Abstract
Background & Aims
Little is known about the prevalence and severity of portal hypertension in patients with non-alcoholic fatty liver disease (NAFLD). We investigated the prevalence and non-invasive predictors of portal hypertension in patients with NAFLD.
Methods
Signs of portal hypertension, including esophageal varices, splenomegaly, portosystemic encephalopathy, and ascites where investigated in 354 patients with NAFLD.
Results
One-hundred patients had portal hypertension at the time of NAFLD diagnosis (28.2%), 88 of these with septal fibrosis or cirrhosis (88%). Fibrosis stage correlated with presence (r=0.41, P<.0001) and number of findings (r=0.48, P=.006) of portal hypertension. Of the 204 patients with no or mild fibrosis (stages 0–2), 12 had portal hypertension (6%); they had a significantly higher grade of steatosis, based on biopsy analysis, compared to the 192 patients without portal hypertension (94%). Thrombocytopenia, hyperbilirubinemia, cirrhosis, and obesity were independently associated with portal hypertension. Esophageal varices were found in 57 of the 128 patients undergoing endoscopic screening (44.5%) and independently associated with thrombocytopenia, type 2 diabetes, and splenomegaly.
Conclusions
Signs of portal hypertension are present in 25% of patients at the time of diagnosis of NAFLD; most had advanced fibrosis or cirrhosis. Portal hypertension can occur in a small proportion of patients with mild or no fibrosis and is associated with the extent of steatosis. Features of advanced liver disease and insulin resistance might identify patients with NAFLD and portal hypertension, and those expected to derive the most benefit from endoscopic screening for esophageal varices.
Keywords: prognostic factor, obesity, disease progression, steatosis
With the increasing prevalence of obesity, diabetes mellitus, and the metabolic syndrome in the general population, non alcoholic fatty liver disease (NAFLD) has become a common liver disease worldwide [1]. NAFLD includes a spectrum of hepatic pathology that ranges from simple, bland steatosis, to steatosis plus features of cellular injury including necrosis, hepatocyte ballooning, and inflammatory infiltrate i.e., nonalcoholic steatohepatitis (NASH) which may or may not be associated with increased liver fibrosis [2].
In some patients, NAFLD progresses to advanced fibrosis and cirrhosis [3] with other features including steatosis, inflammation, and hepatocyte ballooning improving or disappearing [4]. Consequently, liver biopsy may show only cirrhotic stage disease with the disease labeled as ‘cryptogenic’ cirrhosis [5,6]. Patients with cirrhotic stage NAFLD may progress to end-stage liver disease and complications of portal hypertension. The most feared complication of portal hypertension is bleeding from esophageal varices, which is the cause of death in about 20% of patients within the first 6 weeks despite recent progress in its management [7]. However, to date no studies have been performed to determine the prevalence of portal hypertension and gastroesophageal varices in patients with NAFLD.
Fibrosis in NAFLD starts to develop in the pericellular space around the central vein and in the perisinusoidal region in zone 3. This pattern of fibrosis is different from that seen in other forms of chronic liver disease, such as viral hepatitis, cholestatic/autoimmune liver disease, and hemochromatosis in which fibrosis shows an initial portal instead of pericentral distribution. This raises the concern that portal hypertension may occur prior to the development of cirrhosis in patients with NAFLD. However, no studies specifically correlating fibrosis stage with presence of portal hypertension in NAFLD have been performed. Thus, it remains uncertain whether patients with NAFLD without cirrhosis may present with portal hypertension.
Based on current guidelines all patients with cirrhosis should undergo screening endoscopy looking for evidence of esophageal varices [8]. In patients with NAFLD, it remains unclear whether waiting for the development of cirrhosis prior to screening for esophageal varices is appropriate, or if screening endoscopy should be performed also in earlier stages of disease prior to cirrhosis development. In addition, it remains unclear whether noninvasive variables can accurately predict presence of esophageal varices or other signs of portal hypertension in NAFLD. To deal with these issues we performed this study aimed at determining the prevalence of portal hypertension and esophageal varices in a large series of patients with well-characterized and liver biopsy-confirmed NAFLD as well as at identifying non-invasive predictors of portal hypertension and esophageal varices in these patients.
