Abstract
Background
Generalized Social Phobia (GSP) and Generalized Anxiety Disorder (GAD) are both associated with emotion dysregulation. In healthy subjects, research implicates dorsal anterior cingulate (dACC) in both explicit emotion regulation and top-down attentional control. While studies have examined these processes in GSP or GAD, no work compares findings across the two disorders. Moreover, no work examines functioning in cases comorbid for both disorders (GSP/GAD). Here we compare the neural correlates of explicit emotion regulation (EER) and top-down attentional control (TAC) in GSP, GAD, and GSP/GAD.
Method
Medication-free adults with GSP (EER n=19; TAC n=18), GAD (EER n=17; TAC n =17), GSP/GAD (EER n=17; TAC=15), or no psychopathology (EER n=18; TAC n=18). During EER, individuals alternatively viewed, up-regulated, and down-regulated responses to emotional pictures. During TAC, they performed an emotional Stroop task.
Results
For both tasks, significant group-by-condition interactions emerged in dACC and parietal cortices. Healthy adults showed significantly increased recruitment during emotion regulation, relative to emotion-picture viewing. GAD, GSP, and GSP/GAD subjects showed no such increases, with all three groups differing from healthy adults but not from each other. Evidence of emotion-related disorder-specificity emerged in medial prefrontal cortex (MPFC) and amygdala. This disorder-specific responding varied as a function of stimulus emotion content but not emotion-regulatory demands.
Conclusions
GSP and GAD both involve reduced capacity for engaging emotion-regulation brain networks, whether explicitly or via top-down attentional control. A reduced ability to recruit regions implicated in top-down attention might represent a general risk factor for anxiety disorders.
Keywords: imaging, social anxiety, generalized anxiety, emotion regulation, anterior cingulate cortex, top-down attentional control
INTRODUCTION
Generalized Social Phobia (GSP) and Generalized Anxiety Disorder (GAD) are two highly disabling, frequently comorbid conditions (1). GSP involves anxiety to social situations, whereas GAD involves excessive worry, often involving both social and non-social themes. While some imaging suggest that similar neural architecture underlies these two disorders (e.g., 2), few directly compare them. Many imaging studies examine GSP, fewer examine GAD, and none directly compare the two in their non-comorbid state. This study directly compares GSP, GAD, and comorbid GSP/GAD.
Clinical data indicate impaired emotional regulation in both GSP and GAD (e.g., 3, 4). Accordingly, GSP/GAD co-morbidity may reflect shared dysfunction in brain regions supporting emotion regulation. However, emotional regulation is a broad term that subsumes a range of cognitive processes (5). Within this range, it has been argued that emotional regulation can engage two sets of control processes, based in dorsal and ventral brain systems, that are differentially affected across the mood and anxiety disorders (6, 7).
The first type of emotion regulation involves ventral prefrontal systems that represent emotional value and select actions based on these representations. Altering the strength of reinforcement-valence representations may, through reciprocal mechanisms with systems such as the amygdala, reduce emotional responses. Similarly, ventral anterior cingulate cortex (vACC) may specifically support conflict adaptation, a form of emotional regulation (8). These forms of emotional regulation have received minimal attention with respect to GAD and GSP (though, see 8) and will not be the focus of this paper.
The second type involves dorsal prefrontal cortex (both medial and lateral regions). Attention control represents one vital function of these systems, the priming of relevant representations at the expense of irrelevant ones, thereby resolving representational competition (9). Arguably, such control processes can be recruited explicitly within cognitive reappraisal paradigms, where subjects willfully attempt to alter stimulus representations by priming non-emotional features (10). These processes also might be recruited implicitly through attention distraction paradigms (11–13), where the reduction of emotional responding occurs “…without monitoring…without insight and awareness” (p. 401; 5).
The process of cognitive reappraisal recruits lateral and dorsomedial frontal cortices, dorsal anterior cingulate cortex (dACC) and inferior parietal cortex (6, see, for reviews, 14). Distraction paradigms also recruit these systems (13, 15). Patients with GSP show reduced dACC engagement during explicit emotional down-regulation of social emotional stimuli (16, 17). However, a recent study found that a mixed anxiety-prone group, including patients with GSP or GAD, showed increased ventral and dACC during explicit emotion regulation, relative to low-anxiety comparison indivdiuals (18).
