Abstract
Background
Many children with heavy exposure to alcohol in utero display characteristic alterations in brain size and structure. However, the long-term effects of low-to-moderate alcohol exposure on these outcomes are unknown.
Methods
Using voxel-based morphometry and region-of-interest analyses, we examined the influence of lower doses of alcohol on gray and white matter composition in a prospectively recruited, homogeneous, well-characterized cohort of alcohol-exposed (n=11, age 19.5±0.3 yr) and control (n=9, age 19.6±0.5 yr) young adults. A large proportion of the exposed individuals were born to mothers whose alcohol consumption during pregnancy was in the low-to-moderate range.
Results
There were no differences in total brain volume or total gray or white matter volume between the exposed and control groups. However, gray matter volume was reduced in alcohol-exposed individuals in several areas previously reported to be affected by high levels of exposure, including the left cingulate gyrus, bilateral middle frontal gyri, right middle temporal gyrus, and right caudate nucleus. Notably, this gray matter loss was dose dependent, with higher exposure producing more substantial losses.
Conclusions
These results indicate that even at low doses, alcohol exposure during pregnancy impacts brain development and that these effects persist into young adulthood.
Keywords: prenatal alcohol exposure, voxel based morphometry, fetal alcohol spectrum disorders, brain volume
INTRODUCTION
Prenatal alcohol exposure (PNAE) influences fetal as well as postnatal brain development and its impact on behavior can be devastating. Heavy prenatal exposure frequently leads to fetal alcohol syndrome (FAS), with its defining phenotypic triad of facial dysmorphic features, pre and/or post-natal growth deficiency, and central nervous system dysfunction (Benz et al., 2009). Many individuals with a history of alcohol exposure in utero who do not meet the diagnostic criteria for FAS–specifically the facial dysmorphology–nonetheless exhibit one or more of its phenotypic characteristics. These individuals, together with those diagnosed with FAS, meet the broader diagnostic criteria of fetal alcohol spectrum disorder (FASD). With an estimated prevalence of 2-5%, FASD is the leading preventable cause of neurocognitive deficits and mental retardation in the United States (May et al., 2009). Moderate-to-heavy alcohol exposure in utero has been associated with neurocognitive deficits across several domains, including IQ (Streissguth et al., 1990; Jacobson et al., 2004), number processing (Burden et al., 2010; Jacobson et al., 2010), attention (Coles et al., 1997; Burden et al., 2005), verbal learning and memory (Mattson et al., 1996; Willford et al., 2004; O’Leary et al., 2011), visuospatial abilities, executive functioning, fine and gross motor skills, social and adaptive functioning (Guerri et al., 2009; Kodituwakku, 2009), and impaired eyeblink conditioning (Jacobson et al., 2011; Jacobson et al., 2008) .
Neuroimaging studies have consistently shown global volume reductions as well as absolute reductions in the frontal, temporal, and parietal lobes in individuals with a history of heavy in utero exposure (Archibald et al., 2001; Lebel et al., 2008; Li et al., 2008; Mattson et al., 1994; Swayze et al., 1997; Sowell et al., 2002a; Sowell et al., 2002b; Willoughby et al., 2008). Local volume reductions and/or dysmorphisms have been demonstrated in the corpus callosum, caudate nucleus, hippocampus, and cerebellum. Similar structural brain changes have been found in children and adolescents exposed to alcohol in utero who do not meet criteria for FAS, including abnormalities in corpus callosum shape (Bookstein et al., 2007), reductions in caudate volume (Archibald et al., 2001), and decreased cerebellar surface area and volume (Autti-Ramo et al., 2002; O’Hare et al., 2005; Sowell et al., 1996). Astley and colleagues (Astley et al., 2009a; Astley et al., 2009b) further reported that when grouped by severity of phenotype based on growth deficiency, FAS facial phenotype, CNS abnormalities, and prenatal alcohol exposure, there is a linear decrease in absolute volume in the frontal lobes, caudate nucleus, and hippocampus as well as a relative volume decrease in the corpus callosum.
While the effects of heavy prenatal alcohol exposure on brain development have been documented in several studies, the influence of low-to-moderate levels of alcohol exposure on brain structure in humans remains uncertain. Several factors contribute to this uncertainty, including the variable presentation of FASD and the difficulty of establishing an accurate exposure history in older volunteers (May et al., 2009; Rasmussen et al., 2010). Furthermore, the brain undergoes considerable structural remodeling throughout the first two decades of normal development, and little is known about the longer-term impact of low-to-moderate exposure in utero. In this study, we examined the influence of low-to-moderate levels of prenatal alcohol exposure on brain structure in a sample of young African American adults recruited from the Detroit Fetal Alcohol and Drug Exposure Cohort whose in utero exposure and subsequent neurocognitive development from birth to the age of 19 years have been carefully characterized (Jacobson et al., 2002). We hypothesized that in this prospectively recruited, well-documented, racially homogeneous, tightly age-matched study cohort, lower levels of in utero exposure would be associated with dose-dependent structural alterations in one or more of the developmentally vulnerable brain areas identified in previous studies of high exposure.
