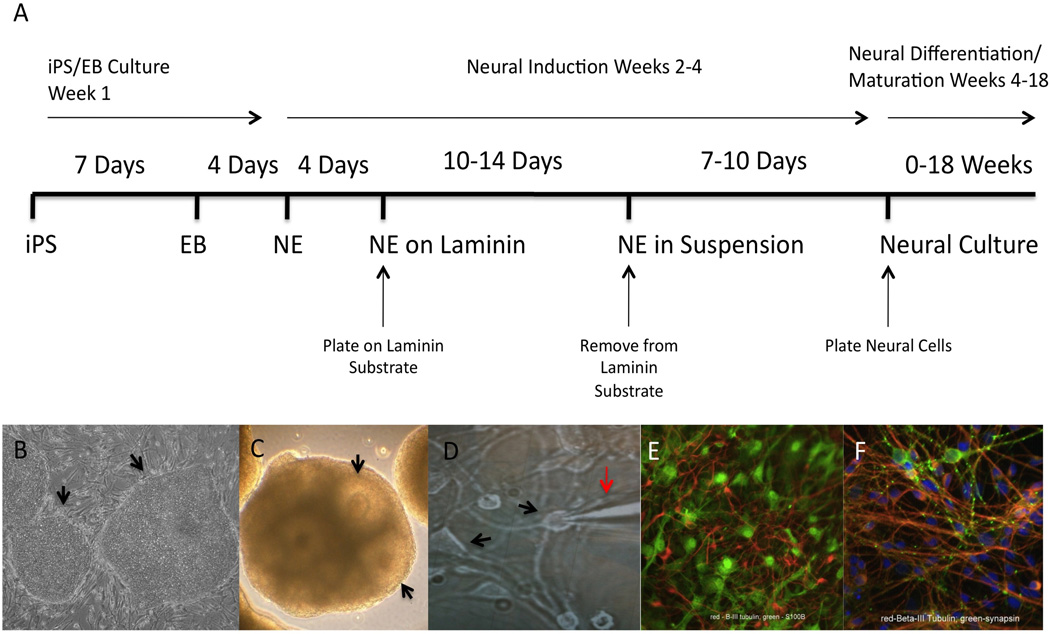
To generate subject-specific tissue, we differentiated iPS cells derived from 7 human subjects, 4 of whom met criteria for current alcohol dependence, including alcohol tolerance, into neural cell cultures using an established protocol for the neural differentiation of hES cells (Figure 1A–F). After 10 weeks in neural differentiation medium, iPS-derived cell cultures contained beta-III tubulin and s100β positive cells (Figure 1E), indicating that human iPS cells were successfully differentiated into cultures containing neurons and glia. Further immunostaining revealed positive synapsin-1 staining (Figure 1F), suggesting that synaptic specializations were forming within iPS-derived neural cultures.
Figure 1. Neural Differentiation Timeline.
(A) Timeline of neural differentiation includes culturing iPS cell colonies (B) to confluency for 7 days, followed by culture in suspension for 4 days to allow for the formation of embryoid bodies, followed by incubation in neural induction media and growth on a laminin substrate to trigger the formation of neuroepithelial cells displaying neural tube-like rosette structures, which are expanded in suspension (C) culture for several days prior to plating dissociated cells on coverslips. Dissociated cells are cultured in a neural differentiation media containing the growth factors BDNF, GDNF, and IGF to promote the differentiation of neural cells (D, arrows indicate neural cell bodies, red arrow indicates recording electrode for performing electrophysiology). Dissociated neural cells were then cultured for 12–18 weeks in neural differentiation media. At 10-weeks post-plating neural cultures displayed neuronal lineage marker beta III-tubulin and glial cell lineage s100β positive cells (E), indicating that iPS cells differentiated into cultures containing a mixed culture of neurons and glia. Focal synapsin-1 immunostaining along beta III tubulin (F) positive processes were observed indicating the formation of synaptic specializations within the culture.