In short, decreased dACC recruitment occurs in GSP during explicit emotional regulation, at least with social stimuli. However, no study examines explicit emotional regulation in GAD, nor do studies examine recruitment of dACC and associated lateral fronto-parietal regions implicated in attentional control. Such recruitment is where emotional responses are implicitly regulated through task-related top-down attention. If patients with GSP, GAD, and GSP/GAD show general dysfunction in regions implicated in top-down attentional control (i.e., lateral frontal, ACC, and parietal regions), one would expect all three patient groups to show reduced recruitment as a function of task demands independent of stimulus valence. In contrast, or additionally, we might see group-by-condition-by emotion interactions. Pessoa has argued that increased amygdala response to emotional stimuli interferes with the recruitment of regions implicated in related top-down attention control (19) and if patients show heightened amygdala responses to negative stimuli specifically, this response might be associated with disrupted recruitment of regions involved in top-down attention control. The current study tests these hypotheses.
METHODS AND MATERIALS
Subjects
Four different patient groups participated in the study: patients with GSP only (n=19 on the explicit emotion regulation [EER] task & n=18 on the top-down attention control [TAC] task), patients with GAD only (n=17 on both tasks), patients with comorbid GSP/GAD (n=17 on EER task & n=15 on TAC task), and healthy comparison (HC) individuals (n=18 on both tasks). Scanning sessions for the subjects completing both tasks were separated by at least 2 weeks. Table 1 shows that subjects were well matched on demographics but differed on symptom ratings.
Table 1.
Subject Characteristics: S.D. in Brackets ()
| GSP | GSP/GAD | GAD | HC | P<* | |
|---|---|---|---|---|---|
| EER | N = 19 | N = 17 | N = 17 | N = 18 | |
| TAC | N = 18 | N = 15 | N = 17 | N = 18 | |
| AGE | |||||
| EER | 29.4 (8.70) | 35.7 (9.54) | 36.1 (11.75) | 33.4 (9.65) | NS. |
| TAC | 31.8 (9.10) | 33.5 (10.57) | 34.9 (10.93) | 30.4 (6.86) | NS. |
| GENDER | |||||
| EER | 10 F/8 M | 12 F/5 M | 13 F/4 M | 10 F/8 M | NS. |
| TAC | 8 F/10 M | 10 F/5 M | 10 F/7 M | 9 F/9 M | NS. |
| IQ | |||||
| EER | 123.6 (9.36) | 116.7 (12.88) | 119.9 (9.20) | 116.0 (10.56) | NS. |
| TAC | 118.5 (11.98) | 118.1 (14.14) | 119.1 (9.34) | 118.5 (11.90) | NS. |
| LSAS-SR | |||||
| EER | 73.2 (24.52) | 67.4 (20.10) | 45.7 (18.23) | 16.8 (10.23) | 0.001 |
| TAC | 75.8 (22.79) | 68.7 (20.34) | 42.3 (17.31) | 15.3 (12.26) | 0.001 |
| BAI | |||||
| EER | 8.6 (7.05) | 12.0 (7.12) | 12.2 (7.34) | 2.3 (2.02) | 0.001 |
| TAC | 8.8 (6.25) | 13.3 (6.18) | 11.6 (6.73) | 1.1 (1.37) | 0.001 |
| STAI-T | |||||
| EER | 49.1 (9.91) | 48.6 (9.12) | 49.1 (5.0) | - | NS |
| TAC | 50.8 (10.14) | 47.5 (10.63) | 44.9 (9.40) | - | NS |
| IDS | |||||
| EER | 16.7 (9.85) | 23.7 (5.92) | 16.9 (7.91) | 3.7 (4.79) | 0.001 |
| TAC | 15.5 (8.27) | 24.4 (6.72) | 19.1 (7.03) | 3.7 (4.96) | 0.001 |
Key to Table 1: M = Male; F = Female; LSAS = Liebowitz Social Anxiety Scale-Self Rated; BAI = Beck’s Anxiety Inventory; IDS = Inventory of Depressive Symptomatology; STAI-T = The Spielberger Trait-State Inventory – Part Trait.
P values refer to the omnibus group tests.