MATERIALS AND METHODS
Participants
Participants were members of the Detroit Fetal Alcohol and Drug Exposure Cohort, which consisted of 480 African American infants whose mothers were recruited at their first prenatal visit to an inner-city maternity hospital (23.4 ± 7.9 wk gestation, mean ± SD)(4). Incidence and daily maternal alcohol and drug use at time of each prenatal visit were determined using a timeline follow-back approach (Jacobson et al., 2002). At recruitment, mothers were also asked to recall day-by-day drinking and substance use around the time they became pregnant (i.e., conception). Prenatal recruitment enabled contemporaneous monitoring of maternal substance use during pregnancy, thus maximizing the accuracy of this assessment. All women who reported alcohol consumption at conception averaging at least 7 drinks/week, a 5% random sample of lower level drinkers and abstainers, and 78 heavy cocaine (> 2 days/week)/light alcohol users (< 7 drinks/week) were recruited. Volume was recorded for each type of beverage consumed daily, converted to absolute alcohol (AA) units (Bowman et al., 1975), where one oz AA/day is the equivalent of two standard drinks of beer, wine, or liquor. AA units were averaged across the clinic visits to provide a contemporaneous report of oz absolute alcohol/day during pregnancy.
Current Sample
The neuroimaging sample was recruited from the first 128 subjects of the larger Detroit Cohort who participated in 19-year neurocognitive and electrophysiological follow-up studies conducted at Wayne State University. The current study used a 3 (prenatal alcohol, prenatal cocaine, nonexposed controls) X 2 (ADHD present/absent) design in which approximately equal numbers of participants from these six groups were invited to Vanderbilt University for neuroimaging. Exclusion criteria included left-handedness, pregnancy, currently not living in metropolitan Detroit, claustrophobia, fear of flying, and body weight and girth exceeding scanner limitations. Written informed consent was obtained from the mothers at recruitment into the longitudinal study and at follow-up visits and from the young adults to participate in this phase of the study. Approval for human research was obtained from the Human Investigation Committee at Wayne State University and the Institutional Review Board at Vanderbilt University.
This paper reports neuroimaging data from 11 participants (10 male, 1 female; age 19.5±0.3) who were exposed to >0.1 oz AA/day in utero (the “exposed” group) and 9 (4 male, 5 female, age=19.6±0.5) whose mothers abstained or drank at very low levels (≤0.1 oz AA/day) during pregnancy (the “control” group), who consented to participate in the neuroimaging studies. Among the exposed group, those whose mothers drank 0.11-0.499 oz AA/day and did not engage in binge drinking (< 4 standard drinks/occasion), were considered to have low exposure (n=4), and those whose mothers drank ≥ 0.5 oz AA/day or showed a pattern of binge drinking were considered moderate-to-heavily exposed (n=7). Only one member of the latter group was very heavily exposed (≥2.0 oz AA/day) and was the only participant showing the FAS phenotype. Mothers of two participants from the control group smoked cigarettes during pregnancy, compared with nine of the light and moderate-to-heavy alcohol users. Three participants from the latter group were also exposed to cocaine during pregnancy (range = 0.3-2.0 days/month); one was exposed to opiates, and none to depressants or stimulants other than cocaine. Mothers of one participant from the control group and three from the exposed group smoked marijuana during pregnancy.
Table 1 summarizes the demographic background characteristics and prenatal exposures of this sample. The exposed and control groups did not differ in age at scan, years of education, or other demographic characteristics except for a disproportionately larger number of males and greater incidence of maternal smoking during pregnancy in the exposed group. Five of the 20 participants reported using alcohol on 1-3 occasions within the previous month and on those occasions alcohol intake ranged from 3-10 drinks (median = 5 drinks). Eight participants reported having tried marijuana at some time, but only three had used it within the previous month: one daily and the others once and twice, respectively. The individual who used marijuana daily was the only participant who had tried opiates or cocaine. Only three individuals reported smoking: two smoked 1 pack of cigarettes/day; the other, 5 cigarettes/day.
Table 1.
Sample characteristics
| Controls (N = 9) |
Exposed (N = 11) |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | t or χ2 | |
| Demographic background | |||||
| Age at scan | 19.6 | 0.5 | 19.5 | 0.3 | 0.86 |
| Gender (% male) | 44.4 | 90.9 | 5.09* | ||
| Years of education | 12.5 | 0.8 | 11.9 | 0.7 | 1.85 |
| Mother’s age at delivery | 28.6 | 5.8 | 27.9 | 5.8 | 0.27 |
| Socioeconomic statusa | 35.4 | 12.1 | 28.1 | 11.1 | 1.40 |
| Primary caregiver’s education (yr) | 13.4 | 2.4 | 12.2 | 1.6 | 1.34 |
| Primary caregiver’s marital status (% married) |
22.2 | 9.1 | 0.67 | ||
| Prenatal exposures | |||||
| AA/day (oz)b | 0.04 | 0.04 | 1.1 | 1.2 | 2.88* |
| AA/occasion (oz) b | 0.7 | 0.5 | 2.9 | 1.6 | 4.24*** |
| Drinking days/week | 0.3 | 0.3 | 2.6 | 1.8 | 4.07*** |
| Cigarettes/day | 2.8 | 5.7 | 17.3 | 14.7 | 3.00** |
| Marijuana (days/month) | 0.3 | 1.0 | 1.7 | 4.5 | 0.89 |
Based on Hollingshead (1975) Four factor index of social status.
AA=absolute alcohol; 1 oz AA ≈ 2 standard drinks.
p < .05
p < .01
p < .001
Structural Image Acquisition
Participants were transported to the airport by our research staff and flown from Detroit to Nashville for the neuroimaging component of this study, where they were met by research staff at the boarding gate upon arrival and transported to and housed at the Vanderbilt University Clinical Research Center. All participants completed a practice MRI session in a mock scanner on the day of arrival for acclimation to the scan environment and introduction to the tasks. All neuroimaging was performed between the hours of 8:00 AM and noon the following morning. Participants were scanned on a Philips 3T Achieva MRI using an 8-channel SENSE head coil. Structural images consisted of whole brain T1-weighted (inversion prepared) 3D turbo gradient echo scans (TI/TR/TE = 923.7/8.0/3.7 ms; flip angle = 5; phase encode SENSE factor = 2; field of view = 256 × 256 × 170 mm; voxel size 1 × 1 × 1 mm isotropic).