PATIENTS AND METHODS
Study Population
The study population was identified from a database prospectively created, starting in 2000, which included 354 untreated, consecutive patients that were scheduled to undergo liver biopsy to confirm the diagnosis of NAFLD. Patients had 1) elevated aminotransferases, 2) liver biopsy consistent with the diagnosis of NAFLD, 3) exclusion of other chronic liver diseases such as viral hepatitis, autoimmune/cholestatic, metabolic or drug-induced liver disease, and 4) average ethanol ingestion of less than 140 g/wk.
In order to study the whole spectrum of the disease, we included patients with liver biopsy proven cryptogenic cirrhosis thought to be secondary to NAFLD (n=41). These 41 patients were diagnosed during the same period of time, and their cryptogenic cirrhosis considered as cirrhotic-stage NAFLD when they had suffered from at least three of the following features of the metabolic syndrome before the diagnosis of cirrhosis was made: hypertension (BP ≥130/≥ 85 mmHg), diabetes mellitus (fasting blood glucose ≥ 126mg/dL), obesity (body mass index [BMI] ≥ 30 kg/m2), hypertriglyceridemia (≥ 150 mg/dl), and low HDL-cholesterol (<50 mg/dl in women, <40 mg/dl in men). Known causes of cirrhosis were ruled out in these 41 patients by standard clinical, laboratory, imaging and liver biopsy features. Cirrhosis was confirmed with a liver biopsy in these 41 patients, but presence of steatosis was not an absolute requirement as steatosis often improves or disappears as cirrhosis develops as described before [4]. This protocol was reviewed and approved by the Mayo Foundation Institutional Review Board, and all patients had given written informed consent for participation in medical research.
The main end point of this study was presence of portal hypertension. Portal hypertension was defined by the presence of at least one of the following findings: 1) esophageal varices documented by endoscopy, 2) portosystemic encephalopathy documented clinically, 3) splenomegaly documented by imaging studies, and 4) ascites documented by imaging studies. Isolated thrombocytopenia (platelet count <150,000/L) was not considered as evidence of portal hypertension, because of the nonspecific nature of this finding.
A detailed medical history and complete physical examination was accomplished in every patient. Data collected in each individual patient included demographic (age, gender, race), clinical (BMI, history of obesity, diabetes mellitus, hypertension, dyslipidemia) clinical features of portal hypertension (splenomegaly, ascites, portosystemic encephalopathy, esophageal varices), and laboratory (AST, ALT, alkaline phosphatase, serum total bilirubin, albumin, prothrombin time, platelet count, fasting glucose, total cholesterol). Abdominal ultrasonography and/or CT scan for examination of the liver parenchyma, spleen, abdominal vasculature, and ascites as well as a percutaneous liver biopsy were performed in all cases. Ultrasound and CT scan images were interpreted by experienced abdominal radiologists, and splenomegaly defined using standard radiological criteria [9–11]. However, the decision whether to perform an endoscopy to screen for esophageal varices was made in an individual basis at the discretion of the treating physician. The presence of esophageal varices and their size, and history of bleeding were recorded. The size of the varices was determined based on the recommendations of the Japanese Research Society for Portal Hypertension [12].
Liver Biopsy
Liver biopsy specimens were read under coded identification by a single liver pathologist who was unaware of the patient clinical details. Biopsies were routinely stained with hematoxylin eosin, and Masson’s trichrome. All biopsies were a minimum of 15 mm in length and had an appropriate number of portal tracts to make a confident evaluation of histological features and diagnosis. Fibrosis was staged on a 5-point scale as proposed by Kleiner et al: [13] stage 0=no fibrosis, stage 1=zone 3 perisinusoidal/perivenular, or portal/periportal fibrosis, stage 2=zone 3 plus periportal fibrosis, stage 3=septal/bridging fibrosis, and stage 4=cirrhosis. Other histological features including steatosis, inflammation, and hepatocyte ballooning were scored as recommended [13]. The features of steatosis (0–3), lobular inflammation (0–3), and hepatocyte ballooning (0–2) were combined in a score that goes from 0 to 8 named the NAFLD activity score [13].
