Abstract
Recent studies have shed light on the connection between elevated EPO production/spleen erythropoiesis and increased susceptibility to Salmonella infection. Herein, we provide another mouse model, SIRPα-deficient (Sirpα−/−) mice, that manifest increased erythropoiesis as well as increased susceptibility to Salmonella infection. Sirpα−/− mice succumbed to systemic infection with attenuated Salmonella, possessing significantly higher bacteria loads in both the spleen and liver. Moreover, Salmonella-specific antibody (Ab) production and antigen (Ag)-specific CD4 T cells were reduced in Sirpα−/− mice compared to those of WT controls. To further characterize the potential mechanism underlying SIRPα-dependent Ag-specific CD4 T cell priming, we demonstrate that lack of SIRPα expression on dendritic cells (DCs) results in less efficient antigen processing and presentation in vitro. Collectively, these findings demonstrate an indispensable role of SIRPα for protective immunity to Salmonella infection.
Keywords: erythropoiesis, dendritic cells, Salmonella, Th1
Introduction
Chronic bacteria infections initiate both innate and adaptive immune response in the host primarily within the secondary lymphoid organs including spleen and lymph nodes (1, 2). Systemic infection of Salmonella enterica, a gram-negative intracellular bacterium, leads to a characteristic pathology/symptom called splenomegaly in both mouse and humans (3, 4). At the peak of infection, the spleen of infected C57BL/6 mice expands to more than 10-fold larger than its naïve state (5). It was commonly thought that the enlarged spleen accompanying Salmonella infection is caused by local leukocyte expansion or recruitment. Indeed, splenic lymphocytes, especially CD4 and CD8 T cells expand after Salmonella infection and a large increase in phagocyte populations is also observed (5-7). While wild-type C57BL/6 mice typically survive and exhibit a self-limiting infection with an attenuated S. typhimurium strain, immunodeficient mice lacking CD4, MHC class-II, IFNγ or T-bet fail to clear a primary Salmonella infection, demonstrating an indispensable role for CD4 Th1 cells in Salmonella protective immunity (8-10). In addition, Ab responses are also known to contribute to resistance to Salmonella infection (11-13), although the mechanism of protection is unclear.
More recently, our laboratory reported that red blood cells (RBCs, defined as CD71− Ter119+) and reticulocyte precursors (CD71+Ter119+) expand greatly during Salmonella infection, increasing from ~20% of naïve splenocytes to over 80% of the infected spleen (5). Given this large erythroid expansion, these observations provide an alternative mechanism to explain Salmonella induced splenomegaly. Importantly, blocking erythropoiesis induced by elevated level of the hormone erythropoietin (EPO) significantly reduced host susceptibility to Salmonella infection (5, 14). These data suggest that increased erythropoiesis is an evasive mechanism that Salmonella adopt to facilitate persistent infection of the host.
In the steady state, erythropoiesis in the bone marrow and RBC turnover in the periphery are tightly regulated (15, 16). An important signaling module that regulates RBC turnover is the SIRPα-CD47 axis. SIRPα is a transmembrane glycoprotein also known as SHPS-1 (Src homology 2 domain-containing protein tyrosine phosphatase substrate-1), CD172a, BIT (brain immunoglobulin–like molecule with a tyrosine-based activation motif), MFR (macrophage fusion receptor) or p84 (17-21). It is expressed primarily on myeloid cells, such as macrophages, granulocytes and myeloid dendritic cells, but barely detectable on B or T lymphocytes (22-26). The extracellular domain of SIRPα comprised three Ig-like domains, which interact with its ligand CD47, another member of the Ig superfamily known as a marker of self on RBCs (24, 27, 28). The binding of CD47 on RBCs with SIRPα on macrophages delivers an inhibitory “do not eat me” signal that prevents unwanted phagocytosis and maintains the peripheral RBC pool (27-31).
Although more recent studies have implied that SIRPα plays an important role in regulating the homeostasis of T cells, NK cells and DCs (32-34), little is known regarding the role that SIRPα plays in bacteria infection and the development of adaptive immunity. In this current study, we have examined the immune response to Salmonella infection in SIRPα-deficient mice, and report an indispensable role of SIRPα in resolving Salmonella primary infection and establishing an effective Salmonella-specific adaptive immune response.
Materials and Methods
Mice
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD) and The Jackson Laboratory (Bar Harbor, ME). Congenic Sirpα−/− mice (B6.129P2-Sirpa<tm1Nog>/Rbrc) were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. These mice had been backcrossed to C57BL/6 for over ten generations. Rag−/− mice were kindly provided by Dr. D. Masopust (University of Minnesota, Minneapolis, MN). CD90.1 or CD45.1 congenic, RAG-deficient SM1 TCR transgenic mice have been previously described (35, 36). Briefly, the SM1 TCR transgenic mice express a monoclonal TCR specific for Salmonella flagellin peptide (427-441)-I-Ab. These mice were backcrossed to RAG-2-defficient C57BL/6 mice to obtain RAG-deficient SM1 offspring that contained SM1 CD4 T cells but no other lymphocytes. Mice used for experiments were 6-12 weeks old, unless otherwise noted. All of the animal experiments were approved by the University of California, Davis Institutional Animal Care and Use Committee.
Salmonella and Chlamydia infection
Salmonella typhimurium strain BRD509 (aroA-aroD-) was grown overnight in LB broth without shaking, and bacterial concentration was estimated using a spectrophotometer (optical density at 600 nm). Chlamydia muridarum strain Nigg (ATCC, Manassas, VA) was cultivated, purified, aliquoted, tittered and stored at −80°C as previously described (37). Mice were infected intravenously with 5 × 105 CFU of S. typhimurium or 1 × 105 IFU of C. muridarum diluted in 200 μl PBS in the lateral tail vein. The actual Salmonella bacterial dose administrated was always confirmed by plating serial dilutions of the bacterial culture onto MacConkey agar plates. A fresh-thawed Chlamydia aliquot from the same stock was used for each experiment. For survival studies, mice were monitored daily for signs of illness and sacrificed at the moribund stage. To determine Salmonella bacterial loads in vivo, spleens and livers were removed from infected mice at various time points. Serial dilutions of organ homogenates were plated on MacConkey agar plates and bacterial counts were calculated per organ.
