Abstract
Background
Infection risk is increased in patients with rheumatoid arthritis (RA), and accurate assessment of infection risk could inform clinical decision-making. The purpose of this study was to develop and validate a score to predict the 1 year risk of serious infections.
Methods
We utilized a population based cohort of Olmsted County, Minnesota residents with incident RA ascertained in 1955–1994 that were followed longitudinally through their complete medical records until January 2000. The validation cohort included residents with incident RA ascertained in 1995–2007. The outcome measures included all serious infections (requiring hospitalization or intravenous antibiotics). Potential predictors were examined using multivariable Cox models. The risk score was estimated directly from the multivariable model and performance was assessed in the validation cohort using Harrell’s c-statistic.
Results
Among the 584 patients with RA (mean age 58 years; 72% female; median follow-up 9.9 years), 252 had ≥ 1 serious infection (646 total infections). The risk score included age, previous serious infection, corticosteroid use, elevated erythrocyte sedimentation rate, extraarticular manifestations of RA and comorbidities (coronary heart disease, heart failure, peripheral vascular disease, chronic lung disease, diabetes mellitus, alcoholism). Validation revealed good discrimination (c-statistic =0.80).
Conclusion
RA disease characteristics and comorbidities can be used to accurately assess the risk of serious infection in patients with RA. Knowledge of risk of serious infections in patients with RA can influence clinical decision making and inform strategies to reduce and prevent the occurrence of these infections.
INTRODUCTION
Patients with rheumatoid arthritis (RA) have increased age-adjusted all-cause mortality (1). In addition, a high frequency of opportunistic and common infections complicates RA, which partially contributes to this increased mortality (2–7). Previous studies have examined potential predictors of infection in RA, including comorbidities, RA disease characteristics and medications (8). However, other potential risk factors for infection, such as lymphocytopenia and neutropenia, which occur commonly over the long disease course, have not been evaluated in RA. The risk of infection is increased by a constellation of comorbidities and is further influenced by RA disease characteristics and treatment (9–11). A risk score that accurately predicts the risk of infection among patients with RA would be useful. The purpose of this study was to develop and validate a score to predict the one-year risk of serious infections among patients with RA.
MATERIALS AND METHODS
Study population
The original cohort consisted of a previously assembled incident cohort of 603 Rochester, Minnesota residents with RA ascertained between January 1, 1955 and December 31, 1994 using the 1987 American College of Rheumatology (ACR) criteria for RA (12), and followed to January 1st, 2000. This cohort was assembled utilizing the resources of the Rochester Epidemiology Project (REP), which is a medical records-linkage system that allows access to the entire inpatient and outpatient medical records of study subjects from all healthcare providers in Olmsted County, Minnesota including Mayo Clinic, Olmsted Medical Center, their affiliated hospitals, and others (13, 14). For the purposes of developing an infection risk score, patients were censored at a diagnosis of cancer and patients with cancer prior to RA incidence were excluded, leaving a total of 584 RA patients in the study group. Due to the very small number and short follow up (January 1, 2000), the few patients who received biologics were also censored at the initiation of biologics for this analysis.
A second cohort was used to validate the risk score. The validation cohort consisted of 464 Olmsted County, MN residents who fulfilled ACR criteria for RA between January 1, 1995 and December 31, 2007 with follow-up until death, migration from Olmsted County, or December 31, 2008. Patients in this cohort were also censored at a diagnosis of cancer, and after excluding patients with cancer prior to RA incidence, the validation cohort comprised a total of 410 RA patients. These patients were not censored at the initiation of biologics.
Data Collection
For the original cohort, the complete medical records of all RA subjects were previously reviewed by trained nurse abstractors (2, 8). For the validation cohort, data collection was performed by the same abstractors using the same variable definitions as the original cohort. The data were collected from the complete (inpatient and outpatient) medical records. Data collection included demographics, comorbidities, disease-related variables, and the use of corticosteroids and disease-modifying antirheumatic drugs (DMARDs) as listed in Table 1, and the daily doses of corticosteroids. In the original cohort, laboratory data included all measured erythrocyte sedimentation rate (ESR) values, the first occurrence of leukopenia (defined as white blood cell counts < 4000/ml on 2 or more occasions), and the first occurrence of lymphocytopenia (defined as lymphocyte counts <1500/ml on 2 or more occasions). In the validation cohort, laboratory data collection included all measured values of ESR and C-reactive protein (CRP), leukocytes, lymphocytes and neutrophils, and data on use of biologic agents was also collected. Leukopenia was defined as white blood cell counts < 3.5 × 109/L, lymphocytopenia was defined as lymphocyte counts < 0.9 × 109/L, and neutropenia was defined as neutrophil counts < 1.7 × 109/L. Note that all blood cell counts were performed in the course of clinical care, predominately to monitor medications, disease activity and other conditions (e.g., anemia). Also, in the validation cohort the most recent test values were assessed at each time point and abnormal values on more than one occasion were not required.