Electrophysiology
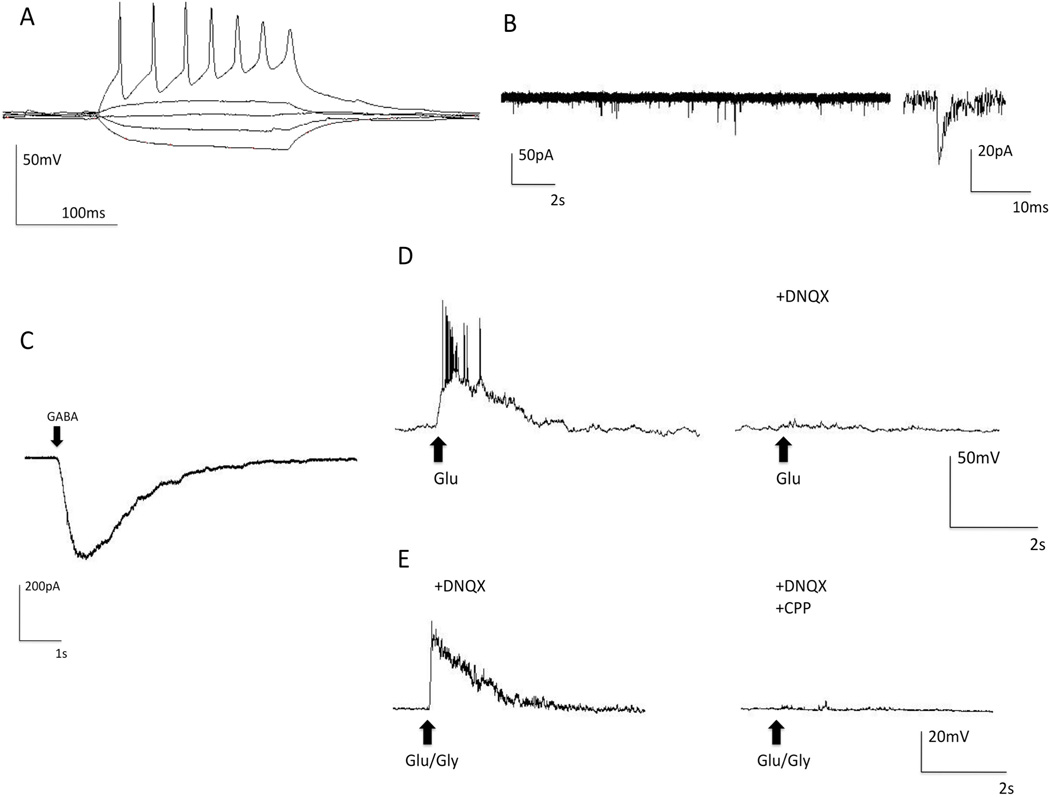
We examined electrophysiological properties of neurons, selected for recording based on morphology (neurite projections and pyramidal-shaped soma), in iPS-derived neural cultures from one non-alcoholic subject, including the ability to generate action potentials and spontaneous synaptic activity. Cells that did not generate action potentials (7 of 58 cells examined) were excluded from further analysis. Consistent with reports on the developmental time course of hES cell-derived neural cells (Johnson et al., 2007), we observed that, after several weeks in culture, neurons developed resting membrane potentials of −50 to −60mV, and beginning at 10–12 weeks post-plating, iPS-derived neurons generated trains of action potentials in response to current injection (Figure 2A). Spontaneous synaptic activity with both fast and slow components typical of glutamatergic synaptic transmission was observed (Figure 2B).
Figure 2. Electrophysiological Properties of iPS-Derived Neurons.
Whole-cell patch recordings from 12-week iPS-derived neurons from a non-alcohol dependent subject demonstrate functional neuronal properties, including generation of action potentials, spontaneous synaptic activity, and ionotropic receptor currents. (A) Neurons generate trains of action potentials in response to current injection. (B) Spontaneous synaptic activity (Inset) close-up of a spontaneous event demonstrates fast and slow kinetic components of the event. (C) GABA receptor current response to a 200 ms puff of 50μM GABA indicates that functional GABA receptors are present (arrow indicates when GABA was applied). (D) Depolarization response to a 200 ms puff of 50μM glutamate in the presence of Mg2+ indicates that functional AMPA receptors are expressed. Response was completely abolished when the AMPA antagonist DNQX (10µM) was perfused into the recording chamber (arrows indicate when glutamate was applied). (E) NMDA channel mediated depolarization in response to a 200 ms puff of 50μM glutamate and 10 μM glycine in a magnesium-free recording solution that contained the AMPA receptor antagonist DNQX (10µM). The response was completely abolished when NMDA receptor antagonist CPP (6µM) was perfused into the recording chamber (Dashed arrows indicate when glutamate plus glycine solution was applied).
