Abstract
Following acute infection in some mouse models, CD4+ memory T cells steadily decline over time. Conversely, in humans CD4+ memory T cells can be maintained for many years at rates similar to CD8+ T cells. Because we previously observed that the longevity of Th1 memory cell survival corresponded to their functional avidity, we hypothesized that secondary challenge, which enriches for high functional avidity Th1 responders, would result in more stable Th1 memory populations. We found that following a heterologous secondary challenge, Th1 memory cells were maintained at stable levels as compared to primary Th1 memory cells, showing little to no decline after day 75 post-infection. The improved stability of secondary Th1 memory T cells corresponded to enhanced homeostatic turnover, enhanced trafficking of effector memory Th1 cells to tissue sites of infection such as the liver and acquisition or maintenance of high functional avidity following secondary challenge. Conversely, a weaker homologous rechallenge failed to induce a stable secondary Th1 memory population. Additionally, homologous secondary challenge resulted in a transient loss of functional avidity by Th1 memory cells recruited into the secondary response. Our findings suggest that the longevity of Th1 memory T cells is dependent, at least in part, on the combined effects of primary and secondary antigen-driven differentiation. Furthermore, they demonstrate that the quality of the secondary challenge can have profound effects on the longevity and function of the ensuing secondary Th1 memory population.
Keywords: T cells, cell differentiation, memory, vaccination
Introduction
A key feature of memory T cells is their ability to self-renew and persist at stable levels for long periods of time. In mouse models of acute infection, CD8+ memory T cells, once established, are maintained with no observable decline throughout the life of the mouse (1, 2). CD8+ and CD4+ memory T cells specific for the smallpox vaccine in humans persist for many years, with population half-lives estimated in the range of 8–15 years (3, 4). Smallpox survivors demonstrate equally robust long-lived immunological memory (5). In contrast, mouse models of acute viral or bacterial infection suggest that the mechanisms that control the stability of CD4+ memory T cell populations are distinct, as in certain cases they have been noted to decline over time (6–8). In at least one study, CD4+ memory T cells became virtually undetectable by two years post-infection (6), although the rate of memory decay may decline over time (8). Understanding the mechanisms that control the generation and survival of CD4+ memory T cell populations that are stable at high frequencies is of critical importance in generating more effective vaccination and immunotherapeutic strategies.
Several factors have been shown to regulate the homeostatic turnover and survival of memory T cell populations. Of these the best described are the cytokines IL-7 and IL-15. Both CD4+ and CD8+ T cells receive signals via these cytokines that regulate cell division and survival, and presumably the relative rates of each process determine the overall stability of the memory population (9–11). It is also possible that the activation and differentiation signals delivered during initial T cell priming also play a key role in regulating the long-term fate of memory T cells. For example, various aspects of CD8+ memory T cell survival and function are programmed through the influence of CD4+ T cell help (12–14) and IL-2 (2, 15, 16).
The differentiation of CD4+ T cells differs from that of CD8+ T cells in several key ways. First, whereas as CD8+ effector and memory T cell differentiation is programmed after a short period (6–24h) of antigen exposure (17–19), CD4+ T cells require longer periods of antigen stimulation (3–4d) for optimal expansion and differentiation (20–22). Second, CD4+ T cell effector differentiation is dependent at least in part on the strength of the antigenic stimulus (23–27). Third, CD4+ T cell repertoires skew to higher avidity responders upon successive antigenic challenges (28, 29). Last, we recently observed that the transition of CD4+ effector T cells into the memory pool, as well as the emergence of very long-lived CD4+ memory T cells, coincided with an increased ability of surviving memory cells to respond to low concentrations of antigen (8). Collectively, these findings suggest that in comparison to CD8+ T cells, CD4+ T cells are subject to a prolonged period of selection on the basis of their ability to bind antigen and that the nature of the antigenic signal impacts all subsequent phases of CD4+ effector and memory T cell differentiation and survival.
