Abstract
Background
Vessels heal after injury and G protein-coupled receptors are involved in the vascular smooth muscle cell (VSMC) proliferation required to form intimal hyperplasia. We have previously identified the role of Gαq in VSMC proliferation in vitro. This study now examines the role of Gαq in the developing intimal hyperplasia in a murine model and the impact of disruption of Gαq signaling on intimal hyperplasia development.
Methods
The murine femoral wire injury model was employed in which a micro wire is passed through a branch of the femoral artery and used to denude the common femoral artery. Specimens were perfusion-fixed and sections were stained with H&E and Movat’s stains such that morphometric analysis could be performed using an Image-Pro system. Additional specimens of femoral artery were also harvested and snap frozen for western blotting or zymography to allow for the study of G protein expression and both protease expression and activity. Contralateral vessels were used as controls. Additional vessels were immersed in pluronic gel containing the chemical Gαq G protein inhibitors GP-2A, siRNA to Gαq or advenovirus containing mutant inactive Gαq.
Results
Gαq expression increased in a time-dependent manner after femoral artery injury. Sham operated on vessels did not produce such a response. Inhibition of Gαq reduced cell proliferation without affecting cell migration. Interruption of Gαq signaling also inhibited the development of intimal hyperplasia. Inhibition of Gαq did not alter peak uPA activity and expression but did increase early PAI-1 activity and expression. Inhibition of Gαq reduced peak MMP-9 activity at day 3 but did not influence peak MMP-2 activity at day 7. Protein expression for MMP-9 was also decreased but that of MMP-2 was not affected. There were no changes in the expression or the activity of the respective inhibitors for MMP-9 and MMP-2, TIMP-1 and TIMP-2.
Conclusions
These data demonstrate that femoral wire injury in the mouse is associated with a time-dependent increase in Gαq expression. Inhibition of Gαq alters cell proliferation and is associated with decreased MMP-9 expression and activity.
Keywords: vascular smooth muscle, response to injury, G proteins, arterial injury
INTRODUCTION
Cell signaling of the vascular smooth muscle cells (VSMC) within the vessel wall is necessary for remodeling during atherogenesis in response to flow and after injury (1). G protein-coupled receptors and receptor-linked tyrosine kinases are the major classes of membrane transduction molecules in vascular smooth muscle cells that participate in transmembrane signaling. G proteins are heterotrimeric proteins consisting of Gα, Gβ and Gγ proteins. Each component protein is a family of proteins. The Gα family in VSMC consists of the Gαi, Gαq and Gαs proteins. G protein-coupled receptors are involved in cell proliferation and migration, which are key elements in remodeling. However, the role of individual G proteins in the mechanisms involved in the development of arterial intimal hyperplasia has not been fully defined. We have previously reported the expression patterns of G proteins in rabbit vein grafts and rat arterial balloon angioplasty models (2–7). Interference with Gβγ inhibited intimal hyperplasia in both models and inhibition of Gαi proteins inhibited intimal hyperplasia in the rat model.
The role of Gαq in vessel remodeling is not known. Gαq participates in angiotensin signaling, which is an important cell-signaling cascade in VSMC that leads to cell proliferation and cell hypertrophy. We previously demonstrated that inhibition of Gαq decreases PLC-β and ERK 1/2 phosphorylation, leading to decreases in VSMC proliferation in vitro. The aim of this study is to characterize the role of the G protein Gαq in the developing intimal hyperplasia in a murine model and the consequences of disruption of this signaling molecule.
MATERIALS AND METHODS
Experimental Design
The murine femoral wire injury model was employed in which a micro wire is passed through a branch of the femoral artery and used to denude the common femoral artery (8, 9). Specimens were perfusion-fixed and sections were stained with Hematoxylin/Eosin (H&E) and Movat’s stains such that morphometry could be performed using an Image-Pro system (Media Cybernetics, Inc., Bethesda, MD). Further specimens of femoral artery were also harvested and snap frozen for western blotting to allow for the study of G protein expression. Contralateral vessels were used as controls. Additional vessels were immersed in pluronic gel with a Gαq chemical inhibitor GP-2A, siRNA to Gαq or an adenovirus containing a dominant negative Gαq. Empty vector or scrambled siRNA served as controls. Animal care and procedures were conducted at the University of Rochester Medical Center in accordance with state and federal laws and under protocols approved by the University of Rochester Animal Care and Use Committee. Animal care complied with the “Guide for the Care and Use of Laboratory Animals” issued by the Institute of Laboratory Animal Resources.
Femoral wire injury
Endoluminal injury to the common femoral artery of C57BL/6 mice was produced by 3 passages of a 0.25-mm-diameter angioplasty guidewire (Advanced Cardiovascular Systems, Santa Clara, CA) as previously described. Control sham-operated arteries underwent dissection, temporary clamping, arteriotomy, and ligature, without passage of the wire. Non-operated normal femoral arteries were used as additional controls. Based on the data derived from the control experiments, additional vessels were immersed in pluronic gel containing the adenovirus with Gβγ inhibitor βARKCT as previously described (2–9). The vessels were harvested for either western blotting to detect G protein expression or final morphometry. Pluronic gel (1 mL of 30% F127 Pluronic gel; Sigma, St. Louis, MO) was applied to the external surface of the femoral artery at the end of the procedure as previously described (8, 10, 11). The pluronic gel remains in a liquid form at 4°C and ra pidly forms a gel on exposure to body temperature. It is dissipated within 4 hrs.
Adenoviral Infection
Adenoviral vectors were constructed by Welgen, Inc. (Worcester, MA) using purified plasmid encoding inactive Gαq obtained from Guthrie cDNA Resource Center (Missouri University of Science and Technology, Rolla, MO). Empty vector served as control. We have previously shown that we can obtain satisfactory transfection (~30% cells) of arterial tissue using adenoviral transfection of vector expressing β-galactosidase (12). The β-galactosidase staining lasts under 3 days and is evident throughout the vessel, with a relative gradient that is maximal with the adventitia and extends into the media. Both VSMC and fibroblasts stain with the vector.
siRNA Transfection
Pre-designed HPLC-purified siRNA for gene knockdown for Gαq expression was procured commercially. Scrambled siRNA served as a control. Prior to in vivo use we confirmed that the siRNA against Gαq produced a concentration-dependent decrease in protein expression that was specific for the protein targeted without altering the expression of the other proteins.
Morphometry
Following perfusion fixation, each vessel was bisected and central segments from each of these halves were embedded in paraffin and cross sections were cut (10 sections, 100 µm apart). Standard morphometric measurements were performed on histological cross sections stained with H&E and Movat’s stains using a SPOT camera system (SPOT Imaging Solutions, Sterling Heights, MI). The luminal, intimal and medial areas of each vessel were determined using an Image-Pro system. Following determination of the dimensions of each vessel, a ratio of the intimal and medial areas was calculated.
Determination of in vivo proliferation and apoptosis
Labeling of proliferating VSMC in specimens was performed using the thymidine analogue 5-bromo-2-deoxyuridine (BrdU; Boehringer-Mannheim Corp., Indianapolis, IN; administered by Intraperitoneal injection 24 hours prior to sacrifice, 60 µg/g body weight). The number of BrdU-stained VSMC nuclei was counted and the BrdU labeling index (% of unlabelled cells) calculated on 10 slides (100 µm apart). Labeling of apoptotic VSMC on adjacent sections from each vessel specimen was evaluated using terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL; Boehringer-Mannheim Corp.) after processing the tissue according to the manufacturer’s guidelines on 10 slides (100 µm apart). Stained VSMC nuclei were counted and the TUNEL labeling index (percentage of unlabelled cells) calculated.
En Face SMC Migration Assay
Femoral arteries were perfusion-fixed as described above, and the vessels were opened longitudinally and pinned down onto agar plates with the luminal surface facing upward. Arteries were stained with hematoxylin for 10 seconds and incubated in Scott’s blue for 2 minutes. Migrated VSMCs were identified as the hematoxylin-stained cells on the luminal surface of the endothelium-denuded area. The length of the femoral artery was visualized under a microscope at ×100 magnification, and the total number of VSMC above and below the internal elastic lamina were counted and results expressed as a percentage of the cells on the luminal surface of the artery.