Table 1.
List of potential predictors included in development of the risk score for serious infections
| Stage of development process | List of potential predictors considered |
|---|---|
| Predictors included in Doran model of serious infections | Age, sex, alcoholism, history of leukopenia, dementia/Alzheimer’s disease, diabetes mellitus, chronic lung disease, extraarticular manifestations of RA (including amyloidosis, Felty’s syndrome, rheumatoid vasculitis and rheumatoid lung disease), rheumatoid factor, functional capacity, corticosteroid use and the interaction between age and diabetes mellitus |
| Additional predictors included in Doran model of objectively confirmed infections | Erythrocyte sedimentation rate, rheumatoid nodules |
| Other predictors considered by Doran | Smoking status, body mass index, and disease modifying-anti-rheumatic medications (methotrexate, azathioprine, hydroxychloroquine, sulfasalazine, intramuscular gold, oral gold, D-penicillamine, leflunomide, and cyclophosphamide) |
| Additional predictors considering in the original cohort | Coronary heart disease, heart failure, peripheral vascular disease, presence of large joint swelling, presence of erosions/destructive changes on radiographs, joint surgeries, RA disease duration, calendar year, and previous serious infections |
| Additional predictors considered in the validation cohort | Leukocytes, lymphocytes, neutrophils, C-reactive protein and biologic agents |
Comorbidities such as chronic lung disease and alcoholism were included based on physician diagnosis. Coronary heart disease (CHD) was defined as myocardial infarction or revascularization procedures (i.e., bypass grafting, percutaneous coronary intervention). Myocardial infarctions were classified as definite or probable, based on the presence of cardiac pain, biomarker values, and the Minnesota electrocardiogram (EKG) coding system (15). Heart failure was defined according to the Framingham criteria (16). Diabetes mellitus was defined as at least 2 measurements of fasting plasma glucose ≥126 mg/dl or a 2-hour plasma glucose ≥200 mg/dl, or a clearly documented history of diabetes mellitus or treatment with hypoglycemic agents(17). Extra-articular manifestations of RA included amyloidosis, Felty’s syndrome, rheumatoid vasculitis and rheumatoid lung disease.
Data were collected on all documented episodes of infection occurring after the RA incidence date as defined previously (2). Minor upper respiratory tract infections and uncomplicated urinary tract infections were not included. For each episode of infection, information was collected regarding accompanying fever, leukocytosis, and findings of relevant investigations, including microbiologic cultures and radiologic findings. Also recorded was whether the infection required treatment with intravenous (I.V.) antibiotics, hospitalization and length of hospital stay. Serious infections were defined as infections requiring hospitalization and/or I.V. antibiotics. Objectively confirmed infections were defined as infections with positive results of microbiologic cultures and/or radiologic imaging.
Statistical Methods
Descriptive statistics (means, proportions, etc.) were used to summarize the data. Variables that developed during follow-up were summarized as “ever” for descriptive purposes only. The outcome of interest was serious infections. Objectively confirmed infections were also examined, but these results were not reported because most serious infections were also objectively confirmed, and the risk models for these 2 outcomes were not meaningfully different.
Since our goal was to predict the risk of infection in the next year for a patient with RA at any time during their disease course, each patient’s follow-up time was divided into 1 year intervals. Covariate values were assessed at the beginning of each yearly interval. The outcome was occurrence of at least 1 serious infection in that year. Subsequent infections in the same year were not included. Andersen-Gill models, which are a variation of Cox models that allows inclusion of multiple events in the same patient, were used (18). The Andersen-Gill model is a marginal model, which assumes the overall effects for each covariate are of interest, rather than the subject specific effects, and then a robust variance corrects the variability to account for the possible correlations within patients. This model assumes events within the same patient are independent, meaning the baseline risk of experiencing a subsequent event does not change when a patient has an event. In the case of infections, having an infection may increase susceptibility to subsequent infections. To address this, we have included covariates for previous infections in the past 1 to 3 years to allow for this increased risk, and we have excluded multiple infections occurring in the same year to minimize this issue. In addition, model assumptions, such as the assumption of proportional hazards, were assessed, and no violations were found (19).
Model building began with the multivariable model for serious infections that was previously defined using this cohort by Doran et al (Table 1) (8). Since the outcomes of objectively confirmed and serious infections were quite similar, we considered the predictors previously included in either model, but otherwise the variables previously assessed during Doran’s modeling process were presumed to be insignificant and were not reconsidered to avoid introducing bias.