Because the emergence of CD4+ memory T cells that are highly sensitive to antigen stimulation corresponds to a decrease in the rate of memory decay (8), and high avidity CD4+ responders are enriched following secondary challenge, we hypothesized that secondary challenge of Th1 memory T cells would result in stable secondary Th1 memory populations that did not undergo decay. To test this hypothesis, we employed a model of heterologous rechallenge using lymphocytic choriomeningitis virus (LCMV)3 and a recombinant Listeria monocytogenes expressing the immunodominant MHC Class II-restricted eptitope from the LCMV glycoprotein (Lm-gp61). This system allows for robust boosting of CD4+ memory T cells without rapid antigen clearance mediated by broadly reactive CD8+ T cells or antibody. While primary memory cells declined for several months after infection with LCMV or Lm-gp61, a strong secondary stimulus induced by heterologous secondary challenge (i.e. LCMV immune mice rechallenged with Lm-gp61 or Lm-gp61 immune mice rechallenged with LCMV) resulted in robust secondary expansion, retention of high-level functionality and long-term stability of the resulting secondary memory populations. In contrast, a weaker secondary stimulus induced by homologous rechallenge (i.e. LCMV immune mice rechallenged with LCMV or Lm-gp61 immune mice rechallenged with Lm-gp61) resulted in poor secondary expansion, a failure to achieve enhanced secondary function and the decay of secondary memory populations with kinetics similar to primary memory cells. Furthermore, while heterologous rechallenge resulted in a relative increase in the distribution of long-lived Th1 memory cells to peripheral sites of infection such as the liver, homologous rechallenge did not result in a similar enrichment. Secondary CD4+ memory T cells induced by heterologous challenge expressed similar levels of homeostatic cytokine receptors and the pro-survival molecule Bcl-2 as compared to primary CD4+ memory T cells. However, long-lived secondary memory cells induced by heterologous rechallenge turned over at a significantly more rapid rate than both their primary memory counterparts and secondary memory cells induced by homologous rechallenge, suggesting an intrinsically enhanced capacity to respond to homeostatic signals from the host. Overall, our findings suggest that while secondary challenge can result in the enrichment of highly functional and stable Th1 memory cells, their overall fate and function are heavily influenced by the nature of the secondary stimulus. Therefore, these findings are directly applicable in the design of vaccination strategies that target CD4+ T cell responses and in validating their efficacy.
Materials and Methods
Mice and Infections
6–8 week old C57BL/6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Thy1.1+ SMARTA TCR transgenic mice were maintained in our colony at the University of Utah (30). All animal experiments were conducted with the approval of the IACUC committee at the University of Utah. LCMV Armstrong 53b was grown in BHK cells and titered in Vero cells (31). For primary challenges and heterologous rechallenges, mice were infected intraperitoneally (i.p.) with 2 × 105 plaque-forming units (PFU). For homologous rechallenges, mice were infected with 2 × 106 PFU intravenously (i.v.). Lm-gp61 (M. Kaja-Krishna, University of Washington) was characterized previously and generated using described methods (8, 32, 33). Prior to infection, the bacteria were grown to log phase and concentration determined by measuring the O. D. at 600 nm (O. D. of 1 = 1 × 109 CFU/ml). For primary infections and heterologous rechallenges with Lm-gp61, mice were injected i.v. with 2 × 105 colony forming units (CFU). For homologous rechallenges, mice were injected i.v. with 2 × 106 CFU. All mice were initially infected when 8–12 weeks of age, and secondary challenges occurred 60–75 days after primary infection in all cases.
Cell preparations and flow cytometry
Splenocytes were placed in single cell suspension in DMEM containing 10% fetal bovine serum (FBS) and supplemented with antibiotics and L-glutamine. Liver lymphocytes were isolated from perfused whole livers following digestion in Collagenase B and DNAse I (Roche) for one hour, followed by Percoll (Sigma) separation and resuspension in the same media as described above. For CFSE experiments, SMARTA splenocytes were labeled using the CellTrace CFSE Labelling Kit (Invitrogen) accrding to the manufacturer’s instructions, followed by i.v. adoptive transfer (1 × 106 SMARTA/mouse). For cell surface staining, cells were incubated with fluorescent dye-conjugated antibodies with specificities as indicated (eBiosciences, San Diego, CA or BDBiosciences, Mountain View, CA)b in PBS containing 1% FBS. MHC Class II tetramers presenting GP66–77 in the context of I-A were obtained from the NIH tetramer core facility (Atlanta, GA). Tetramers were incubated with cells in RPMI containing 2% FBS and 0.1% sodium azide at 37° C for 3 hours, followed by cell surface staining in PBS with 1% FBS. Antibody-stained cells were analyzed on a FACSCanto II flow cytometer (BDBiosciences) and results analyzed using FlowJo software (TreeStar).
Peptide re-stimulation and intracellular staining
Resuspended cells were stimulated for 4 hours with 1 μM of the GP61–80 peptide (GLKGPDIYKGVYQFKSVEFD) in the presence of Brefeldin A (1 μl/ml GolgiPlug). Cells were stained with cell surface antibodies, permeabilized and stained with cytokine antibodies using a kit per manufacturer’s instructions (BDBiosciences). In some cases, cells were also stained with Bcl-2 antibodies (BDBiosciences) at the same time as the cytokine antibodies. For functional avidity assays, cells were restimulated with a range of peptide concentrations (10 μM-0.1 nM) prior to cytokine staining, with the percent maximal response determined by calculating the frequency of IFNγ-producing cells at any given concentration as a percentage of the frequency of IFNγ-producing cells at the highest peptide concentration.
BrdU assays
5-Bromo-2′-deoxyuridine (BrdU)(Sigma-Aldrich, St. Louis, MO) was added to the drinking water of mice at 0.8 mg/ml for two weeks. Splenocytes were harvested and re-suspended in media, followed by peptide restimulation as described above. Cells were surface stained, permeabilized, treated with DNAse I and co-stained with BrdU and cytokine antibodies using a kit per manufacturer’s instructions (BDBiosciences).