Whole Brain Voxelwise Structural Analysis
Voxel-based morphometry (VBM) was used to test for structural differences between brains of the alcohol exposed and control subjects, using the VBM5.1 toolbox (http://dbm.neuro.uni-jena.de/vbm) and SPM5 (Wellcome Department of Imaging Neuroscience, University College London, London, UK; http://www.fil.ion.ucl.ac.uk) running in Matlab R2008a (Mathworks, Natick, MA). Tissue classification (gray matter, white matter, CSF) was performed using VBM on brains extracted from the T1-weighted structural scans (Acosta-Cabronero et al., 2008; Fein et al., 2006). Brain extraction, which significantly decreases tissue misclassification errors (Fagiolo et al., 2008), was performed on each scan using Brain Extraction Tool (BET; http://www.fmrib.ox.ac.uk/fsl) at a fractional intensity threshold of f=0.25. Following the automated BET analysis, individual brain volumes were inspected and any remaining skull tissue was removed manually.
Extracted brain images, excluding cerebellum, were analyzed using the default VBM5 parameters. Images were registered using linear 12-parameter affine and nonlinear (warping) transformations, bias-corrected, and tissue classified in MNI space to the default ICBM template (Ashburner and Friston, 2005). The linear and non-linear components of the tissue class segmentations were multiplied by the Jacobian determinants from the spatial normalization (modulation) to correct for local volume changes and images were resampled to 1×1×1mm. The modulated, warped, and segmented images were then smoothed using an isotropic Gaussian smoothing kernel of 12mm full–width at half–maximum to help control for multiple comparisons (Mechelli et al., 2005). Image segmentations were reviewed prior to statistical analysis to assess the accuracy of the segmentation process.
Whole-brain voxelwise gray and white matter contrast maps comparing the exposed and control groups were individually analyzed using a general linear model to identify local differences in tissue class between the two groups across the entire brain volume. Total intracranial volume, defined as the sum of the gray matter, white matter, and cerebrospinal fluid volumes generated from the VBM segmentations, was included in the models as a covariate. For the whole-brain cortical gray matter contrast, an explicit mask was used to limit the voxels included in the analysis to those within the cerebrum. Given the limited sample and expected small effect sizes, significance thresholds were set at an uncorrected p<0.001 with a cluster size of >50 voxels (Brieber et al., 2007; McAlonan et al., 2007). Icbm2tal.m (http://brainmap.org/icbm2tal/) was used to convert between MNI and Talairach space, and significant voxels were labeled according to the Talairach Client v2.4.2 (http://www.talairach.org/) searching for a gray matter label within 5 voxels of the most significant voxel in the identified volume of interest (VOI).
Whole Brain Volume Analysis
Total gray matter, white matter, and CSF volumes were extracted from the segmentation files using the VBM5 toolbox. Group differences (exposed vs. no/light exposure) in total brain, gray matter, white matter, and CSF volumes and gray matter/white matter ratios were analyzed separately using two-tailed t-tests.
Volume of Interest (VOI) Analysis – Dose Dependence
The effects of prenatal alcohol exposure on the observed structural changes were examined further by testing for a significant dependence of local structural changes on prenatal alcohol exposure severity in each of the regions (VOIs) identified in the whole brain voxel-wise analysis. We used the ‘spm.regions.m’ function (http://www.fil.ion.ucl.ac.uk/spm/doc/manual.pdf) to identify the principal eigenvariate for each VOI, which accounts for >99% of the volumetric variability within the VOI. The eigenvariates were analyzed in two ways to assess the sensitivity of local structural integrity to prenatal exposure dose. First, a one-way ANOVA with post-hoc Tukey analyses (α=0.05) was performed on each VOI to test for local structural differences among individuals with no or very light alcohol exposure and no evidence of binge drinking (≤0.1 oz AA/day across pregnancy, < 2 oz AA/occasion; n=9), low exposure (0.1-0.5 oz AA/day; n=4), and moderate to high dose exposure (>0.5 oz AA/day; n=7). Second, two-tailed Pearson correlation analyses were performed in each VOI to examine dose-dependent effects of a continuous measure of alcohol exposure (ln(oz AA/day during pregnancy + 1)) across all subjects (exposed and controls) and also within exposed subjects only. Pearson correlations were also run to examine the relation between the continuous measure and gray matter volumes in VOIs not identified in the VBM analysis as related to alcohol exposure (negative controls). All statistical testing was performed using Matlab 7.10.0.499 R2010a.
RESULTS
Whole Brain Volumes
There were no significant differences between the prenatally alcohol exposed and control participants in total brain volume, volumes of gray matter, white matter, or CSF or gray matter/white matter ratio in this moderate-exposed sample (Table 2).
Table 2.
Total brain volume (cm3), gray matter volume (cm3), white matter volume (cm3), and gray matter/white matter ratio for control and alcohol-exposed (PNAE) groups
| Total±SD | GM±SD | WM±SD | CSF±SD | GM/WM Ratio | |
|---|---|---|---|---|---|
| Control | 1694±187 | 908±358 | 421±47 | 365±282 | 2.13±0.73 |
| PNAE | 1752±353 | 878±324 | 448±67 | 426±196 | 1.94±0.54 |
| p value | 0.66 | 0.85 | 0.32 | 0.58 | 0.52 |
Influence of Low-to-Moderate Prenatal Alcohol Exposure on Brain Structure – Whole brain VBM Analysis
In contrast, whole brain voxel-wise analyses revealed several specific areas of decreased gray matter volume in participants with prenatal alcohol exposure. These areas included left cingulate gyrus, right middle frontal gyrus, right middle temporal gyrus, and right caudate nucleus (see Table 3 and Fig. 1). One region meeting significance criteria was not within a 5-voxel range of an identifiable gray matter region; expansion of the distance range to 7 voxels identified the closest gray matter region as left middle frontal gyrus.
