Abstract
Immunological diseases like IBD are infrequent in less developed countries possibly because helminths provide protection by modulating host immunity. In IBD murine models, the helminth Heligmosomoides polygyrus bakeri (Hpb) prevents colitis. It was determined if Hpb mediated IBD protection by altering DC function. We used a Rag IBD model where animals were reconstituted with IL10-/- T cells making them susceptible to IBD and with OVA antigen-responsive OT2 T cells allowing study of a gut antigenic response. Intestinal DC from Hpb-infected Rag mice added to lamina propria mononuclear cells (LPMC) isolated from colitic animals blocked OVA IFNg/IL17 responses in vitro through direct contact with the inflammatory LPMC. DC from uninfected Rag mice displayed no regulatory activity. Transfer of DC from Hpb-infected mice into Rag mice reconstituted with IL10-/- T cells protected animals from IBD, and LPMC from these mice lost OVA responsiveness. After DC transfer, OT2 T cells populated the intestines normally. However, the OT2 T cells were rendered antigen-nonresponsive through regulatory action of LPMC non-T cells. The process of regulation appeared to be Treg independent. Thus, Hpb modulates intestinal DC function, rendering them tolerogenic. This appears to be an important mechanism through which Hpb suppresses colitis. IFNg and IL17 are colitogenic. The capacity of these DC to block a gut antigen-specific IFNg/IL17 T cell response also is significant.
Keywords: helminths, dendritic cells, mucosa, colitis
INTRODUCTION
Many immune-mediated diseases like inflammatory bowel disease (IBD) are rare in less developed countries. Helminths are worm-like organisms common in the geographic regions with a low prevalence of these conditions. Helminths strongly interact with the immune system of their hosts to modulate immune reactivity. A growing body of data suggest that helminthic infections can protect the host from developing these immunological diseases (1). Helminths also are being tested in clinical trials as therapy for IBD and other conditions.
Several animal models of human IBD have shown that various helminth species can prevent or reverse ongoing colitis. IL10 is important for maintenance of intestinal immune homeostasis. Mice lacking IL10 develop colitis spontaneously. Mice with defective IL10 production are commonly used to study IBD. T. muris or Heligmosomoides polygyrus bakeri (Hpb) infection, or schistosome ova exposure prevents or reverses the Th1/Th17-type colitis of IL10 deficient (IL10-/-) mice (2,3). In trinitrobenzene sulfonic acid (TNBS)-induced colitis, animals receiving intestinal helminths like Trichiura muris (2), Trichinella spiralis (4), Hpb or Hymenolepis diminuta (5) or that receive non-viable schistosome ova (6) are protected from IBD.
The mechanisms through which helminths function to alleviate disease remain incompletely understood. The protection probably involves induction of several independent immune regulatory processes. At least part of the protection depends on parasite induction of regulatory-type cytokines in the host. After Hpb infection, lamina propria (LP) T cells from healthy wild-type (WT) mice make much more regulatory cytokines like IL10 and TGFb (7). Regulatory T cells also are important. T cells from the mesenteric lymph nodes (MLN) of Hpb-infected, IL10-/- mice abrogate established colitis when transferred into IL10-/- recipients (3). Helminth colonization induces FoxP3 expression in MLN and LP T cells. In a Rag-IL10-/- T cell transfer colitis model of IBD, Hpb acquire CD8+ T cells to reverse the disease process (8). Hpb infection also elicits a regulatory T cell population that improves allergen-induced, lung pathology (9).
Interactions with cells of the innate immune system may be part of the regulatory process. Schistosomes protect BALB/c mice from DSS enteritis via a macrophage-dependent mechanism not requiring regulatory T cells (10). Alternatively activated macrophages may protect animal models from asthma (11).
We previously showed that Rag mice briefly exposed to Hpb only before T cell reconstitution are rendered resistant to colitis (12). This suggested that interactions of Hpb just with cellular components of innate immunity are sufficient to protect these animals from IBD. LP DC from Hpb-infected Rag mice present antigen poorly compared to the DC from the uninfected animals. Following Hpb infection, intestinal DC of Rag mouse show changes in expression of cell surface molecules. This raised the possibility that alterations in DC function were part of the protective process.
The current study found that intestinal and mesenteric lymph node (MLN) DC from Rag mice infected with Hpb function as immune regulatory cells that can limit antigenic responses in the gut. Moreover, transfer of these DC into colitis-prone mice is sufficient to protect the animals from IBD even in the absence of Hpb infection. The tolerogenic DC require direct contact with the pro-inflammatory LPMC to block antigenic responses and do not appear to function through altering the frequency of Foxp3+ T cells in the gut or MLN, or by stimulating IL10 or TGFb production.
MATERIALS AND METHODS
Mice and Hpb infection
This study used C57BL/6 Rag2 mice, OT2 and IL10-/- mice (Jackson Laboratory, Bar Harbor, ME). In some experiments, we used C57BL/6 OT2 CD45.1 mice (a gift of Dr. Fuhlbrigge, BWH, Boston, MA) or IL10 KO Foxp3 eGFP reporter mice (gift from Dr. Cathryn Nagler, University of Chicago, IL). Breeding colonies were maintained in SPF facilities at Tufts University. Animals were housed and handled following national guidelines and as approved by our Animal Review Committee.
For these experiments, 5- to 6-wk-old mice were colonized with 125 Hpb third stage larvae by oral gavage. Infective, ensheathed Hpb L3 (U.S. National Helminthological Collection no. 81930) were obtained from fecal cultures of eggs by the modified Baermann method and stored at 4°C. To de-worm mice, animals were given a single dose of pyrantel pamoate (0.5 mg/mouse, Sigma, St. Louis, MO) via oral gavage. De-worming was confirmed by the absence of adult worms in the duodenum at time of animal sacrifice and by preliminary experiments that showed the absence of worms in the GI track of mice 1 wk after taking pyrantel pamoate.
Dispersion of splenocytes or MLN cells, and splenic T cell enrichment
Single cell suspensions of splenocytes or MLN cells were prepared by gentle teasing in RPMI 1640 medium (GIBCO, Grand Island, NY). The cells were washed three times in RPMI. Splenic T cells or CD4+ T cells were isolated by negative selection using the EasySep mouse T cell Enrichment Kit as outlined by the manufacturer (Stemcell Technologies, #19751, Vancouver, Canada). Viability was determined using exclusion of trypan blue dye.
LPMC isolation and LP cell fractionation
Gut LPMC were isolated from the terminal ileum or colon as described (3). Cell viability was 90% as determined by trypan blue exclusion. Dendritic cells (DC)(CD11c+) were isolated from dispersed LPMC or MLN cells with mouse CD11c positive selection Kit #18758 from Stem Cell Technologies that was used according to kit directions. The beads used to isolate CD11c+ cells from the MLN and gut recovered about 85% of these cells at about 95% purity as determined by FACS. The beads displayed equal efficiency at isolating both the CD11chi and CD11clo subsets.