Flow Cytometry
Spleens were harvested from naïve and infected mice. Single cell preparations were prepared in PBS with 2% FCS. Aliquots of single cell suspension were stained with a panel of Abs (listed below) and analyzed on a FACS Canto or an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). Antibodies used included FITC-CD71, APC-Ter119, eFlour450-CD3, FITC-CD11a, APC-IFNγ, PE-CD45.1, APC-eF780-CD45R, APC-CD19, FITC-CD69, PE-CD62L, PE-Cy7-CD44, APC-Cy7-CD25 (eBioscience, San Diego, CA), and PerCP-CD4, APC-Cy7-CD8 (BD Biosciences, San Diego, CA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Salmonella-specific serum Ab ELISA
Mice were bled retro-orbitally at various time points post infection. Serum was collected and analyzed by enzyme-linked immunosorbent assay (ELISA) for Salmonella-specific antibody, as previously described (13). Briefly, serial dilutions of serum samples were added to HKST-coated microtiter plates (Costar, Corning, NY). Salmonella-specific Abs were detected using biotinylated isotype-specific Abs (eBioscience, San Diego, CA) andExtrAvidin peroxidase substrate (Sigma-Aldrich, St. Louis, MO).
Salmonella-specific ELISPOT assay
Two weeks after Salmonella infection, mice were treated with Enrofloxacin (Baytril) at 2mg/ml in drinking water to eradicate bacteria. After removal of antibiotic water, mice were rested for an additional week before spleens were harvested and single cell suspensions prepared. After RBC lysis, CD4+ T cells were enriched via magnetic selecting LS MACS columns and CD4 magnetic beads (Miltenyi Biotec, Auburn, CA). Purified CD4+ T cells were co-incubated with irradiated APCs of naïve mice in the presence of 10μM Salmonella specific peptide (SseI, SseJ, FliC or PagC, Table 1) in 96-well ELISPOT plates (Millipore, Billerica, MA) pre-coated with purified anti-IFNγ (BD Biosciences, San Diego, CA). SseI, SseJ and FliC epitopes were previously described (38). The PagC MHC classII epitope was identified using an overlapping peptide screen using a PagC-specific T cell line. After 20 h incubation at 37°C, cells were washed and cytokine spots developed using biotinylated anti-IFNγ (BD Biosciences, San Diego, CA),AKP Streptavidin (BD Biosciences, San Diego, CA), and 1-Step NBT/BCIP substrate (Thermo Scientific, Waltham, MA). Cytokine spots were counted using an ImmunoSpot S5 Core Analyzer (C.T.L. Shaker Heights, OH) and the total number of IFNγ-producing CD4+ T cell per spleen calculated.
Table 1. Salmonella-specific MHC Class-II epitopes.
| Salmonella peptide | Salmonella protein | Epitope Sequence |
|---|---|---|
| FliC 427-441 | Bacterial flagellum | VQNRFNSAITNLGNT |
| SseI 268-280 | SPI2 secreted effector protein | LIYYTDFSNSSIA |
| SseJ 329-341 | SPI2 secreted effector protein | CYYETADAFKVIM |
| PagC 163-174 | phoP-activated gene C | GYEGSNISSTKI |
Salmonella-specific cytokine ELISA
Mice were infected and treated with antibiotics as described in Salmonella-specific ELISPOT assay. After RBC lysis, one million splenocytes were incubated in the presence of 10μM Salmonella specific peptide (SseI, SseJ, FliC or PagC, Table 1) or serial diluted HKST in 96-well round-bottom plates. After 24-48 h incubation at 37°C, the supernatants were collected and added to 96-well ELISA plates (Costar, Corning, NY) pre-coated with purified anti-IFNγ, anti-IL-2 or anti-IL-4 (eBiosciences, San Diego, CA). Cytokine production was detected using biotinylated anti-IFNγ, anti-IL-2 and anti-IL-4 (eBiosciences, San Diego, CA), respectively, followed by ExtrAvidin peroxidase substrate (Sigma-Aldrich, St. Louis, MO). Plates were analyzed using a spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA) and cytokine concentration were calculated according to standard curves.
Generation of bone marrow chimeras
Bone marrow cells from donor mice were isolated under sterile conditions and 1×106 donor cells were injected i.v. into recipient mice via the tail vein. Recipient mice were given 1000 rads irradiation 16 hrs before injection using a cesium irradiator. Recipient mice were treated with antibiotic water for the first six weeks after bone marrow reconstitution and chimerism was used at eight weeks after reconstitution.
DC enrichment and in vitro stimulation
Salmonella flagellin was purified as described previously (39). Flagellin peptide (flagellin427-441) was purchased from Invitrogen (Carlsbad, CA). Spleens were harvested and digested with collagenase D (Roche Diagnostics, Indianapolis, IN) (39) and DCs were enriched to greater than 85-95% purity via magnetic selecting LS MACS column and CD11c magnetic beads (Miltenyi Biotec, Auburn, CA). Enriched DCs were seeded at 1×105 cells/well in a 96-well plate and co-cultured with 1×105 purified SM1 T cells plus antigen. SM1 T cell were recovered 16 hrs later, stained with surface markers and analyzed by flow cytometry.
Detection of Chlamydia muridarum-specific CD4 T cells
The C. muridarum-specific epitope PmpG-1 has been previously described (40). PE-labeled MHC class II tetramers (I-Ab) containing Cm Polymorphic membrane protein G-1 (PmpG-1) residue 303-311 were made in our laboratory (to be published elsewhere). Single-cell preparations from spleens were incubated for 1 h at room temperature with 10 nM of PmpG-1 tetramer in Fc block. Tetramer-specific cells were enriched via magnetic selecting LS MACS column and anti-PE magnetic beads (Miltenyi Biotec, Auburn, CA) (41). The enriched cells were stained with surface markers and analyzed by flow cytometry.