Table 3.
Regions identified by optimized VBM analysis where gray matter volume is greater in control compared to prenatal alcohol exposed participants (p<0.001, cluster size kE ≥ 50 voxels). Labels represent the closest gray matter region within 5 voxels of the cluster voxel of highest significance.
| MNI Coordinates | |||||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | kE | unc. p | Brain Region |
| −13 | −12 | 36 | 330 | <.001 | Left cingulate gyrusa |
| −16 | −30 | 35 | <.001 | Left cingulate gyrusa | |
| 22 | −1 | 45 | 176 | <.001 | Right middle frontal gyrus |
| −25 | 39 | −3 | 210 | <.001 | Left middle frontal gyrusb |
| 41 | −53 | 8 | 77 | <.001 | Right middle temporal gyrus |
| 25 | 28 | 31 | 51 | <.001 | Right middle frontal gyrus |
| 22 | 11 | 21 | 96 | 0.001 | Right caudate |
Coordinates represent two local maxima within a single cluster
Closest gray matter for this region was 7 voxels from the cluster voxel of highest significance
Figure 1.
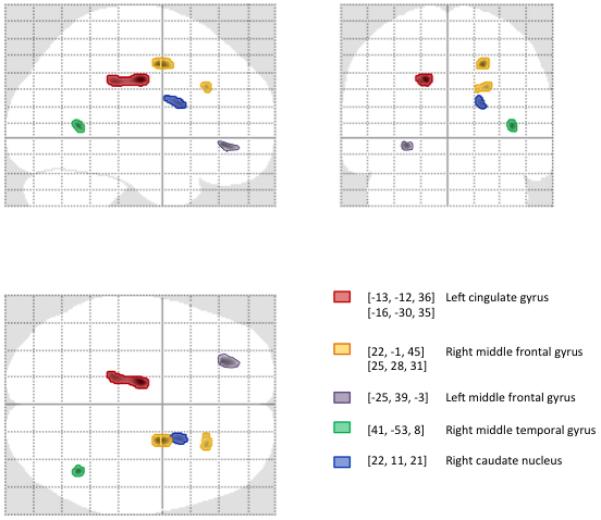
Glass brain projections showing areas of significant (p<0.001; cluster size kE>50 voxels) gray matter volume reduction associated with exposure to alcohol in utero (exposed vs. controls) identified using optimized VBM. Gray matter regions were identified by converting MNI coordinates to Talairach space and labeled using Talairach Daemon searching for a gray matter label nearest the most significant voxel in the cluster.
There were no areas of white matter volume reduction in the alcohol-exposed group compared to controls, and there were no regions of gray or white matter reduction in control participants compared to alcohol-exposed participants.
Dependence of Gray Matter Change on Exposure Severity - VOI Analysis
Eigenvariate analysis confirmed that the principal eigenvector accounted for >99% of the variance in gray matter structure within the VOIs identified as significant by VBM. When participants were stratified into three groups based on in utero alcohol exposure: no/light exposure, low dose, and moderate-to-high dose, one way ANOVA identified significant differences in gray matter volume between exposure groups in multiple VOIs. Post-hoc analyses (see Fig. 2) showed significant gray matter reductions in the moderate-to-high dose exposure group compared to participants with no/light exposure and between the low dose exposure and no/light exposure in four of the six VOIs. Finally, gray matter volumes in left cingulate gyrus and right caudate nucleus were lower in the moderate-to-heavy than in the low alcohol exposure group (p < 0.1) but fell short of statistical significance.
Figure 2.
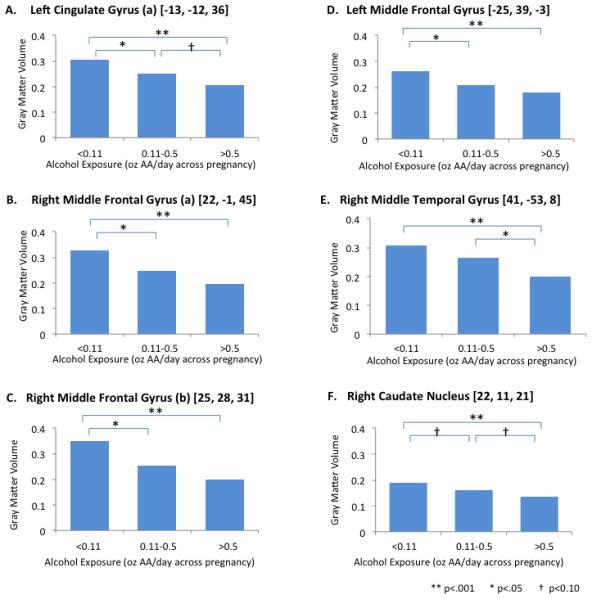
Group analysis comparing local gray matter volumes for subjects with no or very light (0-0.1 oz AA/day with no evidence of binge drinking), low (0.11-0.5 oz AA/day) and moderate-to-heavy (>0.5 oz AA/day) prenatal alcohol exposure.
Correlation analyses including all subjects (exposed and control) revealed a significant dependence of local gray matter volume reduction on exposure severity, for all VOIs previously identified by whole brain VBM (see Fig. 3). In contrast, no significant dose-dependent gray matter changes were present in 20 VOIs centered on randomly selected gray matter voxels. When the analysis was limited only to those subjects with documented prenatal alcohol exposure, the dose dependence of the observed gray matter changes remained significant for right middle frontal gyrus (r = −0.614, p=0.045) and right caudate nucleus (r = −0.62, p=0.038), and the trend towards correlation in right middle temporal gyrus remained (r = −0.57, p=0.069). Exclusion of the subject with heavy alcohol exposure from these analyses did not alter the findings.