To further characterize iPS-derived neurons, we examined whether functional GABAA, AMPA, and NMDA ionotropic receptors were expressed. Functional GABAA receptors were observed using voltage-clamp mode where a 50µM GABA puff produced an inward current response (Figure 2C). In total, 20 of 20 cells tested responded to GABA. Functional AMPA receptors were observed in current-clamp mode where 50µM glutamate puffs produced a depolarization of the membrane in the presence of 1mM Mg2+. In total, 9 of 9 cells tested responded to glutamate administration under these conditions. This response to glutamate was blocked by the administration of the AMPA antagonist DNQX (Figure 2D). To identify NMDA glutamate receptor responses, a Mg2+ free recording solution containing DNQX to block AMPA receptor activity was used. The puffing pipette contained 50µM glutamate plus 10µM glycine and produced a depolarization of the membrane. This response was blocked when the NMDA antagonist CPP (6µM) was administered in the bath solution (Figure 2E). In total, 27 of 29 cells examined demonstrated functional NMDA responses.
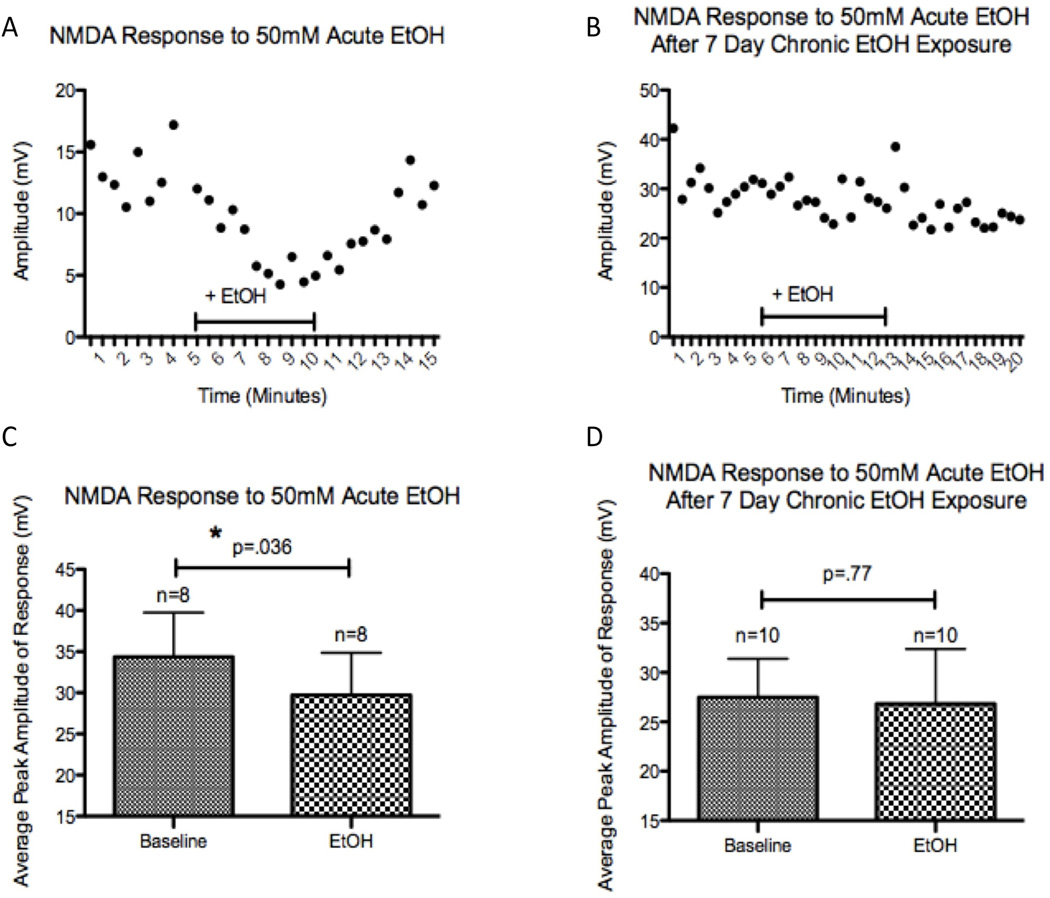
We next explored the effects of acute alcohol exposure on the NMDA response, which in other model systems was attenuated by acute exposure to alcohol (Nie et al., 1994, Puglia and Valenzuela, 2010). In iPS-generated neurons, an acute exposure to 50mM alcohol significantly attenuated the peak amplitude of NMDA responses (p=0.036, Figure 3C). To further explore the use of human iPS-derived neural cells as a model to examine the effects of alcohol exposure, we explored whether tolerance to acute alcohol exposure could be observed in the culture after chronic