Immunoblotting
Following wire injury, femoral arteries were rapidly retrieved, cleaned once in ice-cold dPBS and then placed in ice-cold kinase buffer (200 µL; 25 mM Hepes, pH 7.5, 10% glycerol, 5 mM EDTA, 5 mM EGTA, 150 mM NaCl, 100 mM benzamidine, 0.1% 2-mercaptoethanol, 1% Triton X-100, 1 mM pepstatin A, 2 mg/mL leupeptin and 20 kallikrein inhibitor units/mL aprotinin). Protein determinations were performed using a bicinchonic acid assay (Bio-Rad Laboratories, Inc., Richmond, CA) with bovine serum albumen as the standard. Equal amounts of protein lysates (20 µg) were loaded onto a 10% polyacrylamide gel, separated by electrophoresis and then transferred to a nitrocellulose membrane (Bio-Rad) (13). The membrane was blocked in 5% non-fat milk. The blot was then immunostained with antibodies against the target proteins. Protein bands were visualized using an anti-mouse IgG horseradish peroxidase-conjugated antibody followed by enhanced chemi-luminescence (ECL, Amersham Bioscience, Piscataway, NJ) using the manufacturer’s protocols. Band intensity was measured using Gel-Imaging software (Kodak, Rochester, NY). All experiments were performed at least three times and normalized to GAPDH levels.
Plasminogen Zymography
Casein zymography was performed as described previously (14) with minor modifications. Equal amounts of proteins were mixed with SDS sample buffer without reducing agent and subjected to 12.5% SDS-PAGE containing casein (0.25 mg/mL) and plasminogen (20 µg/mL). After electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 min each at room temperature to remove SDS and then incubated for 18 hrs at 37°C in buffer containing 100 mM Glycine, pH 8.0. The proteins in the gel were stained with 0.2% Coomassie Brilliant Blue in 30% methanol: 10% acetic acid solution for 2–3 hrs and then destained in 30% methanol: 10% acetic acid.
Reverse Plasminogen Zymography
Reverse plasminogen zymography was performed as described previously (14) with minor modifications. Equal amounts of proteins were mixed with SDS sample buffer without reducing agent and subjected to 12.5% SDS-PAGE containing casein (0.25 mg/mL), plasminogen (20 µg/mL) and urokinase-type plasminogen activator (uPA; 0.03 U/mL). After electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 min each at room temperature to remove SDS and then incubated for 18 hrs at 37°C in buffer containing 100 mM Lysine, pH 8.0. The proteins in the gel were stained with 0.2% Coomassie Brilliant Blue in 30% methanol: 10% acetic acid solution for 2–3 hrs and then destained in 30% methanol: 10% acetic acid.
Gelatin Zymography
For assay of MMP-2 and MMP-9 activity, vessels were isolated and snap frozen after being rinsed in ice cold PBS. Segments were then pulverized on liquid nitrogen using a mortar and pestle and extracted with a guanidine-based buffer (0.05 M Tris, pH 7.5, 0.01 M CaCl2, 2.0 M guanidine and 0.2% Triton X-100). The suspension was centrifuged and dialyzed against 0.05 M Tris and 0.2% Triton for 48 hours. A BCA protein assay (Pierce, Rockford, IL) was performed and 10 µg protein was loaded per lane. Gelatin zymography for matrix metalloproteinases (MMPs) was performed as described previously (15).
Reverse Gelatin Zymography
Gelatin zymography was performed as described previously (16) with minor modifications. Equal amounts of proteins were mixed with SDS sample buffer without reducing agent and subjected to 7.5% SDS-PAGE containing gelatin (2.5 mg/mL) and pro-MMP-2 (1.2 µg/mL) or pro-MMP-9 (1.2 µg/mL). After electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 min each at room temperature to remove SDS and then incubated for 18 hrs at 37°C in buffer containing Tris-HCl (40 mM), CaCl2 (10 mM) and NaCl (50 mM). The proteins in the gel were stained with 0.2% Coomassie Brilliant Blue in 30% methanol: 10% acetic acid solution for 2–3 hrs and then destained in 30% methanol: 10% acetic acid. TIMP activity was detected as blue bands against a clear background of MMP digestion.
Data and Statistical Analysis
All data are presented as the mean ± standard error of the mean (s.e.m.) of six observations and statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate. A p-value less than 0.05 was regarded as significant. Non-significant p-values were expressed as p=ns.
RESULTS
G protein Expression
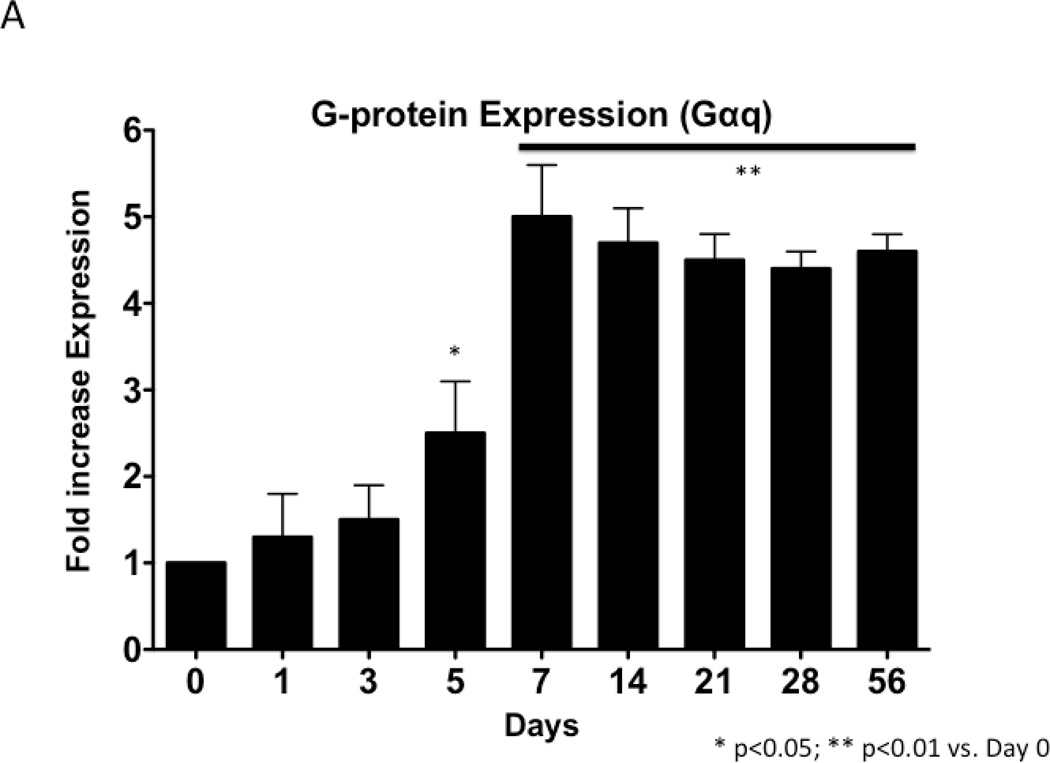
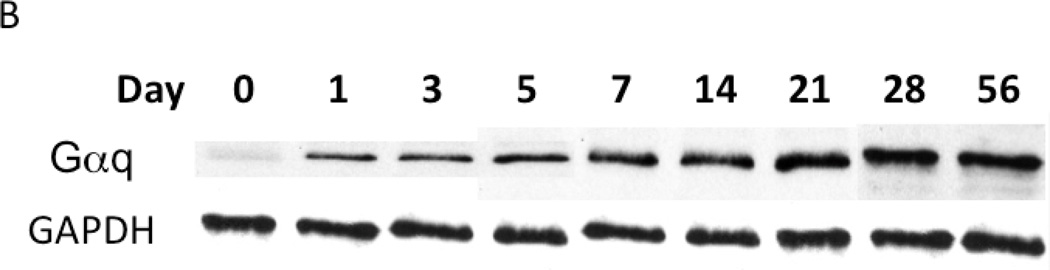
To examine the changes in Gαq expression, samples of injured artery were examined by western blotting and normalized to GAPDH. Gαq expression increased in a time-dependent manner after injury, which peaked at 7 days (Fig 1). Sham operated on vessels did not produce such a response.
Figure 1.
Time course of Gαq expression normalized to α-actin following wire injury in the murine femoral artery (A) with a representative Western blot (B). Values are the mean± s.e.m. (n=6 per group). Statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate.