Subjects were explicitly recruited to suffer from particular types of anxiety to facilitate direct comparisons among groups. Thus, patients with GSP could only meet criteria for GSP, subjects with GAD only met criteria for GAD, and subjects with comorbid GSP/GAD only met criteria for GSP and GAD, based on the Structural Clinical interview for DSM-IV Axis I disorders (SCID) (20) and a confirmatory clinical interview. No patient had any other current Axis I diagnosis; all were medication-free for at least six months. HCs had no psychiatric illness. All subjects were in good physical health and were recruited from NIMH Institutional Review Board (IRB) approved fliers and advertisements.
Tasks
For subjects receiving both tasks, task order was counter-balanced both across subjects and across groups.
Explicit Emotion Regulation (EER)
Procedures followed those used in prior research on explicit emotion regulation through reappraisal (e.g., 10, 21). Subjects viewed 30 positive and 30 negative IAPS pictures. As in prior studies, neutral pictures were not used to minimize confusion. Each picture was viewed three times, where subjects were asked either to simply view the pictures, to reappraise them by thinking about them in a way that would make their content more positive (i.e., down-regulating a negative image; up-regulating a positive image), or to reappraise them in a way that would make their content more negative (i.e., down-regulating a positive image; up-regulating a negative image). There were thus two factors: a “Condition” factor with three levels (View, Up-regulate [i.e., making the negative more negative and the positive more positive], Down-regulate [i.e., making the negative less negative and the positive less positive]) and an “Emotion” factor with two levels (Negative, Positive). Pictures were viewed in random order. The normative mean (±S.E.) valence and arousal values on a 9-point scale were respectively 3.08±0.12 and 5.43±0.14 for negative and 7.21±0.08 and 5.15±0.16 for positive pictures.
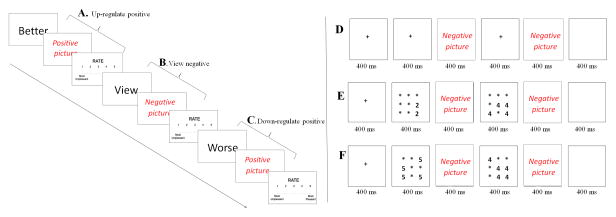
The task used a rapid-event-related design, as depicted in Figure 1. On each 10-second trial, a one-word instruction was presented for 2 seconds, followed by 5 seconds of picture exposure where subjects either viewed or reappraised the pictures. A rating scale then appeared for 3 seconds, and subjects rated their emotional response (1=most unpleasant and 5=most pleasant). Prior to scanning, subjects were trained to successfully perform the task. The 180-event task involved six runs, each with 30 trials, and 30 fixation points to generate a baseline.
Figure 1.
Example EER and TAC trial sequences. (A) Up-regulate positive trial; (B) View negative trial; (C) Down-regulate positive trial; and (D) Negative view trial; (E) Negative congruent trial; (F) Negative incongruent trial.
Top-down Attention Control (TAC)
The full task details can be found elsewhere (13). Briefly, as shown in Figure 1, positive, negative, and neutral pictures were presented either on their own (View trials) or bracketed by two number displays that either involved congruent or incongruent number displays trials. Congruent trials involved matching numbers; e.g., two 2s and four 4s. Incongruent trials involved numbers with discordant value; e.g., four 5s and five 4s. No response was required on View trials. On Congruent and Incongruent trials, subjects decided which of the two numerical displays contained the greater value. Thus, the Congruent and Incongruent-trial examples in Figure 1 required the same response. The normative mean (±S.E.) valence and arousal values on a 9-point scale were respectively 2.71±0.11 and 5.85±0.11 for negative, 7.30±0.11 and 5.01±0.10 for positive, and 4.96±0.07 and 2.78±0.08 for neutral pictures.
There were nine trial types: view, congruent, and incongruent trials involving neutral, negative, and positive events. Subjects completed four runs, generating 360 picture-trial events (40 in each 9 trial category) and 88 fixation points to generate a baseline.
Image acquisition
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired using a 1.5 Tesla Siemens MRI scanner. Functional T2* weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a 64×64 matrix (TR=3000ms, TE=30ms, FOV=240mm, 3.75×3.75×4mm), followed by a high-resolution anatomical image.
fMRI analysis
Data were analyzed in whole-brain random effects general linear models in AFNI (22) as in prior studies (23–25). Briefly, this involved generation of subject-level data in an initial step followed by second-level random-effects models.