Results
Heterologous boosting results in stably maintained secondary Th1 memory cells
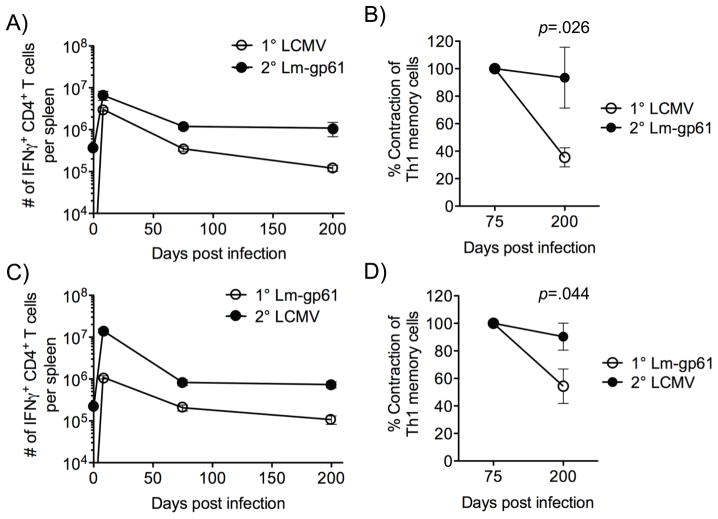
We infected B6 mice with either LCMV or Lm-gp61 to induce CD4+ effector and memory T cells under distinct infectious conditions. These pathogens share a single MHC Class II-restricted epitope (GP61–80), along with a sub-dominant Class I-restricted epitope (GP67–77)(34). Following the establishment of memory (>60 days post-infection), LCMV-immune mice were heterologously rechallenged with Lm-gp61 and Lm-gp61-immune mice were heterologously rechallenged with LCMV. GP61–80-specific primary and secondary IFNγ-producing Th1 responders in the spleen were then measured at effector and memory time points over the next 200 days by ex vivo peptide restimulation followed by intracellular cytokine staining. GP61–80-specific CD4+ T cells exhibited a vigorous expansion after either LCMV or Lm-gp61 infection, followed by contraction and the development of primary Th1 memory cells. In agreement with previous reports, primary Th1 memory cells gradually declined over time following either infection. Heterologous rechallenge also resulted in a massive expansion of primary Th1 memory cells during the first week of the recall response, followed by the development of secondary Th1 memory cells with a significantly increased frequency, as compared with that of primary memory cells (Fig. 1A, C).
Figure 1.
Heterologous rechallenge results in the stable maintenance of secondary Th1 memory cells. A, C, We infected B6 mice with LCMV or Lm-gp61 and measured the number of GP61–80-specific IFNγ-producing cells in the spleen at the indicated time points. At day 75 post-infection, mice received a heterologous rechallenge with Lm-gp61 or LCMV, respectively, and we measured the number of GP61–80-specific IFNγ-producing cells in the spleen at the indicated time points. B, D, The percent contraction of IFNγ-producing CD4+ T cells specific for GP61–80 between day 75 and 200 post-infection was measured after primary challenge with LCMV or Lm-gp61, or after heterologous rechallenge with Lm-gp61 or LCMV. The error bars indicate the standard error of the mean, and p-values were calculated using a Student’s t-test (SEM)(n=4–5 mice/group). Results are representative of three separate experiments.
To precisely compare their stability, we measured the rate of decline of primary and secondary Th1 memory cells. While primary Th1 memory cell populations gradually decayed throughout the first 6–7 months post-infection by 60–80%, there was no statistically significant reduction in the number of secondary Th1 memory cells during the same time period. Additionally, secondary Th1 memory cells showed elevated stability regardless of the order of prime-boost infection (Fig. 1B, D).
A weak secondary challenge induced by homologous boosting results in poorly maintained secondary Th1 memory cells
We previously observed that the most long-lived Th1 memory cells skew to a higher functional avidity (8). Additionally, secondary challenge has been shown to induce the selective outgrowth of high avidity clones (29). We therefore hypothesized that the strength of the secondary stimulus, as defined by its duration and antigenic load, could impact the differentiation and function of secondary Th1 memory cells. As compared to heterologous rechallenge, homologous rechallenge induces a relatively poor secondary Th1 response (21), presumably due to rapid clearance by pre-existing antibodies and/or memory CTL. We confirmed that homologous rechallenge results in rapid antigen clearance as compared to heterologous rechallenge. CFSE-labeled TCR transgenic SMARTA cells, which are specific for LCMV GP61–80, did not undergo cell division when transferred two or three days after homologous rechallenge. In contrast, SMARTA cells underwent several cell divisions when transferred into heterologously challenged hosts at similar time points (Supp. Fig. 1). Therefore, even though heterologous rechallenge boosts the response to a single Class II-restricted and a single Class I-restricted epitope, it provides a more robust boost than homologous rechallenge, which is rapidly cleared by broadly acting CTL and antibody responses. We therefore employed a model of homologous rechallenge (>60 days post-infection) to assess the maintenance and function of secondary Th1 memory cells following a weak secondary challenge.