alcohol treatment. Neural cultures were treated daily with 50mM alcohol for 7 days and alcohol withdrawn for 1 hr following transfer of coverslips to the recording chamber, and the acute effects of alcohol were measured. In neurons exposed to alcohol for 7 days, re-exposure to 50mM alcohol did not attenuate the NMDA response (p=0.77, Figure 3D). In contrast to results for NMDA receptors, no significant changes were observed following acute 50 mM alcohol exposure for GABAA (n=4, p=0.27, data not shown) or AMPA receptors (n=5, p=0.19, data not shown). As we did not observe significant changes in responses of these receptors to acute alcohol exposure, we did not examine the effect of a 7-day chronic alcohol treatment on GABAA or AMPA responses to acute alcohol.
Figure 3. Acute Alcohol Attenuation of NMDA Response is Not Observed After 7-Days of Alcohol Exposure.
Whole-cell patch clamp recordings from neurons derived from a non-alcohol dependent subject. (A) Example of the time course illustrating the inhibition of the NMDA response by acute alcohol exposure (50 mM) in a naïve neuron. (B) Example of the lack of effect of alcohol on NMDA receptors following 7-day exposure to 50 mM alcohol. (C) Group data for the effect of acute alcohol which significantly reduced the amplitude of the NMDA response (n=8, paired t-test, p= .036). (D) Group data for 7-day alcohol pre-treated cells showing no significant effect of bath applied alcohol on NMDA response (n=10, paired t-test, p= .77). Differences in the mean amplitude of the baseline depolarization between naïve and chronically treated cells (panels C and D) relate in large measure to variation in the distance of the puffing pipette from the patched cells examined.