Statistical Analysis
Data were compared between patients with and without portal hypertension. Continuous data were analyzed by standard parametric or nonparametric statistics where appropriate. Categorical data were analyzed by chi square test or Fisher’s exact test where appropriate. Spearman’s rank correlation coefficient was used as a measure of association. Variables associated with portal hypertension (two-tailed p-value < 0.05) were analyzed by multiple logistic regression to determine their association with presence of portal hypertension. The frequency of esophageal varices was recorded, and patients with and without varices compared. Multiple logistic regression was performed to determine the independent association of significant variables with presence of esophageal varices.
RESULTS
Characteristics of the patient population
Table 1 summarizes the clinical, laboratory, and liver biopsy data of the patient population.
Table 1.
Characteristics of the patient population (n = 354)
| Variable | Mean (± SD) or number (%) |
|---|---|
| Age (years) | 49±12 |
| Gender (female) | 225(64%) |
| Obesity (BMI ≥30 kg/m2) | 238(67%) |
| Diabetes Mellitus | 152(43%) |
| Hypertension | 124/339(37%) |
| Hypertriglyceridemia | 174/327(53%) |
| HDL-cholesterol mg/dL | 43±13 |
| Albumin <3.5 g/dL | 58(16%) |
| Bilirubin >1.0mg/dL | 95(27%) |
| AST/ALT ratio | 1.2±1.7 |
| Prothrombin time (seconds) | 11±2.6 |
| Platelets < 150,000/L | 103(29%) |
| Cirrhosis | 105(30%) |
| Fibrosis stage | |
| 0 | 95(27%) |
| 1–2 | 109(30%) |
| 3 | 45(13%) |
| 4 | 105(30%) |
|
| |
| Finding of portal hyopertension | 100/354 |
| Splenomegaly | 88 (88%) |
| Ascites | 42 (42%) |
| Gastroesophageal varices | 57 (57%) |
| Hepatic encephalopathy | 24 (24%) |
|
| |
| Number of findings of portal hypertension | N (%) [with thrombocytopenia] |
| 1 | 37 (37%) [23] |
| 2 | 27 (27%) [22] |
| 3 | 23 (23%) [19] |
| 4 | 13 (13%) [13] |
FOOTNOTE: Data are presented as mean ± SD, or number (proportion) of patients with a condition. Obesity defined as BMI ≥30kg/m2; diabetes as fasting glucose ≥126 mg/dl or requiring treatment; hypertension as blood pressure ≥130/≥85 mm/Hg or requiring treatment; hypertriglyceridemia as fasting triglyceride ≥150 mg/dl; and low HDL-cholesterol as <40mg/dl in men, <50mg/dl in women. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Prevalence and indicators of portal hypertension
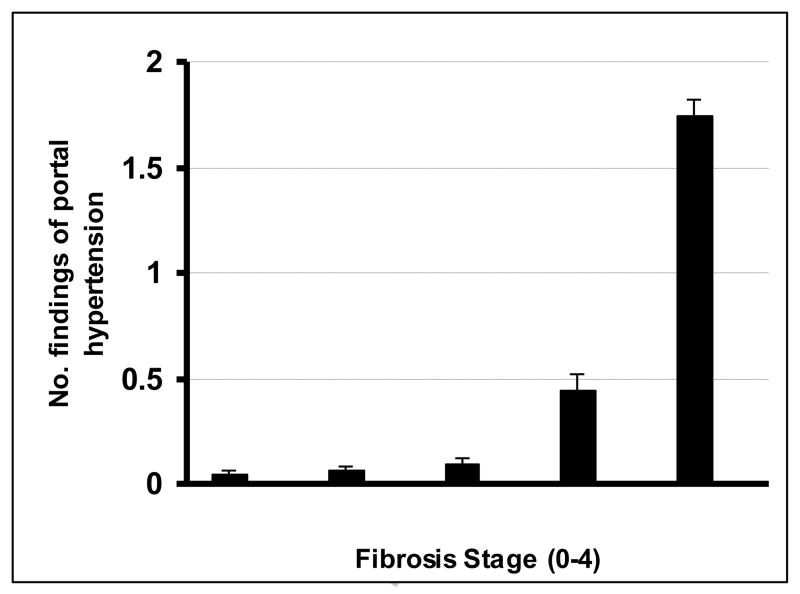
Table 1 also summarizes the number and distribution of findings of portal hypertension in the patient population. One hundred (28%) of the 354 patients had findings of portal hypertension at the time of diagnosis of NAFLD. The spleen was greater than 12 cm in the 88 patients with splenomegaly. Fibrosis stage correlated significantly with presence of portal hypertension (r = 0.41, p < 0.0001), and the number of findings of portal hypertension (r = 0.48, p = 0.006) as illustrated in Figure 1. Supplementary Table 1 summarizes the comparison between patients with and without portal hypertension. As shown, age, BMI in the obese category, history of type 2 diabetes, hypertension, thrombocytopenia, hypoalbuminemia, hyperbilirubinemia, AST/ALT ratio, prothrombin time, presence of cirrhosis, and fibrosis stage were positively, while hypertriglyceridemia was negatively associated with the presence of portal hypertension.