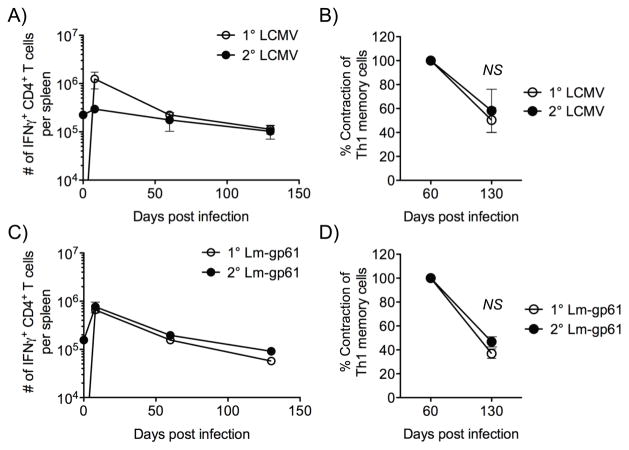
Similar to what we previously reported (21), homologous rechallenge of either LCMV-immune or Lm-gp61-immune mice resulted in little boosting of the Th1 response at either effector or memory time points, as compared to the primary Th1 response to the same pathogen (data not shown). Furthermore, the resulting memory population declined with kinetics similar to the primary Th1 memory population. Between days 60 and 120 post-challenge both primary Th1 memory cells and secondary Th1 memory cells generated by homologous rechallenge were reduced in number by 50–70% (Fig. 2). Importantly, the rechallenge doses used were sufficient to effectively induce a robust secondary CD8+ T cell response (data not shown), highlighting the differences in antigenic requirements in the generation of primary and secondary CD4+ or CD8+ T cell responses. In sum, these data indicated that the strength or duration of the secondary stimulus influenced the long-term survival of secondary Th1 memory cells.
Figure 2.
Homologous rechallenge results in poor maintenance of secondary Th1 memory cells. A, C, We again infected B6 mice with LCMV or Lm-gp61 and measured the number of GP61–80-specific IFNγ-producing cells in the spleen at the indicated time points by peptide restimulation and intracellular cytokine staining. At day 75 post-infection, mice received a homologous rechallenge with LCMV or Lm-gp61, respectively, and we measured the number of GP61–80-specific IFNγ-producing cells in the spleen at the indicated time points. B, D, The percent contraction of IFNγ-producing CD4+ T cells specific for GP61–80 between day 75 and 200 post-infection was measured after primary challenge with LCMV or Lm-gp61, or after homologous rechallenge with Lm-gp61 or LCMV. The error bars indicate the SEM, and p-values were calculated using a Student’s t-test (n=4 mice/group). Results are representative of two separate experiments.
Strength of stimulus impacts function and localization of secondary Th1 responses
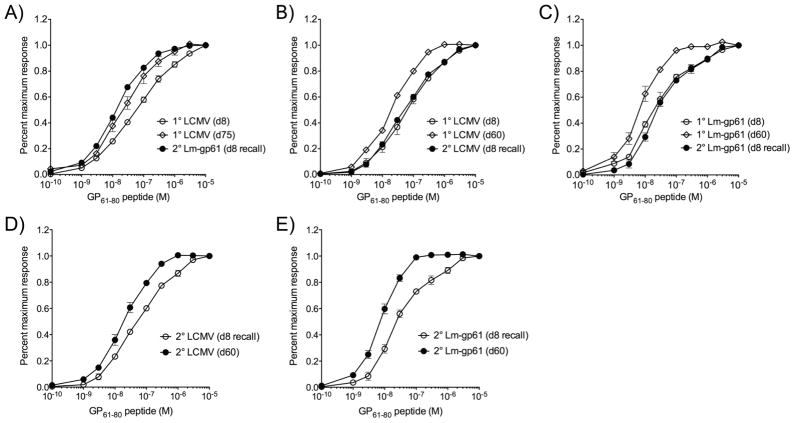
Previous studies have shown that secondary challenge results in the selective expansion of responders with high avidity for antigen. We found that long-lived Th1 memory cells that are maintained most stably also skew to a high functional avidity (as measured by the antigen dose required to elicit a functional response, such as IFNγ production)(8). We hypothesized that the induction of secondary Th1 responses with high antigen sensitivity would correspond to increased stability of the ensuing memory population. We assessed the functional avidity of primary and secondary Th1 responders following either homologous or heterologous rechallenge. The development of long-lived Th1 memory in LCMV-immune mice was associated with an overall increase in functional avidity, as previously reported. Secondary challenge with Lm-gp61 resulted in secondary effector Th1 cells with similarly high functional avidity (Fig. 3A). A homologous rechallenge with LCMV, however, led to an overall decrease in functional avidity as compared to the memory population prior to rechallenge, leaving them with a relatively low antigenic sensitivity that was similar to primary Th1 responders (Fig. 3B–C). While secondary Th1 memory cells eventually skewed once again to high functional avidity after homologous rechallenge (Fig. 3D–E), this corresponded to secondary Th1 memory decline that was similar to the decline seen in primary memory (Fig. 2). The eventual re-acquisition of high functional avidity by secondary Th1 memory cells induced by homologous rechallenge may therefore come at a cost of decreased secondary memory maintenance. Re-acquisition of high functional avidity may reflect cell-specific changes in functional avidity or the preferential population-based outgrowth of high functional avidity responders.