Colitis model
Rag mice of similar age (usually 6 to 7 wks old) were reconstituted i.p. with 4×106 IL10-/- splenic T cells. In some experiments, mice also received 2×106 OT2 splenic T cells given ip. One wk later, the animals were administered piroxicam mixed into their feed for 2 wks (Piroxicam at 40mg/250g chow wk 1, and 60mg/250 g chow wk 2). Two wks after induction of colitis, the piroxicam (Sigma) was stopped, and the colitis was studied 1 wk later (week 4 after cell transfer). The colons then were microscopically examined and scored for severity of colitis, and LPMC were isolated for culture (e.g. Fig 1 and 3). In some experiments, Rag mice were infected with Hpb (125 larvae) for 2 wks, then treated with pyrantel pamoate to terminate the Hpb infection, rested 1 wk, and then reconstituted with T cells as described above.
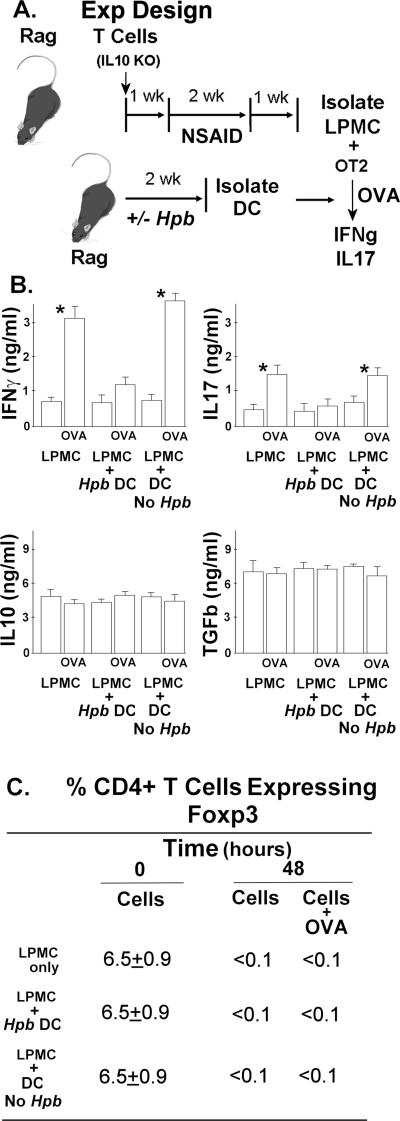
Figure 1. Intestinal DC from Hpb-Infected Rag Mice Inhibit IFNg and IL17 Production.
A. The experiment design. Rag mice were reconstituted with 106 IL10 KO T cells or 106 IL10 KO/Foxp3eGFP T cells, given i.p., and exposed to piroxicam (NSAID) to induce colitis as outlined above in panel A and in the methods section. One wk after stopping the piroxicam, Rag mice were infected with Hpb for 2 wks and then the Hpb were eliminated by treating the animals with a single orally dose of pyrantel pamoate. Age-matched control mice received the same drug treatment, but were not given the infection. FACS was used to determine the percentage of CD4+ T cells expressing Foxp3.
B. Cytokines analysis. Panel B shows that adding intestinal DC from Hpb-infected mice (Hpb DC) inhibited OVA-induced cytokine secretion. Colitis LP cells cultured at 2×105 cells/well in RPMI complete medium for 48h in 96-well round-bottomed plates with or without OVA (10 ug/ml) to stimulate IFNg and IL17 release. However, adding DC from mice with no prior infection (Dc No Hpb) had no such effect. Data are mean ± SE of 3-4 independent experiments For IFNg and IL17: *LPMC±OVA or LPMC + DC (No Hpb)±OVA, p<0.01.
C. FACS analysis .The table in panel C shows that LPMC maintained in culture loose most of their Foxp3+ T cells and that adding Rag intestinal DC to the LPMC cultures does not maintain Foxp3+ T cell content. Data are means of 3 experiments ±SE.
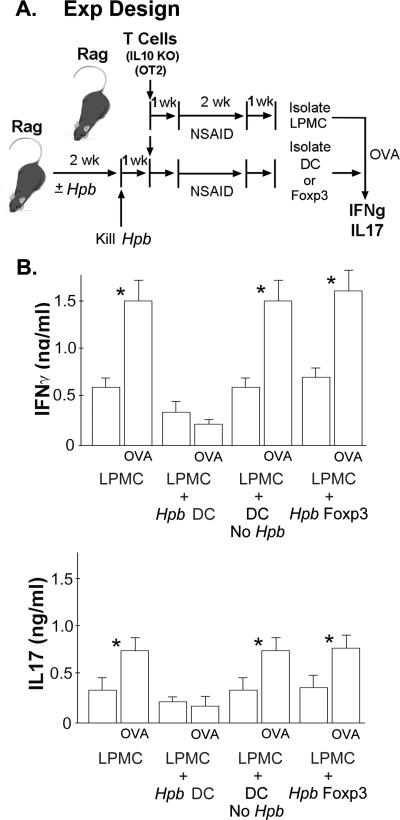
Figure 3. Hpb Infection Induces Tolerogenic DC in the Gut upon Suppression of the Colitis.
A. The experiment design. To obtain LPMC from colitic mice that would respond to OVA, Rag mice first were reconstituted i.p. with 106 splenic T cells from IL10 KO mice and 3×105 OT2 T cells from WT OT2 mice. Then, the Rag mice were given piroxicam (NSAID) to induce colitis. One wk after stopping the piroxicam, LPMC were isolated from the TI. To produce DC that could block the LPMC antigenic response, a second group of Rag mice were exposed to Hpb for 2 wks before reconstitution with IL10 KO T cell or IL10 KO/Foxp3eGFP reporter T cells and OT2 T cells. Then they were give piroxicam. DC or Foxp3+ T cells were isolated from the TI one wk after stopping the piroxicam.
B. Cytokines analysis. Colitis LP cells cultured at 2×105 cells/well in RPMI complete medium for 48h in 96-well round-bottomed plates with or without OVA (10 ug/ml) to stimulate IFNg and IL17 release. To determine if these intestinal DC or Foxp3+ T cells could modulate the LPMC cytokine response to antigen (OVA), the DC (Hpb DC) or Foxp3+ T cells (Hpb Foxp3) were added to some of the LPMC cultures at the ratio of 1:5. DC isolated from the TI of mice devoid of the infection, but otherwise manipulated similarly to the mice that received the infection, served as a source for control DC (DC No Hpb). Data are mean ± SE of 3 independent experiments. *LPMC ±OVA, LPMC+DC no Hp b ±OVA, or LPMC+Foxp3 ±OVA, p<0.01.
Cell culture
LPMC from IL10-/- T cell- and OT2 T cell-reconstituted Rag mice were cultured (2 × 105 cells per well) for 48h in 96-well round-bottomed plates. Cells were cultured alone or with OVA (10 μg/ml) (Sigma). The culture medium was RPMI 1640 containing 10% FCS, 25 mM HEPES buffer, 2 mM L-glutamine, 5 × 10–5 M 2-ME, 1 mM sodium pyruvate, 100 U/ml penicillin, 5 mg/ml gentamicin, and 100 mg/ml streptomycin (complete medium) (all Life Technologies, Gaithersburg, MD). After culture, the supernatants were assayed for IFNγ and IL17A using ELISA (described below).