For the EER task, a 4(Group: GSP, GSP/GAD, GAD, HC) by 3(Condition: View, Up-regulate, Down-regulate) by 2(Emotion: Negative, Positive) ANOVA was applied to the individual subject BOLD data. For the TAC task, a 4(Group: GSP, GSP/GAD, GAD, HC) by 3(Condition View, Congruent, Incongruent) by 3(Emotion: Neutral, Negative, Positive) ANOVA was used. The same models were used to analyze behavioural data. For both tasks, group-level analyses first generated whole-brain maps initially thresholded at p<0.005. To generate corrected statistical thresholds, these initial maps were then subjected to spatial clustering operations using AlphaSim with 1,000 Monte Carlo simulations taking into account the entire EPI matrix (p<0.005), with a map-wise false-positive probability of p<0.05.
This approach produced functional regions of interest (ROI) where activation clusters surpassed the map-wise, whole-brain corrected threshold. To facilitate interpretation of these results, the average percent signal change was extracted for each of the regressors across all voxels within each of the functional ROI identified by the ANOVAs so that subsequent group-level statistics could be performed within SPSS.
For results ANOVAs involving the GAD and GSP groups only, see Supplement.
RESULTS
Explicit Emotion Regulation (EER)
Behavioral Data: Affective Ratings
No interactions emerged involving group (group-by-condition, group-by-emotion, or group-by-condition-by-emotion [F range=0.193 to 2.14; ns]). Means appear in Table S2 in the Supplement. However, as expected there was a significant condition-by-emotion interaction (F=160.95; p<0.001); negative pictures were rated as significantly more unpleasant when subjects up-regulated, and significantly less unpleasant when subjects down-regulated, their responses compared to viewing the pictures (p<0.001 in all cases). Similarly, positive pictures were rated as significantly more pleasant when subjects up-regulated, and significantly less pleasant when subjects down-regulated, their responses compared to viewing the positive pictures (again p<0.001 in all cases). In short, reappraisal was successfully associated with change in self-reported experienced affect.
EPI Data
We present the main interaction with respect to our predictions; see Table S3 in the Supplement. No activations survived corrections for multiple comparisons at p<0.005 for the group-by-emotion or group-by-condition-by-emotion interactions. For an overview of condition main effects, see Supplement.
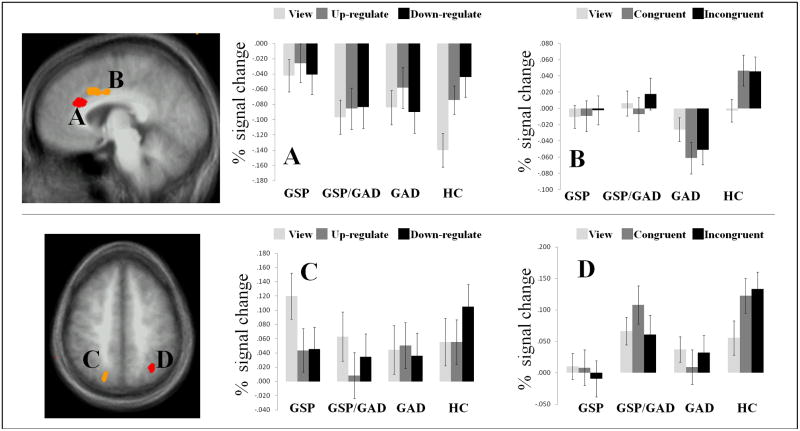
Our principal interest was to test the hypothesis that patients with GSP, GSP/GAD, and GAD show reduced BOLD responses within regions implicated in top-down attentional control (i.e., lateral frontal, ACC, and parietal regions) during explicit emotional regulation, though we also tested whether these task effects were valence specific. Results supported predictions on task effects but not task-by-emotion interactions in three regions. These manifested group-by-condition interaction: the dACC (x,y,z=−5,12,35) and left inferior (BA40) (x,y,z=−59, −39,28) and right superior (BA7) parietal cortices (x,y,z=20, −75,50). In all three regions, the HC group showed a significantly greater increase in response on the down-regulate (reduce emotional response) relative to the view trials that was greater than that seen in any of the three patient groups (t range=2.38 to 4.64; p<0.05 to 0.001); see Figure 2. In addition, for the down-regulate (decrease emotional response) trials, relative to the view trials, the HC group also showed increased responding in both the dACC and superior parietal cortex relative to the patients with GSP and GSP/GAD (t range=2.23 to 2.91; p<0.05 to 0.01).