Figure 3.
Homologous boosting results in a loss of antigen sensitivity by secondary Th1 effector cells. Splenocytes were restimulated with GP61–80 peptide at the indicated concentrations, and then stained for the production of IFNγ. Results are represented as the percent maximal response, with the maximal response defined as the frequency of CD4+ T cell responders at the highest peptide concentration. A, Graph displays response across a range of peptide concentrations after primary infection with LCMV (day 8 and day 75 post-infection) or after rechallenge with Lm-gp61 (day 8 post-rechallenge). B, C, Graphs display response at the indicated time points after primary challenge with LCMV or Lm-gp61 (day 8 and day 60 post-infection) or after homologous rechallenge (day 8 post-infection). D, E, Graphs display the indicated response at the peak of the secondary effector response (day 8 post-rechallenge) and following the establishment of memory (day 60 post-rechallenge) after homologous rechallenge with LCMV or Lm-gp61. Error bars indicate the SEM (n=4–5 mice/group). Results are representative of two separate experiments.
We also determined whether the differences in function were a T cell-intrinsic response to homologous challenge or whether their function was dictated by the stimulatory environment of the challenge itself. We transferred Lm-gp61-immune (Thy1.1+) CD4+ memory T cells into Lm-gp61-immune or LCMV-immune secondary hosts (Thy1.2+). The transferred CD4+ memory T cells were then given a “homologous” rechallenge with Lm-gp61. The functional avidity of the ensuing recall response depended on the environment of the rechallenge. Lm-gp61-induced memory cells maintained high functional avidity when rechallenged in LCMV-immune hosts (homologous challenge in a heterologous environment), while they demonstrated lower functional avidity when rechallenged in Lm-gp61-immune hosts (homologous challenge in a homologous environment)(Supp. Fig. 2). Additionally, it is possible that newly arising naïve cells with specificity for GP61–80 could complicate the interpretation of the functional avidity assays following rechallenge. However, homologous and heterologous rechallenges were also given to B6 mice containing LCMV-induced memory SMARTA cells with similar results, indicating that differences in functional avidity were due to bona fide differences in recall responses and not the influence of newly arising naïve cells (data not shown).
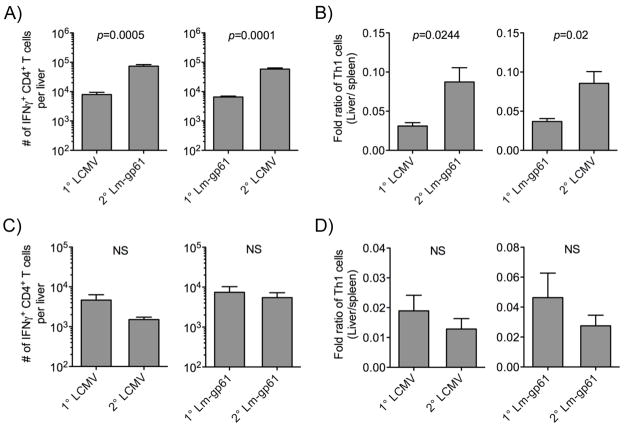
We next tested the impact of secondary challenge on the relative distribution of Th1 memory cells in the secondary lymphoid organs versus a peripheral site of infection for both LCMV and Lm-gp61, the liver. While both primary and secondary Th1 memory cells in the spleen expressed similar levels of CD62L (Supp. Fig. 3), the relative ratio of Th1 responders in the liver versus the spleen significantly increased following heterologous rechallenge. Following heterologous rechallenge of LCMV-immune mice with Lm-gp61 or Lm-gp61-immune mice with LCMV, Th1 responses in the liver were boosted significantly at both effector (data not shown) and memory (Fig. 4A) time points. Furthermore, the relative ratio of Th1 memory cells in the liver versus the spleen following secondary challenge also increased significantly, as compared to primary challenge (Fig. 4B). These findings suggest that robust boosting of Th1 responses can result in not only an increase in the overall number of Th1 memory cells, but also a relative shift towards tissue homing effector memory Th1 cells. In contrast, homologous rechallenge failed to boost the number of Th1 memory cells in the liver or increase the ratio of Th1 memory cells residing there (Fig. 4C–D). In summary, while heterologous boosting resulted in enhanced numbers, survival, function and tissue homing by secondary Th1 memory cells, homologous rechallenge resulted in neither boosting nor functional enhancement of Th1 memory populations. In fact, homologous boosting resulted in a loss of functional avidity by Th1 memory cells recruited into the response. In all ways that we measured, secondary Th1 responses induced by homologous rechallenge displayed functions characteristic of primary Th1 responses.
Figure 4.