Modulation of Cell Kinetics
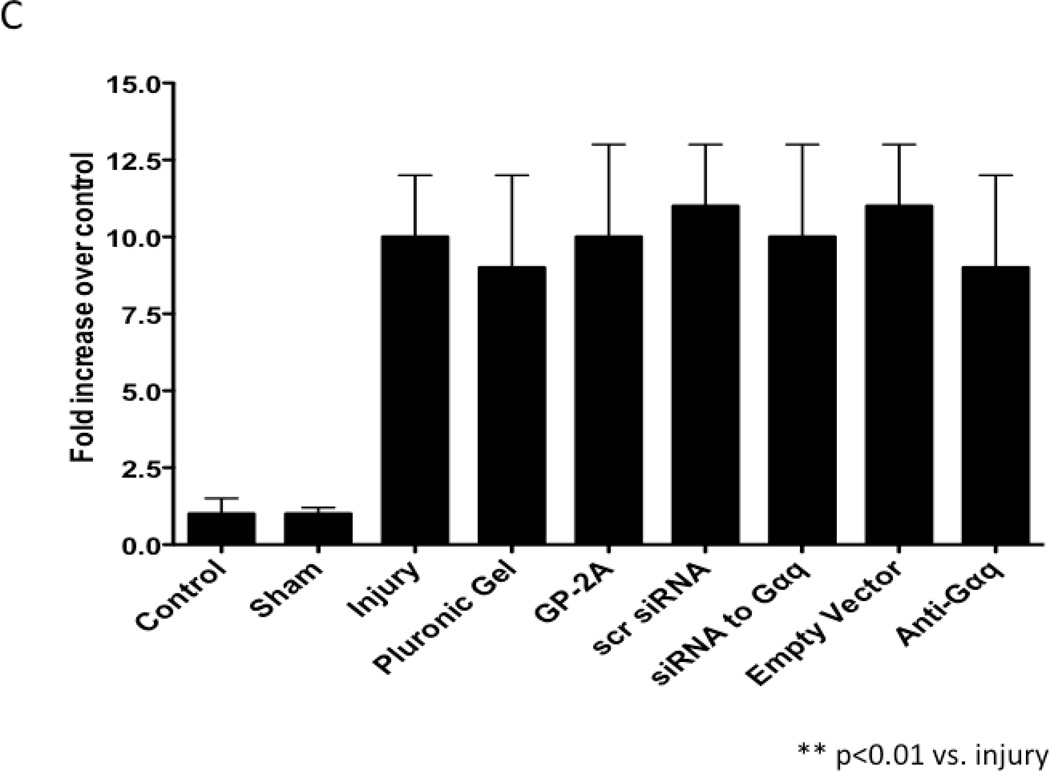
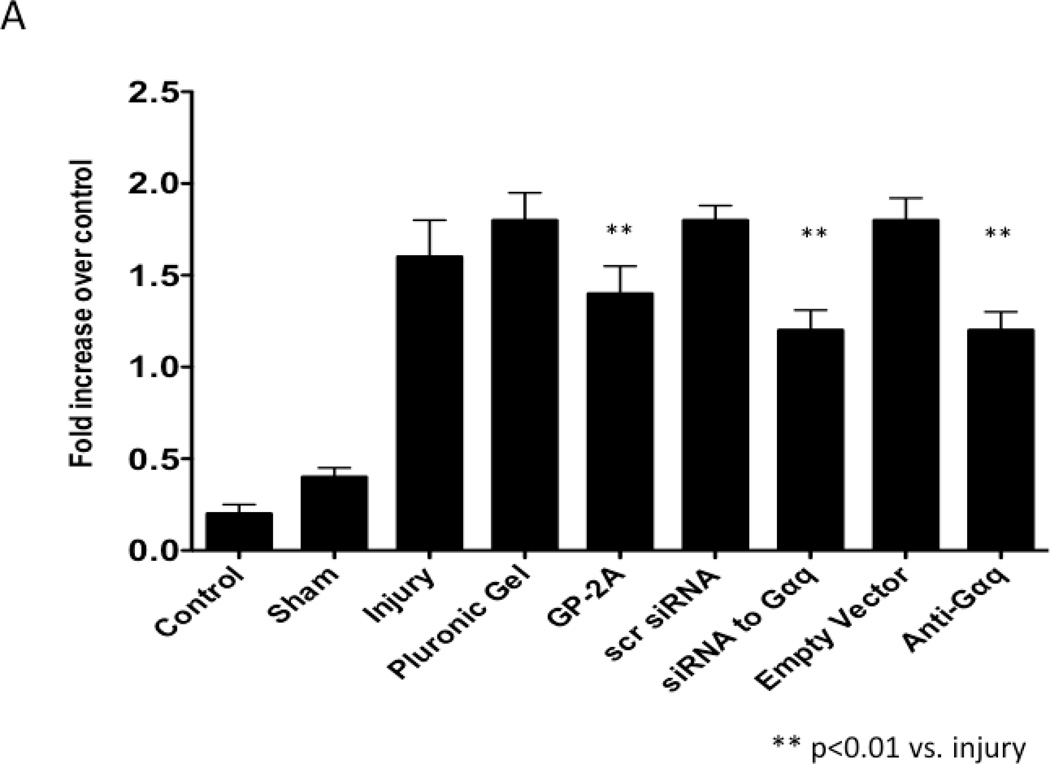
VSMC apoptosis using TUNEL as a marker peaked within 1–3 days after injury with detection of apoptosis in the adventitia and in the media early and in the intima and media late (Fig 2A). VSMC proliferation using BrdU as a marker peaked at 3–5 days after injury (Fig 2B). VSMC migration peaked at 7 days after injury (Fig 2C). To determine the effect of chemical and molecular inhibition of Gαq, the injured vessels were encased in pluronic gel containing GP-2A, siRNA to Gαq or dominant negative Gαq. Inhibition of Gαq by application of the Gαq chemical inhibitor, GP-2A, or siRNA to Gαq increased early VSMC apoptosis as measured by TUNEL staining in the vessels. Transfection with adenovirus of a dominant negative Gαq had a similar effect (Fig 2A). Application of GP-2A, the chemical Gαq inhibitor, decreased cell proliferation as measured by BrdU staining but not migration as measured by enface microscopy (Fig 2B and Fig 2C). Inhibition of Gαq by adenoviral transfection of dominant negative Gαq also reduced cell proliferation but did not influence cell migration (Fig 2B and Fig 2C). Application of siRNA to Gαq also reduced cell proliferation without affecting cell migration (Fig 2B and Fig 2C). Transfection with empty virus or application of scrambled siRNA had no effect on cell proliferation (Fig 2B and 2C).
Figure 2.
Changes in cell apoptosis (A), cell proliferation (B) and cell migration (C) in wire-injured femoral vessels in the presence of pluronic gel with and without GP-2A, siRNA to Gαq or adenovirus with dominant negative Gαq. Values are the mean± s.e.m. (n=6 per group). Values are the mean± s.e.m. (n=6 per group). Statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate.