In vitro cell mix experiments
In some experiments DC isolated from the TI or MLN of Rag mice with a 2 wk Hpb infection were mixed in vitro with LPMC isolated from the TI of T cell-reconstituted colitic Rag animals to determine if these DC cells could modulate the in vitro OVA response. In these experiments, freshly isolated splenic OT2 T cells were mixed with the isolated LPMC from colitic Rag mice at the ratio of (1:4) before the cells (2×105) were cultured in RPMI complete medium for 48 h. Some cultured contained OVA at up to 10 μg/ml to stimulate cytokine release. Supernatants were assayed for IFNg and IL17 after the incubation using ELISA (see figure 1 and 2).
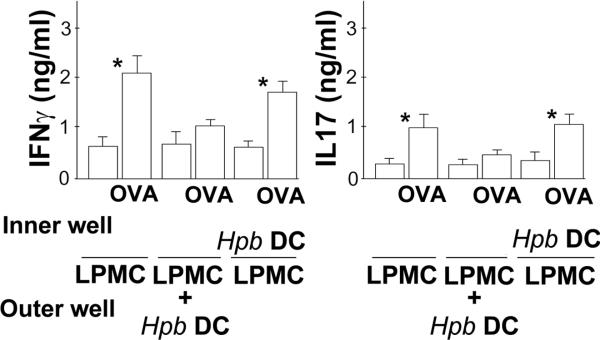
Figure 2. DC Regulation Requires Cell Contact.
The experiment was designed as outlined in figure 1, and 96 well Transwell plates were used in this experiment. Each well contained complete RPMI medium (200 ul/well). LPMC were mixed with splenic OT2 T cells (ratio 2:1) and placed in the outer chamber (2×105 cells/well). In some cases, the outer chamber also contained DC (4×104 cells/well) derived from TI of Rag mice infected with Hpb for 2 wks (Hpb DC). Still other wells contained Hpb DC placed in the inner chamber with the LPMC + OT2 T cells in the outer chamber separated by a 0.4 um semi-permeable membrane. Data are mean ± SE of 3 independent experiments. *LPMC±OVA; or LPMC±OVA cultured without contact with Hpb DC, p<0.01.
Transwell experiments used the Corning Transwell-96 well permeable support system with a 0.4 um pore size (#3381).
In other experiments, it was determined if the LP of mice protected from colitis by Hpb infection contained DC capable of blocking the OVA response of LPMC isolated from colitic mice. These experiments are outlined in Figure 3.
DC adoptive transfer experiments
Rag mice were infected with Hpb for 2 wks and then the Hpb were eliminated by treating the animals with a single orally dose of pyrantel pamoate. Age-matched control mice received the same drug treatment, but were not given the infection. One wk after drug treatment, DC were isolated from the MLN or intestine, and the DC from either Hpb-infected or control mice were adoptively transferred by i.p. injection (4×104 cells/mouse) into Rag mice along with 4×106 splenic T cells from IL10 KO mice and 2×106 OT2 T cells from WT OT2 mice. A third group of Rag mice was reconstituted with IL10 KO and OT2 T cells, but received no DC. Animals then were treated with piroxicam (NSAID), as outlined above, to induce colitis. One wk after stopping the piroxicam, the colon was examined and scored blinded for severity of colitis on a 4-point scale (13). LPMC were isolated from the gut for cell culture with or without OVA to measure IFNg and IL17 release, or they were subject to flow analysis (see figure 4).
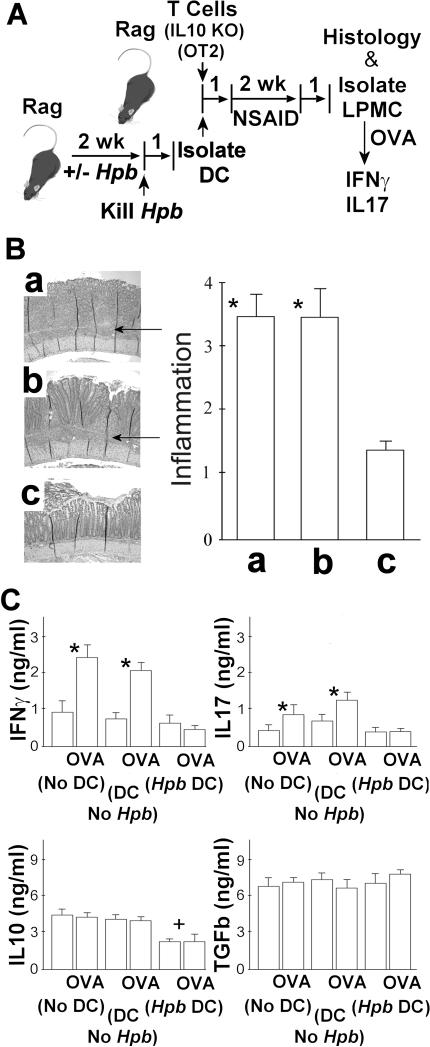
Figure 4. Adoptive Transfer of Intestinal DC from Hpb-Infected Rag Mice Protects the Recipients from Colitis.
A. The experimental design. Rag mice were infected with Hpb for 2 wks and then the Hpb were eliminated by treating the animals with a single orally dose of pyrantel pamoate. Age-matched control mice received the same drug treatment, but were not given the infection. One wk after drug treatment, DC were isolated from the MLN, and the DC from either Hpb-infected or control mice were adoptively transferred by i.p. injection (4×104/mouse) into Rag mice along with 4×106 splenic T cells from IL10 KO mice and 2×106 OT2 T cells from WT OT2 mice. A third group of Rag mice was reconstituted with IL10 KO and OT2 T cells, but received no DC. Animals then were treated with piroxicam (NSAID), as outlined above, to induce colitis.
B. The histology analysis. At the end of the experiment, colonic tissue was examined microscopically to score the severity of the colitis using a 4-point scale. The data in the panel B represents the severity of inflammation in colons of mice receiving a) no DC, or were adoptively transferred b) DC from mice with no prior Hpb infection (DC No Hpb), or c) DC from mice after Hpb infection (Hpb DC). The arrows point to the intense lymphocytic infiltration in the mucosa and LP that is not present in C. Data are means ± SE from 4 separate experiments each containing 4-5 mice/group. * a or b vs. c, p<0.01. C. Cytokines analysis. Also, studied was the effect of cell transfer on mucosal cytokine production (panel C). LPMC were isolated from the TI of these very same mice. The cells were cultured in vitro at 2×105 cells per well in RPMI complete medium for 48h with or without OVA (10 μg/ml) to stimulate cytokine release. Cell culture supernatants were assayed for IFNg, IL17, IL10 and TGFb using ELISAs after the 48h culture period. Data are mean ± SE of 4 independent experiments. For IFNg and IL17: *LPMC (No DC) ±OVA or LPMC (DC No Hpb ±OVA, p<0.01; LPMC (Hpb DC) ±OVA, p NS. For IL10: +Hpb DC±OVA vs. No DC or DC No Hpb, p<0.01.