Results
Sirpα−/− mice exhibit a marked increase in splenic erythroid cells
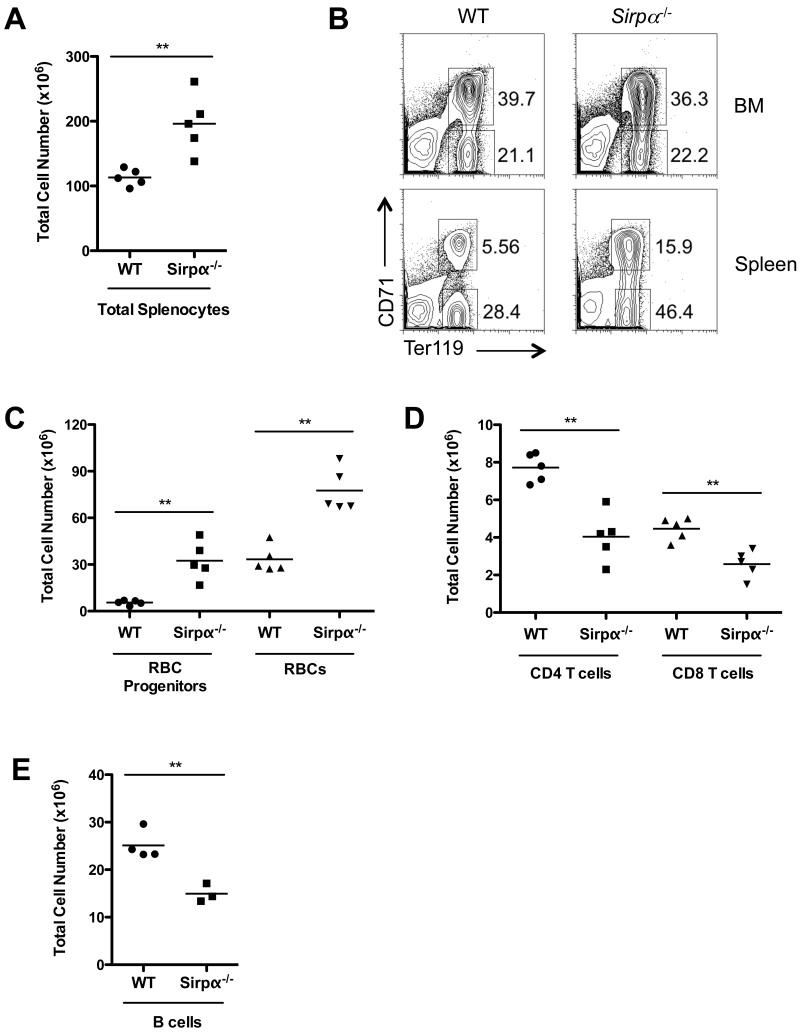
We first examined the splenic cellular profile of naïve Sirpα−/− mice. Consistent with previous reports, Sirpα−/− mice exhibited mild splenomegaly, with a total cell number ~2 fold that of age-matched wild type controls (Fig. 1A) (30). Further analysis demonstrated that the increased number of splenic cells in Sirpα−/− mice is mostly, if not completely, due to higher percentages and total cell numbers of RBCs (CD71−Ter119+) and RBC progenitors (CD71+Ter119+) (Fig. 1B and 1C). Notably, although we observed a marked increase in the erythrocyte frequency and total cell number in the spleen of Sirpα−/− mice, we did not detect any difference in the bone marrow, indicating that erythropoiesis in the bone marrow of Sirpα−/− mice is normal (Fig. 1B). The marked increase in RBCs in Sirpα−/− mice also correlated with decreased lymphocyte cell numbers. CD4 T cell, CD8 T cell and B cell numbers were ~2 fold reduced in Sirpα−/− naïve mice compared to WT controls (Fig. 1D and 1E). These results explain the previous finding that spleen white pulps were reduced in Sirpα−/− mice (30, 33).
Fig. 1. Increased splenic erythropoiesis in Sirpα−/− naïve mice.
A, Total splenocytes recovered from the spleens of WT and Sirpα−/− mice. B, The percentages of RBCs (Ter119+CD71−) and RBC precursors (Ter119+CD71+) in the bone marrow and spleen of WT and Sirpα−/− mice as measured by flow cytometry. C, Total RBCs and RBC precursors recovered from the spleens of WT and Sirpα−/− mice. D, Total CD4 and CD8 T lymphocytes recovered from the spleen of WT and Sirpα−/− mice. E, Total B lymphocytes recovered from the spleen of WT and Sirpα−/− mice. Data shown are representative of three similar experiments. Error bars represent the mean ± SEM; **p < 0.01
SIRPα is indispensable for protective immunity to S. typhimurium infection
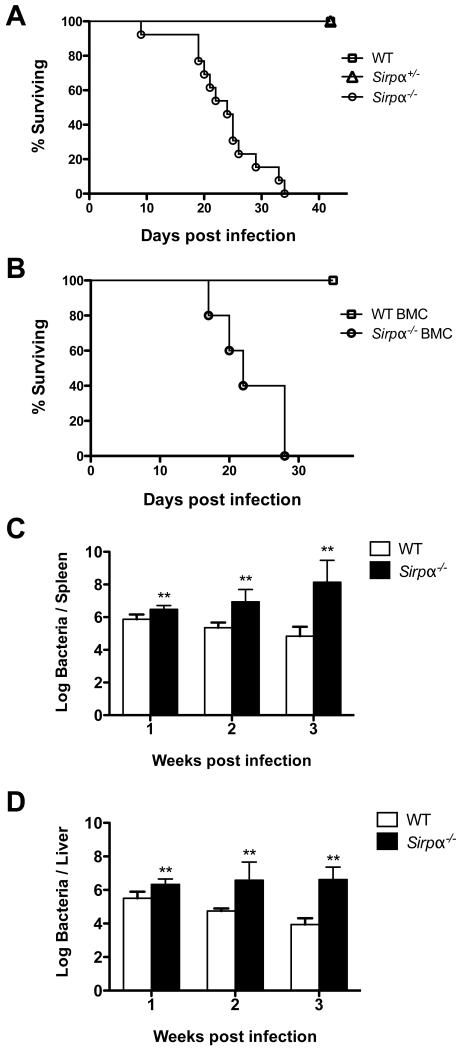
To examine the role that SIRPα plays in Salmonella resistance, we infected WT, Sirpα+/- and Sirpα−/− mice (littermates) i.v. with 5 × 105 attenuated S. typhimurium (BRD509) and monitored infected mice for sign of disease. Given the previous association between erythropoietin and susceptibility to Salmonella infection (5), we hypothesized that increased erythropoiesis in Sirpα−/− mice would increase susceptibility to Salmonella infection. Indeed, while WT and Sirpα+/- mice survived for months after Salmonella infection, all Sirpα−/− mice died between 2-4 weeks post infection (Fig. 2A). The inability of Sirpα−/− mice to resolve Salmonella infection was mapped to a deficiency in hematopoietic cell lineages since Sirpα−/− bone marrow chimeras exhibit a similar survival pattern (Fig. 2B).