Figure 2.
Group-by-condition. dACC as identified by the (A) EER task, and (B) TAC task, and superior parietal lobule as identified by the (C) EER task, and (D) TAC task.
Affective Stroop task
Behavioral Data
For the reaction time data, expected main effects emerged for congruency (F=27.06; p<0.001) and emotion (F=4.80; p<0.01); subjects responded slower to incongruent than congruent trials and to negative relative to neutral and positive trials. There were no significant interactions involving group (group-by-condition, group-by-emotion, or group-by-condition-by-emotion) (F range=0.57 to 1.89; ns). Means for each trial type appear in Table S4 in the Supplement. Groups were similarly accurate (F(3,65)=0.094; p=0.963).
EPI Data
The primary analysis focused on the key interaction with respect to the main study hypothesis, as tested in the group-by-condition interaction. Subsequent analyses considered emotion-specific group effects; see Table S3 in the Supplement. No clusters survived correction for multiple comparisons for the group-by-condition-by-emotion interaction or group main effects. For overview of condition main effects, see Supplement.
The group-by-condition interaction identified four regions: rostral ACC (x,y,z=−5,26,24) and bilateral inferior (BA40) (x,y,z=57, −33,21 & −63, −42,31) and left superior (BA7) parietal (x,y,z=−31, −58,44) cortices. In all four regions, the HC group showed a significantly greater increase in response to incongruent relative to view trials that was greater than that seen in any of the three patient groups (t range=1.97 to 4.54; p<0.06 to 0.001); see Figure 3/4. The HC responses to congruent and incongruent trials did not differ.
With respect to the group-by-emotion interaction, two regions were identified: medial prefrontal cortex (MPFC) (x,y,z=−6,64,20) and the amygdala (x,y,z=20, −7, −18); see Table S3 in the Supplement. In the MPFC, findings reflected abnormal responding in GSP but not GAD. Namely, both GSP groups, those with GSP alone and those with comorbid GSP/GAD, showed significantly increased response to negative relative to positive trials compared to both the HC and GAD groups (t=2.08 to 2.83; p<0.05 to 0.01) who did not significantly differ. Within the amygdala, the GAD group showed a significantly decreased BOLD response to negative trials, relative to the HC group (t=2.84; p<0.01) and GSP group (t=3.66; p<0.001) but not the comorbid GAD/GSP group (t=1.21; n.s). While the comorbid GAD/GSP group did not show a significantly decreased BOLD response to negative trials, relative to the HC group (t=1.52; n.s.) they did relative to the GSP group (t=2.31; p<0.05). Importantly, the group-by-emotion was driven, in all cases, by the response to the negative stimuli; there were no significant group difference between any of the groups to either neutral or positive trials.
DISCUSSION
The current study examined neural correlates in GSP and GAD engaged by explicit emotional regulation and top-down-attentional-control tasks. On the explicit emotion regulation task, the healthy individuals exhibited expected increases in dACC and parietal cortices previously implicated in emotion regulation, but this was absent in at least portions of these regions in patients. On the top-down-control task, parallel findings emerged, with healthy adults once again exhibited greater expected increases in dACC and parietal cortices.
GSP and GAD are separate diagnoses in DSM-IV despite their frequent co-occurrence (26) and shared features, such as signs of emotion dysregulation (e.g., 3, 4). Such shared features might arise from shared neural correlates relating to emotion regulation abilities, with therapeutic implications. Novel therapies targeting shared emotion regulation deficiencies might be effective both in GSP and GAD.
Shared deficits in GSP and GAD could relate to explicit emotion regulation alone or a more general difficulty in the recruitment of regions implicated in top-down attentional control. The specific nature is important: for example, deficits in explicit emotional regulation might indicate cognitive therapies designed to train emotion regulation explicitly, while deficits in both capacities encourage a more general focus on attentional training. Previous fMRI studies examine explicit emotional regulation in GSP to aversive social stimuli (16, 17). The current study implicates dACC dysfunction, as found previously, as well as associated parietal cortex dysfunction in emotion regulation while also implicating these regions in top-down attentional control. Moreover, this dysfunction is seen in both GSP and GAD. In short, a reduced ability to recruit regions implicated in top-down attentional control may reflect an underlying risk factor for anxiety generally (cf. 27). This reduced ability manifests when task instructions require explicit emotion control and when such control emerges as a function of task demands.