Sandwich ELISAs
ELISAs were performed using paired antibodies (R & D Systems, Minneapolis, MN) according to manufacturer's instructions. IL17 ELISA was done using primary capture antibody from (R&D Systems, Inc) and biotinylated anti-IL17A antibody (R&D Systems). IL10 was captured with anti-IL10 mAb (R&D Systems) and detected with biotinylated mAb (R&D Systems). To measure IFNγ, plates were coated with a mAb to IFNγ (HB170, ATCC) and incubated with supernatant. IFNγ was detected with polyclonal rabbit anti-IFNγ (gift from Dr. Mary Wilson, University of Iowa) followed by biotinylated goat anti-rabbit IgG (AXcell, Westbury, NY). Total TGFβ was measured using acid-treated supernatant and mAb240 for capture and biotinylated chicken IgY BAF240 for detection (both R&D Systems)
Flow cytometry analysis
LPMC were washed twice and adjusted to 107 cells/ml in FACS buffer (LGM) and stained with saturating amounts of conjugated mAb for 30 min at 4°C. Following staining, cells were washed twice and re-suspended in LGM for analysis on a FACSCalibur using Cell Quest software (BD Biosciences, Mountain View, CA). Before adding labeled mAb, each tube received 1 μg of anti-Fc mAb (eBioscience, San Diego, CA) to block nonspecific binding of conjugated Abs to FcRs. The mAbs used for staining or cell sorting were anti-Thy1.2-FITC, or -PECy5, or -APC; anti-CD11c-FITC; anti-CD4-PE or -PE-Cy5; anti-CD45.1-APC; anti-CD45.2-APC; anti-CD8-APC or-PE; CD103-PE (all from eBioscience).
Statistical analysis
Data are means ±SE of multiple determinations. Difference between two groups was compared using Student's t-test. Multiple group comparisons used analysis of variation and Dunnett's t-test. P values <0.05 were considered significant.
Results
To study colitis, experiments employed a well established Rag IL10-/- T cell transfer model of IBD, which renders these animals susceptible to colitis (13) (14). This model is relevant to human IBD since defects in the IL10- signaling pathway predispose people to IBD (15). Many such models develop colitis inconsistently. To enhance expression of disease, 1 wk after cell transfer, mice were fed a NSAID (piroxicam) for 2 wks. This resulted in all animals developing severe colitis that was evident 1 wk thereafter stopping the NSAID. (Four wks after cell transfer.) The NSAID disrupts the production of protective arachadonic acid metabolites in the mucosa (13) making the animals more prone to IBD. This is relevant to human IBD, since administration of many types of NSAIDs worsen the disease (16) (17). We also adoptively transferred transgenic OT2 T cells bearing MHC Class II dependent TCR that recognize OVA into the Rag mice concomitantly with the other cells so that we could study an antigen specific T cell response in the gut LP. Isolated LPMC from these T cell reconstituted Rag animals respond to OVA with IFNg and IL17 release. These cytokines were of particular interest, since these cytokines help drive the disease in human IBD and in many animal models of this condition (18)(19)(20).
Rag mice exposed to Hpb only before T cell reconstitution are protected from colitis (12). Since Rag mice lack functional T and B cells, this suggested that interactions of Hpb just with cellular components of innate immunity are sufficient to provide this protection. To extend this observation, these experiments tested the hypothesis that infection with Hpb induces tolerogenic DC in the gut, which are an important component of the protective process.
DC isolated from Rag mice infected with Hpb cultured in vitro with LPMC block OVA-induced cytokine secretion
In the initial experiments, it was determined if DC from Hpb-infected Rag mice could impair the natural interaction of intestinal pro-inflammatory DC with their effector T cells such as to impede antigenic responses. Rag mice were reconstituted with IL10-/- splenic T cells, and treated with piroxicam to induce colitis. LPMC were isolated from these animals, mixed with splenic OT2 T cells and cultured in vitro with or without OVA antigen to stimulate cytokine production. Some wells also received supplemental DC isolated from the intestines of a separate group of Rag mice that had been infected with Hpb for 2 wks before sacrifice (Hpb DC). The control for Hpb infection was wells given DC isolated from the gut of age-matched Rag mice that never experienced Hpb infection (DC no Hpb). (Fig 1, panel A)
Isolated LPMC from the mice with colitis produced IFNg and IL17 constitutively and secreted substantially more when cultured with OVA (Fig 1, panel B). Addition of Hpb DC, given at a ratio of 1:5 (DC:LPMC), did not affect constitutive cytokine secretion, but totally blocked OVA-induced, cytokine stimulation. Addition of the DC no Hpb control cells did not block cytokine release (Fig 1, panel B).
Supernatants from these cell cultures also were examined for IL10, TGFb and IL4 content, which are cytokines that can regulate or alter antigenic responses. IL10 or TGFb were secreted in similar amounts under all culture situations (Fig 1, panel B). OVA added to the cultures did not increase either IL10 or TGFb release. No IL4 was detected in any cultures.
Other experiments used DC isolated from the MLN of Rag mice with or without Hpb infection. DC from the MLN of Hpb-infected mice when added to the isolated LPMC from the colitic animals (1:5 cell ratio) also blocked OVA-induced, cytokine responses equally as well as gut DC. The OVA response was not affected by the addition of MLN DC from Rag mice who never received Hpb-infection (data not shown).
DC regulation of the OVA-response requires cell contact
Using Transwell plates, it next was determined if DC regulation of OVA-induced, cytokine production required cell contact. Once more, LPMC isolated from colitic animals were mixed with splenic OT2 T cells and cultured in the outer wells of a Transwell plate alone or with gut Hpb DC. Some wells also receive OVA to stimulate cytokine release. As expected, OVA induced strong cytokine responses only in the absence of the supplemental DC (Fig 2). However, Hpb DC did not alter OVA-induce cytokine secretion when the Hpb DC were placed in the inner well separated by a semi-permeable membrane from the OT2 T cells and colitic LPMC present in the outer well.
The intestines of Rag mice protected from colitis by Hpb infection contain tolerogenic DC
The above experiments suggested that infection of Rag mice with Hpb induces DC within the gut that can block the intestinal OVA response in vitro. To further test the significance of this observation, it next was determined if animals protected from colitis by Hpb infection display tolerogenic type DC within their intestines.
Rag mice were reconstituted with IL10-/- T cells and OT2 T cells, and then treated with piroxicam to induce colitis. One week after stopping the piroxicam, the mice were sacrificed, and the intensity of the colitis was scored by examining histological tissue sections. Also, intestine was dissociated to isolate the LPMC, which were cultured in vitro with or without OVA to stimulate IFNg and IL17 release.
In parallel, a second group of age-matched Rag mice were exposed to Hpb for 2 wks and then de-wormed by pharmaceutical means before they received similar T cell-reconstitution and piroxicam exposure. One week after stopping the piroxicam, the mice were sacrificed, and their colitis also was scored for intensity of inflammation by examining histological sections. In these mice, intestine was dissociated to isolate DC, which were added to LPMC from the severely colitic mice and then cultured in vitro to see if these DC would affect the LPMC OVA response. (Fig 3, panel A)
IL10 KO T cell-reconstituted, Rag mice developed severe colitis after piroxicam treatment, unless they were infected with Hpb before the T cell reconstitution. (No Hpb infection vs. Hpb infection: inflammatory score 3.7+/-0.4 vs. 1.2+/-0.3, mean+/-SE, N=3 separate experiments, p<0.01) Isolated LPMC from the colitic mice (no Hpb infection) cultured in vitro produced IFNg and IL17 whose production was enhanced by OVA stimulation (Fig 3, panel B). When the LPMC were mixed with DC from the Rag mice protected from colitis by Hpb infection and cultured, OVA stimulation failed to excite more cytokine release. However, mixing LPMC with intestinal DC from mice never infected with Hpb did not affect either constitutive or OVA-induced IFNg or IL17 production (Fig 3, panel B).