Each of the additional potential predictors was assessed individually to determine whether it would add significantly (p<0.05) to the previously established multivariable model (Table 1). In addition, daily dosage of corticosteroids was assessed. Age, RA disease duration and ESR were also modeled using smoothing splines to allow non-linear effects, and these analyses were used to inform the choice of cutpoints for age and ESR. Two-way interactions between predictors in the model were also assessed. Particularly, interactions with previous infections were assessed to determine whether separate models were warranted to predict the risk of infection among patients with and without previous infections, but no significant interactions were found.
The general formula for obtaining a risk estimate from a Cox model is: where S0(t) is baseline infection-free rate at follow-up time t (here t=1 year), βi is the estimated regression coefficient, Xi is the value of the ith risk factor, and p is the number of risk factors.
Validation was performed by assessing the performance of the risk score in the validation cohort. Discrimination, which is the ability to accurately rank risk levels to distinguish low risk from high risk, was assessed using the concordance statistics (20). For a binary outcome, the c statistic is analogous to the area under the receiver operating characteristic curve. The c statistic ranges from 0.5 to 1 with 0.5 indicating the risk score is uninformative. This method has been extended for use in Cox models (21). Calibration is the ability to accurately predict the absolute risk level. To assess calibration, the cohort was divided into deciles and the risk score estimates were plotted beside the Kaplan-Meier estimates to allow visual assessment of agreement. In addition, standardized incidence ratios (SIRs), which are the ratio of observed to predicted events, were used to assess overall calibration. Ninety-five percent confidence intervals for SIRs were computed assuming the observed number of events follows a Poisson distribution.
Recalibration is often necessary when a risk score is translated from one population to another. Our risk score was developed in one time period and validated in another time period. Since the underlying risks of first and subsequent serious infections had changed over time, the predicted risks systematically over-estimated the observed risks. The risk score was re-calibrated to the new cohort by modifying the baseline infection-free rate (i.e., S0(t) in the risk score equation above) and the coefficients for previous infections. Coefficients of all other factors in the risk model were held fixed when estimating the re-calibrated coefficients.
Following recalibration, a few potential predictors that were not available in the original cohort were assessed in the validation cohort (Table 1). For each yearly interval, the most recently measured value was used, and each potential predictor was added to the previously developed risk score model individually to assess its potential significance. Non-linear effects of the continuous variables were also examined using smoothing splines.
RESULTS
Of the 584 RA patients in the original cohort (patients diagnosed in 1955–1994), the mean age was 58 years and the majority of patients (72%) were female (Table 2). The median follow up was 9.9 years (7,096 total person-years) during which 252 had ≥1 serious infection (646 total infections, 491 of which were used in the models which included only 1 infection per patient per year). In the validation cohort (patients diagnosed in 1995–2007), the mean age was 55 years and 69% were female. The median follow up was 5.2 years (2,292 total person-years) during which 55 patients had ≥1 serious infection (166 total infections, 103 were the first infection in a given year). Characteristics of both cohorts are reported in Table 2. Due to the difference in length of follow-up between the cohorts, formal comparisons of characteristics were not performed.
Table 2.
Patient characteristics among 994 incident patients with rheumatoid arthritis (RA) diagnosed between 1955 and 2007
| Characteristic | Original cohort 1955–1994 (n=584) | Validation cohort 1995–2007 (n=410) |
|---|---|---|
| At RA incidence | ||
| Age, years | 57.5 ± 15.1 | 54.9 ± 15.5 |
| Female sex | 422 (72%) | 284 (69%) |
| Body mass index, kg/m2 | 25.6 ± 4.9 | 28.6 ± 6.4 |
| Erythrocyte sedimentation rate, mm/hr | 34.4 ± 25.9 | 23.1 ± 19.1 |
| C-reactive protein, mg/L‡ | -- -- | 27.4 ± 57.9 |
| Ever* | ||
| Presence of rheumatoid factor | 331 (65%) | 267 (65%) |
| Extraarticular manifestations of RA** | 50 (9%) | 33 (8%) |
| Chronic lung disease | 149 (26%) | 118 (29%) |
| Leukopenia | 87 (15%) | 40 (10%) |
| Lymphocytopenia | 303 (52%) | 136 (33%) |
| Neutropenia | -- -- | 35 (9%) |
| Diabetes mellitus | 60 (10%) | 63 (15%) |
| Alcoholism | 40 (7%) | 37 (9%) |
| Heart failure | 160 (27%) | 10 (2%) |
| Peripheral vascular disease | 91 (16%) | 36 (9%) |
| Coronary heart disease | 57 (10%) | 25 (6%) |
| Ever use of medications* | ||
| Methotrexate | 122 (21%) | 263 (64%) |
| Hydroxychloroquine | 214 (37%) | 259 (63%) |
| Other DMARDs | 211 (36%) | 92 (22%) |
| Corticosteroids | 250 (43%) | 323 (79%) |
| Biologic agents | -- -- | 83 (20%) |
| Length of follow-up, years | 14.2 ± 9.5 | 5.6 ± 3.5 |
Values in table are mean ± s.d. or Number (%)
C-reactive protein measures were available in 366 (89%) of patients in the validation cohort.