Heterologous rechallenge boosts the frequency of tissue homing Th1 effector memory cells. Lymphocytes were isolated from perfused livers following digestion in Collagenase B and DNAse I. Total numbers of CD4+ IFNγ-producing cells were calculated following ex vivo restimulation with GP61–80. A, Bar graphs indicate the number of IFNγ-producing Th1 cells in the liver at day 75 after primary challenge or day 75 after heterologous rechallenge. B, Bar graphs indicate the relative distribution of Th1 memory cells in the spleen and liver at day 75 after primary challenge or heterologous secondary rechallenge. Data are represented as the fold ratio of Th1 cells in the liver versus the spleen. C, Bar graphs indicate the number of IFNγ-producing Th1 cells in the liver at day 75 after primary challenge or day 75 after homologous rechallenge. D, Bar graphs indicate the relative distribution of Th1 memory cells in the spleen and liver at day 75 after primary challenge or homologous secondary rechallenge. Data are represented as the fold ratio of Th1 cells in the liver versus the spleen. Error bars indicate the SEM, and p-values were calculated using a Student’s t-test (n=4–5 mice/group). Results are representative of two separate experiments.
Stable maintenance of Th1 memory corresponds to enhanced homeostatic turnover
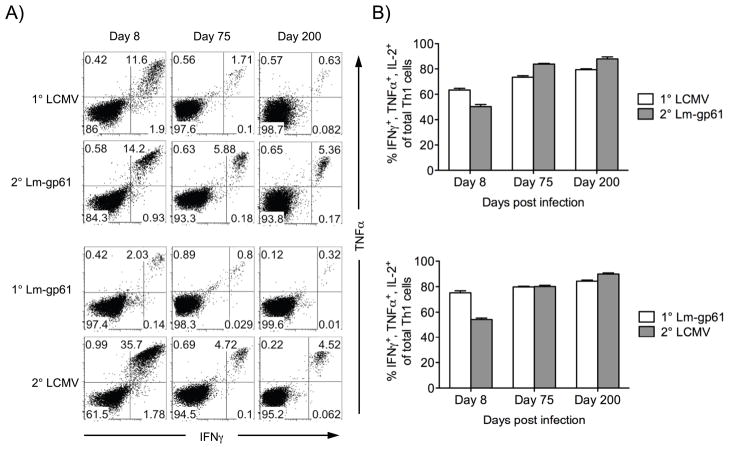
We next sought to identify the characteristics of the secondary response that might explain the ability of secondary Th1 memory populations induced by heterologous rechallenge to maintain themselves at stable levels long-term. Primary Th1 responses induced by either LCMV or Lm-gp61 are characterized by the expansion of CD4+ T cells with the ability to produce multiple cytokines upon antigen stimulation, and the presence of multiple cytokine producers is a strong correlate of CD4+ T cell-mediated protection (35, 36). At the peak of the response to either infection, >60% of IFNγ-producing Th1 cells also produced IL-2 and TNFα. This number was enriched to ~80% during memory maintenance. Heterologous rechallenge resulted in an initial enrichment of secondary Th1 effector cells producing only IFNγ. However, the resulting secondary Th1 memory cells once again skewed towards multiple cytokine producers and were not significantly different in their cytokine production profile than primary Th1 memory cells (Fig. 5).
Figure 5.
Secondary Th1 memory cells induced by heterologous challenge are highly functional. A, We measured the polyfunctionality of Th1 effector and memory cells following infection with LCMV or Lm-gp61, or following heterologous secondary challenge with either Lm-gp61 or LCMV. After peptide restimulation in the presence of Brefeldin A, cells were permeabilized and stained with antibodies to IFNγ, TNFα and IL-2. Representative flow plots at each time point indicate the frequency of CD4+ T cells that co-stained with antibodies to IFNγ and TNFα. B, The bar graphs indicate the frequency of IFNγ-producing cells that co-stained with antibodies to TNFα and IL-2 at the indicated time points after primary or secondary challenge. Error bars indicate the SEM (n=4–5 mice/group). Results are representative of three separate experiments.
We next considered the hypothesis that secondary Th1 memory cells responded to homeostatic or survival signals more effectively than primary Th1 memory cells, thus resulting in more stable maintenance. However, secondary Th1 memory cells expressed similar levels of the homeostatic cytokine receptors CD122 (IL-15Rβ) and IL-7Rα (Supp. Fig. 4A–B). Similarly, both primary and secondary Th1 memory cells expressed similar levels of the pro-survival molecule Bcl-2 (Supp. Fig. 4C).