Figure 1.
Correlation of findings of portal hypertension and fibrosis stage in 354 patients with NAFLD. Bars represent mean values, and error bars represent standard errors.
As shown in supplementary Table 1, 77 of the 100 (77%) patients with portal hypertension had cirrhosis on liver biopsy, whereas 23 (23%) did not. Among patients with portal hypertension, 88/100 (88%) had advanced fibrosis as either cirrhosis (stage 4, n=77) or septal fibrosis (stage 3, n=11) with only 12/100 (12%) patients having no (stage 0) or mild (stage 1–2) fibrosis.
Of the 12 patients with portal hypertension and fibrosis stage 0–2, 10 had splenomegaly and 1 had esophageal varices as the only finding of portal hypertension; 1 patient had both ascites and splenomegaly. Four of the 12 patients had thrombocytopenia as well. As compared to the 192 patients with stage 0–2 fibrosis without portal hypertension, these 12 patients had a significantly higher mean steatosis grade (2.3 ± 0.5 vs. 1.9 ± 0.7, p < 0.04), whereas no significant difference was found in other histological features including lobular inflammation, hepatocyte ballooning, fibrosis stage, or NAFLD activity score (supplementary Table 2). The 12 patients had normal serum albumin and prothrombin time, and AST/ALT ratio below 1; only three patients had elevated serum bilirubin levels to 1.2, 1.3, and 2.0 mg/dL. Platelet count was below the normal range in only three of the 12 patients, including the patient with serum bilirubin level of 1.2 mg/dl. Otherwise, no clinical or other laboratory features were significantly different between patients with or without portal hypertension (supplementary Table 2).
Conversely, when compared to the 12 patients with stage 0–2 fibrosis and portal hypertension, the 88 patients with stage 3–4 fibrosis and portal hypertension were significantly older, and had more advanced liver disease as indicated by significantly higher levels of bilirubin, AST/ALT ratio and prothrombin time, and significantly lower levels of albumin and platelet count (supplementary Table 3). Also, patients with portal hypertension and stage 3–4 fibrosis had a significantly lower NAFLD activity score and lower ALT levels as compared to those with stage 0–2 (supplementary Table 3).
Independent indicators of portal hypertension
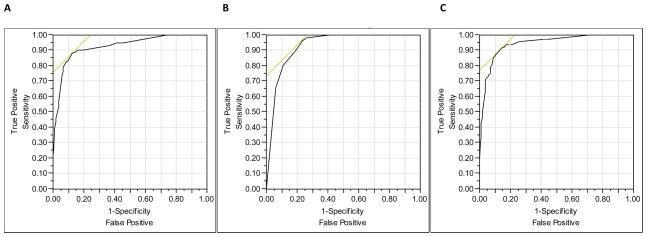
By multivariate analysis, thrombocytopenia, presence of cirrhosis, hyperbilirubinemia and obesity were significantly and independently associated with presence of portal hypertension (Table 2). The area under the ROC for this model was 0.93 (Figure 2A). After removal of cirrhosis from the model to look for noninvasive indicators of portal hypertension, diabetes and hypoalbuminemia became significant along with thrombocytopenia and hyperbilirubinemia (Table 2). The area under the ROC for this multivariate model was 0.91 (Figure 2B).