Fig. 2. SIRPα expression is required for survival after Salmonella infection.
A, Survival curve of Sirpα−/−, Sirpα+/- and WT littermate control mice infected i.v. with 5 × 105 live attenuated Salmonella (BRD509). Data shown are combined results from three independent experiments with at least ten mice per group. B, Survival curve of WT and Sirpα−/− bone marrow chimeras infected i.v. with 5 × 105 live attenuated Salmonella (BRD509). Data represents at least five mice per group. C and D, Spleens (C) and livers (D) from infected WT and Sirpα−/− mice were recovered 1, 2 and 3 weeks post infection. The bacterial counts per organ were determined by serial dilution and plating. Data shown are representative of three independent experiments. Error bars represent the mean ± SEM; *p < 0.05; **p < 0.01.
We next accessed the bacterial burden in WT and Sirpα−/− mice at various time points after Salmonella infection. Wild type C57BL/6 mice typically resolve attenuated Salmonella infection ~5 weeks post infection. At one-week post infection, Sirpα−/− mice have a slightly higher bacteria burden than WT mice in both spleen and liver (Figure 2C and 2D). This small, but statistically significant difference, indicates that innate immune response in Sirpα−/− mice is impaired in cleaning bacteria infection. A much greater difference in bacteria counts between WT and Sirpα−/− mice became obvious as infection progressed to week two (Figure 2C and 2D). While many Sirpα−/− mice died between 2 to 3-week, surviving Sirpα−/− mice exhibited a dramatic increase in Salmonella colonization in both spleen and liver at three weeks post infection (Fig. 2C and 2D). In conclusion, Sirpα mice fail to control Salmonella replication in vivo and ultimately succumb to an infection that is self-limiting in WT controls.
Defects in Salmonella-specific CD4 T cell response in Sirpα−/− mice
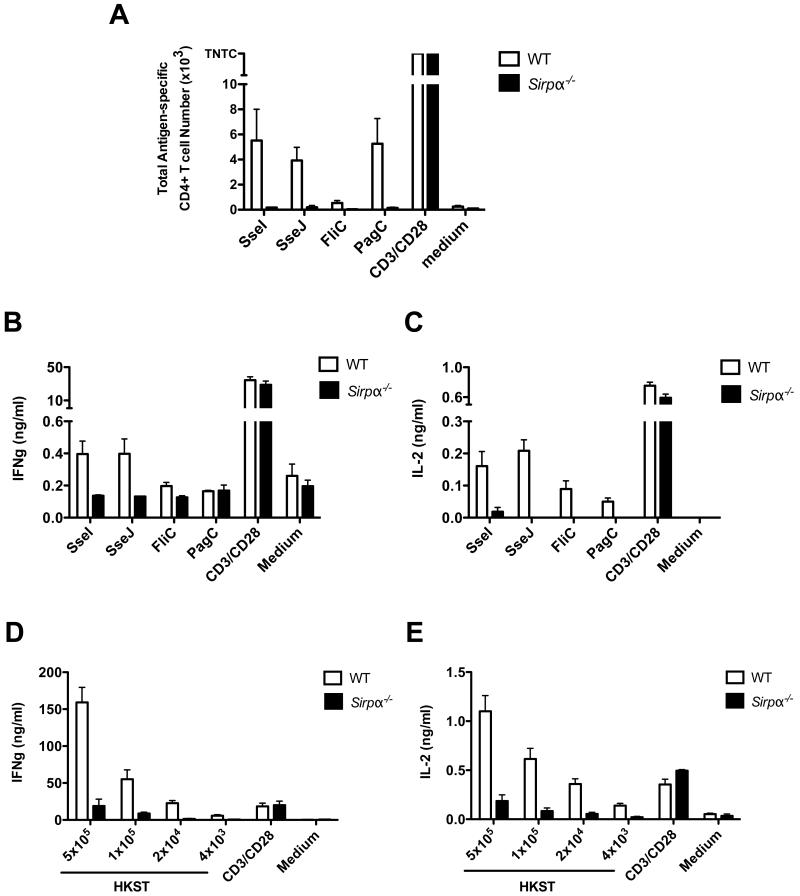
Our laboratory and others have previously documented a similar inability to resolve Salmonella infection in MHC class-II-deficient, CD28−/− and T-bet−/− mice (8, 10, 13), suggesting that SIRPα deficiency may affect the CD4 T cell response to Salmonella infection. To test this hypothesis, we examined Salmonella epitope-specific Th1 responses in Sirpα−/− mice using IFNγ ELISPOTs and four different natural Salmonella epitopes (Table 1). In order to allow time for Salmonella-specific CD4 T cell responses to develop in vivo while keeping Sirpα−/− mice alive, mice were treated with antibiotics during the 3rd and 4th weeks post infection. Five weeks after initial infection, we observed that SseI, SseJ, FliC and PagC-specific CD4 T cell responses were diminished in Sirpα−/− mice (Fig. 3A). In contrast, WT mice maintained an elevated frequency of CD4 T cells specific for each of these Salmonella epitopes (Fig. 3A).