Figure 3.
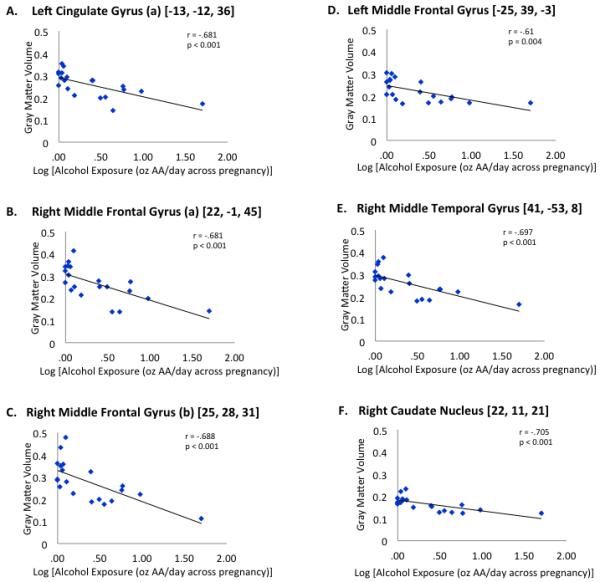
Dependence of gray matter volume (principal eigenvariate) on level of alcohol exposure in utero in VOIs identified by VBM. Dose-response graphs of regions of interest showing a significant difference in gray matter volume reduction in prenatally alcohol-exposed participants compared to the children of abstainers or light drinkers with no evidence of binge drinking.
Confirmatory Analyses
To control for the potential influence on brain development of alcohol consumption by the volunteers themselves (Sasaki et al., 2009) we performed a separate VBM analysis including participants’ drinks/occasion within the last month as a covariate of no interest. These and subsequent VOI analyses confirmed that participant alcohol exposure did not alter the demonstrated dose-dependent regional gray matter volume reductions. Also, removal of the only volunteer with a history of heavy marijuana use and reported past cocaine use from analyses did not alter our findings.
In view of the significantly higher level of maternal smoking in the alcohol exposed subjects, we repeated these analyses including maternal cigarette use as a winsorized covariate (Winer, 1971) and found that alcohol exposure severity remained a significant predictor of gray matter volume reduction in all the VOIs identified by VBM. Consistent with this finding, VBM identified the same areas of gray matter volume reduction with alcohol in exposed compared with no/light exposure subjects when maternal cigarette use was included as a covariate in the VBM analysis, and a VBM analysis contrasting nicotine-exposed and nicotine non-exposed children revealed no differences in regional brain volume. Inclusion of cocaine and marijuana exposure as covariates did not change any of the findings.
Finally, given the unbalanced composition of the exposed and control groups with respect to gender and the possible contribution of gender dimorphisms on the observed effects, we confirmed that inclusion of gender as a covariate did not significantly alter our findings. While significance in the left middle temporal gyrus was lost, the decreases in gray matter volume were maintained in the right cingulate gyrus, right middle frontal gyrus, left middle frontal gyrus, and right caudate (punc<0.001). We also tested for exposure dose dependence in males and females separately. This analysis confirmed a significant effect of exposure dose on severity of gray matter loss in males in left cingulate gyrus, right anterior cingulate cortex, right middle frontal gyrus, right middle temporal gyrus, and right caudate nucleus (r ≤ −0.63, p ≤ 0.02) and non-significant trends that were similar in magnitude in the smaller number of females.
DISCUSSION
Whole brain structural analyses of young adult African Americans recruited from the Detroit Fetal Alcohol and Drug Exposure Cohort revealed areas of reduced gray matter volume in left cingulate gyrus, right caudate, right middle temporal gyrus, and right and left middle frontal gyri in participants with a history of low dose in utero alcohol exposure, when compared with their no/light exposure peers. Using detailed fetal alcohol exposure information obtained from the mother during gestation, we found that the severity of gray matter loss in these areas increased with fetal exposure dose, both in analyses that included all participants (exposed and no/light exposure) and in analyses of exposed participants alone. These differences were evident despite the absence of differences in total intracranial, gray matter, or white matter volumes between the exposed and control groups or any local differences in white matter volumes at these relatively low levels of exposure. Further, the significance of the findings survived subsequent analyses examining gender, maternal smoking during pregnancy, and the young adult’s current alcohol and drug use. Our findings are consistent with previous studies regarding the sites of particular gray matter vulnerability to prenatal alcohol exposure, but this is, to our knowledge, the first study to document a dose-dependent reduction in gray matter in these vulnerable areas or structural effects at such low levels of exposure. The nature, possible origins, and potential impacts of these differences are explored more fully below.
Reduced caudate volume was one of the earliest (Mattson et al., 1992) and remains one of the most robust findings in individuals with FAS and high exposure FASD (Archibald et al., 2001; Astley et al., 2009a; Cortese et al., 2006; Mattson et al., 1994; Norman et al., 2009), while gray matter abnormalities in right frontal cortex, as well as bilateral perisylvian areas (inferior parietal and posterior temporal cortices), assessed using a variety of measures of gray matter integrity, are a consistent finding in an FASD cohort with heavy exposure (Sowell et al., 2010; Sowell et al., 2008b; Sowell et al., 2001b; Sowell et al., 2002a; Sowell et al., 2002b). In addition, a recent study identified significant effects of heavy prenatal alcohol exposure on cingulate gyrus morphology, but in contrast to the present study, these were explained by the influence of alcohol exposure on white matter volume (Bjorkquist et al., 2010). We found no significant impact of mild to moderate PNAE on white matter in these structural analyses, although our data do not exclude the possibility of meso-structural changes in white matter integrity that may be revealed by diffusion weighted MRI.