Hpb DC are sufficient to prevent colitis
The next series of experiments ascertained if DC from Hpb-infected Rag mice could adoptively transfer protection from colitis. Rag mice received IL10-/- and OT2 T cells administered i.p. Some mice also received DC isolated from the MLN of Rag mice that had been infected with Hpb for 2 wks. An additional group of animals received DC from the MLN of age-matched Rag mice that never experienced Hpb infection. After NSAID administration, the animals were sacrificed at the appropriate time to assess severity of colitis and the responsiveness of isolated LPMC to OVA stimulation (Fig 4, panel A).
Fig 4, panel B shows, as expected, that severe colitis developed in mice receiving no DC. Mice receiving DC from Hpb-infected animals displayed much less colonic inflammation. Adoptive transfer of DC from animals never infected with Hpb did not affect the intensity of the inflammatory response.
LPMC isolated from mice that received no DC or DC from uninfected mice produced both IFNg and IL17 constitutively when cultured in vitro and even more after OVA stimulation. However, LPMC from Rag mice receiving DC from the infected animals lost their responsiveness to OVA stimulation (Fig 4, panel C).
Culture supernatants also contained IL10. OVA stimulation did not increase IL10 secretion. LPMC isolated from the colons of mice that received no DC or DC from uninfected mice produced comparable amounts of this cytokine. However, LPMC from mice receiving DC from the infected animals actually made much less IL10 (Fig 4, panel C).
Also measured were TGFb and IL4. All cultures produced comparable amounts of TGFb, and OVA did not stimulate more TGFb release (Fig 4, panel C). IL4 was not detected in any cell culture.
Additional experiments were conducted as described above except that mice received DC isolated from the TI of Rag mice with or without Hpb infection. Comparable to DC from MLN, gut DC from Hpb-infected animals transferred into Hpbnaïve recipients protection the mice from colitis and blocked mucosal responsiveness to OVA. Once again, intestinal DC from uninfected mice had no effect (data not shown).
Adoptive transfer of DC from Hpb-infected mice into Rag recipients does not impair OT2 T cell accumulation in the gut and MLN
The loss of LPMC OVA responsiveness after adoptive transfer of DC from Hpb-infected mice could have signified that DC transfer simply interfered with normal OT2 T cell accumulation in the LP. T cells from C57BL/6 mice express the molecule CD45.2. We thus reconstituted Rag mice with OT2 T cells from transgenic C57BL/6 mice expressing CD45.1 so that OT2 cells within the isolated LPMC could be distinguished from the IL10-/- T cells through differential CD45 display. In this mouse, transgenic TCR expressed on the CD4+ OT2 T cell subset recognize the OVA.
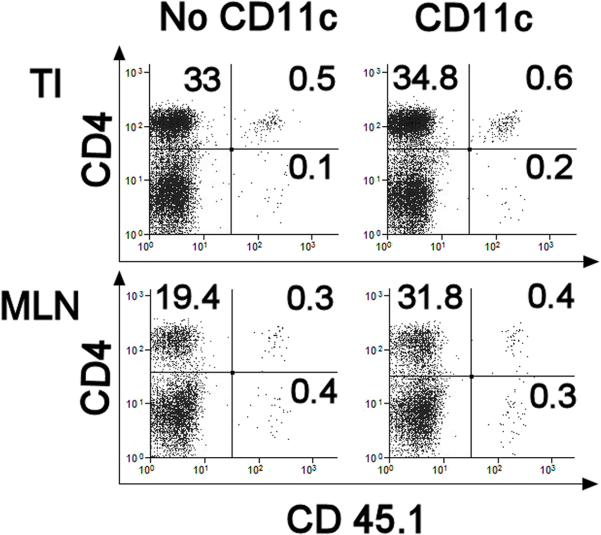
One group of Rag mice received CD45.1+ OT2 T cells and CD45.2+ IL10-/- T cells, and then were exposed to piroxicam to induce colitis. Another group of Rag animals were reconstituted with T cells and treated as above except they also received DC from the MLN of Rag mice infected with Hpb to abolish the intestinal response to OVA. In the colitic mice, about 0.5% of the LP or MLN CD4+ T cells displayed CD45.1 (Fig 5). The relative number of LP or MLN CD4+ T cells expressing CD45.1 did not diminish in Rag mice receiving the DC.
Figure 5. DC Transfer Does not Diminish the Number of OT2 T Cells that Accumulate in the Gut or MLN.
Rag mice received 4×106 splenic IL10 KO T cells displaying CD45.2 and 2×106 OT2 T cells expressing CD45.1 given by i.p injection. Some mice also received DC (CD11c) (4×104/mouse) from the MLN of Rag mice previously infected with Hpb, while other animals did not receive DC (No CD11c). At the end of the experiment, LPMC were isolated from the TI of either the control or CD11c transfer group. Also studied were dispersed MLN cells. Cells in the lymphoid gate were subject to flow analysis to determine the relative number of LP T cells expressing CD45.1. For each group, flow analysis was performed on pooled LPMC or MLN cells isolated from 4 individual mice. Data are representative of 3 independent experiments.
After Hpb DC transfer, isolated intestinal OT2 T cells display retained capacity to produce cytokines after OVA stimulation when cultured in a permissive environment
Since OT2 T cells readily accumulated in the gut even after tolerogenic DC transfer, it next was determined if the unresponsive intestinal OT2 T cells could regain OVA responsiveness in a more permissive environment. Once again, one group of Rag mice received CD45.1+ OT2 T cells and CD45.2+ IL10-/- T cells, and then were exposed to piroxicam to induce colitis. Another group of Rag animals were reconstituted with T cells and treated as above except they also received DC from the MLN of Rag mice infected with Hpb to abolish the intestinal response to OVA. One wk after stopping the piroxicam, LPMC were isolated from the TI of both groups, and CD45.1+ OT2 T cells were separated from the dispersed LPMC using FACS (Hpb OT2 and OT2). Also, the residual LPMC were depleted of T cells (Hpb non-T and non-T). OT2 T cells were mixed with either one or the other non-T cell preparation and cultured with or without OVA. All cultures produced comparable amounts of IFNg and IL17 without OVA stimulation (Table I). OT2 cells from either source did not respond to OVA if the cells were mixed with T cell-depleted, LPMC isolated from the intestines of Rag mice that received DC (Table I). However, OT2 cells responded well to OVA if they were co-cultured with T cell-depleted, LPMC from mice that did not receive DC. These experiments suggested that OT2 T cell antigen unresponsiveness was at least partly secondary to the presence of the non-T cell element of the LP and that they do not lose the capacity to respond to OVA.
Table I.
Intestinal OT2 T cells rendered inert by DC transfer retain the capacity to respond to OVA
| Hpb OT2 + Non-T | Hpb OT2 + Hpb Non-T | OT2 + Non-T | OT2 + Hpb Non-T | |
|---|---|---|---|---|
| IFNg | ||||
| Cells | BD* | BD | BD* | BD |
| Cells +OVA | 93±5 | BD | 72±10 | BD |
| IL17 | ||||
| Cells | 67±5* | 70±6 | 67±10* | 73±9 |
| Cells +OVA | 167±26 | 82±4 | 142±22 | 80±6 |
Intestinal OT2 T cells isolated from Rag mice who received DC from Hpb-infected animals (Hp OT2) respond to OVA when cultured with T cell-depleted LPMC (Non-T) from Rag mice who did not receive DC. Conversely, the OT2 cells fail to respond to antigen when they are cultured with their own LPMC (Hp OT2 + Hp Non-T). Moreover, intestinal OT2 T cells that normally respond to OVA (OT2 + Non-T) will fail to do so if they are cultured with Hpb Non-T.