Fig. 3. Impaired Ag-specific CD4+ T cell response in Sirpα−/− mice after Salmonella infection.
WT and Sirpα−/− mice were infected i.v. with 5 × 105 attenuated Salmonella (BRD509) for a total of five weeks. All mice were treated with antibiotics during week 3 and 4. A, Purified splenic CD4+ T cells from infected mice were co-cultured with Salmonella specific CD4 T cell antigen (SseJ, SseI, FliC or PagC) in the presence of irradiated APCs for 20 hrs in ELISPOT plate pre-coated with anti-IFNγ. Antigen-specific CD4 T cell number were determined. Data represents three similar experiments with at least three mice per group. B and C, One million total splenocytes from infected WT and Sirpα−/− mice were co-cultured with Salmonella specific CD4 T cell antigens (SseJ, SseI, FliC or PagC) for 24-48 hrs. Cytokine productions in culture supernatants were determined by cytokine ELISA. Data represents three similar experiments with at least three mice per group. D and E, One million total splenocytes from infected WT and Sirpα−/− mice were co-cultured with serial diluted heat-killed Salmonella (HKST) for 24-48 hrs. Cytokine productions in culture supernatants were determined by cytokine ELISA. Data represents three similar experiments with at least three mice per group.
It was possible that CD4 T cells in Sirpα−/− mice produced other Th1 cytokines such as IL-2 instead of IFNγ, or that they polarized down the Th2 pathway by producing IL-4. To test these possibilities, we measured IFNγ, IL-2 and IL-4 production in response to Salmonella antigens by cytokine ELISA. Similar to what we discovered in IFNγ ELISPOT assay, Sirpα−/− mice produced less IFNγ and IL-2 overall (Figure 3B and 3C). In contrast, none of the mice produced IL-4, indicating that CD4 T cells do not skew toward the Th2 lineage in either WT or Sirpα−/− mice (data not shown).
To assess the total polyclonal Salmonella-specific CD4 T cell response to Salmonella antigens, we next measured multiple cytokine productions in response to heat-killed Salmonella (HKST). Similar to single antigen stimulations, HKST induced high levels of IFNγ and IL-2 production by WT mice whereas these cytokines were only marginally produced by Sirpα−/− mice (Figure 3D and 3E). Again, no IL-4 production was detected regardless the dose of HKST used (data not shown).
Taken together, we conclude that Sirpα−/− mice develop an antigen-specific deficiency in CD4 T cell responses to single Salmonella antigens (SseI, SseJ, flagellin and PagC), and the overall polyclonal Salmonella-specific Th1 response is also reduced.
SIRPα deficiency on DCs leads to defect in antigen presentation
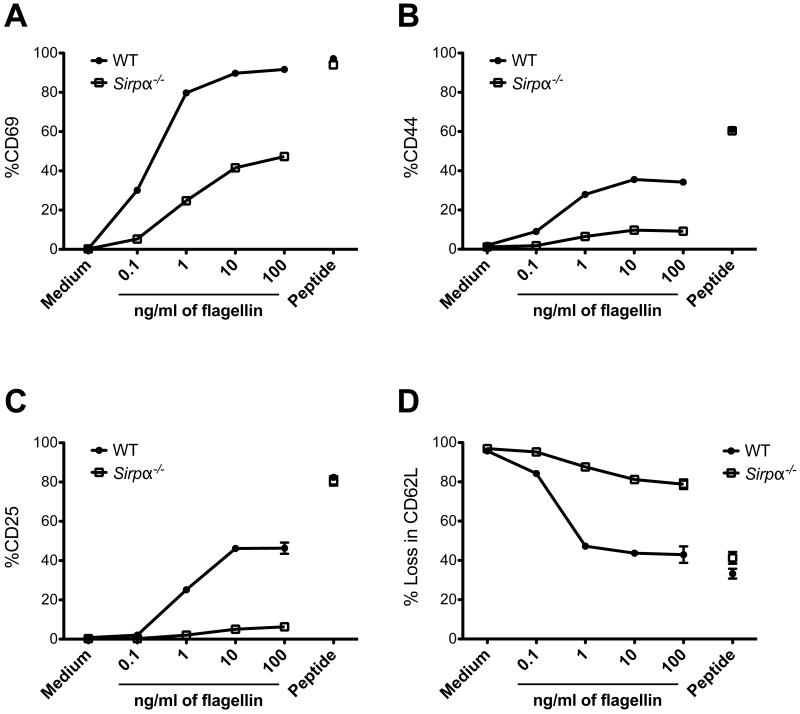
SIRPα is expressed by a sub-population of APCs in mice (22). It seemed possible that the defect we detected in peptide-specific CD4 T cell responses in Sirpα−/− mice was due to the lack of SIRPα expression by DCs. To test this hypothesis, we examined the ability of SIPRα-deficient DCs to activate Ag-specific CD4 T cells in vitro. We co-cultured Salmonella flagellin-specific SM1 T cells (35) with WT or SIPRα-deficient DCs in the presence or absence of crude (flagellin) or processed (flagellin peptide) antigen. Sixteen hours after co-incubation, SM1 T cells upregulated the activation markers CD69, CD44, CD25 and downregulated CD62L in a dose-dependent manner when flagellin was processed and presented by wild-type DCs (Fig. 4, A to D). However, the activation level of SM1 T cells was considerably lower in the presence of SIRPα-deficient DCs when compared to WT DCs (Fig. 4, A to D), indicating that SIRPα-deficient DCs failed to process and present flagellin to the same level as WT DCs. In contrast, when SM1 T cells were activated by flagellin peptide, expression of SIRPα on DCs was not essential (Fig. 4, A to D). Taken together, we conclude that SIPRα expression on DCs plays a crucial role in antigen processing and presentation to CD4 T cells.
Fig. 4. Reduced activation of Salmonella-specific CD4 T cells by SIRPα-deficient DCs in vitro.