Our finding of an exposure dose dependent decrease in right caudate gray matter volume is consistent with the increased caudate volume asymmetry (R<L) observed in young adults with a history of moderate prenatal alcohol exposure (Willford et al., 2010). This asymmetry is opposite to that found in normal children and young adults (R>L; (Giedd et al., 1996)).
The dose dependent decrease in the cortical gray matter volume observed in caudate in the present study is consistent with findings in previous studies of high exposure cohorts. However, in those studies, in which prenatal alcohol exposure levels were four times greater than the current study, exposure was also associated with locally increased gray matter volume, density and cortical thickness bilaterally in perisylvian areas, particularly inferior parietal cortices and temporal lobes, and in frontal cortex (Sowell et al., 2010; Sowell et al., 2008b; Sowell et al., 2001b; Sowell et al., 2002a; Sowell et al., 2002b). Interestingly, at those higher levels of exposure, these areas also exhibit considerable gross dysmorphology, parameterized as the point distance from center by Sowell and colleagues (Sowell et al., 2002a), and also are epicenters of white matter loss (Sowell et al., 2001b) and/or microstructural abnormalities (Sowell et al., 2008a).
Several differences between the study cohorts may account for these apparently disparate findings. The participants in the present study comprised a racially homogeneous group of young African American adults of uniform age (18.9-20.8 years), whose prenatal alcohol exposure was well characterized prospectively. A large proportion of the exposed group (63.6%) were born to mothers whose alcohol intake during pregnancy was in the low-to-moderate range, and removal of the one subject with very high exposure and the FAS phenotype did not alter any findings. Consistent with the relatively low levels of in utero exposure in this sample, there were no significant differences in total, gray, or white matter volumes between the exposed and control groups. These features may mitigate the long-term impact of prenatal alcohol exposure on brain development and structure.
Studies of the trajectory of normal brain development from newborns to young adults reveal initial expansion and thickening of cerebral cortex, with a subsequent reduction in cortical gray matter, beginning around puberty (Giedd et al., 1996) (Giedd and Rapoport, 2010; Gogtay et al., 2004), which has been attributed to cortical pruning (Huttenlocher, 1994; Bourgeois et al., 1994; Giedd et al., 1996). This pattern of cortical expansion followed by later reduction is spatially and temporally inhomogeneous: primary sensory brain areas mature earlier, while higher order association and multisensory areas, as well as phylogenetically newer frontal areas mature later (Giedd et al., 1999; Sowell et al., 2001; Jernigan et al., 1991). This process of maturational change in normal developing cortex, particularly in frontal and perisylvian cortices, represents a potential complication in the assessment and interpretation of PNAE’s long term impact on brain morphology, particularly in cohorts whose members span the window of ages during which cortical development occurs. Age-associated changes in GM composition and local morphology (surface curvature, cortical thickness, etc.), both in the normally developing and PNAE-exposed brain, significantly increase the variance in these measures. While the effects of age can be added as a covariate, the long-term effects of PNAE on the temporal trajectory of development are likely to differ from no/light exposure subjects both globally and locally. Inclusion of both pre- and post-pubertal volunteers, whose trajectories of gray matter change are opposite (Giedd and Rapoport, 2010; Giedd et al., 1996) may, if the effects of PNAE include a slowing of the overall process of gray matter maturation, lead to the observation that PNAE is associated with increases in measures of cortical gray matter (representing a delay in the cortical pruning), but may not be indicative of the nature of the differences in local cortical GM composition once these early phases of brain development are complete. Our study mitigates this potential confound by examining young adult volunteers in a narrow age range. Our observation of significant dose-dependent reductions in gray matter volume in areas previously shown to be particularly vulnerable to PNAE indicate that mild-to-moderate alcohol exposure leads to long term reductions in local gray matter volume. These findings suggest that the increases in measures of cortical gray matter reported in previous large cohort studies may reflect an impact of PNAE on the rate of brain maturation during childhood and adolescence.
White matter loss (Archibald et al., 2001; Bjorkquist et al., 2010; Sowell et al., 2001b) and structural dysmorphology are hallmarks of heavy PNAE. Complete or partial agenesis of the corpus callosum (Riley et al., 1995; Swayze et al., 1997; Bhatara et al., 2002) and abnormalities in corpus callosum shape and location are also observed (Sowell et al., 2001a; Bookstein et al., 2002). However, most of these studies focused on children with heavy prenatal alcohol exposure, and only one utilized voxel-based morphometry (Sowell et al., 2001a). Further, when the VBM study compared children with heavy prenatal alcohol exposure but no facial features of FASD to controls, no white matter differences were observed.
Diffusion tensor MRI, which probes the micro-structural integrity of white matter does reveal significant differences between children with FASD and no/light exposure controls (Lebel et al., 2010; Wozniak et al., 2009; Lebel et al., 2008; Makris et al., 2008; Sowell et al., 2008a; Wozniak et al., 2006). Whether these effects represent a developmental delay, permanent microstructural deficits, or a combination of both is unclear, since there is little information available for adults with a history of PNAE, and the normal trajectory of white matter development varies considerably. Given the resolution and structural analytic methods of diffusion tensor imaging required to detect tract-specific diffusion alterations, whole-brain voxel based analyses of brain volume may not be sufficient to characterize the white matter changes seen with in utero alcohol exposure or, alternatively, there may be dose-dependent white matter alterations that are too subtle to identify in individuals with low-dose alcohol exposure. Further analyses with manual tracing and diffusion tensor methods are necessary in order to define the structural white matter abnormalities in individuals with low-dose in utero alcohol exposure.