The experimental design is outlined in figure 4, panel A, except that the recipient mice received CD45.1+ OT2 T cells to allow OT2 T cell isolation via FACS. Some mice also received DC from the MLN of Rag mice infected with Hpb for 2 wks (Hpb DC), whereas other mice received no DC. At the time of sacrifice, OT2 T cells were isolated from dispersed LPMC using FACS and anti-CD45.1 mAb. LPMC deleted of T cells (Non-T) were obtained using FACS and anti-Thy 1.2 mAb. OT2 T cells (106) from the TI of either the Hpb DC recipients or control mice (No DC) were mixed with 2×106 Non-T from the TI of either experimental group (Hpb non-T or non-T, no DC) and cultured without or with OVA (10 ug/ml) for 48h. Data are mean pg/ml±SE of three independent determinations.
cytokine vs. cytokine + OVA, p<0.01
Hpb infection does not increase the number of T cells expressing Foxp3 in LPMC cultures, the intestines or MLN
Using our murine model of IBD, the above experiments showed that Hpb infection induced a regulatory type intestinal DC that could inhibit colitis and antigenic specific T cell responses in the gut. DC can drive expansion of Foxp3+ T cells in tissues. Thus, it was deemed important to investigator if DC regulation likely was working through induction of Foxp3+ T cells.
It first was determined if mixing tolerogenic DC with LPMC expanded the number of Foxp3+ T cells in LPMC cultures. For these experiments, Rag mice were reconstituted with IL10 KO/Foxp3eGFP reporter T cells before induction of colitis so that Foxp3+ T cells could be monitored, by their endogenous expression of eGFP, in the subsequent LPMC cell cultures. Fig 1, panel C shows that LPMC from the mice with colitis when cultured in vitro will lose most of their Foxp3+ T cells. Culturing LPMC with intestinal DC from Hpb infected mice did not expand the number Foxp3+ T cells within these mixed cell cultures.
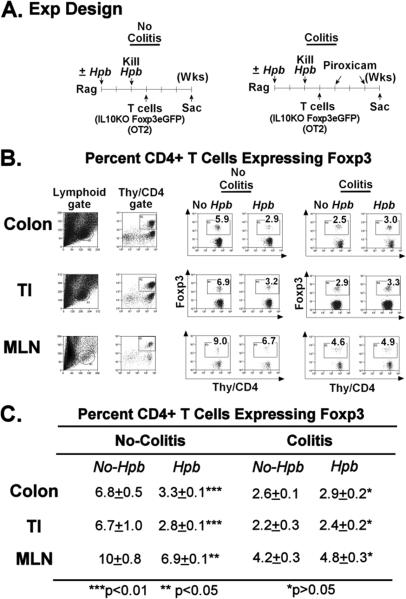
Employing one of the experimental protocols used to generate tolerogenic intestinal DC, it also was ascertained if the frequency of Foxp3+ T cells in the TI, colon or MLN would increase if Rag mice were infected with Hpb before T cell reconstitution. Rag mice were infected with Hpb for 2 wks and then de-wormed by treatment with pyrantel pamoate. One wk later, the mice received Foxp3eGFP IL10-/- reporter T cells and OT2 T cells. In some experiments, mice received piroxicam to induce colitis, whereas, others received no such treatment and remained free of colitis (Fig 6, panel A). At the time of sacrifice, dispersed MLN cells, and LPMC isolated from the TI and colon were analyzed for Foxp3 T cell expression using flow analysis to detect eGFP+ T cells. About 98% of the Foxp3+ T cells in the MLN, TI and colon were CD4+. In mice that did not receive piroxicam (no colitis), Hpb infection induced a substantial decrease in the relative number of CD4+ T cells expressing Foxp3 in the three tissues studied (Fig 6, panel B and C, no colitis). In the mice fed piroxicam to induce colitis, colonic inflammation was associated with a drop in the proportion of CD4+ T cells expressing Foxp3. Moreover, Hpb exposure protected the animals from colitis (inflammatory score: no Hpb vs. Hpb, 3.5±0.5 vs. 1.0±0.2, ±SE, N=3) without significantly altering the proportion of CD4+ T cells expressing Foxp3 (Fig 6, panel B and C, colitis).
Figure 6. Compared to WT Controls, T Cell-Reconstituted Rag Mice Infected with Hpb Do Not Display Increased Numbers of Foxp3+ CD4+ T Cells in the TI, Colon or MLN.
A. The experiment design. 1) Some Rag mice were infected with Hpb (125 larvae) for 2 wks and then de-wormed by giving pyrantel pamoate. Control mice received the same drug, but no infection. Mice then received 4×106 IL10KO/Foxp3 eGFP reporter T cells and 2×106 OT2 T cells, by i.p. injection, one wk after pyrantel pamoate treatment. Three wks after T cell reconstitution, dispersed MLN cells as well as LPMC isolated from the colon and TI were examined using flow cytometry to assess the relative number of Foxp3+ CD4+ T cells in these cell preparations. None of these mice developed colitis, since they did not receive piroxicam (No Colitis). 2) In other experiments, mice were prepared as described above, except that the mice received 2 wks of piroxicam treatment to stimulate colitis, which only appeared in a substantial degree in the mice without prior Hpb exposure. One wk after stopping the piroxicam, the MLN cells and LPMC from the TI and colon were examined for eGFP+ Foxp3+ CD4+ T cells using flow cytometry (Colitis model).
B. shows that in the no colitis model, Hpb infection actually decreased the proportion of CD4+ T cells expressing Foxp3 in the TI, colon and MLN. Compared to the no colitis group, the percentage of CD4+ T cells expressing Foxp3+ diminished following induction of colitis. Exposing mice to Hpb before induction of colitis only minimally altered the proportion of CD4+ T cells expressing Foxp3 within these tissues. Data are representative of three separate experiments. For each group, flow analysis was performed on pooled LPMC isolated from 3-4 individual mice. C. are the mean flow data from 3 individual experiments ±SE.
Unlike intestinal DC, intestinal Foxp3+ T cells from Hpb-infected mice display no regulatory activity
Studies also examined if co-culturing Foxp3+ T cells, isolated from the gut of mice protected from colitis by Hpb infection, with LPMC from the colitic animals would suppress IFNg or IL17 secretion. These experiments once again used IL10 KO/Foxp3eGFP reporter T cells to allow identification and isolation of gut Foxp3+ T cells using FACS. Unlike the isolated DC, intestinal Foxp3+ T cells added to LPMC from colitic mice did not alter the capacity of the LPMC to produce IFNg or IL17 (Fig 3).