Effect of Repeated Alcohol Exposure on NMDA Subunit mRNA Expression
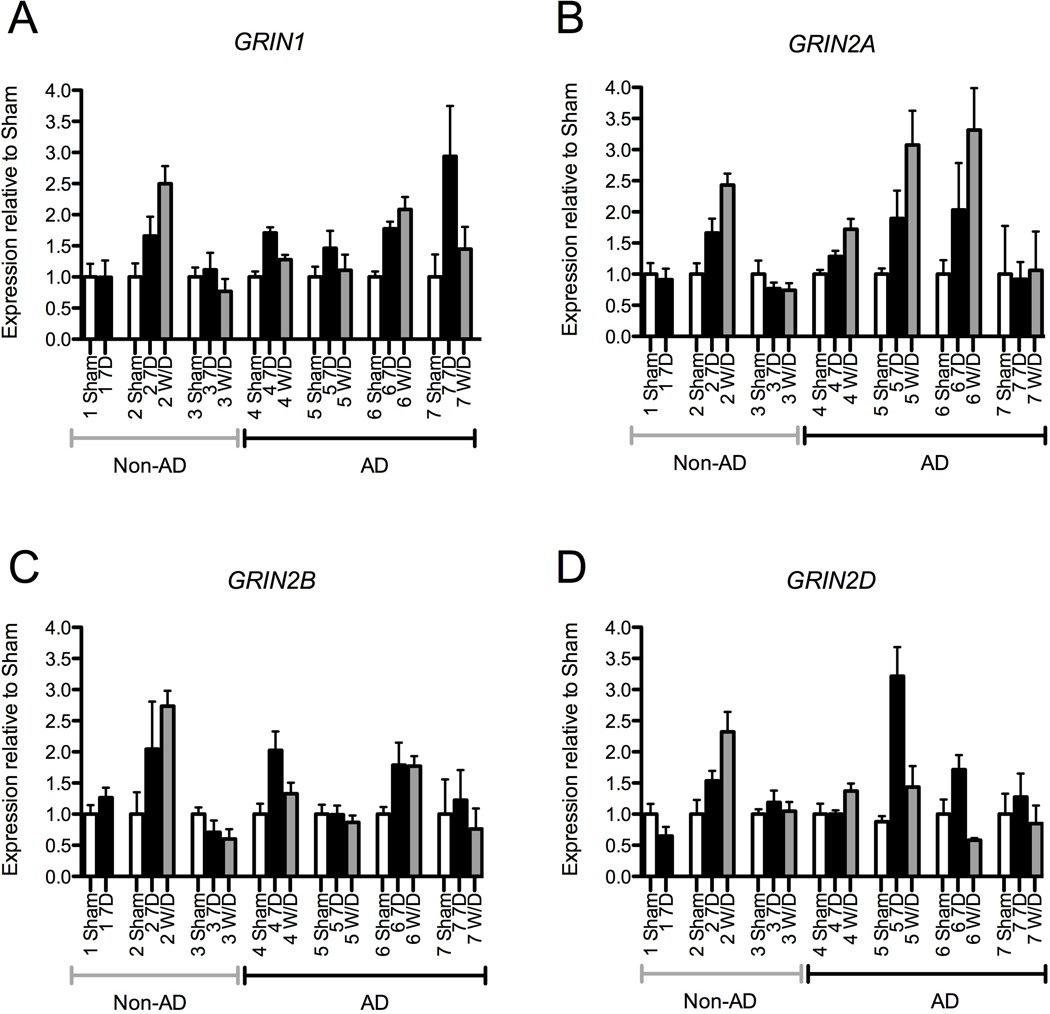
To examine whether chronic alcohol treatment produced changes in NMDA subunit mRNA expression, 15–18 week old human iPS-derived neural cultures derived from 4 alcoholic and 3 non-alcoholic donor subjects were fed daily with media containing 0 or 50mM alcohol and the levels of expression relative to sham treated cells were examined for the NMDA receptor subunit genes GRIN1 (NR1 subunit), GRIN2A, (NR2A subunit), GRIN2B, (NR2B subunit) and GRIN2D (NR2D subunit) (Figure 4). For neural cultures derived from alcoholic subjects, significant treatment effects were seen after 7 days of alcohol exposure for GRIN1 (F(1,37)=10.8, p=0.002), GRIN2A (F(1,39)=13.5, p=0.001), and GRIN2D (F(1,37)=16.1, p<0.001) mRNA levels. A trend towards significance was seen for GRIN2B (F(1,37)=3.7, p=0.06). Following a 24-hour withdrawal period, the expression of GRIN1 in alcoholic-derived cultures remained significantly elevated (F(1,40)=5.0, p=0.03), the expression of GRIN2A continued to increase (F(1,40)=33.6, p<0.001), and the expression of GRIN2B and GRIN2D returned to baseline levels (F(1,43)=0.2, p=0.64, and F(1,40)=0.6, p=0.44, respectively). In contrast, following 7-day alcohol exposure of neural cultures derived from non-alcohol dependent subjects there was no change in the expression of the four NMDA subunit genes (GRIN1 F(1,29)=1.0, p=0.32, GRIN2A F(1,25)=0.06, p=0.82, GRIN2B F(1,29)=0.75, p=0.39, GRIN2D F(1,27)=0.26, p=0.62). Withdrawal effects on mRNA concentration were available for only 2 of 3 cultures derived from non-alcoholics, precluding a meaningful analysis of that condition.