The current findings indicate that the dACC pathophysiology in GAD and GSP is complex. Thus, various emotional provocations in prior studies engage ACC in GSP (25, 28–30) more consistently than in GAD where intact (25), or even reduced (31) responses have been noted. Nevertheless, two studies find that rostral ACC responses (32, 33) predicts response to treatment in GAD, though other work finds signs of deficient dACC function during explicit emotional down-regulation of social emotional stimuli (in GSP: 16, 17) and dorsomedial frontal cortex during emotional conflict (in GAD; 8). These results possibly suggest that patients with anxiety disorders show enhanced recruitment of systems involved in attentional control on the basis of emotionally evocative stimuli but a reduced capacity to recruit these regions as a function of task demands.
Interestingly, one dominant model proposes that lateral frontal cortex guides top-down attentional processing and that later dACC activity reflects response conflict-related processing (34, 35). The current data suggest intact lateral frontal cortex function in top-down attentional control among patients with GSP and GAD. Rather, later conflict-related processing appears to be disrupted, as instantiated in dACC. If these findings are confirmed, it may be fruitful to pursue novel anxiety-disorder therapies that focus on improving these later, conflict-related information-processing functions, as they facilitate emotional regulation.
It is important to note that the current data do find differences among GSP, GAD and comorbid GSP/GAD. Indeed, a supplemental analysis (see Supplement) suggested that patients with GAD may show greater difficulties than patients with GSP in the recruitment of regions implicated in top-down attentional control. Moreover, the full clinical picture of the three conditions is complex, and emotion regulation dysfunction, as assessed here, undoubtedly only represents one small component of these conditions. Thus, it is very likely that GSP and GAD differ in terms of other neuro-circuitry, such as systems underlying self-referential processing (23, 24, 36) or the regulation of negative self-beliefs (16, 17), which may be particularly relevant for GSP, or stimulus-reinforcement based decision-making which may be particularly relevant to GAD (37, for review of disorder specific findings in GSP vs. GAD see 38). The current data may reflect one underlying shared factor may interact with other, disorder-specific factors to produce each unique clinical picture.
Consistent with this view, even in the current study, evidence of disorder-specific findings emerged. Significant group-by-emotion interactions arose on the TAC task, with the GSP and GSP/GAD groups showing increased MPFC activation, and the GAD group showing decreased amygdala activation to negative stimuli; see also Supplement. Atypical responding within MPFC has been reported in the context of other paradigms investigating socio-emotional processing in patients with GSP (23, 24, 36, 39–42). The current study extends these findings by showing the findings to manifest in GSP but not GAD. As such, the current data support the view that part of the unique pathophysiology of GSP may relate to dysfunctional processing within this region (23, 24, 36).
In terms of the decreased amygdala response in the GAD group, it is notable that one previous study found decreased amygdala responses to negative stimuli in GAD (25), and four others found no group differences to negative stimuli (31–33, 43), though one, Nitschke et al., found increased responses in GAD to neutral stimuli (32), and a fifth study similarly found increased amygdala responses (8). This apparent lack of enhanced amygdala responses to threat in adult GAD contrasts with findings in other anxiety disorders and possibly reflect the cognitive process of worrying occuring in this population. Although worry remains incompletely understood, consistent with the current data, Hoehn-Saric et al. showed that increased worry is associated with a suppression of the amygdala response in healthy individuals (44). Thus, it is possible that decreased amygdala response is linked to worry in GAD.
Pessoa (2009) has argued that increased amygdala responses to emotional stimuli may hamper top-down attention control (19). Thus, group-by-condition-by-emotion interactions might be expected in the current study, reflecting heightened amygdala responses to negative stimuli disrupting attention control. However, neither heightened amygdala responses to negative stimuli (indeed they were reduced in patients with GAD) nor significant group-by-condition-by-emotion interactions were seen here. Notably, given the absence of significantly increased amygdala responses, tests of the Pessoa model are limited. However, we can conclude on the basis of the current results that any increased interference would reflect an exacerbation of a general difficulty in the recruitment of these regions in patients with GSP, GAD and GSP/GAD.