Morphological Response
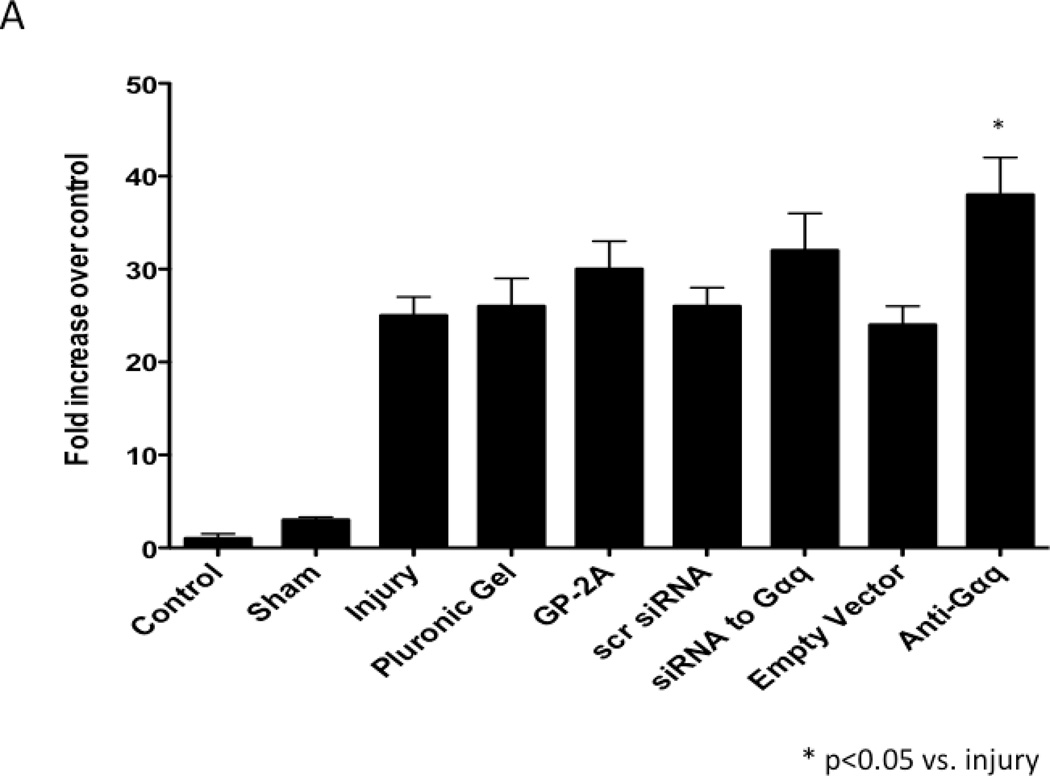
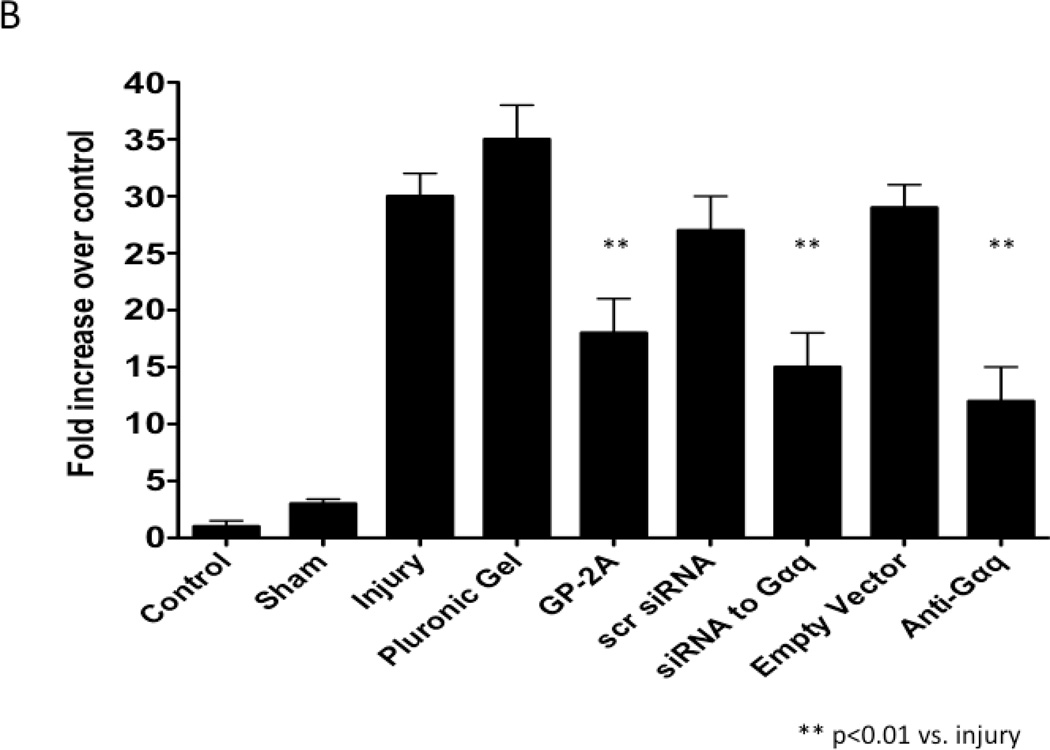
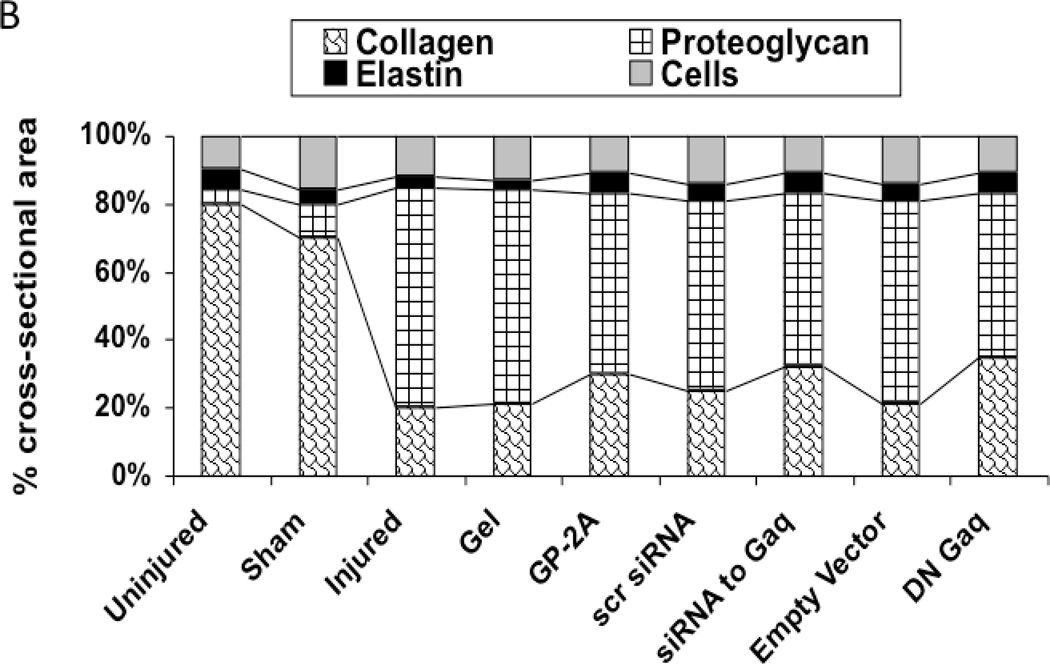
The development of intimal hyperplasia was determined by cross-section stained with H&E. Following wire injury, the injured femoral arteries heal with the development of intimal hyperplasia, which levels off at 14 days and is maximal at 28 days and does not change substantially between day 28 and day 56. Vessels subjected to a sham procedure do not produce such a response. When the vessels were encased in pluronic gel in vivo, there was no significant change in intimal hyperplasia development (Fig 3A). A similar response was seen when an empty vector was placed in the gel. Application of GP-2A, the Gαq inhibitor, decreased the intimal hyperplastic response (Fig 3A). Transfection with dominant negative Gαq or application of siRNA also reduced intimal hyperplasia development (Fig 3A). The cross-sections were stained with Movat’s stain to evaluate the composition of the extracellular matrix. The percent area of the wall composed of proteoglycans increased significantly after wire injury (p<0.05) and this increase was decreased by transfection with Gαq, with a significant increase in collagen deposition (p<0.05) (Fig 3B). There was no difference in the inflammatory infiltrate between the vessel treated with a Gαq inhibitor or control.
Figure 3.
A comparison of the effect of pluronic gel with and without GP-2A, siRNA to Gαq or adenovirus with dominant negative Gαq on intimal hyperplasia development at 14 days after wire injury (A). Changes in the area of cells, collagen, proteogylcan and elastin in wire-injured femoral vessels in the presence of pluronic gel with and without GP-2A, siRNA to Gαq or adenovirus with dominant negative Gαq (B). The percent area of the wall composed of proteoglycans increased significantly after wire injury (p<0.05) and this increase was decreased by transfection with Gαq, with a significant increase in collagen deposition (p<0.05). Values are the mean± s.e.m. (n=6 per group). Statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate.