DC phenotypes in the MLN and gut after Hpb infection
DC in the MLN and gut of Rag mice were partially characterized before and after Hpb infection using CD11c and CD103. In the mice without infection, the MLN were composed of about 1.3±0. 1% CD11c + cells that separated into two distinct subsets based on their intensity of CD11c expression. The CD11clo subset was more numerous (CD11chi 45±0.6% vs. CD11clo 56±1.0%, mean ±SE, N=3 experiment). The relative number of CD11c cells in MLN did not change after Hpb infection (1.1±0.1%). However, Hpb infection decreased the number of CD11chi cells (33.7±0.7%), while increasing the CD11clo cells (67.3±0.9%), (mean±SE, 3 separate experiments) (CD11chi or CD11clo, uninfected vs. infected, both p<0.01.). CD103 expression was noted almost exclusively in the CD11chi subset (CD11chi 14.6±2.1%; CD11clo 1.7±0.6% N=3, ±SE), and the relatively number of CD11chi and CD11clo cells expressing CD103 did not significantly change after Hpb infection (data not shown).
In the gut, only one CD11c+ cell subset could be readily distinguished by the expression of CD11c staining intensity. The percentage of LPMC expressing CD11c was not affected by Hpb infection (uninfected 2.6 ±0.7%, vs. infected 2.3 ±0.8%, mean ±SE, P>0.05, N=3). CD103 expression on the CD11c+ population were no changed by Hpb infection (uninfected 25.69% ± 1.4 vs. infected 24.0% ± 1.8, mean ± SE. P>0.05, N=3)
The beads (StemCell) used to isolate CD11c+ cells from the MLN and gut recovered about 85% of these cells at about 95% purity. The beads displayed equal efficiency at isolating both the CD11chi and CD11clo subset.
DISCUSSION
A critical point made by this investigation is that exposure of Rag mice to Hpb induces tolerogenic-type DC in the intestines. This implies that Hpb does not require direct interaction with T or B cells to render intestinal DC regulatory. These tolerogenic DC appear to be an important mechanism in Hpb-induce protection from colitis, since DC transfer is sufficient to render animals resistant to IBD even if the recipient mice never experienced Hpb infection.
The experiments presented here showed that adoptive transfer of DC isolated from the MLN or intestines of Rag mice infected with Hpb could block colitis and intestinal OVA-induced cytokine responses in vivo. Transfer of DC from uninfected Rag mice had no effect. Thus, the process of infection altered the function of the DC in the gut and MLN of Rag mice, without the aid of T or B cell interactions, rendering these DC capable of modulating mucosal immunity and protecting animals from IBD.
The loss of LPMC antigenic responsiveness after transfer of DC from Hpb-infected mice could have signified that DC transfer interfered with normal OT2 T cell population of the LP. Our studies using OT2 T cells that expressed CD45.1, which allowed visualization of the OT2 T cells in the LP, showed that this was not the case.
Since OT2 T cells populated the LP appropriately, it also was determined if the LP CD45.1+ OT2 T cells, which were not responsive to OVA stimulation in their natural environment after DC transfer, would respond to antigen if placed in a more permissive environment. Our data suggest that the OT2 T cells retained the capacity to respond to antigen, but were being held in check by the non-T cell elements of the LP.
Various additional in vitro experiments revealed that OT2 T cells mixed with LPMC from colitic mice produced large amounts of IFNg and IL17 upon OVA stimulation. However, adding intestinal or MLN DC only from the Hpb-infected mice blocked this response. While helminths interacting with DC can drive a Th2 response (21,22,23), the cultures contained no measurable IL4 suggesting that the loss of IFNg and IL17 secretion in response to OVA was not simply a shift from a Th1/Th17 response to that of Th2.
The composite of all these data support the contention that Hpb infection modulates DC function, rendering these cells highly regulatory. Since inappropriate T cell activation to luminal antigens is believed to underlie the etiology of human IBD, the capacity of these DC to block an antigen-specific T cell response in the gut could be an important mechanism through which these cells work to suppress colitis.
The in vitro DC/LPMC mix experiments suggested that these tolerogenic DC interfered with effector T cell/pro-inflammatory DC interacts. The Transwell experiments showed that the tolerogenic DC required direct physical interaction with other LPMC to block the OVA-induced, cytokine response.
We previously showed that Hpb infection had a prominent effect on the capacity of intestinal DC in Rag mice to display various surface proteins (12). This included a decreased expression of the co-stimulatory molecules CD80 and CD86. More widely expressed were PDCA-1, a marker of plasmacytoid DC (24), and CD40. Studying MLN, we observed an increase in DC that were CD11clo. This shift was previously reported using WT mice with Hpb infection (25). In our study, a CD11clo subset was not observed in the intestine of our Rag mice before or after Hpb infection. DC in MLN, Peyer's patches and the LP are continuously exposed to a multitude of luminal antigens. In different regions of the intestines, various DC subsets have been characterized based on the expression of cell surface molecules like CD103, CX3Cr1 and CD11b (26). It is yet to be determined if these Hpb-induced, tolerogenic DC can be identified through a unique display of cell surface molecules.
Intestinal DC also can produce regulatory cytokines like IL10 and TGFb or can induce regulatory-type T cells to make these cytokines (26), which in turn can modulate T cell responses. Adding tolerogenic DC to the LPMC/OT2 T cell cultures did no enhance either IL10 or TGFβ production. Isolated LPMC from Rag mice that received tolerogenic DC in vivo to prevent colitis actually displayed a decreased capacity to produce IL10. Under all culture conditions, the release of IL10 and TGFβ was independent of OVA stimulation and thus assumed to be independent of the T cell response. There even was no response to anti-CD3/CD28 mAb stimulation. Except for the OT2 T cells, the T cells used to reconstitute Rag mice were derived from IL10-/- mice further supporting this contention. The failure of Hpb-induced DC to increase IL10 or TGFb production in the cultures suggests that rising levels of these soluble regulatory cytokines were not a critical part of the regulatory mechanism. We previously showed that DC isolated from the gut of Hpb-infected Rag mice, compared to DC from uninfected control animals, do not produce more IL10 or TGFβ when cultured in vitro with or without LPS or CpG oligo stimulation (12).
A diverse array of DC phenotypes has been reported to help induce and maintain immune tolerance. CD11clo DC that lack the usual markers of plasmacytoid DC (e.g. CD8, CD103, PDCA and Siglec-H) expand in the MLN of immunologically intact mice after Hpb infection. This subset can drive antigen-specific CD4+ Foxp3+ T cell expression in cell cultures (25). Hpb infection expands the number of CD4+ Foxp3+ T cells in the MLN of infected mice suggesting that this CD11clo DC has relevance to the natural infection. It also is reported that Hpb and other helminths secrete proteins that can induce de novo Foxp3 expression in T cells without the aid of DC (27). There are reports that CD11chi DC expressing CD103 also can induce Treg expression in vitro (28)(29) although there is some dispute whether they can function in this capacity in the colon (30). We did not detect a relative increase in the number of DC expressing CD103. While our DC isolation technique was able to recover both the CD11c hi and CD11clo subsets from the MLN, we did not determine which specific DC subset was responsible for control of colitis.