CD11c+ DCs (1×105) purified from wild type or SIRPα-deficient mice were co-cultured with 1×105 flagellin-specific SM1 T cells in the presence or absence of various dose of antigen flagellin or 10μg/ml flagellin peptide. Graphs depict the percent expression of CD69 (A), CD44 (B), CD25 (C) or loss of CD62L (D) on gated SM1 T cells 16 hours post stimulation. Error bars represent mean ± SEM.
Defects in ab response in Sirpα−/− mice
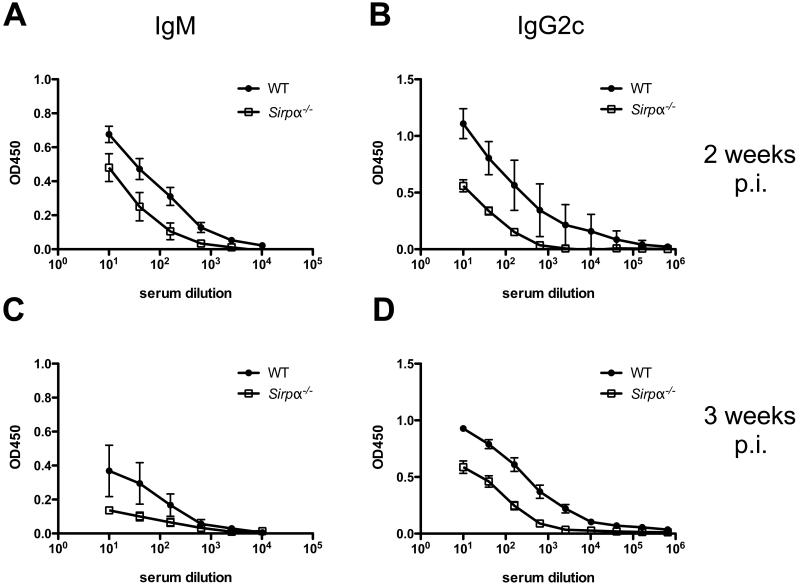
Although Salmonella is an intracellular bacterium, it is known that antibody responses play an important role in protective immunity (11-13). We therefore examined Salmonella-specific serum Ab responses after infection of WT and Sirpα−/− mice. Both WT and Sirpα−/− mice developed elevated titers of Salmonella-specific IgM and IgG2c response at 2 and 3 weeks post infection, however, the overall antibody titers were lower in Sirpα−/− mice compared to WT controls (Fig. 5A to 5D). No Salmonella-specific IgG1 response was detected in either WT or Sirpα−/− mice (data not shown). Together, these data demonstrate that mice deficient in SIRPα generate Salmonella-specific antibody responses after Salmonella infection but that these responses are lower than WT mice.
Fig. 5. Salmonella-specific Ab responses in WT and Sirpα−/− mice.
WT and Sirpα−/− mice were infected i.v. with 5 × 105 attenuated Salmonella (BRD509). Serum was collected 2 (A and B) and 3 (C and D) weeks post infection. Salmonella-specific IgM (A and C) and IgG2c (B and D) titer were determined by ELISA. Graphs show OD readings (mean ± SEM) of IgM and IgG2c responses. Data represents three independent experiments with at least four mice per group.
SIRPα deficiency does not affect the development of Chlamydia-specific CD4 T cells
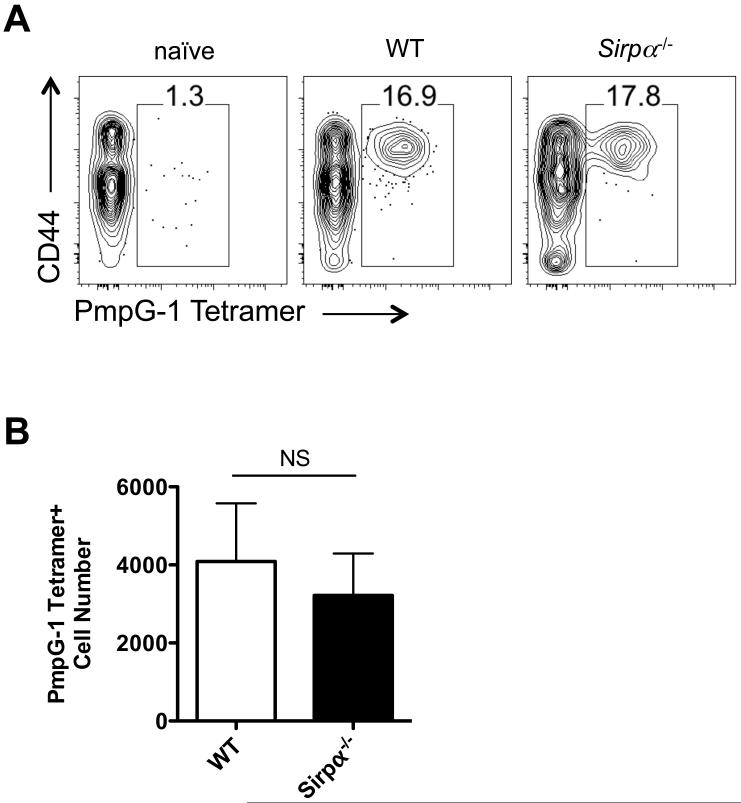
We next asked whether the failure to develop pathogen-specific CD4 T cell responses in Sirpα−/− mice was a peculiar feature of Salmonella infection. We infected WT and Sirpα−/− mice i.v. with 1 × 105 Chlamydia muridarum (Cm), another intracellular bacteria that requires a Th1 response for resolution (42, 43). Interestingly, we did not observe any defect in Sirpα−/− mice when comparing Chlamydia epitope-specific CD4 T cell responses between WT and Sirpα−/− mice using MHC class-II tetramers (Fig. 6A and 6B). Indeed, Sirpα−/− mice developed similar clonal expansion of Chlamydia (PmpG-1)-specific CD4 T cells following infection. Therefore, the defects in CD4 T cell responses we detected in Salmonella infection were not universal but appear restricted to the CD4 T cell response to Salmonella.