Protease Response
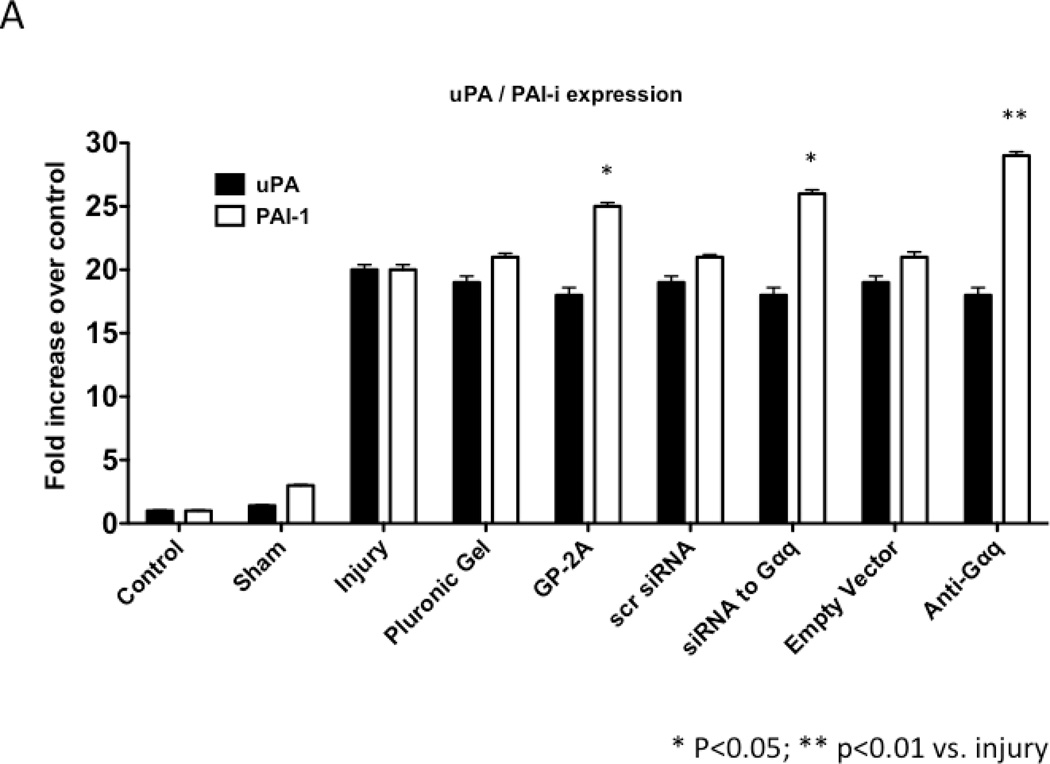
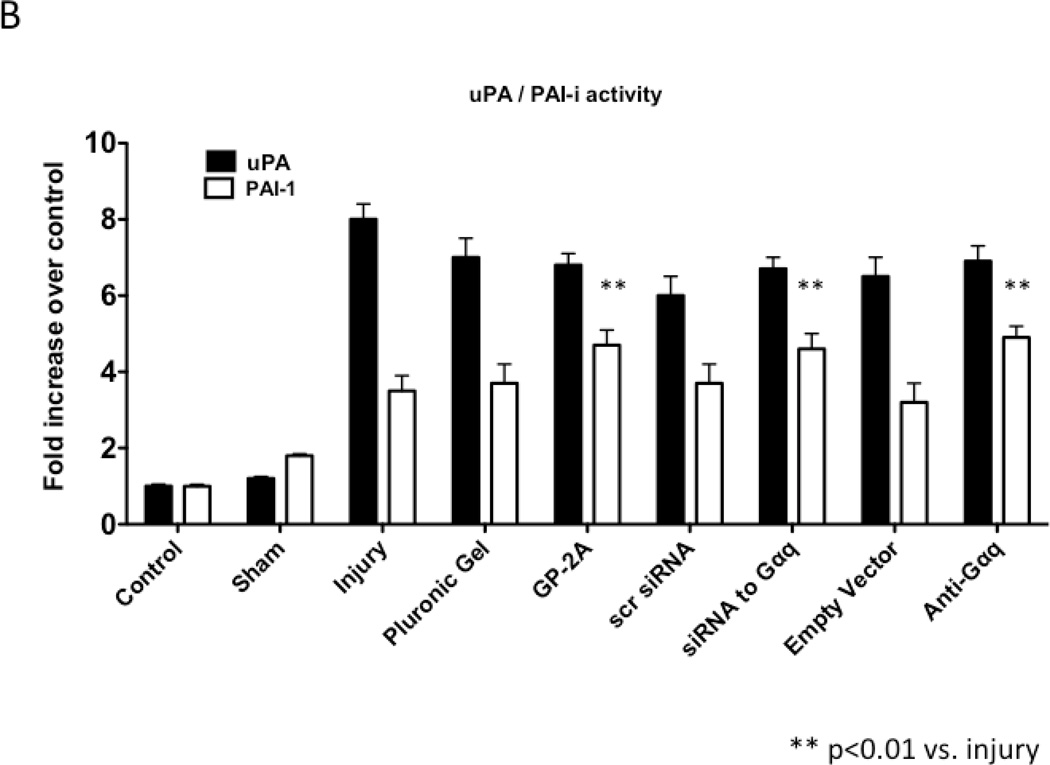
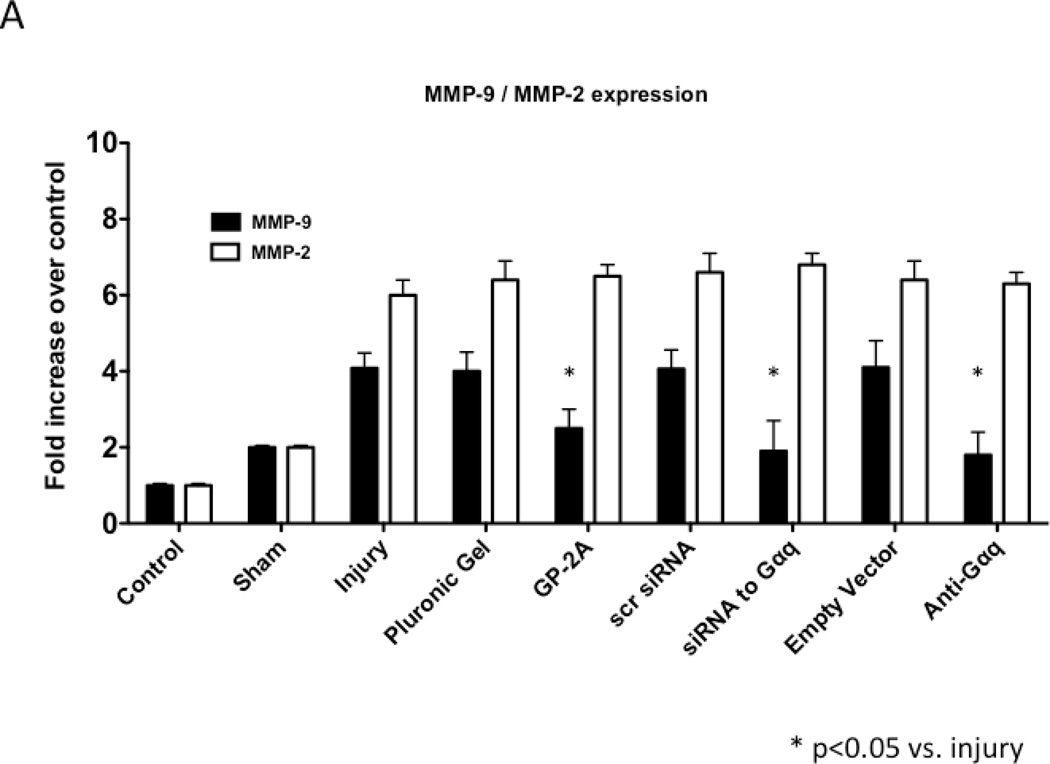
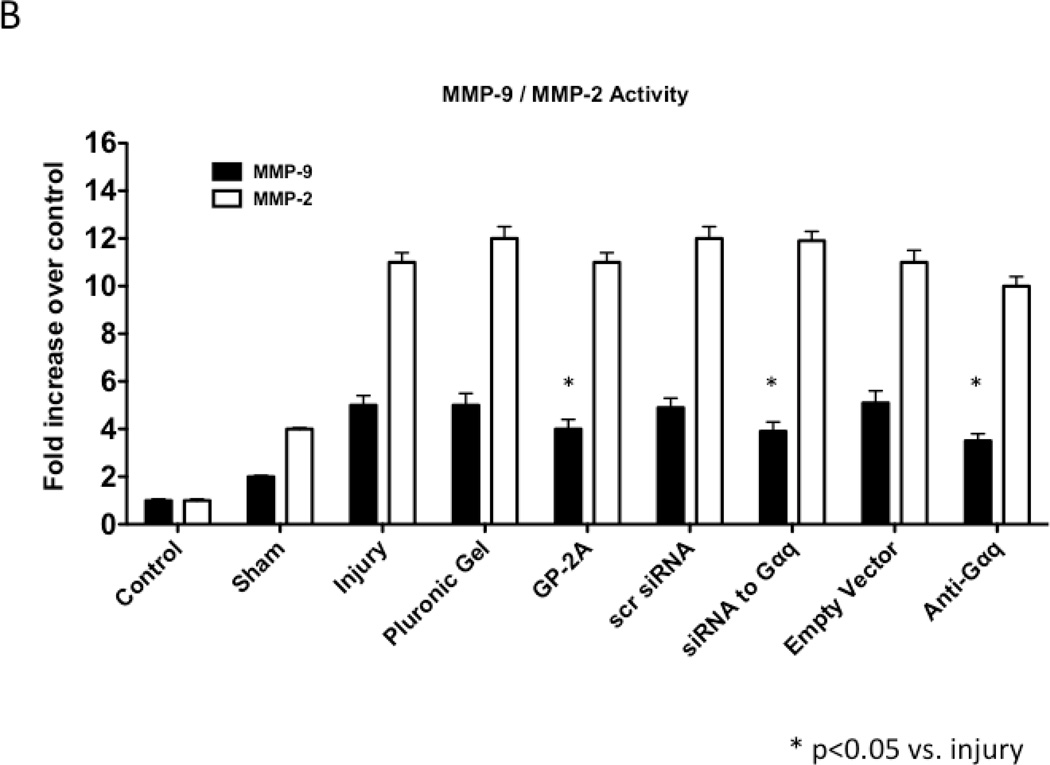
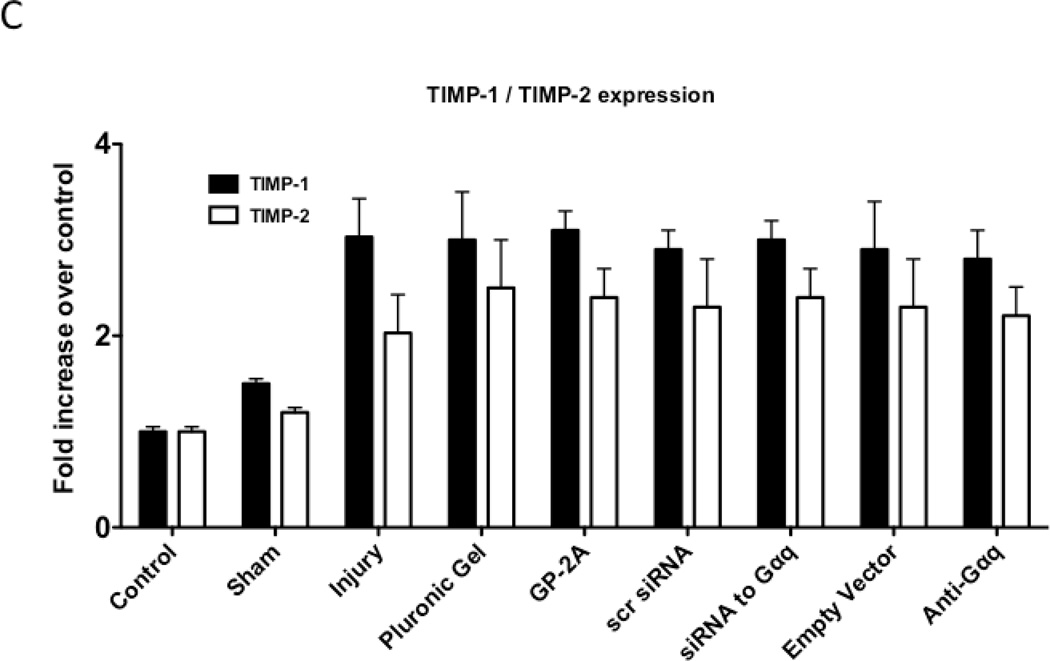
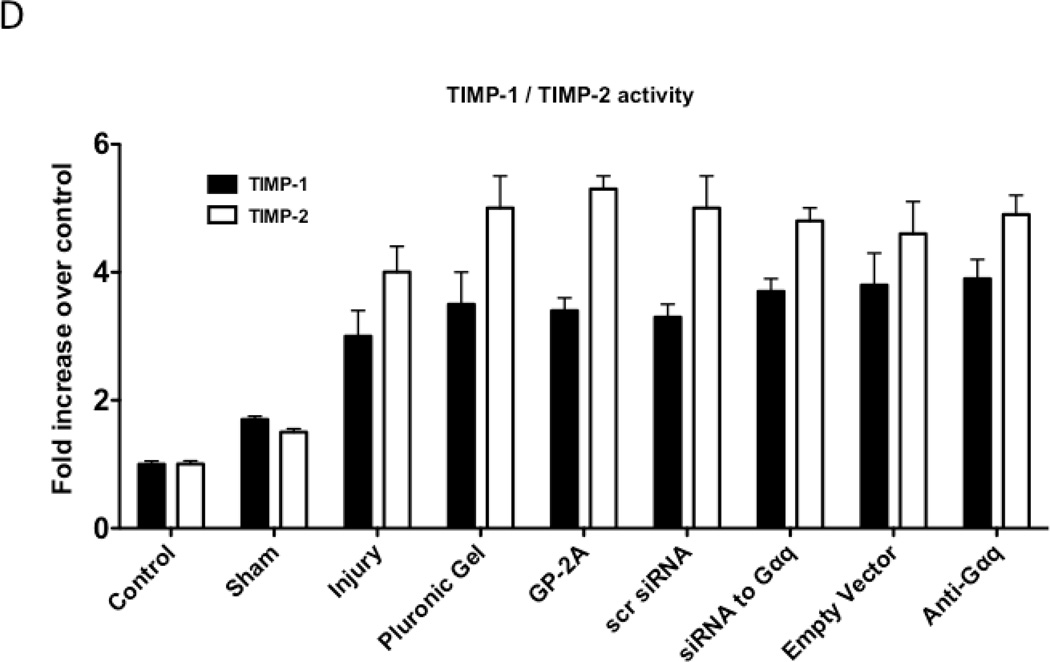
We determined the activity of uPA and PAI-1 activity by casein and reserve casein zymography, respectively. uPA activity peaked at 7 days while plasminogen activator inhibitor-1 (PAI-1) activity was decreased early from 0 to 3 days and returned to baseline thereafter. There were time-dependent changes in expression of uPA and PAI-1 by western blotting after injury. PAI-1 expression was increased early from 0 to 3 days and returned to baseline thereafter. In contrast, uPA protein expression showed a steady increase that rose at day 7 and peaked at day 28. Inhibition of Gαq did not alter peak uPA activity and expression (Fig 4A and 4B), but did increase early PAI-1 activity and expression (Fig 4A and 4B). We determined the activity of MMP-2/MMP-9 and TIMP-1/TIMP-2 by gelatin and reverse gelatin zymography, respectively. MMP-9 activity peaked early at day 3 and declined thereafter. MMP-2 activity increased significantly at day 1 and peaked again at day 7 and then showed a continual decline in activity to day 56 in the untreated wire-injured vessels (Fig 5A). Protein expression for both MMP-9 and MMP-2 increased significantly after injury and were both maximal at day 14 (Fig 5B). Inhibition of Gαq reduced peak MMP-9 activity at day 3 but did not influence peak MMP-2 activity at day 7 (Fig 5A). Protein expression for MMP-9 was also decreased but that of MMP-2 was not affected (Fig 5B). There were no changes in the expression or the activity of the respective inhibitors for MMP-9 and MMP-2, TIMP-1 and TIMP-2 (Fig 5C and 5D).