As presented here, Hpb-induced, intestinal DC that block colitis in our IL10-/-Rag model of IBD, and which inhibit antigen-induced cytokine secretion in the gut, do not appear to mediate their action through expansion of the proportion of T cells expressing Foxp3. This was shown in several different experiments. Among these experiments included a demonstration that intestinal Treg could not substitute for tolerogenic DC in our in vitro regulation experiments. The previous reports suggesting that Hpb promotes Foxp3 expression in the MLN are not necessarily at odds with our findings. The IBD model used here is unique in that the Rag mice, which lack functional T and B cells, are only exposed to Hpb before T cell reconstitution. Hpb infection after T cell reconstitution can enhance Foxp3 expression in Rag mice (31). It is likely that helminths function through several independent regulatory pathways to protect mice from IBD. Tolerogenic DC can limit T cell responses via Treg independent mechanisms including induction of effector T cell anergy (32)(33) or apoptosis through upregulation of Fas ligand expression (34). Thus, there is precedence for our observation.
DC can promote immune tolerance and protect mice from colitis. Tolerance induction can occur through DC induction of regulatory-type T cells that can express Foxp3 and/or produce IL10 or TGFβ (35)(36). The DC also can induce effector T cell deletion or anergy as discussed above. The Hpb-induced, tolerogenic DC appear to function in our IBD model via the latter mechanism. While the effector T cells are not lost following exposure to the tolerogenic DC, it will be interesting to explore if the pro-inflammatory DC that drive the immune response are subject to apoptosis, which is a newly appreciated mechanism that can lead to immune tolerance (37). As shown here and elsewhere, tolerogenic-type DC induced by Hpb exposure can be adoptively transferred to murine recipients to prevent IBD (38), and tolerogenic DC can do likewise in other immunologic diseases (39). It is tempting to speculate that induction of potent tolerogenic DC in vitro through exposure to helminthic products will afford a powerful new approach for the treatment of IBD.
Acknowledgments
Supported by NIH grants DK38327, DK058755, DK091987, and Broad Foundation, Schneider Family, Friedman Family, Gilman Family
Abbreviations
- APC
Antigen presenting cells
- DC
Dendritic cells
- Hpb
Heligmosomoides polygyrus bakeri
- Hpb DC
DC from Hpb-infected mice
- DC no Hpb
DC from mice never infected with Hpb
- IBD
Inflammatory bowel disease
- LP
Lamina propria
- LPMC
Lamina propria mononuclear cells
- NSAID
Non-steroidal anti-inflammatory drug
- RPMI
RPMI 1640 medium
- TI
Terminal ileum
Reference List
- 1.Weinstock JV, Summers R, Elliott DE. Helminths and harmony. Gut. 2004;53:7–9. doi: 10.1136/gut.53.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott DE, Urban JF, Jr., Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease? FASEB J. 2000;14:1848–1855. doi: 10.1096/fj.99-0885hyp. [DOI] [PubMed] [Google Scholar]
- 3.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr., Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 4.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Inf. Immun. 2002;70:5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon C, Sanchez A, Hogaboam CM, McKay DM. Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Inf. Immun. 2001;69:4417–4423. doi: 10.1128/IAI.69.7.4417-4423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr., Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 7.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr., Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Inf. Immun. 2007;75(9):4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2006;291(2):G253–259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. :1199–1212. doi: 10.1084/jem.20042572. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith P, Mangan NEWCM, Fallon RE, McKenzie ANJ, van Rooijen N, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 11.Erb KJ. Helminths, allergic disorders and IgE-mediated immune responses: where do we stand? Eur. J. Immunol. 2007;37(5):1170–1173. doi: 10.1002/eji.200737314. [DOI] [PubMed] [Google Scholar]
- 12.Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, Reinecker HC, Weinstock JV. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J. Immunol. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 14.Blum AM, Metwali A, Elliott DE, Berg DJ, Weinstock JV. CD4+ T cells from IL-10-deficient mice transfer susceptibility to NSAID-induced Rag colitis. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2004;287(2):G320–325. doi: 10.1152/ajpgi.00527.2003. [DOI] [PubMed] [Google Scholar]
- 15.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clinical Gastroenterology & Hepatology. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 17.Chan SS, Luben R, Bergmann MM, Boeing H, Olsen A, Tjonneland A, Overvad K, Kaaks R, Kennedy H, Khaw KT, Riboli E, Hart AR. Aspirin in the aetiology of Crohn's disease and ulcerative colitis: a European prospective cohort study. Alimentary Pharmacology & Therapeutics. 2011;34:649–655. doi: 10.1111/j.1365-2036.2011.04784.x. [DOI] [PubMed] [Google Scholar]
- 18.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm. Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 19.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 20.Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, Ince MN, Bazzone LE, Stadecker MJ, Urban JF, Jr., Weinstock JV. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J. Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. PNAS. 2009;106(33):13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur. J. Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KA, Hochweller K, Hämmerling GJ, Boon L, MacDonald AS, Maizels RM. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103- dendritic cells. J. Immunol. 2011;186:40–49. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng SC, Kamm MA, Stagg AJ, Knight SC. Intestinal dendritic cells: their role in bacterial recognition, lymphocyte homing, and intestinal inflammation. Inflamm. Bowel Dis. 2010;16:1787–1807. doi: 10.1002/ibd.21247. [DOI] [PubMed] [Google Scholar]
- 27.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF- pathway. J. Exp. Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui KR, Powrie F. CD103+ GALT DCs promote Foxp3+ regulatory T cells. Mucosal immunology. 2008;1:34–38. doi: 10.1038/mi.2008.43. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr., Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 32.Tan JK, Periasamy P, O'Neill HC. Delineation of precursors in murine spleen that develop in contact with splenic endothelium to give novel dendritic-like cells. Blood. 2010;115:3678–3685. doi: 10.1182/blood-2009-06-227108. [DOI] [PubMed] [Google Scholar]
- 33.Wilson HL, Ni K, O'Neill HC. Identification of progenitor cells in long-term spleen stromal cultures that produce immature dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4784–4789. doi: 10.1073/pnas.080278897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu x., Yi H, Guo Z, Quan C, Xia S, Yao Y, Cao X. Splenic stroma-educated regulatory dendritic cells induce apoptosis of activated CD4 T cells via Fas ligand-enhanced IFN-g and nitric oxide. J. Immunol. 2012;188:1168–1177. doi: 10.4049/jimmunol.1101696. [DOI] [PubMed] [Google Scholar]
- 35.Yamanishi H, Murakami H, Ikeda Y, Abe M, Kumagi T, Hiasa Y, Matsuura B, Onji M. Regulatory dendritic cells pulsed with carbonic anhydrase I protect mice from colitis induced by CD4+CD25- T cells. J. Immunol. 2012;188:2164–2172. doi: 10.4049/jimmunol.1100559. [DOI] [PubMed] [Google Scholar]
- 36.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological Reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [erratum appears in Immunol Rev. 2006 Oct;213:257].
- 37.Kushwah R, Hu J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J. Immunol. 2010;185:795–802. doi: 10.4049/jimmunol.1000325. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen AE, Schmidt EG, Gad M, Poulsen SS, Claesson MH. Dexamethasone/1alpha-25-dihydroxyvitamin D3-treated dendritic cells suppress colitis in the SCID T-cell transfer model. Immunology. 2009;127:354–364. doi: 10.1111/j.1365-2567.2008.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Wan Y. Tolerogenic dendritic cells and their potential applications. Immunology. 2011;132:307–314. doi: 10.1111/j.1365-2567.2010.03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]