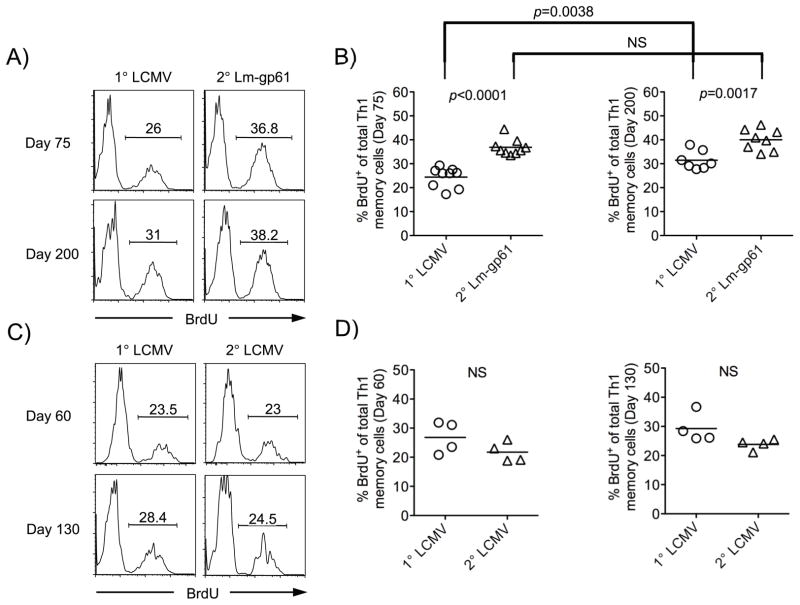
To address definitively whether secondary Th1 memory cells enjoyed a homeostatic advantage over primary Th1 memory cells, we administered the nucleotide analog BrdU into the drinking water of mice over a two week time period and measured its incorporation into dividing cells. Following heterologous rechallenge, secondary Th1 memory cells incorporated BrdU at a significantly higher rate at days 75 and 200 post-rechallenge, as compared to primary Th1 responders (Fig. 6A–B). Enhanced homeostatic turnover corresponded to memory stability, as secondary Th1 memory cells induced by homologous rechallenge, which are not maintained stably, demonstrated no increase in homeostatic turnover following either a homologous LCMV rechallenge (Fig. 6C–D) or a homologous Lm-gp61 rechallenge (data not shown). We concluded that secondary Th1 memory cells induced by robust heterologous rechallenge developed an enhanced intrinsic capacity to divide in response to homeostatic signals despite the fact that they were present in much higher numbers and therefore were competing for a more limited supply of these signals.
Figure 6.
Secondary Th1 memory cells induced by heterologous rechallenge display an increased rate of homeostatic division. Mice were fed BrdU in their drinking water for two weeks beginning at the indicated time points after primary or secondary challenge, after which splenocytes were restimulated with peptide and stained for expression of IFNγ and BrdU. A–B, Representative flow plots (A) indicate BrdU staining by IFNγ-producing Th1 memory cells at the indicated time points after primary infection with LCMV or heterologous rechallenge, while scatter plots indicate the frequency of BrdU-positive cells at day 75 or day 200 (B) after primary or heterologous secondary rechallenge. C–D, Similar plots display the results obtained after primary challenge with LCMV or homologous rechallenge. p-values were calculated using a Student’s t-test (NS, not significant) and results are representative of two separate experiments.
Discussion
Our findings demonstrate that enhanced recall responses induced by robust secondary challenge improve the stability, size and early acquisition of increased effector function by Th1 memory populations. They also suggest that the context of effector Th1 differentiation has profound consequences for the long-term fate of ensuing memory populations. While the concept of early commitment by developing CTL to a memory differentiation program has long been established, more recently convincing evidence has begun to accumulate that subsets of effector CD4+ T cells are similarly fated for subsequent memory differentiation (37). In this context, it seems likely that not only the ability to enter the memory pool but also the long-term survival of ensuing memory populations is dependent on the nature of activation signals received during the primary response.
We have previously shown that even while primary Th1 memory cells decline, as a population they acquire heightened sensitivity to antigen. Acquisition of a higher sensitivity to antigen in turn corresponds to a decrease in the rate of decline (8). One possible explanation for these observations is that Th1 memory cells acquire heightened sensitivity to antigen throughout memory maintenance. A second possibility, however, is that T cell clones that successfully acquire a heightened ability to translate antigenic stimulation into a functional response during primary activation enjoy a selective survival advantage during memory maintenance. Prior data has shown that secondary challenge results in the selective outgrowth of CD4+ T cells with high TCR avidity for antigen (29). In our studies, we found that long-lived Th1 memory cells (>75 days post-infection) were of high functional avidity as compared to primary Th1 effector cells, and that heterologous rechallenge resulted in the expansion of secondary Th1 effector cells whose functional avidity reflected that of the memory population from which they arose. Furthermore, high functional avidity during the secondary Th1 response corresponded to enhanced stability of the ensuing memory population, supporting a model in which the acquisition of high functional avidity during the effector response is predictive of long-term survival within the memory pool.