Figure 4.
Alterations in the peak expression (A) and peak activity (B) of uPA at day 7 and PAI-1 at day 3 in wire-injured femoral vessels in the presence and absence of GP-2A, siRNA to Gαq or adenovirus with dominant negative Gαq. Values are the mean±s.e.m. (n=6 per group) (8, 9). Statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate.
Figure 5.
Alterations in the peak activity (A) at day 7 and peak expression at day 14 (B) of MMP-9 / MMP-2 in wire-injured femoral vessels in the presence and absence absence of GP-2A, siRNA to Gαq or adenovirue with dominant negative Gαq. Peak activity (C) at day 7 and peak expression at day 14 (D) of TIMP-1 / TIMP-2 in wire-injured femoral vessels in the presence and absence of GP-2A, siRNA to Gαq or adenovirus with dominant negative Gαq is also shown. Values are the mean± s.e.m. (n=6 per group) (8, 9). Statistical differences between groups were tested with a Kruskal-Wallis non-parametric test with post hoc Dunn’s multiple comparison correction, where appropriate.
DISCUSSION
This study demonstrates that Gαq expression increased in a time-dependent manner after femoral artery injury and was sustained out to 56 days, Interruption of Gαq reduced cell proliferation and increased cell apoptosis without affecting cell migration. Interruption of Gαq signaling early also inhibited the development of intimal hyperplasia and altered the extracellular matrix within, increasing the collagen content at 14 days. Associated with these long-term changes in the extracellular matrix, inhibition of Gαq increased early PAI-1 activity and expression without changing uPA activity or expression and reduced peak MMP-9 activity without a change in the MMP-9 inhibitor TIMP-1. The MMP-2/TIMP-2 system was unaffected.