Table 2.
Predictors of portal hypertension by multivariate analysis (n = 354)
| Variable | Odds ratio (95%CI) | p-value | Noninvasive
|
|
|---|---|---|---|---|
| Odds ratio (95%CI) | p-value | |||
| Thrombocytopenia | 8.1 (3.8, 17.8) | <0.001 | 16.9 (8.5,35.1) | <0.001 |
| Cirrhosis | 8.4 (3.9, 18.4) | <0.001 | - | - |
| Hyperbilirubinemia | 7.6 (3.5, 17.2) | <0.001 | 5.6 (2.7,12.0) | <0.001 |
| Obesity (BMI ≥30 kg/m2) | 2.5 (1.1, 6.2) | 0.04 | - | - |
| Diabetes Mellitus | - | - | 3.0 (1.5, 6.2) | 0.003 |
| Hypoalbuminemia | - | - | 3.9 (1.6,9.7) | 0.003 |
FOOTNOTE: Thrombocytopenia: platelet count <150,000/L; hyperbilirubinemia: total bilirubin >1.0mg/dL; hypoalbuminemia: albumin <3.5g/dL; BMI, body mass index; diabetes, fasting glucose ≥126 mg/dl or requiring treatment.
Figure 2.
Receiver operating characteristic (ROC) curves (A) to predict portal hypertension including fibrosis stage in the model (AUC = 0.93); (B) to predict portal hypertension excluding fibrosis stage from the model (AUC = 0.91); and (C) to predict presence of esophageal varices (AUC = 0.94).
Prevalence and indicators of gastroesophageal varices
Screening gastroesophageal endoscopy was performed in 128 of the 354 patients, including 77 (60%) patients with advanced fibrosis or cirrhosis (stage 3–4), and 51 (40%) with no or mild fibrosis (stage 0–2). Esophageal varices were present in 57 patients including 35 with small varices and 20 with large varices (variceal size was not recorded in 2 patients). Among these 57 patients with esophageal varices, 55 (97%) had advanced fibrosis either septal fibrosis (stage 3) or cirrhosis (stage 4) on biopsy. A history of variceal bleeding was documented in 33/57 patients. The same variables significantly associated with presence of portal hypertension described in supplementary Table 1 were significantly associated with presence of gastroesophageal varices as summarized in Table 3 along with splenomegaly, ascites and hepatic encephalopathy.
Table 3.
Association between clinical and laboratory predictors and the presence of gastroesophageal varices (unadjusted) n = 128
| Variable | Varices (n=57) | No varices (n=71) | p-value |
|---|---|---|---|
| Age (years) | 56±11 | 48±12.5 | <0.001 |
| Gender (female) | 36(63%) | 48(68%) | NS |
| Obesity (BMI ≥30 kg/m2) | 52(91%) | 53(75%) | 0.02 |
| Diabetes Mellitus | 47(82%) | 18(25%) | <0.001 |
| Hypertension | 30/54(56%) | 25/69(36%) | 0.04 |
| Splenomegaly | 46(81%) | 13(18%) | <0.0001 |
| Ascites | 30(53%) | 5(7%) | <0.0001 |
| Hepatic encephalopathy | 20(35%) | 3(4%) | <0.0001 |
| Thrombocytopenia | 51(89%) | 14(20%) | <0.001 |
| Hypertriglyceridemia | 6/55(11%) | 44/65(68%) | <0.001 |
| Albumin <3.5 g/dL | 25(44%) | 11(16%) | <0.0004 |
| Total Bilirubin >1.0 mg/dL | 34(60%) | 8(11%) | <0.001 |
| AST/ALT ratio | 1.5±1.7 | 0.9+0.5 | <0.001 |
| Prothrombin time (sec) | 15.5±22.6 | 10.5±1.1 | <0.001 |
| Cirrhosis | 50(88%) | 14(20%) | <0.0001 |
| Fibrosis stage | <0.0001 | ||
| 0 | 0 | 26(37%) | |
| 1–2 | 2(3%) | 23(33%) | |
| 3 | 5(9%) | 8(11%) | |
| 4 | 50(88%) | 14(20%) |
FOOTNOTE: Continuous variables are presented as mean ± SD. Obesity defined as BMI ≥30kg/m2; diabetes as fasting glucose ≥126 mg/dl or requiring treatment; hypertension as blood pressure ≥130/≥85 mm/Hg or requiring treatment; hypertriglyceridemia as fasting triglyceride ≥150 mg/dl; and low HDLcholesterol as <40mg/dl in men, <50mg/dl in women. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