At RA incidence or during follow-up
amyloidosis, Felty’s syndrome, rheumatoid vasculitis, and rheumatoid lung disease
The overall rate of serious infections in the validation cohort (7.2 per 100 person-years) was significantly lower than in the original cohort (9.1 per 100 person-years; rate ratio = 0.80; 95% CI: 0.69, 0.93); p<0.001) reflecting the general decline in hospitalization rates that occurred over this extensive time period. Similarly the rate of first infection was lower in the validation cohort (2.6 per 100 person-years) than in the original cohort (3.9 per 100 person-years; p<0.001). However, among those who experienced a first infection, the rate of second infections was higher in the validation cohort (36.5 per 100 person-years) than in the original cohort (13.6 per 100 person-years; p<0.001).
A multivariable model for serious infections was developed using the original cohort as outlined in the statistical methods. This model, which defines our risk score for serious infections, is shown in Table 3. Increasing age was associated with a high infection risk, with the greatest risk in those ≥80 years (HR 2.36 [95% CI 1.71, 3.24]) compared to patients with age < 60 years. Another risk factor found to be a strong predictor of infection risk was having had a previous serious infection in the past year, with a HR of 3.48 (95% CI 2.67, 4.54). Elevated ESR was also associated with infection risk, with the greatest risk in those with ESR > 50 mm/h (HR 1.84 [95% CI 1.45, 2.34]). Corticosteroid use was associated with a higher risk for serious infection in a dose-dependent fashion. For corticosteroid use up to 10 mg daily prednisone equivalent, the HR was 1.74 (1.35, 2.24) compared to patients not taking corticosteroids. With doses greater than 10 mg daily, the HR increased to 3.60 (95% CI 1.90, 6.81) compared to those not using corticosteroids.
Table 3.
Multivariable Models for One-year Risk of Serious Infections in Patients with Rheumatoid Arthritis Developed in the Original Cohort and Assessed in the Validation Cohort
| Predictor | Level | Model developed in Original Cohort† Hazard Ratio (95% CI) | Model assessed in Validation Cohort‡ Hazard Ratio (95% CI) |
|---|---|---|---|
| Age | Age< 60 years | Reference | Reference |
| 60 ≤ age < 80 years | 1.50 (1.15, 1.95) | 1.53 (0.98, 2.40) | |
| age ≥ 80 years | 2.36 (1.71, 3.24) | 2.18 (1.21, 3.91) | |
| Previous serious infection | Never or >3 years ago | Reference | Reference |
| In the past year | 3.48 (2.67, 4.54) | 10.13 (6.00, 17.12) | |
| In the past 2–3 years | 1.95 (1.45, 2.63) | 5.59 (2.69, 11.64) | |
| Extraarticular manifestation of RA* | 1.86 (1.33, 2.60) | 1.59 (0.88, 2.87) | |
| Erythrocyte sedimentation rate (ESR) | ESR < 30 mm/hr | Reference | Reference |
| 30 ≤ ESR ≤ 50 mm/hr | 1.20 (0.93, 1.55) | 1.50 (0.98, 2.32) | |
| ESR > 50 mm/hr | 1.84 (1.45, 2.34) | 1.05 (0.54, 2.03) | |
| Corticosteroid dose | None | Reference | Reference |
| ≤ 10 mg daily | 1.74 (1.35, 2.24) | 2.14 (1.42, 3.23) | |
| > 10 mg daily | 3.60 (1.90, 6.82) | 3.97 (1.79, 8.82) | |
| Comorbidities** | None | Reference | Reference |
| One | 1.96 (1.55, 2.49) | 1.25 (0.77, 2.05) | |
| More than one | 2.79 (2.17, 3.58) | 2.67 (1.45, 4.90) |
RA=rheumatoid arthritis; ESR=erythrocyte sedimentation rate
amyloidosis, Felty’s syndrome, rheumatoid vasculitis, and rheumatoid lung disease
diabetes mellitus, chronic lung disease, alcoholism, coronary heart disease, heart failure, and peripheral vascular disease
Several comorbidities were associated with the risk of serious infections, including chronic lung disease (HR: 1.56; 95% CI: 1.24, 1.95; adjusted for all other predictors in the risk score), diabetes mellitus (HR: 1.35; 95% CI: 1.01, 1.81), alcoholism (HR: 1.50; 95% CI: 1.05, 2.16), coronary heart disease (HR: 1.47; 95% CI: 1.08, 2.01), heart failure (HR: 1.70, 95% CI: 1.34, 2.16), and peripheral vascular disease (HR: 1.50; 95% CI: 1.13, 2.10). In order to simplify the model, comorbidities were combined and modeled as none, one or more than 1 comorbidity; the c-statistic did not change with this simplification. The presence of one comorbidity was associated with an increased risk of infection (HR: 1.96; 95% CI: 1.55, 2.49), with an additional risk for more than 1 comorbidity (HR: 2.79; 95% CI: 2.17, 3.58).