Figure 4. NMDA Receptor Subunits are Upregulated After Chronic Alcohol Exposure in Neural Cells Derived from Alcoholics.
Neural cells (age 12–15 weeks) derived from 7 different subjects, 3 non-alcoholics and 4 alcoholics, were treated daily with 50 mM alcohol for 7 days and levels of GRIN1, GRIN2A, GRIN2B, and GRIN2D mRNA expression was analyzed using RT-PCR. No significant changes were observed in the expression of these NMDA receptor subunit genes after 7-day alcohol exposure (relative to the sham condition) in neural cells derived from the group of non-alcoholic donor subjects (left group of data panels A–D). In contrast significant increases in mRNA levels compared to the sham condition were seen for neural cells derived from the alcoholic subject group for NMDA subunit genes (A) GRIN1, (B) GRIN2A, and (D) GRIN2D, while a trend towards significance was observed for (C) GRIN2B (p=0.06). Scatter plot symbols represent results from individual culture wells together with mean and SEM (significance level compared with sham condition * < 0.05, ** <0.01, *** <0.001).
Subject-to-subject differences in responses were noted (Fig. 5 A–D), with a greater proportion of alcoholic subjects (4 out of 4) showing at least a 50% increase in expression for one or more NMDA receptor subunits compared to non-alcoholics (1 of 3) after a 7-day exposure.
Figure 5. Variability Between Subjects in NMDA Subunit mRNA changes following Alcohol Exposure and Withdrawal.
Individual subject data normalized to each subject’s average sham NMDA receptor gene expression normalized to GUSB. iPS-derived neural cells from non-alcohol dependent (Non-AD) subjects are indicated on the left side of the x-axis, while neural cell generated from alcohol-dependent (AD) subjects are indicated on the right. Only one non-AD subject demonstrated an increase in NMDA mRNA expression following 7 days of alcohol exposure. In contrast, under these conditions, all four AD subjects demonstrated increased expression of GRIN1, which returned to baseline levels in three of the four subjects. Three AD subjects demonstrated increased GRIN2A expression, which continued to increase after 24 hours of withdrawal in all three subjects. Two AD subjects showed increases in GRIN2B and GRIN2D expression; with the exception of GRIN2B in one AD subject, these changes returned to baseline after withdrawal. (Symbols: open bars − sham condition; solid bars − 7-day of alcohol exposure; gray bars − 7-day alcohol + 24-hour withdrawal; error bars represent SEM).
Discussion
The objective of this study was to explore the potential utility of human iPS cells as a model to examine the biological actions of alcohol on human neural cells. We found that NMDA receptor function and mRNA expression in human iPS-derived neural cells responded to acute and chronic alcohol exposure similarly in several respects to that seen in rodent models. Specifically, acute exposure attenuated the NMDA but not AMPA receptor response, consistent with previous findings (Puglia and Valenzuela, 2010, Nie et al., 1994, Nagy, 2004, Dodd et al., 2000). In contrast we did not observe acute alcohol enhancement of GABAA responses, which has been observed in some, but not all, reports of alcohol effects in dissociated cell rodent cultures (Kumar et al., 2009, Yamashita et al., 2006). GABAA responses to acute alcohol may in part be dependent on indirect effects of alcohol on GABAA receptors mediated by neuroactive steroids (Sanna et al., 2004). We also observed that 7-day alcohol exposure significantly increased NMDA receptor subunit mRNA expression, which parallels previous findings in rodent models (Nagy, 2008b, Hu et al., 1996, Follesa and Ticku, 1995). Our results suggest that human iPS-derived neural cells may provide a useful experimental model to examine mechanisms underlying changes in NMDA function and expression in response to acute and chronic alcohol, which remain incompletely understood (Hu et al., 1996, Hanchar et al., 2006).