By multivariate analysis (excluding ascites and hepatic encephalopathy from the multivariate model as their presence highly correlates with presence of varices), thrombocytopenia (odds ratio [OR] 16.8; 95% confidence intervals [CI] 5.0, 66.4; p<0.001); diabetes mellitus (OR 5.1; 95% CI 1.5, 18.3; p=0.009) and splenomegaly (OR 5.0; 95% CI 1.4, 19.5; p=0.01) were the only variables significantly associated with presence of esophageal varices (area under the ROC curve 0.94, Figure 2C). In this multivariate analysis, the presence of cirrhosis was not associated with esophageal varices. The diagnostic accuracy of these three variables is described in Table 4. Thrombocytopenia had the highest sensitivity, specificity, and prositive and negative predictive values as compared to the other two variables. The prevalence of esophageal varices was 88% (35/40) when these three variables were present, 68% (17/25) when two variables were present, 29% (5/17) when only one variable was present, and 0% (0/46) when none of these three variables was present.
Table 4.
Diagnostic accuracy of variables identified by multivariate analysis associated with gastroesophageal varices
| Variable | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Thrombocytopenia | 89.5 | 81.7 | 79.7 | 90.6 |
| Type 2 diabetes | 82.5 | 76.1 | 73.4 | 84.4 |
| Splenomegaly | 80.7 | 81.7 | 78 | 84.1 |
FOOTNOTE: Thrombocytopenia: platelet count <150,000/L; diabetes, fasting glucose ≥126 mg/dl or requiring treatment
DISCUSSION
This large series of patients with the full spectrum of NAFLD demonstrates that 1) signs of portal hypertension are found in about a fourth of patients at the time of NAFLD diagnosis confirmed by liver biopsy, and directly related to fibrosis stage; 2) although most patients (88%) with NAFLD and portal hypertension have advanced fibrosis either septal fibrosis (stage 3) or cirrhosis (stage 4), a small proportion of patients (12%) with NAFLD and portal hypertension have mild or no fibrosis (stage 0–2) on liver biopsy; 3) more severe steatosis is present in patients with NAFLD with mild or no fibrosis (stage 0–2) who present with portal hypertension; 4) development of esophageal varices is not uncommon among patients with NAFLD with the vast majority of NAFLD patients with esophageal varices having either septal fibrosis or cirrhosis on liver biopsy; and 5) clinical and laboratory features associated with advanced liver disease along with overt insulin resistance may help identify the subgroup of NAFLD patients with portal hypertension, and those in whom endoscopic screening for esophageal varices is expected to provide the most diagnostic information.
We found that 28% (100/354) of patients had signs of portal hypertension with a clear positive relationship between fibrosis stage and both presence and number of signs of portal hypertension. Splenomegaly was the most common sign of portal hypertension in NAFLD found in 25% (88 of the total 354 patients), with ascites and portosystemic encephalopathy present in 12% and 7%, respectively. This suggests that splenomegaly per se may be an early indicator of presence of portal hypertension in NAFLD, or an indicator of further development of overt portal hypertension over time. Thus, spleen size should be routinely measured during abdominal imaging studies performed as part of the diagnostic evaluation of patients with suspected NAFLD. Finding a spleen of increased size along with the other variables identified by multivariate analyses may prompt clinicians to specifically look for other signs of portal hypertension (Table 2) and esophageal varices (Table 4).