Four caveats should be noted in relation to the current study. First, while comparable to other studies involving GSP and GAD, and roughly in line with our power analysis which suggested a sample size of 17 in each of the groups (which we achieved in 7 of the 8 group cells), our sample size for each group (N=15 to 19) was no more than moderate. It is thus possible that the lack of group-by-emotion interaction on the EER task and group-by-condition-by-emotion interactions on both the EER and TAC tasks is in part attributable to inadequate power and that larger sample sizes would result in additional, possibly disorder-specific, results. Nevertheless, the fact that between-group differences in key regions for the group-by-condition interaction did emerge suggests that the sample size was adequately powered to detect at least some key associations.
Second, it should be noted that we did not observe behavioural group differences on either the EER (perhaps as a result of the strong demand characteristics of this task; see (45)) or TAC task. This absence of behavioral effect has been seen in previous investigations of explicit emotional regulation in patients with GSP and GAD (16–18), though it does contrast with clinical data indicating impaired emotional regulation in both disorders (e.g., 3, 4). Moreover, it is notable in the current study that the main effects of task (documented in Table S1 and Figures S1–S2 in the Supplement) revealed extensive recruitment of lateral frontal, dorsomedial and parietal cortices in all four groups. All four groups recruited these regions as a function of explicit and top-down attentional control. These main effect data are important for two main reasons: First, they indicate the recruitment of lateral frontal cortex in top-down attention is intact in GSP and GAD; and second, they indicate that it was only portions of dorsomedial and parietal cortices that are disrupted in the two disorders (particularly, dACC rather than more general dorsomedial frontal cortex).
Third, the current data do not preclude a different interpretation. Patients with anxiety disorders could be superior in their recruitment of top-down attention control systems, potentially due to increased practice in their recruitment in the context of emotional distracters. According to this hypothesis, the patients could show reduced recruitment of dACC and parietal cortices because less recruitment was required to prime task-relevant representations to achieve emotional regulation. While this hypothesis is inconsistent with clinical data indicating impaired emotional regulation in GSP and GAD (e.g., 3, 4), it cannot be ruled out on the basis of the current data.
Fourth, patients were excluded from the current study if, in addition to their disorder, they presented with a range of common comorbidities, including other anxiety disorders, substance use disorders, and severe mood disorders. Our goal was to determine the pathophysiology of GAD and GSP in a dataset where results could not be attributed to the presence of comorbid conditions. However, this means that the patients studied were atypical to many anxiety patients presenting clinically and it may prove useful to determine the extent to which these findings apply to patients presenting with significant co-morbidity. Further, it would be beneficial to consider patients with other anxiety disorders for comparison. So, for example, recent fMRI work has indicated that trauma exposed individuals with BPD and PTSD also show decreased ACC activation during emotion regulation (46, 47). In addition, other work has suggested that trauma resistant individuals show increased ACC and lateral prefrontal cortex activation relative to healthy controls during emotion regulation (48, 49). Thus, it is possible that a reduced ability to recruit regions implicated in top-down attention represent a general risk factor for other anxiety disorders in addition to GAD and GSP. Notably, such future research should be designed to allow for the probing of complementary processes across emotion regulation brain networks, something that unfortunately was not possible within our current study design.