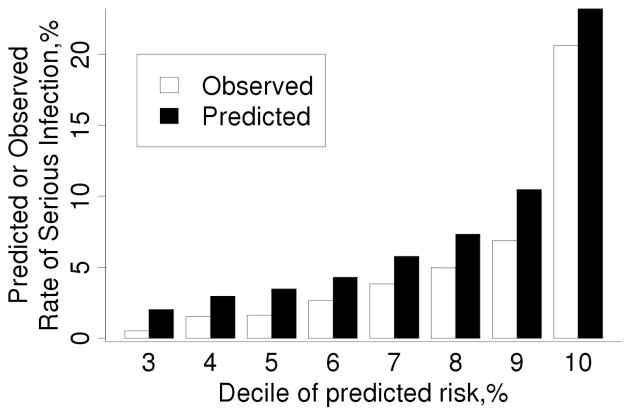
When the risk score developed on the original cohort was tested in the validation cohort, it showed excellent discrimination (c-statistic =0.80; 95% CI: 0.74, 0.85). Figure 1 shows that the observed risk increases as the predicted risk increases. However, the calibration of the risk score was poor in that the risk score predicted a higher risk of infection than was observed, due to the lower event rate in the validation cohort (103 observed serious infections vs. 148.0 predicted infections; standardized incidence ratio [SIR]: 0.70; 95% CI: 0.57, 0.84).
Figure 1.
Comparison of observed and predicted 1 year risk of serious infection in the validation cohort according to deciles of predicted risk obtained from our risk score for serious infections developed in the original cohort. The observed risk was obtained using Kaplan-Meier methods. Note that predicted risk for patients in deciles 1–3 was identical, so these 3 deciles of patients are included in the bars labeled decile 3.
The calibration was explored further by re-fitting the model from the original cohort in the validation cohort (Table 3). In most cases, the coefficients in the model fit in the validation cohort closely agreed with those in the original cohort (i.e., were within the 95% CI in the original model). However, some of the coefficients were not statistically significant in the validation cohort (e.g., age between 60 and 80 years, extraarticular manifestations of RA, ESR between 30 and 50 mm/hr, and presence of 1 comorbidity), likely due to the smaller sample size or shorter follow-up of this cohort which somewhat limited statistical power. Striking differences in coefficients were noted for previous infections due to the increased risk of subsequent infections in the validation cohort. Recalibration, which involved replacing the 1-year baseline infection-free rate from the original cohort (S0(1)=0.980) with that from the validation cohort (S0(1)=0.989) and modifying the coefficients for previous infections, improved the calibration of the risk score (SIR: 0.96; 95% CI: 0.78, 1.16) and had little impact on the discrimination (c-statistic =0.81; 95% CI: 0.75, 0.86).Figure 2 shows the improved performance of the re-calibrated score
Figure 2.
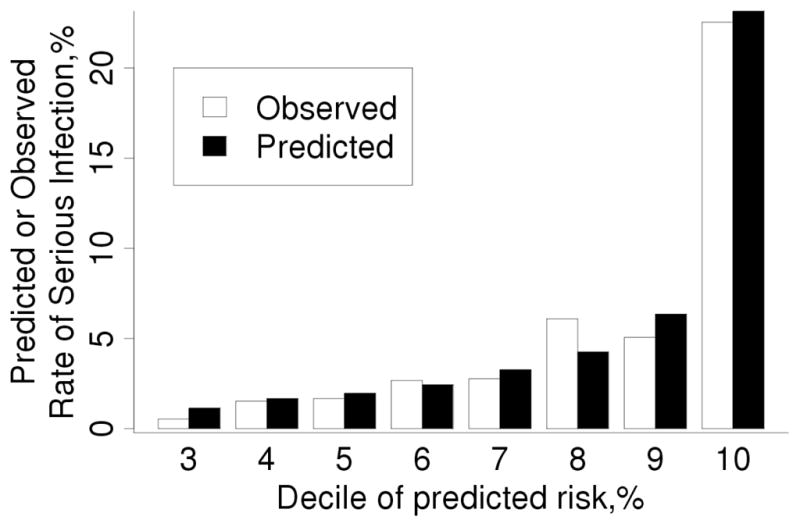
Comparison of observed and predicted 1 year risk of serious infection in the validation cohort according to deciles of predicted risk obtained from our re-calibrated score for serious infections. The observed risk was obtained using Kaplan-Meier methods. Note that predicted risk for patients in deciles 1–3 was identical, so these 3 deciles of patients are included in the bars labeled decile 3.