Signs of portal hypertension correlated positively with fibrosis stage, but portal hypertension was not exclusive of patients with cirrhotic stage NAFLD. Although 88 of the 100 (88%) patients with portal hypertension had advanced liver fibrosis as either cirrhotic stage disease or septal fibrosis at the time of diagnosis, the remaining 12 (12%) patients had either no fibrosis (stage 0) at all, or mild (stage 1–2) fibrosis on liver biopsy. It is possible that sampling variability on liver histology did not allow the identification of more advanced fibrosis in these 12 patients, but this possibility seems unlikely given the similarly well-preserved liver function of patients with stage 0–2 fibrosis with or without signs of portal hypertension (supplementary Table 2). The only clear difference between patients with and without portal hypertension was the significantly more severe steatosis among those with portal hypertension (supplementary Table 2). Although mean serum bilirubin level was significantly higher in patients with portal hypertension than in those without, mean bilirubin levels were within the normal range in both groups. Thus, aside from severity of steatosis, no clinical, laboratory or other liver histology data were significantly different between the 12 patients with portal hypertension and the 192 patients without portal hypertension with fibrosis stage 0–2 (supplementary Table 2). This finding suggests that obstruction of sinusoids and/or obstruction of the terminal hepatic venules by steatotic hepatocytes impede the blood flow through the sinusoids and terminal veins increasing the intrahepatic resistance. Increased intrahepatic resistance to blood flow is one of the most important elements of the syndrome of portal hypertension [14]. Consistent with the notion that steatotic hepatocytes may induce portal hypertension in NAFLD without advanced liver fibrosis are the findings from some animal studies of non-alcohol induced fatty liver [15,16]. For instance, microvascular dysfunction and marked impaired sinusoidal perfusion has been demonstrated in mice fed a methionine-, choline-deficient diet (a model of NASH) even in an early stage of NASH development when liver fibrosis did not appear [15]. In another study [16], disturbance of hepatic circulation developed when septal fibrosis was associated with steatosis, but not in animals developing septal fibrosis alone.
Non-invasive findings indicative of advanced liver disease including thrombocytopenia and increased bilirubin levels are also independent indicators of presence of portal hypertension in patients with NAFLD. Thrombocytopenia and hyperbilirubinemia were associated with a 16.9-fold and 5.6-fold increased risk of having portal hypertension, respectively, and both remained statistically significant even when cirrhosis was present in the multivariate model. Further, thrombocytopenia was associated with a 16.8-fold increased risk of having esophageal varices, and independent of presence of cirrhosis. These findings are in agreement with several other large series that have consistently identified thrombocytopenia as an independent indicator of presence of portal hypertension and esophageal varices in patients with liver disease of a variety of causes [17].
Presence of obesity was also independently associated with portal hypertension, and diabetes was independently associated with both portal hypertension and esophageal varices. Given the high prevalence of obesity and diabetes in NAFLD, it may no be appropriate to use these two comorbidities alone to screen for portal hypertension and esophageal varices in all patients with NAFLD seen in clinical practice. Presence of cirrhosis per se on liver biopsy should prompt screening for esophageal varices and hepatocellular cancer based on current guidelines [8,18,19]. However, if a noninvasive algorithm were to be used for predicting portal hypertension, it should be platelet count followed by bilirubin and albumin which had higher odds ratio than diabetes (Table 2). For variceal screening, again platelet count had the highest odds ratio and was three-fold more accurate than diabetes or splenomegaly for predicting presence of esophageal varices. Thus, although both diabetes and obesity are well known predictors of more advanced liver disease [6,20–23], they by themselves do not seem to be the strongest predictors of presence of portal hypertension and esophageal varices in NAFLD and should not be used alone in prompting screening for portal hypertension or varices in these patients.
Interestingly, when thrombocytopenia and diabetes were in the multivariate model, presence of cirrhosis was not significantly associated with presence of esophageal varices. This suggests that in patients with NAFLD, platelet count, diabetes and splenomegaly may be clinically more accurate than cirrhotic stage disease on liver biopsy in predicting presence of esophageal varices. Although further studies are necessary to validate these results, a platelet count below 150,000/L along with presence of splenomegaly and type 2 diabetes may be used in clinical practice to perform screening endoscopy in patients with NAFLD regardless of fibrosis stage on liver biopsy, and even when liver biopsy is not performed.