One limitation of this study is that, given the small sample size, these findings must be considered preliminary. Nevertheless, as noted above, the racial homogeneity and narrow age range of the participants, as well as the absence of significant gross differences in total intracranial, gray matter, white matter, or CSF volumes between the control and alcohol-exposed arms, enabled us to identify areas of significant dose-dependent regional gray matter loss in the VBM analysis. Our statistical thresholds were chosen to reflect the likelihood of a smaller effect size than in larger cohorts of more severely exposed volunteers and are similar to those used by other groups (e.g., Brieber et al., 2007; McAlonan et al., 2007).
Another potential confound in the present study, given the gender imbalance between the exposed and no/light exposure groups, is the possibility that the structural differences reflect sexual dimorphisms rather than effects of in utero alcohol exposure. Dimorphism has been reported in several cortical and subcortical areas including greater cortical thickness in posterior temporal/inferior parietal regions in females relative to males (Sowell et al., 2007), greater caudate volume in females, and an age-associated decrease in caudate volume in males but not females (Giedd et al., 1996). These effects may contribute to the difference between the exposed and no/light exposure groups in caudate GM volume, but our finding that the difference in caudate gray matter volume is lateralized to the right hemisphere is consistent with the dose-dependent increase in caudate asymmetry reported by Willford and colleagues (Willford et al., 2010). Also, the level of in utero exposure to alcohol accounts for a similar fraction of the total variance in GM volume in the areas identified as significantly different by VBM not only in the whole cohort, but also within the subgroup of subjects with documented exposure, while there is no such correlation in other gray matter or white matter VOIs, including those previously identified as having significant differences between males and females. Perhaps most telling, this effect remained significant in male subjects (exposed and no/light exposure) analyzed separately, and an effect of similar magnitude, albeit statistically weaker, was present in the smaller group of females. These findings support the idea that PNAE underlies the differences in local gray matter volume observed in the present study.
Our finding of areas of significant gray matter loss in the setting of low to moderate alcohol exposure in utero is consistent with studies in animals. Thus, reduced brain and/or gray matter volume, increased neuronal loss, and associated neurobehavioral deficits are a consistent finding in rodents and non-human primates exposed to alcohol during the neuro-developmental window corresponding to human in utero exposure (Farber et al., 2010; Ikonomidou et al., 2000; Schneider et al., 2001). Alcohol appears to disrupt neurodevelopment via multiple molecular and cellular mechanisms. It has NMDA-antagonist and GABAA-mimetic activity, promoting both excitotoxic and natural apoptotic cell death, particularly during the period of synaptogenesis (Olney et al., 2001). Prenatal alcohol exposure alters radial glial migration (Miller and Robertson, 1993), impairing neuroblast migration (Gressens et al., 1992). This impaired migration may arise in part from alcohol’s ability to alter neuronal outgrowth by interfering with normal L1-mediated cell-cell adhesion and activation of extracellular receptor kinases in the central nervous system (Yeaney et al., 2009). Alcohol also disrupts growth factor regulation of differentiation and survival, promotes the formation of and damage by reactive oxygen species, and impairs midline serotonergic neuron development and signaling (Goodlett et al., 2005). Many of these early developmental events have distinct spatio-temporal expression patterns (Olney et al., 2000; Olney, 2004) and this, coupled with the differential vulnerability of different brain areas (Derauf et al., 2009) and variations in the timing and nature of alcohol use during pregnancy, may underlie the variable neuro-anatomical and behavioral impact of prenatal alcohol exposure.
Coles et al. (2011) recently reported that compared to unexposed controls, young adult African-Americans with a history of prenatal alcohol exposure and strong phenotypic characteristics of fetal alcohol exposure show significant volume reductions in frontal and temporal brain regions and even those with less pronounced phenotypic features of PNAE had significantly smaller hippocampal volumes. The present study is consistent with and extends the findings of Coles et al.: .we too find that prenatal alcohol exposure is associated with long-term loss of gray matter volume in specific brain regions. Indeed we find that this loss is evident in areas beyond the frontal and temporal areas identified by Coles et al.; furthermore, as a result of the prospective manner in which prenatal alcohol exposure was determined throughout the pregnancy, we were able to demonstrate a significant dose-dependent impact of alcohol exposure in utero on regional brain gray matter volumes that persists into young adulthood. It is striking that this dose dependence is present even in individuals with a history of low-to-moderate in utero exposure who show no significant reduction in total brain volume compared to no/light exposure control subjects. That low levels of exposure may have an impact is supported by the observation that as little as a single exposure to alcohol during periods of rapid brain expansion induces significant and widespread neuroapoptosis in rodents (Ikonomidou et al., 2000) and non-human primates (Farber et al., 2010). Taken together with these findings, the present study suggests that even low levels of alcohol consumption during pregnancy have an impact on brain development and that these effects persist into young adulthood.
Acknowledgments
SUPPORT:
The recruitment and longitudinal follow-up of the Detroit Prenatal Alcohol and Drug Exposure Cohort was funded by grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA06966 and R01 AA09524) and from the Joseph R. Young, Sr., Fund, State of Michigan. The 19-year neurocognitive and neuroimaging follow-up assessment was funded by the National Institute on Drug Abuse (R21 DA021034) and the National Center for Research Resources (Vanderbilt CTSA grant UL1 RR024975). We thank Robert J. Sokol, M.D., who collaborated in the recruitment and infant and school-age phases of the study; and Renee Sun and Audrey Morrison, M.A., who coordinated recruitment and transport of the sample for the 19-year neuroimaging assessments at Vanderbilt. We also thank Chris Gatenby, Ph.D., Joanna Blankner, Ph.D., Jennifer Pryweller, B.S., and Efrain Garcia, Ph.D., for assistance in protocol development and study coordination, Robin Avison CNMT, RT (N, MR) and Donna Butler, RT for performing all the MRI studies, and Lynda Lane and the nurses and staff of the Vanderbilt Clinical Research Center for their clinical support. Finally, we appreciate the contribution of the young adults and their mothers who participated in this follow-up assessment.