In conclusion, the current study implicates perturbed dACC and parietal function in the emotion regulatory deficits that occur in anxiety disorders, findings that extend previous work on the neural correlates of healthy emotion regulation. These deficits occur in both GAD and GSP, suggesting that they predispose to at least two anxiety disorders that share many other features. Finally, because the findings extend basic-science models of top-down attention control (9), they provide a context for cross-species research on the manner in which improvements in attention control facilitate successful responding to emotionally challenging circumstances. As such, the findings provide the context for using neuroscience in the service of generating ideas about novel therapeutics.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
Footnotes
FINANCIAL DISCLOSURES
All authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. 2009;26(12):1066–85. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- 2.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin Lab Med. 2010;30(4):865–91. doi: 10.1016/j.cll.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to Generalized Anxiety Disorder. Clin Psychology: Science and Practice. 2002;9:85–90. [Google Scholar]
- 4.McClure EB, Pine DS. Social anxiety and emotion regulation: A model for developmental psychopathology perspectives on anxiety disorders. In: Cicchetti DEC, Donald J, editors. Developmental Psychopathology: Theory and Method. Wiley; 2006. [Google Scholar]
- 5.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25(3):400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 8.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 10.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 11.Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–55. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33(8):1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66(2):170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biol Psychiatry. 2009;66(12):1091–9. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage. 2011;54(1):689–96. doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pessoa L. How do emotion and motivation direct executive control. TICS. 2009;13(4):160–6. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 21.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Blair KS, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJR, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry. 2008;65(10):1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair KS, Geraci M, Hollon N, Otero M, DeVido J, Majestic C, Jacobs M, Blair RJR, Pine DS. Social norm processing in adult Social Phobia: Atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. Am J Psychiatry. 2010;167(12):1526–32. doi: 10.1176/appi.ajp.2010.09121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair KS, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney D, Blair RJR, Drevets W, Pine DS. Response to emotional expressions in Generalized Social Phobia (GSP) and Generalized Anxiety Disorder (GAD): Evidence for separate disorders. Am J Psychiatry. 2008;165(9):1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12(1):92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 28.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57(9):975–81. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Blair KS, Geraci M, Korelitz K, Otero M, Towbin K, Ernst M, Leibenluft E, Blair RJR, Pine DS. The Pathology of Social Phobia Is Independent of Developmental Changes in Face Processing. Am J Psychiatry. 2011;168(11):1202–9. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palm ME, Elliott R, McKie S, Deakin JF, Anderson IM. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol Med. 2010;41(5):1–11. doi: 10.1017/S0033291710001455. [DOI] [PubMed] [Google Scholar]
- 32.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin ND. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166(3):302–10. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63(9):858–63. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33(5):613–30. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex. 2006;16(6):827–34. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- 36.Blair KS, Geraci M, Otero M, Majestic K, Odenheimer S, Jacobs M, Blair RJR, Pine DS. Atypically reduced modulation of medial prefrontal cortex to self-referential comments in Generalized Social Phobia. Psych Research: Neuroimaging. 2011;193(1):38–45. doi: 10.1016/j.pscychresns.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeVido J, Jones M, Geraci M, Hollon N, Blair RJR, Pine DS, Blair KS. Stimulus-reinforcement Based Decision-making and Anxiety: Impairment in Generalized Anxiety Disorder (GAD), but not in Generalized Social Phobia (GSP) Psychol Med. 2008;39(7):1153–61. doi: 10.1017/S003329170800487X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair KS, Blair RJR. A cognitive neuroscience approach to Generalized Anxiety Disorder and Social Phobia. Emot Review (in press) [Google Scholar]
- 39.Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sripada CS, Angstadt M, Banks S, Nathan PJ, Liberzon I, Phan KL. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport. 2009;20(11):984–9. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakao T, Sanematsu H, Yoshiura T, Togao O, Murayama K, Tomita M, Masuda Y, Kanba S. fMRI of patients with social anxiety disorder during a social situation task. Neurosci Res. 2011;69(1):67–72. doi: 10.1016/j.neures.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Ghen G, Blair RJR, Leibenluft E, Fox N, Erns M, Pine DS, Nelson E. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65(11):1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Research. 2004;131(1):11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Hoehn-Saric R, Lee JS, McLeod DR, Wong DF. Effect of worry on regional cerebral blood flow in nonanxious subjects. Psychiatry Res. 2005;140(3):259–69. doi: 10.1016/j.pscychresns.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Cognitive-emotional interactions - Attention regulation and monitoring in meditation. TICS. 2008;30:47–50. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koeningsberg HW, Fan KN, Ochsner KN, Liu X, Guise K, Pizzarello C, Dorantes C, Tecuta L, Guerreri S, Goodman M, New A, Flory J, Siever L. Neural correlates of using distancing to regulation emotional responses to social situations. Neuropsychologia. 2009;48:1813–22. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang S, Kotchoubey B, Frick C, Spitzer C, Grabe JJ, Barnow S. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2012;59(2):1727–34. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 48.New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Cherney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):656–64. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Scaramozza M, Mondillo K, Pine DS, Charney DS, Blair RJR. Vulnerability and resilience to PTSD are both associated with cognitive control of attention. Psych Medicine. doi: 10.1017/S0033291712000840. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.