G proteins are involved in the signal transduction processes of seven membrane spanning receptors such as angiotensin II and the angiotensin II type 1 receptor has been found to be associated with Gαq proteins (17, 18). Angiotensin II and the angiotensin system have been shown to be intimately involved in the vessel’s response to injury and its remodeling (19). Angiotensin II is a G protein agonist that activates a Gαq-coupled receptor (20) and stimulates smooth muscle cell proliferation (21, 22). Angiotensin II signaling is associated with increases in MMP-9 levels and an increase in PAI-1 expression or activity (23, 24). The Gαq family of G proteins has been shown to mediate receptor stimulation of PLC-β activity at the plasma membrane. PLC-β and Regulators of G protein signaling (RGS) function as GTPase activating kinases (GAP) on Gαq to control the amplitude and duration of stimulation (25). Receptor activation of PLC-β via G proteins can be classifed as pertussis toxin sensitive and pertussis toxin insensitive (26). The α̃ subunits of the Gαq family are presumed to mediate the toxin-insensitive pathway (26, 27). We have previously demonstrated that GP-2A can effectively inhibit both PLC-β phosphorylation and reduce ERK1/2 activation in response to angiotensin II stimulation (28).
We have previously shown that there were time-dependent changes in G protein expression in two models of vessel remodeling: vein grafting in the rabbit and balloon injury in the rat. Vein grafts harvested at 28 days postoperatively display an increased expression of G proteins (Gαs, Gαi2 and Gβγ subunits) and de novo expression of Gαi3 (3). In addition, receptor-coupled responses change from being pertussis toxin insensitive in the jugular vein to pertussis toxin sensitive in the vein graft (2). After arterial balloon injury, there is a specific increase in Gαi3 expression at 3 days while both Gαq and Gβγ expression increase in a time-dependent manner over the 28 days (7). Several inhibitor studies have strengthened the contention that G proteins are involved in the genesis of intimal hyperplasia (4, 29). We have shown that inhibition of Gαi proteins will decrease intimal hyperplasia in a rat arterial injury model (7) and specific blockade of the Gβγ-subunit in balloon-injured rat carotids with a single exposure to the Gβγ inhibitor βARKCT will also reduce the intimal hyperplastic response (6). We have seen a similar response in rabbit vein grafts (5).
We have shown that following femoral wire injury in the mouse, there was a consistent model of intimal hyperplasia and this remodeling was associated with a time-dependent increase in the activity of MAPK signaling kinases (ERK1/2, p38MAPK and JNK) (8). After injury, there were also time-dependent alterations in the activity and expression of the protease/inhibitor combinations MMP-2 / TIMP-2, MMP-9 / TIMP-1 and urokinase / PAI-1 (8, 9). Interruption of the MAPK kinase activity with chemical inhibitors decreased the intimal hyperplastic response and interrupted the expression and activity of the uPA / PAI-1, MMP-2 / TIMP-2 and MMP-9 / TIMP-1 pathways (8, 9). More recently we demonstrated that inhibition of Gβγ alters in vivo VSMC proliferation and migration with associated reductions in MMP-2, MMP-9 and uPA expression and activity. In the current study we saw an increase in the expression of Gαq associated with the development of intimal hyperplasia that was sustained out to 56 days. Early inhibition of Gαq resulted in a decrease in intimal hyperplasia early at 14 days. Gαq appears to be associated with the VSMC phenotype involved in the development and persistence of intimal hyperplasia.
uPA and PAI-1 expression is linked to vessel remodeling with elevated uPA and decreased PAI-1 levels having been shown to be predictors for angiographic coronary restenosis (30). In our previous report, we saw a steady rise in uPA protein, which stabilized at 14 days, and a peak in uPA activity at 7 days in this model (8). Both responses were blunted by the presence of MAPK inhibitors and inhibition of Gβγ. A similar pattern in the uPA / PAI-1 system was seen after injury to the rat carotid artery (31, 32). If uPA is placed in a pluronic gel around an injured carotid, there is a significant increase in the earlier development of intimal hyperplasia due to enhanced migration and proliferation of smooth muscle cells (33). Increased expression of PAI-1 will promote proliferation when VSMCs are exposed to tumor necrosis factor (34). Over-expression of uPA or a deficiency of PAI-1 in transgenic mice has been shown to promote neointimal formation in response to transmural electrical injury. A lack of uPA leads to a reduction in neointimal hyperplasia (35). Inhibition of Gβγ decreased peak uPA activity and expression without altering the early peaks in PAI-1 activity and expression, resulting in a change in the relative activities, which may in part explain the decease in intimal hyperplasia. In contrast, inhibition of Gαq increased PAI-1 without effecting uPA expression or activity.
MMP-9 is not constitutively expressed in VSMC but is induced after arterial injury and this increase in activity coincides with the onset of cell proliferation (14, 36–38). We have shown that the stress kinases play a role in MMP-9 expression and that peak MMP-9 expression and activity is associated with VSMC proliferation within the vessel wall (8). MMP inhibitors decrease smooth muscle cell proliferation and migration (9, 32, 36). Inhibition of Gαq reduced peak MMP-9 activity at day 3 without a change in MMP-2 activity. This change in protease activity is associated with a decrease in cell proliferation. Cell migration was not significantly affected by Gαq inhibition and the associated reduction in MMP9. This likely reflects the fact that the main driver for migration in this model is MMP2. In the rat model MMP-9 overexpression is associated with increased migration (39) and MMP-9 inhibition is associated with decreased migration (40). This dichotomy in the results may suggest a species and strain-specific effect, but also may suggest that other compensatory mechanisms have occurred with Gαq suppression.
In this study, we noted an increase in PAI-1 and an increase in MMP-9 after vessel injury. There is an apparent link between PAI-1 and MMP-9 expression in the vessel wall. The distribution of latent and active MMP-9 has been monitored in aorta extracts and in serum-free conditioned cell culture medium obtained from wild-type mice and from mice with deficiency of tissue-type plasminogen activator (tPA), uPA, PAI-1 or plasminogen (41). In aorta extracts, the contribution of active MMP-9 to the total MMP-9 level ranged from 17% to 57% for smooth muscle cells, with the exception of plasminogen (−/−) smooth muscle cells, which had undetectable levels of active MMP-9. Addition of plasminogen to the cell culture medium of fibroblasts resulted in an approximately two-fold enhancement of the contribution of active MMP- 9. Activation of pro-MMP-9 was enhanced in the presence of plasminogen but active MMP-9 was also detected in fibroblasts of plasminogen (−/−) mice, indicating that in vivo activation may occur via plasminogen-independent mechanisms (41). In experimental deep vein thromboses (DVTs), inhibition of plasmin activation using aprotinin resulted in significantly increased levels of MMP-9 (42). These reports confirm our data and suggest that a Gαq-PAI-1 pathway is involved in the upregulation of MMP-9.
Conclusions
These data demonstrate that femoral wire injury in the mouse is associated with a time-dependent increase in Gαq expression and that inhibition of Gαq will decrease intimal hyperplasia development. Inhibition of Gαq alters cell proliferation and is associated with decreased MMP-9 expression and activity.
ACKNOWLEDGEMENTS
This research was supported by U.S. Public Health Service grants HL086968 and HL067746 to Mark G. Davies, M.D., Ph.D. The authors thank Daynene Vykoukal, Ph.D. for critical reading of the manuscript.