Several additional risk factors were available in the validation cohort (i.e., leukopenia, lymphocytopenia, neutropenia, CRP and use of biologic agents). Each of these potential predictors was assessed by adding it to the recalibrated risk score model. Elevated infection risks of 20–80% were associated with each of the following: leukopenia (HR; 1.23; 95% CI: 0.34, 4.47), lymphocytopenia (1.45; 95% CI: 0.86, 2.43), and neutropenia (HR: 1.76; 95% CI: 0.42, 7.37). However, these associations did not reach statistical significance, likely due to the low prevalence of these abnormalities, as well as the inclusion of previous infections in the risk score models. Analyses of grades of leukopenia, lymphocytopenia, and neutropenia, as well as non-linear effects did not reveal improved associations with risk of serious infections. Abnormal CRP was not significantly associated with the risk of serious infection (HR: 1.04; 95% CI: 0.64, 1.67). Similar results were seen for continuous (linear and non-linear) and log-transformed CRP.
In the validation cohort, 20% of patients were exposed to biologic agents. The majority (95%) of biologic exposures were TNF antagonists. The use of biologic agents was not associated with the risk of serious infections (HR: 1.27; 95% CI: 0.76, 2.12) compared to non-biologic users.
Table 4 lists the coefficients of the risk score, which include the re-calibrated coefficients for previous infections and the coefficients from the model developed in the original cohort for all other risk factors. Since all items in the score are either present or absent, the coefficients are simply added together to obtain a patient’s score. To convert the score into a risk percentage, refer to the cutoffs in the footnote of Table 4. For example, a 70 year old woman with RA who has ESR = 35 mm/hr, is diabetic and has a 15 mg/day dose of prednisone with no previous serious infection has a risk score of 2.54 which corresponds to a risk of 10–15% of developing an infection in the next year. If her prednisone dose was reduced to 5 mg/day, her risk score would be 1.81, corresponding to a 5–10% risk of serious infection within the next year.
Table 4.
Risk Assessment for One-year Risk of Serious Infections in Patients with Rheumatoid Arthritis*
| Predictor | Level | Coefficient |
|---|---|---|
| Age | Age< 60 years | 0 |
| 60 ≤ age < 80 years | 0.404 | |
| age ≥ 80 years | 0.857 | |
| Previous serious infection | Never or >3 years ago | 0 |
| In the past year | 2.138 | |
| In the past 2–3 years | 1.670 | |
| Extraarticular manifestation of RA | 0.620 | |
| Erythrocyte sedimentation rate (ESR) | ESR < 30 mm/hr | 0 |
| 30 ≤ ESR ≤ 50 mm/hr | 0.180 | |
| ESR > 50 mm/hr | 0.611 | |
| Corticosteroid dose | None | 0 |
| ≤ 10 mg daily | 0.553 | |
| > 10 mg daily | 1.281 | |
| Comorbidities | None | 0 |
| One | 0.675 | |
| More than one | 1.024 |
1-year risk of serious infection (%) = [1 – 0.989 (exp[A])] × 100% where A = 0.404 (if age 60–79 years) + 0.857 (if age ≥ 80 years) + 2.138 (if serious infection in the past year) + 1.670 (if serious infection in the past 2–3 years) + 0.620 (if extraarticular RA) + 0.180 (if 30 ≤ ESR ≤ 50 mm/hr) +0.611 (if ESR > 50 mm/hr + 0.553 (if corticosteroids ≤ 10 mg/day) + 1.281 (if corticosteroids > 10 mg/day) + 0.675 (if one comorbidity) + 1.024 (if >1 comorbidity)
to use the risk score, add the coefficients for the relevant items, then refer to the following cutoffs:
- 5% risk: score = 1.53;
- 10% risk: score=2.25;
- 15% risk: score=2.69;
- 20% risk: score=3.00
DISCUSSION
In this study we developed a risk score for serious infections, which utilized age, comorbidities, measures of RA disease severity (e.g., extraarticular RA, abnormal RF) and activity (e.g., increased ESR), corticosteroid use and previous infection. We expanded upon knowledge of risk factors for infection determined previously with this RA cohort and demonstrated that cardiovascular comorbidities also increased the risk for infection (8). We validated the risk score using patients who developed RA more recently (since 1995), and demonstrated that the risk score had good discrimination in the validation cohort.
Although it is recognized that RA is associated with an increased risk of infection, few risk scores exist to help clinicians assess infection risk. Franklin et al. created a score using a population of patients with new-onset polyarthritis, though this strategy may not accurately predict risk for patients with longer disease duration (22). Additionally, the risk score was based on a multivariable model with a limited set of predictors (i.e., history of smoking, presence of rheumatoid factor, and use of corticosteroids) which was less comprehensive than ours. Recently the German biologics register RABBIT also investigated infection risk. Their data allowed a more comprehensive assessment of biologic risk than ours did, but their findings regarding the impact of other risk factors were similar to ours.