What, then, are the signals that induce the outgrowth of secondary Th1 effector and memory cells with high functional avidity? Importantly, a weak homologous rechallenge, even though it created an environment of increased competition for antigen, failed to result in a highly functional secondary Th1 effector response. In fact, the Th1 memory population displayed a decline in functional avidity after recruitment into the secondary response. The failure to acquire enhanced function corresponded to poor stability of the ensuing memory populations. These observations lead to two conclusions. First, the context of secondary stimulation impacts the long-term fate and survival of the ensuing secondary memory population. Second, the generation of high functional avidity responders following secondary challenge can’t be entirely explained by the selection of high TCR avidity clones due to competition for limited amounts of antigen. Rather, the selection of effective secondary Th1 effector and memory cells likely depends not only on the quantity of the secondary stimulation but also its quality. This is supported by findings that both CD4+ and CD8+ monoclonal T cell populations can shift their functional avidity during the primary response (38, 39). We have also found that monoclonal populations of CD4+ memory T cells can display a broad spectrum of functional avidity in response to antigen stimulation (8). We propose a model in which a weak secondary challenge results in poor quality activation events and the subsequent decrease in antigen sensitivity by CD4+ memory T cells recruited into the secondary response. However, our findings do not rule out the possibility that selection of long-lived and stable secondary Th1 memory is at least in part clonal (e.g. dependent on the strength of TCR signaling), as a high quality secondary stimulus may be required to provide the appropriate context for selective outgrowth of highly functional clones and mediate their entry into the memory pool.
While the precise mechanisms allowing enhanced survival of secondary Th1 memory cells are unknown, we made two key observations. First, secondary Th1 effector and memory cells induced by heterologous rechallenge maintained a higher functional avidity phenotype than either primary Th1 effector cells or secondary Th1 effector cells induced by homologous rechallenge. The stability of the ensuing memory populations corresponded directly to the emergence of high functional avidity Th1 memory cells, suggesting that those responders able to acquire high functional avidity also enjoyed a selective advantage for survival within the memory compartment. Second, secondary Th1 memory cells induced by heterologous rechallenge turned over at a higher rate than primary Th1 memory cells. IL-15 and IL-7 are required for the maintenance and homeostatic proliferation of primary CD4+ memory T cells (9, 11, 40). While primary and secondary Th1 memory cells express similar levels of the IL-15 and IL-7 receptors, it is possible that secondary Th1 memory cells are better equipped to transmit these cytokine signals into a biological response. Future studies are needed to determine the extent to which the stable maintenance of secondary Th1 memory cells is dependent on IL-7 and IL-15.
These studies have clear implications for the design of vaccination strategies aimed at the generation of protective CD4+ memory T cells. Additionally, it is likely that successful vaccination to a variety of infections, including HCV and HIV, will require coordinated mobilization of all aspects of adaptive immunity, including CTLs, B cells, Th1 and follicular helper T cells. Our findings suggest that the success of simultaneous boosting of CTL and Th1 responses may hinge on the ability to adequately stimulate the formation of stable and highly functional secondary Th1 memory cells.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of J. Cassiano.
Footnotes
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Numbers R01AI080830 and T32AI055434 (Training Program in Microbial Pathogenesis). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this manuscript: LCMV, lymphocytic choriomeningitis virus; Lm-gp61, Listeria monocytogenes recombinantly engineered to secrete GP61–80; B6, C57BL/6; i.p., intraperitoneal; i.v., intravenous; PFU, plaque forming units; CFU, colony forming units; FBS, fetal bovine serum; BrdU, 5-Bromo-2′-deoxyuridine.
References
- 1.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current Opinion in Immunology. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 3.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 4.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 5.Hammarlund E, Lewis MW, Hanifin JM, Mori M, Koudelka CW, Slifka MK. Antiviral immunity following smallpox virus infection: a case-control study. J Virol. 2010;84:12754–12760. doi: 10.1128/JVI.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homann D, Teyton L, Oldstone MBA. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 7.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surh CD, Sprent J. Homeostasis of naïve and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 13.Shedlock DJ, Shen H. Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 14.Sun JC, Bevan MJ. Defective CD8 T Cell Memory Following Acute Infection Without CD4 T Cell Help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 16.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Stipdonk MJB, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 20.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravkov EV, Williams MA. The Magnitude of CD4+ T Cell Recall Responses Is Controlled by the Duration of the Secondary Stimulus. The Journal of Immunology. 2009;183:2382–2389. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams MA, Bevan MJ. Shortening the Infectious Period Does Not Alter Expansion of CD8 T Cells but Diminishes Their Capacity to Differentiate into Memory Cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 23.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 24.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 26.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, Jones SC, Kamperschroer C, Lee WH, McKinstry KK, Roman E, Strutt T, Weng NP. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 29.Savage PA, Boniface JJ, Davis MM. A Kinetic Basis For T Cell Receptor Repertoire Selection during an Immune Response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 30.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slifka MK, Shen H, Matloubian M, Jensen ER, Miller JF, Ahmed R. Antiviral cytotoxic T-cell memory by vaccination with recombinant Listeria monocytogenes. J Virol. 1996;70:2902–2910. doi: 10.1128/jvi.70.5.2902-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homann D, Lewicki H, Brooks D, Eberlein J, Mallet-Designe V, Teyton L, Oldstone MB. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology. 2007;363:113–123. doi: 10.1016/j.virol.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 36.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 37.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 39.Whitmire JK, Benning N, Whitton JL. Precursor Frequency, Nonlinear Proliferation, and Functional Maturation of Virus-Specific CD4+ T Cells. J Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 40.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.