Supported by: U.S. Public Health Service HL086968 and HL67746
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: Presented at the 4th Academic Surgical Congress, Sanibel, FL, Feb. 2009
REFERENCES
- 1.Davies MG, Hagen P-O. Pathobiology of Intimal Hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 2.Davies MG, Ramkumar V, Gettys T, Hagen P-O. The expression and function of G-proteins in experimental vein grafts. J Clin Invest. 1994;94:1680–1689. doi: 10.1172/JCI117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MG, Ramkumar V, Hagen P-O. Temporal expression of G-proteins in intimal hyperplasia. J Surg Res. 1996;63:115–122. doi: 10.1006/jsre.1996.0233. [DOI] [PubMed] [Google Scholar]
- 4.Davies MG, Huynh TTT, Fulton GJ, Lefkowitz RJ, Svendsen E, Hagen P-O, et al. G-protein signaling and vein graft intimal hyperplasia: Reduction of intimal hyperplasia in vein grafts by a Gβγ inhibitor suggests a major role of G-protein signalling in lesion development. Arterioscler Thromb Vasc Biol. 1998;18:1275–1280. doi: 10.1161/01.atv.18.8.1275. [DOI] [PubMed] [Google Scholar]
- 5.Huynh TTT, Iaccarino G, Peppel KC, Davies MG, Koch WJ, Hagen P-O. Adenoviral mediated inhibition of Gβγ subunit limits the formation of intimal hyperplasia in vein grafts. Surgery. 1998;124:177–186. [PubMed] [Google Scholar]
- 6.Iaccarino G, Smithwick LA, Lefkowitz RJ, Koch WJ. Targeting Gβγ signaling in arterial vascular smooth muscle cell proliferation: a novel strategy to limit restenosis. Proc Natl Acad Sci USA. 1999;96:3945–3950. doi: 10.1073/pnas.96.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MG, Mason DP, Tran PK, Hawkins SA, Clowes AW. G-protein expression and intimal hyperplasia after arterial injury: a role for Gαi proteins. J Vasc Surg. 2001;33:408–418. doi: 10.1067/mva.2001.111748. [DOI] [PubMed] [Google Scholar]
- 8.Zou Y, Qi Y, Roztocil E, Nicholl SM, Davies MG. Patterns of kinase activation induced by injury in the murine femoral artery. J Surg Res. 2007;141:332–340. doi: 10.1016/j.jss.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Y, Qi Y, Roztocil E, Nicholl SM, Lumsden AB, Davies MG. Patterns of gelatinase activation induced by injury in the murine femoral artery. J Surg Res. 2009;154(1):135–142. doi: 10.1016/j.jss.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Edelman ER, Dekeyser J-L, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 11.Fulton GJ, Davies MG, Koch WJ, Dalen H, Svendsen E, Hagen P-O. Antisense oligonucleotide to proto-oncogene c-myb inhibits the formation of intimal hyperplasia in experimental vein grafts. J Vasc Surg. 1997;25:453–463. doi: 10.1016/s0741-5214(97)70255-6. [DOI] [PubMed] [Google Scholar]
- 12.Davies MG, Annex BH, Channon KM, Denning SM, Taylor DA, Fulton GJ, et al. Alteration of arterial vasomotor function by gene transfer with a replication deficient adenovirus. Vascular Surgery. 1997;31:131–136. [Google Scholar]
- 13.Tanski WJ, Fegley AJ, Roztocil E, Davies MG. Domain dependent actions of urokinase on smooth muscle cell responses. J Vasc Surg. 2004;39:214–222. doi: 10.1016/s0741-5214(03)01031-0. [DOI] [PubMed] [Google Scholar]
- 14.Lijnen HR, VanHoef B, Lupu F, Moon L, Carmeliet P, Collen D. Function of the plasminogen/plasmin and MMP systems after vascular injury in mice with targeted inactivation of fibrinolytic genes. Arterioscler Thromb Vasc Biol. 1998;18(7):1035–1045. doi: 10.1161/01.atv.18.7.1035. [DOI] [PubMed] [Google Scholar]
- 15.Kenagy RD, Nikkari ST, Welgus HG, Clowes AW. Heparin inhibits the induction of three metalloproteinases (stomelysin 92kD gelatinase and collagenase) in primate arterial smooth muscle cells. J Clin Invest. 1994;93:1987–1993. doi: 10.1172/JCI117191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver GW, Leferson JD, Stetler-Stevenson WG, Kleiner DE. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymogram. Anal Biochem. 1997;244(1):161–166. doi: 10.1006/abio.1996.9895. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Ortega M, Ruperez M, Esteban V, Egido J. Molecular mechanisms of angiotensin II-induced vascular injury. Curr Hypertens Rep. 2003;5(1):73–79. doi: 10.1007/s11906-003-0014-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284(18):11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81(3):999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 20.Gutkind JS. Regulation of MAPK signaling networks by G-protein-coupled receptors. Science STKE. 2000;40:re1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- 21.Dubey RK, Roy A, Overbeck HW. Cultures of renal arteriolar smooth muscle cells. mitogenic responses to angiotensin II. Circ Res. 1992;71(5):1143–1152. doi: 10.1161/01.res.71.5.1143. [DOI] [PubMed] [Google Scholar]
- 22.Briand V, Riva L, Galzin AM. Characterization of the angiotensin II AT1 receptor subtype involved in DNA synthesis in cultured vascular smooth muscle cells. Br J Pharmacol. 1994;112(4):1195–1201. doi: 10.1111/j.1476-5381.1994.tb13210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaghooti H, Firoozrai M, Fallah S, Khorramizadeh MR. Angiotensin II induces NF-κB, JNK and p38 MAPK activation in monocytic cells and increases matrix metalloproteinase-9 expression in a PKC- and Rho kinase-dependent manner. Braz J Med Biol Res. 2011;44(3):193–199. doi: 10.1590/s0100-879x2011007500008. [DOI] [PubMed] [Google Scholar]
- 24.Cipollone F, Fazia M, Iezzi A, Pini B, Cuccurullo C, Zucchelli M, et al. Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2-dependent matrix metalloproteinase activity. Circulation. 2004;109(12):1482–1488. doi: 10.1161/01.CIR.0000121735.52471.AC. [DOI] [PubMed] [Google Scholar]
- 25.Litosch I. Novel mechanisms for feedback regulation of PLCb activity. IUBMB. 2002;54(5):253–260. doi: 10.1080/15216540215673. [DOI] [PubMed] [Google Scholar]
- 26.Boyer JL, Graber SG, Waldo GL, Harden TK, Garrison JC. Selective actiation of phospolipase C by recombinant G-protein alpha and beta gamma subunits. J Biol Chem. 1994;269(4):2814–2819. [PubMed] [Google Scholar]
- 27.Boyer JL, Waldo GL, Harden TK. Beta-gamma subunits activation of G-protein regulated phospholipase C. J Biol Chem. 1992;267 26451-5466. [PubMed] [Google Scholar]
- 28.Tanski WJ, Roztocil E, Hernady E, Williams JP, Davies MG. Role of Gαq in smooth muscle cell proliferation. J Vasc Surg. 2004;39:639–644. doi: 10.1016/j.jvs.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 29.Fulton GJ, Svendsen E, Hagen P-O, Davies MG. Oral D-limonene therapy (perillyl alcohol) reduces vein graft intimal hyperplasia. J Surg Res. 1997;69:128–134. doi: 10.1006/jsre.1997.5047. [DOI] [PubMed] [Google Scholar]
- 30.Strauss BH, Lau HK, Bowman KA, Sparkes J, Chisholm RJ, Garvey MB, et al. Plasma urokinase antigen and PAI-1 antigen levels predict angiographic coronary restenosis. Circulation. 1999;100(15):1616–1622. doi: 10.1161/01.cir.100.15.1616. [DOI] [PubMed] [Google Scholar]
- 31.Clowes AW, Reidy MA, Clowes MM, Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid. Circ Res. 1990;67:61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- 32.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 33.Plekhanova OS, Parfenova EV, Bibilashvily RS, Stepanova VV, Erne P, Bobik A, et al. Urokinase plasminogen activator enhances neointima growth and reduces lumen size in carotid arteries. J Hypertens. 2000;18(8):10065–10069. doi: 10.1097/00004872-200018080-00011. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Budd RC, Kelm RJJ, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26(8):1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 35.Shen GX. Vascular cell-derived fibrinolytic regulators and atherothrombotic vascular disorders. Inter J Mol Med. 1998;1(2):399–408. [PubMed] [Google Scholar]
- 36.Zempo N, RD K, Au YPT, Bendeck M, Clowes MM, Reidy MA, et al. Matrix metalloproteinase of vascular wall cells are increased in balloon injured rat carotid artery. J Vasc Surg. 1994;20(2):209–217. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 37.Bassiouny HS, Song RH, Hong XF, Singh A, Glagov S. Reduced flow enhances in vivo collagenase IV transcription after arterial injury. Circulation. 1996;94(suppl) I-349(abstract). [Google Scholar]
- 38.Southgate KM, Fisher M, Banning A, Thurston VJ, Baker AH, Fabunmi RP, et al. Upregulation of basement membrane degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ Res. 1996;79:1177–1187. doi: 10.1161/01.res.79.6.1177. [DOI] [PubMed] [Google Scholar]
- 39.Mason DP, Kenagy RD, Hasentab D, Bowen-Pope DF, Seifert RA, Coats S, et al. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 40.Galis Z, Johnson C, Godin D, Magid R, Shipley J, Senior R, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 41.Lijnen HR, Silence J, Lemmens G, Frederix L, Collen D. Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb Haemost. 1998;79(6):171–176. [PubMed] [Google Scholar]
- 42.Dewyer NA, Sood V, Lynch EM, Luke CE, Upchurch GRJ, Wakefield TW, et al. Plasmin inhibition increases MMP-9 activity and decreases vein wall stiffness during venous thrombosis resolution. J Surg Res. 2007;142(2):357–363. doi: 10.1016/j.jss.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]