Fig. 6. SIRPα expression is not required for Ag-specific CD4 T cell response to Chlamydia muridarum infection.
A, FACS plots of PmpG-1 MHCII tetramer specific CD4 T cell staining in naïve, WT and Sirpα−/− mice 5 weeks after Cm i.v. infection. B, Total PmpG-1 specific CD4 T cell numbers in WT and Sirpα−/− mice.
Discussion
In an earlier study, we reported a marked increase in splenic erythropoiesis after systemic Salmonella infection of mice (5). We concluded that massive expansion of erythrocytes accounts for Salmonella-induced splenomegaly and also increased susceptibility to infection (5). As a key regulatory molecule for peripheral RBC turnover, SIRPα is widely expressed on myeloid lineage cells such as macrophages (27). Consistent with previous observations (30), we found that Sirpα−/− mice exhibit an increase in the number of erythroid cells in the spleen. Surprisingly, we found that Sirpα−/− mice are considerably more susceptible to Salmonella infection, as demonstrated by uncontrolled bacteria replication and increased mortality after challenge with attenuated bacteria. Thus, our current study provides another example of a correlation between increased erythropoiesis and increased susceptibility to Salmonella infection.
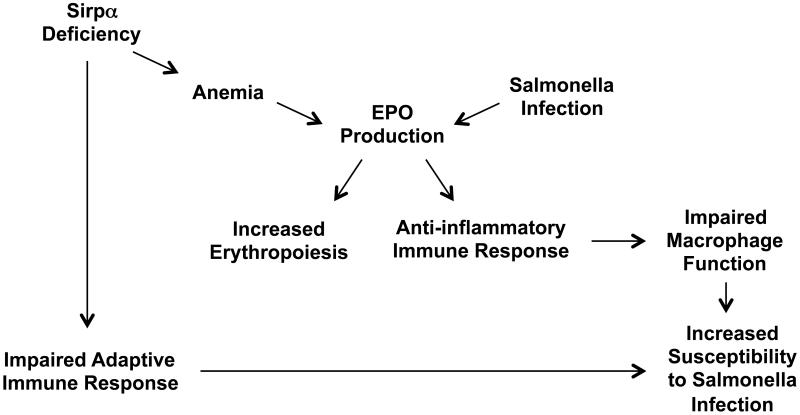
There are likely to be multiple mechanisms by which erythropoiesis, and SIRPα affect susceptibility to Salmonella (Fig. 7). We previously demonstrated that increased EPO production encourages bacterial growth and speculated that this was due to an alteration in the architecture of the spleen that inhibited the adaptive immune response (5). An alternative possibility is that EPO can directly inhibit macrophage bactericidal activity, thus increasing susceptibility to Salmonella (14). Our new data suggest that SIRPα deficiency also induces erythropoiesis, impairs host immunity and encourages Salmonella growth. Together these processes suggest a growing link between erythroid development and susceptibility to bacterial infection.
Fig. 7. Predicted model of how SIRPα deficiency leads to increased susceptibility to Salmonella infection.
The increased susceptibility of SIRPα-deficient mice to bacteria infection may also be independent of increased erythropoiesis and directly due to defects in antigen-specific CD4 T cell responses. Our ELISPOT results show that five weeks post Salmonella infection, Sirpα−/− mice lack Ag-specific CD4 T cells of all known Salmonella epitopes: SseI, SseJ, FliC and PagC. These epitopes represent the only well-defined epitopes in the Salmonella mouse model and therefore suggested a broad reduction in the Th1 response to Salmonella (38, 44). The overall Th1 cytokine production by Sirpα−/− mice is also largely diminished as stimulation of Sirpα−/− splenocytes with single or bulk Salmonella antigen (HKST) leads to only minimal IFNγ or IL-2 production. Indeed, the survival curve of Sirpα−/− mice after Salmonella infection parallels that observed in mice lacking the Th1 specific transcription factor T-bet, suggesting a similar deficiency in these mouse models (10). Similar requirement for SIRPα in induction of Th1 responses has also been reported in a recent study using the parasite Leishmania major infection model (45).
In addition to macrophages, SIRPα is also abundantly expressed on DCs (22). CD8α− CD11c+DCs in the spleen express a much higher level of SIRPα than that of CD8α+CD11c+DCs (26, 46, 47). Lack of SIRPα or its ligand, CD47 expression results in reduced number of CD8α−CD11c+DCs (26). Thus, it is possible that the diminished Ag-specific CD4 T cell responses in Sirpα−/− mice is due to the reduced number of this CD8α− CD11c+DC population and/or less efficient antigen presentation by SIRPα-deficient DCs. Indeed, our data clearly show that the ability of Ag-specific CD4 T cell priming by SIRPα-deficient DCs in vitro was reduced when compared to that of WT DCs. These results provide a mechanistic explanation for the reduced Salmonella ag-specific CD4 T cell response in Sirpα−/− mice in vivo. Similar in vitro studies have also reported that interaction of SIRPα on DCs with CD47 on T cells contributes to the activation of antigen-specific cytotoxic T cells (25).
In addition to reduced cell-mediated immunity, the development of Salmonella-specific Ab responses was also reduced in Sirpα−/− mice. As described earlier, increased erythropoiesis in Sirpα−/− mice disrupts the splenic architecture after Salmonella infection. The enlarged spleen could lead to disruption of efficient B cell and T helper cells interactions thus making antibody production less efficient.
Overall, our present study demonstrates an indispensable role for SIRPα in protective immunity against Salmonella infection (Fig. 7). This defect correlated with a reduction of CD4 Th1 responses to Salmonella-specific antigens, inefficient antigen presentation and reduced Salmonella-specific Ab. Our data also strengthen the association between susceptibility to Salmonella and erythroid development and suggest that focusing on this interaction may assist the development of vaccines or therapeutics for bacterial infection.
Acknowledgements
We thank Dr. S.S. Way (University of Minnesota) for helpful discussion.
This work was supported by Grants R01AI055743 and R01AI073672 from the National Institutes of Health.