REFERENCES
- Acosta-Cabronero J, et al. The impact of skull-stripping and radio-frequency bias correction on grey-matter segmentation for voxel-based morphometry. Neuroimage. 2008;39:1654–1665. doi: 10.1016/j.neuroimage.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Archibald SL, et al. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Astley SJ, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009a;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, et al. Magnetic resonance spectroscopy outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Magn Reson Imaging. 2009b;27:760–778. doi: 10.1016/j.mri.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Ramo I, et al. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Benz J, Rasmussen C, Andrew G. Diagnosing fetal alcohol spectrum disorder: History, challenges and future directions. Paediatr Child Health. 2009;14:231–237. doi: 10.1093/pch/14.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatara VS, et al. Brain function in fetal alcohol syndrome assessed by single photon emission computed tomography. S D J Med. 2002;55:59–62. [PubMed] [Google Scholar]
- Bjorkquist OA, et al. Cingulate gyrus morphology in children and adolescents with fetal alcohol spectrum disorders. Psychiatry Res. 2010;181:101–107. doi: 10.1016/j.pscychresns.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL, et al. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. doi: 10.1111/j.1530-0277.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, et al. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior. I. On measuring patterns of alcohol consumption. II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 1975;36:1154–1172. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Brieber S, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, et al. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, et al. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Coles CD, et al. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, et al. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Cortese BM, et al. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicol Teratol. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Derauf C, et al. Neuroimaging of children following prenatal drug exposure. Semin Cell Dev Biol. 2009;20:441–454. doi: 10.1016/j.semcdb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolo G, Waldman A, Hajnal JV. A simple procedure to improve FMRIb Software Library Brain Extraction Tool performance. Br J Radiol. 2008;81:250–251. doi: 10.1259/bjr/12956156. [DOI] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW. Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol Dis. 2010;40:200–206. doi: 10.1016/j.nbd.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, et al. Statistical parametric mapping of brain morphology: sensitivity is dramatically increased by using brain-extracted images as inputs. Neuroimage. 2006;30:1187–1195. doi: 10.1016/j.neuroimage.2005.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Gressens P, et al. Ethanol-induced disturbances of gliogenesis and neuronogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcohol. 1992;27:219–226. [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; 1975. Four factor index of social status. [Google Scholar]
- Huttenlocher PR. Synaptogenesis in human cerebral cortex. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. Guilford Press; 1994. pp. 137–152. [Google Scholar]
- Ikonomidou C, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, et al. Number processing in adolescents with prenatal alcohol exposure and ADHD: Differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 2010;35:431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, et al. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, et al. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, et al. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, et al. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, et al. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, et al. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, et al. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Li Z, et al. Occipital-temporal Reduction and Sustained Visual Attention Deficit in Prenatal Alcohol Exposed Adults. Brain Imaging Behav. 2008;2:39–48. doi: 10.1007/s11682-007-9013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Mattson SN, et al. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, et al. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16:1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol. 1994;16:283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- May PA, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Mechelli A, et al. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Miller MW, Robertson S. Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J Comp Neurol. 1993;337:253–266. doi: 10.1002/cne.903370206. [DOI] [PubMed] [Google Scholar]
- Norman AL, et al. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare ED, et al. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- O’Leary CE, et al. Verbal learning and memory in fetal alcohol spectrum disorder: Findings from Cape Town and Detroit. Alcohol Clin Exp Res. 2011;35 in press. [Google Scholar]
- Olney JW. Fetal alcohol syndrome at the cellular level. Addict Biol. 2004;9:137–49. doi: 10.1080/13556210410001717006. discussion 151. [DOI] [PubMed] [Google Scholar]
- Olney JW, et al. Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect. 2000;108(Suppl 3):383–388. doi: 10.1289/ehp.00108s3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, et al. Glutamate signaling and the fetal alcohol syndrome. Ment Retard Dev Disabil Res Rev. 2001;7:267–275. doi: 10.1002/mrdd.1037. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, et al. The impact of an ADHD co-morbidity on the diagnosis of FASD. Can J Clin Pharmacol. 2010;17:e165–76. [PubMed] [Google Scholar]
- Riley EP, et al. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, et al. Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage. 2009;46:505–510. doi: 10.1016/j.neuroimage.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25:1383–1392. [PubMed] [Google Scholar]
- Sowell ER, et al. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, et al. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008a;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, et al. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008b;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, et al. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001a;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, et al. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001b;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002a;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, et al. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002b;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Sowell ER, et al. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Swayze V.W.n., et al. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Willford J, et al. Caudate asymmetry: a neurobiological marker of moderate prenatal alcohol exposure in young adults. Neurotoxicol Teratol. 2010;32:589–594. doi: 10.1016/j.ntt.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willford JA, et al. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, et al. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. McGraw-Hill; New York: 1971. [Google Scholar]
- Wozniak JR, et al. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, et al. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaney NK, et al. Ethanol inhibits L1 cell adhesion molecule tyrosine phosphorylation and dephosphorylation and activation of pp60(src) J Neurochem. 2009;110:779–790. doi: 10.1111/j.1471-4159.2009.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