In addition to the patient and disease characteristics included in our risk score, we examined the effect of medications used in the treatment of RA. In our analysis, only corticosteroid use was associated with higher risk, as has been reported by us and others (3, 8, 23, 24).
There is conflicting information regarding the increased risk for infection with traditional DMARD therapy. In the current study, traditional DMARD therapy was assessed, but not found to be significantly associated with infection risk. In our original cohort, use of methotrexate was not associated with an increased risk, consistent with other studies(8, 25). In contrast, Smitten et al found a reduced risk of infections, and other investigators have found an increased risk of infections associated with methotrexate use (23, 24, 26). In our original cohort, cytotoxic medications (e.g., cyclophosphamide, azathioprine, etc.) were assessed and appeared to confer increased risk of infections, but these associations did not reach statistical significance due to low prevalence of use of these medications (8).
We found no evidence of an increased risk of serious infections associated with biologic therapy, which is consistent with the findings of some others (11). Still other authors have noted an increased risk of serious infections in patients taking biologics (9, 10, 27, 28). The recent assessment of infection risk performed using the German biologics register RABBIT helped to make sense of these conflicting reports (29). An increased risk of infection during the first year of treatment with biologic agents was found, with a subsequent decline in infection risk due to improvement in disease activity, reduction in concomitant corticosteroid use and discontinuation of biologics among patients with high risk of infections. The low prevalence of biologic use in our cohort did not allow detailed examination of patient selection and time-varying effects of biologic use.
Another objective of this study was to identify predictors of infection that were not considered previously (8). Lymphocytopenia occurred in ~80% of patients in our original cohort during the follow-up (mean 15 years) (30), and may have been a useful indicator of increased infection risk. However, lymphocytopenia may also be associated with previous infection or corticosteroid use. While lymphocytopenia was modestly associated with the risk of serious infection after adjustment for the other predictors in our risk score, this association did not reach statistical significance. Our finding of a relatively weaker association between lymphocytopenia and infection risk is consistent with a prior analysis of morbidity and mortality in a small cohort of 53 patients with RA who received a lymphocytotoxic monoclonal antibody, CAMPATH-1H/alemtuzumab, which resulted in prolonged therapy-induced lymphocytopenia (31, 32). Twelve year outcome data from this cohort did not reveal increased infection-related mortality, or excess mortality when compared to matched controls with long term follow-up. Possible explanations for this lack of association include immune sufficiency at other sites (e.g., lymph nodes) or preserved immunologic memory (31, 32).
Although we did not have data on disease activity scores (e.g., disease activity score 28 [DAS28], we were able to assess measures reflecting RA severity (e.g., RF positivity, rheumatoid nodules, extraarticular manifestations of RA, and poor functional status) and markers of inflammation (e.g., ESR and CRP) as predictors of infection. Additionally, we were able to demonstrate that cardiovascular comorbidities, which have also been linked to measures of disease severity (33), are associated with an increased risk for infection. Other authors have also noted an increased infection risk with cardiovascular comorbidities and with increased disease activity based on the clinical disease activity index (CDAI) and DAS28 utilizing RA patients from the CORRONA registry (18).
Application of our risk score in the clinic may alert physicians to an increased risk of infection, triggering heightened vigilance with follow-up, education regarding preventative measures for infection and possible alteration of treatment decisions. In fact, quantifying the risk of infection could help with decision making regarding antimicrobial prophylaxis. There are currently immunization guidelines, but no guidelines for whom prophylaxis might be useful in patients with RA. Further research is needed to determine the level of infection risk at which patients receive the most benefit from prophylaxis, and how formal infection risk assessment might influence choice of DMARD, including biologic therapy in RA.
A limitation of the original cohort was that the patients studied did not reflect current treatment patterns for RA, since follow up was only through the year 2000. The validation cohort included patients more recently diagnosed with RA, but confounding by indication in this observational study may still affect our ability to draw conclusions about the effects of medication on infection risk. Another limitation is that potential risk factors with low prevalence (<5%) would have less likelihood of inclusion in the risk score due to limited statistical power for identifying an association with infection. Our validation cohort had shorter follow-up than the original cohort. While we did not find disease duration to be associated with risk of serious infection in the original cohort, the shorter disease duration in the validation cohort would allow less time for development of comorbidities; lower prevalence of comorbidities could affect our ability to estimate their effects in the validation.
A further potential limitation is the retrospective nature of the study. Since our definition of serious infection required hospitalization or I.V. antibiotics, it is unlikely that we have failed to capture any of these infections due to the comprehensive nature of the REP resources. In contrast, some of the risk factors, such as lymphocytopenia, may have been missed, as it is possible some may not have come to medical attention. We believe, however, that the majority of clinically relevant risk factors would have been assessed clinically and would therefore be captured by our extensive medical-records review. Lastly, the majority of the Olmsted County population is white, which may limit the generalizability of our study results.