Abbreviations
- BMC
bone marrow chimera
- DC
dendritic cell
- HKST
heat-killed Salmonella typhimurium
- LMC
litter mate control
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Tam MA, Rydstrom A, Sundquist M, Wick MJ. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran R, McSorley SJ. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology. 2005;114:450–458. doi: 10.1111/j.1365-2567.2005.02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 4.Griffin AJ, McSorley SJ. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal immunology. 2011;4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson A, Nanton MR, O’Donnell H, Akue AD, McSorley SJ. Innate immune activation during Salmonella infection initiates extramedullary erythropoiesis and splenomegaly. Journal of immunology. 2010;185:6198–6204. doi: 10.4049/jimmunol.1001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan A, Foley J, McSorley SJ. Massive number of antigen-specific CD4 T cells during vaccination with live attenuated Salmonella causes interclonal competition. Journal of immunology. 2004;172:6884–6893. doi: 10.4049/jimmunol.172.11.6884. [DOI] [PubMed] [Google Scholar]
- 7.Mittrucker HW, Kohler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infection and immunity. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. Journal of immunology. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 9.Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. Journal of immunology. 1990;145:1265–1269. [PubMed] [Google Scholar]
- 10.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. Journal of immunology. 2005;175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 11.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(-/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infection and immunity. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. Journal of immunology. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 13.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infection and immunity. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-kappaB-inducible immune pathways. Immunity. 2011;34:61–74. doi: 10.1016/j.immuni.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. Journal of immunology. 2005;175:7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano S, Ohnishi H, Omori A, Hasegawa J, Kubota M. BIT, an immune antigen receptor-like molecule in the brain. FEBS Lett. 1997;411:327–334. doi: 10.1016/s0014-5793(97)00724-2. [DOI] [PubMed] [Google Scholar]
- 20.Comu S, Weng W, Olinsky S, Ishwad P, Mi Z, Hempel J, Watkins S, Lagenaur CF, Narayanan V. The murine P84 neural adhesion molecule is SHPS-1, a member of the phosphatase-binding protein family. J Neurosci. 1997;17:8702–8710. doi: 10.1523/JNEUROSCI.17-22-08702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, Vignery A. MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol. 1998;18:6213–6223. doi: 10.1128/mcb.18.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahoud MH, Proietto AI, Gartlan KH, Kitsoulis S, Curtis J, Wettenhall J, Sofi M, Daunt C, O’Keeffe M, Caminschi I, Satterley K, Rizzitelli A, Schnorrer P, Hinohara A, Yamaguchi Y, Wu L, Smyth G, Handman E, Shortman K, Wright MD. Signal regulatory protein molecules are differentially expressed by CD8-dendritic cells. Journal of immunology. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 23.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 24.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, Kanz L, Ullrich A, Buhring HJ. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(-) hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- 26.Okajo J, Kaneko Y, Murata Y, Tomizawa T, Okuzawa C, Saito Y, Ishikawa-Sekigami T, Okazawa H, Ohnishi H, Matozaki T, Nojima Y. Regulation by Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 of alpha-galactosylceramide-induced antimetastatic activity and Th1 and Th2 responses of NKT cells. Journal of immunology. 2007;178:6164–6172. doi: 10.4049/jimmunol.178.10.6164. [DOI] [PubMed] [Google Scholar]
- 27.Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Current opinion in immunology. 2009;21:47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 29.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara-Yokoo M, Nishiyama U, Ohnishi H, Matozaki T, Nojima Y. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 31.Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H, Hayashi Y, Matozaki T, Takeda K, Akira S, Kasuga M. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–39839. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- 32.Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick-Marchand H, de Geus SJ, Pouw SM, Bohne M, Voordouw A, Weijer K, Di Santo JP, Spits H. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer-(NK) cell homeostasis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13224–13229. doi: 10.1073/pnas.1101398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato-Hashimoto M, Saito Y, Ohnishi H, Iwamura H, Kanazawa Y, Kaneko T, Kusakari S, Kotani T, Mori M, Murata Y, Okazawa H, Ware CF, Oldenborg PA, Nojima Y, Matozaki T. Signal regulatory protein alpha regulates the homeostasis of T lymphocytes in the spleen. Journal of immunology. 2011;187:291–297. doi: 10.4049/jimmunol.1100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito Y, Iwamura H, Kaneko T, Ohnishi H, Murata Y, Okazawa H, Kanazawa Y, Sato-Hashimoto M, Kobayashi H, Oldenborg PA, Naito M, Kaneko Y, Nojima Y, Matozaki T. Regulation by SIRPalpha of dendritic cell homeostasis in lymphoid tissues. Blood. 2010;116:3517–3525. doi: 10.1182/blood-2010-03-277244. [DOI] [PubMed] [Google Scholar]
- 35.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose Salmonella infection evades activation of flagellin-specific CD4 T cells. Journal of immunology. 2004;173:4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infection and immunity. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, McLachlan JB, Kurtz JR, Fan D, Winter SE, Baumler AJ, Jenkins MK, McSorley SJ. Temporal expression of bacterial proteins instructs host CD4 T cell expansion and th17 development. PLoS Pathog. 2012;8:e1002499. doi: 10.1371/journal.ppat.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, Vella AT, McSorley SJ. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. Journal of immunology. 2007;179:6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. Journal of immunology. 2009;182:1602–1608. doi: 10.4049/jimmunol.182.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infection and immunity. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roan NR, Gierahn TM, Higgins DE, Starnbach MN. Monitoring the T cell response to genital tract infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12069–12074. doi: 10.1073/pnas.0603866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. Journal of immunology. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto N, Murata Y, Motegi S, Suzue K, Saito Y, Okazawa H, Ohnishi H, Kotani T, Kusakari S, Ishikawa O, Matozaki T. Requirement of SIRPalpha for protective immunity against Leishmania major. Biochem Biophys Res Commun. 2010;401:385–389. doi: 10.1016/j.bbrc.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 46.Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. Journal of immunology. 2006;176:5772–5778. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- 47.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8-dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. Journal of immunology. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]