Perhaps more significantly, iPS-derived neural cells may provide a model to examine between-subject differences in molecular and physiologic responses to acute or chronic alcohol exposure, and in gene function with respect to between-subject genetic variation. Although preliminary, our results suggest that patterns of NMDA mRNA expression change following repeated alcohol treatments vary from subject to subject. Further, increases in expression may be more common in neural cells derived from alcoholic subjects than from non-alcoholics. This finding suggests that heritable traits may render gene expression in the NMDA system more reactive to effects of alcohol in persons predisposed to AD and may thereby contribute to the development of tolerance to the inhibitory effects of alcohol on the NMDA receptor activity. Although the list of genetic markers associated with alcohol dependence is expanding (Edenberg and Foroud, 2006, Enoch et al., 2009, Kimura and Higuchi, 2011), the biological correlates of polymorphisms reported to be associated with alcohol dependence are in large part unknown. iPS cell technologies may provide a powerful tool with which to study the functional effects on neural tissue of alleles identified with alcohol dependence risk.
Future research might also use iPS cells as models to examine drug therapies for the treatment of alcohol use disorders or symptoms associated with alcohol withdrawal. A promising potential use for iPS cell technologies is to identify new drugs and drug targets, as well as to screen drugs for off-target toxicities (Cundiff and Anderson, 2011). Three of the most widely prescribed medications to treat alcohol use disorders include disulfiram, which disrupts alcohol metabolism, naltrexone, which antagonizes opioid receptors, and acamprosate, which disrupts glutamate signaling. However, each of these drugs has adverse effects associated with their use, and treatment efficacy varies among individuals, and this variation may be associated with different genotypes (Miller, 2008, Johnson et al., 2011, Ray et al., 2009). Additional research is currently focusing on additional drugs for the treatment of alcohol use disorders, such as topiramate, which targets glutamate and GABAA receptors (Miller, 2008, Johnson, 2010). Our results show that functional glutamate and GABAA receptors are expressed in iPS-derived neural cultures. Because the use of iPS cell technologies allows us to obtain neural cultures from individuals with different genotypes, we propose that these cells could be used as models to evaluate drug therapies and their adverse effects in the treatment of alcohol use disorders. While the use of iPS cells to model pathophysiology and to screen potential drug therapies is still in its early stages, further research can be expected to clarify the potential benefits and limitations of this approach.
The use of human iPS cells to study the action of alcohol is a novel and promising means to evaluate group differences in the response to alcohol exposure, However, much remains to be learned about this approach and additional research is needed to address our study’s limitations. Our electrophysiologic studies were limited to the examination of cells from a single non-alcoholic subject. Examination of receptor responses from cultures derived from multiple alcoholic and non-alcoholic subjects are needed to provide electrophysiologic validation of the expression results. Secondly, we isolated NMDA responses using a low magnesium solution containing DNQX, which allowed the natural agonists (glutamate and glycine) to activate the receptor. Others have reported that inhibition of NMDA receptor currents in response to alcohol is greater in the presence of magnesium when the synthetic agonist NMDA is used (Martin et al., 1991). Future studies may benefit from investigating the effect of alcohol under such conditions. Third, our puff application of neurotransmitters produced a local dilution of alcohol during recordings to examine alteration of receptor responses by bath-applied alcohol. Use of a multi-barrel pipette for puff application could limit dilution of local alcohol concentration during receptor activation in such experiments. Fourth, as with any in vitro study, the neural cells we examined were not in their native environment and variation in culture conditions or well-to-well variability may impact patterns of gene expression (Newman and Cooper, 2010). Fifth, although the genetic sequence differences contributing to risk of alcohol dependence in neural cells derived from AD individuals are likely to be maintained in iPS cells, the environmental effect of chronic alcohol exposure in AD donor subjects (e.g. epigenetic, transcriptional or post-translational effects of alcohol on neural cells) are not likely to be preserved during reprogramming. Sixth, the results generated from our pilot data do not identify whether changes in mRNA expression directly lead to changes in NMDA receptor activity. Post-translational effects of alcohol on NMDA receptors have also been reported. Fyn kinase-mediated phosphorylation of tyrosine residues on the long intracellular tail of the NR2B subunit is stimulated by acute alcohol exposure and is thought to contribute to the development of acute tolerance to the inhibitory effects of alcohol on the NMDA receptor (Yaka et al., 2003, Miyakawa et al., 1997). Finally, although our pilot study has focused on an examination of the effects of alcohol on the expression of NMDA receptor genes, study of other neurotransmitter systems, including GABAA, in iPS derived neurons will also be important.