Our study has some limitations. First, our study population included patients seen in a tertiary referral center and all patients underwent liver biopsy to confirm the diagnosis of NAFLD. Given the lack of effective treatment along with the high prevalence of NAFLD, most patients seen in clinical practice or even in the average academic medical center do not undergo liver biopsy. Thus, our results may not be extrapolated to patients from the community or those seen in non-referral practice who are expected to have a milder liver disease. Consistent with this referral bias towards more advanced liver disease is the fact that 33 of the 57 patients who underwent endoscopy had bled from esophageal varices and the average AST/ALT ratio in the patient population was 1.2. Second, screening endoscopy was performed in some, but not all cases, and at the discretion of the treating physician. Thus, further prospective studies that include endoscopy are necessary to validate our results. In fact, patients undergoing screening endoscopy had laboratory evidence of more advanced liver disease, and more advanced fibrosis stage on liver biopsy as compared to those patients who were not scoped (data not shown). This simply illustrates the higher likelihood to screen for esophageal varices those patients with NAFLD with more advanced liver disease. Finally, further studies of portal hypertension in NAFLD should correlate the hepatic venous pressure gradient (HVPG) and Doppler characteristics to the clinical, endoscopic and histological data.
In summary, portal hypertension and esophageal varices in our patients with NAFLD were common findings at the time the diagnostic liver biopsy was performed. Signs of portal hypertension correlated significantly with more advanced fibrosis, but they occurred also in patients without or only mild fibrosis who had more severe steatosis on liver biopsy. Besides features indicative of more advanced liver disease, comorbid conditions indicative of more severe insulin resistance such as type 2 diabetes and obesity were important indicators of presence of portal hypertension and esophageal varices in our patients. These clinical indicators may allow the identification of patients with NAFLD with portal hypertension, and those who are expected to benefit the most from endoscopic screening for esophageal varices.
Supplementary Material
Acknowledgments
Grant support: This study was supported by an NIH R01 DK82426 grant to Dr. Paul Angulo.
Footnotes
Disclosures: None of the authors have any conflicts or financial arrangements to disclose related to this study.
Contribution to the manuscript: Conception and design of the study: P. Angulo
Generation, collection, assembly, analysis and/or interpretation of data: F. Mendes; A. Suzuki; S. Sanderson; K. Lindor; P. Angulo.
Drafting and revision of the manuscript: F. Mendes; A. Suzuki; S. Sanderson; K. Lindor; P. Angulo.
Approval of the final version of the manuscript: F. Mendes; A. Suzuki; S. Sanderson; K. Lindor; P. Angulo.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Sanderson SO, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–9. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 6.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–92. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 9.Niederau C, Sonnenberg A, Müller JE, Erckenbrecht JF, Scholten T, Fritsch WP. Sonographic measurements of the normal liver, spleen, pancreas, and portal vein. Radiology. 1983;149:537–540. doi: 10.1148/radiology.149.2.6622701. [DOI] [PubMed] [Google Scholar]
- 10.Leopold GR, Asher WM. Fundamentals of abdominal and pelvic ultrasound. Philadelphia, PA: WB Saunders; 1975. [Google Scholar]
- 11.Bezerra AS, D’Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol. 2005;184:1510–1513. doi: 10.2214/ajr.184.5.01841510. [DOI] [PubMed] [Google Scholar]
- 12.Inokuchi K. The general rules for recording endoscopic findings on esophageal varices. Jpn J Surg. 1980;10:84–7. doi: 10.1007/BF02468653. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Shah V, Garcia-Cardena G, Sessa WC, et al. The hepatic circulation in health and disease: report of a single-topic symposium. Hepatology. 1998;27:279–288. doi: 10.1002/hep.510270141. [DOI] [PubMed] [Google Scholar]
- 15.McCuskey RS, Yahiya I, Robertson GR, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. doi: 10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 16.Shibayama Y, Nakata K. Role of septal fibrosis in development of hepatic circulatory disturbance in the presence of liver cell enlargement. Liver. 1992;12:84–89. doi: 10.1111/j.1600-0676.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico G, Morabito A. Noninvasive markers of esophageal varices: another round, not the last. Hepatology. 2004;39:30–34. doi: 10.1002/hep.20018. [DOI] [PubMed] [Google Scholar]
- 18.de Franchis R. Portal hypertension. Proceedings of the fifth Baveno international consensus workshop; West Sussex, UK. Wiley-Blackwell; 2011. [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:356–362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 21.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 22.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.