Conclusion
Our risk score for serious infection has potential clinical utility for predicting infection within the next year in patients with RA. Validation of this risk score in patients with RA diagnosed since 1995 has enhanced understanding of the infection risk in these patients, and demonstrated excellent performance of the risk score. Assessment of the risk of serious infections in patients with RA can influence clinical decision-making and inform strategies to reduce and prevent the occurrence of these infections.
Acknowledgments
Grant Support: This study was funded by a grant from Genentech, Inc. and made possible by a grant from the National Institutes of Health (NIAMS R01 AR46849) and the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging).
References
- 1.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 2.Doran M, Crowson C, Pond G, O’Fallon W, Gabriel S. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis & Rheumatism. 2002;46(9):2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. Br Med J (Clin Res Ed) 1985;290(6484):1797–9. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myllykangas-Luosujarvi R, Aho K, Kautiainen H, Isomaki H. Shortening of life span and causes of excess mortality in a population-based series of subjects with rheumatoid arthritis. Clin Exp Rheumatol. 1995;13(2):149–53. [PubMed] [Google Scholar]
- 5.Prior P, Hassall C, Cross KW. Causes of death associated with psychiatric illness. J Public Health Med. 1996;18(4):381–9. doi: 10.1093/oxfordjournals.pubmed.a024534. [DOI] [PubMed] [Google Scholar]
- 6.Reilly PA, Cosh JA, Maddison PJ, Rasker JJ, Silman AJ. Mortality and survival in rheumatoid arthritis: a 25 year prospective study of 100 patients. Ann Rheum Dis. 1990;49(6):363–9. doi: 10.1136/ard.49.6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prior P, Symmons DP, Scott DL, Brown R, Hawkins CF. Cause of death in rheumatoid arthritis. Br J Rheumatol. 1984;23(2):92–9. doi: 10.1093/rheumatology/23.2.92. [DOI] [PubMed] [Google Scholar]
- 8.Doran M, Crowson C, Pond G, O’Fallon W, Gabriel S. Predictors of infection in rheumatoid arthritis. Arthritis & Rheumatism. 2002;46(9):2294–300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 9.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius N, et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Annals of the rheumatic diseases. 2007;66(10):1339–44. doi: 10.1136/ard.2006.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JR, Xie F, Chen L, Baddley JW, Beukelman T, Saag KG, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Annals of the rheumatic diseases. 2011;70(8):1401–6. doi: 10.1136/ard.2010.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheumatic diseases clinics of North America. 2004;30(4):819–34. vii. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Gill R. Understanding Cox’s regression model. Experientia Suppl. 1982;41:187–99. [PubMed] [Google Scholar]
- 19.Therneau TM, Grambsch PM. Multiple events per subject. Modeling survival data: extending the Cox model. New York: Springer-Verlag; 2000. pp. 189–229. [Google Scholar]
- 20.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell FE., Jr . Regression Modeling Strategies. New York: Springer-Verlag; 2001. [Google Scholar]
- 22.Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis. 2007;66(3):308–12. doi: 10.1136/ard.2006.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–91. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 24.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35(3):387–93. [PubMed] [Google Scholar]
- 25.Lacaille D, Guh DP, Abrahamowicz M, Anis AH, Esdaile JM. Use of nonbiologic disease-modifying antirheumatic drugs and risk of infection in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(8):1074–81. doi: 10.1002/art.23913. [DOI] [PubMed] [Google Scholar]
- 26.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford) 2007;46(7):1157–60. doi: 10.1093/rheumatology/kem076. [DOI] [PubMed] [Google Scholar]
- 27.Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50(1):124–31. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Ustianowski AP, Helbert M, et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Annals of the rheumatic diseases. 2011;70(10):1810–4. doi: 10.1136/ard.2011.152769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strangfeld A, Eveslage M, Schneider M, Bergerhausen HJ, Klopsch T, Zink A, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70(11):1914–20. doi: 10.1136/ard.2011.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Icen M, Nicola PJ, Maradit-Kremers H, Crowson CS, Therneau TM, Matteson EL, et al. Systemic lupus erythematosus features in rheumatoid arthritis and their effect on overall mortality. J Rheumatol. 2009;36(1):50–7. doi: 10.3899/jrheum.080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaacs JD, Greer S, Sharma S, Symmons D, Smith M, Johnston J, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis and rheumatism. 2001;44(9):1998–2008. doi: 10.1002/1529-0131(200109)44:9<1998::AID-ART348>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzi AR, Clarke AM, Wooldridge T, Waldmann H, Hale G, Symmons D, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis and rheumatism. 2008;58(2):370–5. doi: 10.1002/art.23122. [DOI] [PubMed] [Google Scholar]
- 33.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]