In summary, this study demonstrates that human iPS-derived neural cells provide a promising new model system to examine the molecular and biological effects of alcohol on human nervous tissue. To our knowledge, this is the first study to examine the effects of acute and chronic alcohol exposure on human neurons derived from iPS cells. Using this subject-specific tissue, future research should aim to identify and examine molecular differences in neural cultures derived from individuals differentiated by the presence of alcohol tolerance and genetic markers of risk for alcohol dependence.
Acknowledgments
The authors would like to thank Linda Burian for all of the help that she provided throughout the conduct of this research. This work would not have been possible without her constant support and guidance. This study was supported by NIH grants T32 NS041224-08, P60 AA03510, M01 RR06192, R0
References
- Bigge CF. Ionotropic glutamate receptors. Curr Opin Chem Biol. 1999;3:441–447. doi: 10.1016/S1367-5931(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38:16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, Chen PF, Ng KY, Bourgois-Rocha F, Lemtiri-Chlieh F, Levine ES, Lalande M. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc Natl Acad Sci U S A. 2010;107:17668–17673. doi: 10.1073/pnas.1004487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, Li XJ, Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–235. doi: 10.1007/s10048-008-0147-z. [DOI] [PubMed] [Google Scholar]
- Cundiff PE, Anderson SA. Impact of induced pluripotent stem cells on the study of central nervous system disease. Curr Opin Genet Dev. 2011;21:354–361. doi: 10.1016/j.gde.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan QP, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as Independent Predictors for Alcoholism in Two Populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. Journal of Pharmacology and Experimental Therapeutics. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Lewohl JM, Scott HL, Dodd PR. Biochemical and molecular studies using human autopsy brain tissue. Journal of Neurochemistry. 2003;85:543–562. doi: 10.1046/j.1471-4159.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167:630–639. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD. Pharmacogenetic Approach at the Serotonin Transporter Gene as a Method of Reducing the Severity of Alcohol Drinking. American Journal of Psychiatry. 2011;168:265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kimura M, Higuchi S. Genetics of alcohol dependence. Psychiatry Clin Neurosci. 2011;65:213–225. doi: 10.1111/j.1440-1819.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Expression of the alpha 1, alpha 2 and alpha 3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997;751:102–112. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, Bleich S, Ruther E, Kornhuber J. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052:156–162. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Martin D, Morrisett RA, Bian XP, Wilson WA, Swartzwelder HS. Ethanol inhibition of NMDA mediated depolarizations is increased in the presence of Mg2+ Brain Res. 1991;546:227–234. doi: 10.1016/0006-8993(91)91486-k. [DOI] [PubMed] [Google Scholar]
- Miller G. Psychopharmacology. Tackling alcoholism with drugs. Science. 2008;320:168–170. doi: 10.1126/science.320.5873.168. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: Relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Nagy J. The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr Drug Targets CNS Neurol Disord. 2004;3:169–179. doi: 10.2174/1568007043337409. [DOI] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Current Neuropharmacology. 2008a;6:39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol. 2008b;6:39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005;1048:69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Newman AM, Cooper JB. Lab-Specific Gene Expression Signatures in Pluripotent Stem Cells. Cell Stem Cell. 2010;7:258–262. doi: 10.1016/j.stem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- Preece P, Cairns NJ. Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Molecular Brain Research. 2003;118:60–71. doi: 10.1016/s0169-328x(03)00337-1. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Ethanol acutely inhibits ionotropic glutamate receptor-mediated responses and long-term potentiation in the developing CA1 hippocampus. Alcohol Clin Exp Res. 2010;34:594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, MacKillop J, McGeary J, Tidey JW, Rohsenow DJ, Gwaltney C, Swift RW, Monti PM. A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp Clin Psychopharmacol. 2009;17:122–129. doi: 10.1037/a0015700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Ho AM, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann N Y Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tomberg C. Alcohol Pathophysiology Circuits and Molecular Mechanisms. Journal of Psychophysiology. 2010;24:215–230. [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]