Abstract
The rat dorsal striatum exhibits domain-dependent kinetics of dopamine release and clearance. The present report describes the domain-dependent actions of nomifensine (20 mg/kg i.p.), a competitive dopamine uptake inhibitor, on evoked dopamine responses recorded by voltammetry during electrical stimulation of the medial forebrain bundle. In slow domains, nomifensine increases the initial rate of evoked overflow, increases response overshoot, does not affect the slope of the linear segment of the dopamine clearance profile, and slows the non-linear segment of the clearance profile. In fast domains, nomifensine does not affect the initial rate of overflow, increases the end-of-stimulus overshoot, and decreases the slope of the linear segment of the dopamine clearance profile. Collectively, these findings do not concur with existing models of evoked dopamine release that describe the effect of nomifensine as an increase in the effective KM of dopamine uptake. These findings suggest that dopamine clearance after evoked release is affected by both dopamine uptake and a restricted extracellular diffusion process.
Keywords: dopamine, dopamine transporter, autoinhibition, evoked release, voltammetry, dorsal striatum
Introduction
Dopamine (DA) is a neurotransmitter that participates in multiple aspects of normal brain function (Roitman et al. 2004, Obeso et al. 2008) and a variety of brain disorders (Salahpour et al. 2008, de la Fuente-Fernandez et al. 2011, Kim et al. 2011). Consequently, drugs that act on DA have wide-ranging uses, some therapeutic (Gottwald & Aminoff 2011, Schlochtermeier et al. 2011) and some illicit (Phillips et al. 2003, Hollander & Carelli 2007, Ramsson et al. 2011). Understanding the actions of such drugs, including their impact on extracellular DA concentrations, is highly significant. Drugs such as cocaine, methylphenidate, and nomifensine, which inhibit DA uptake (Jones et al. 1995, Jones et al. 1998, Makos et al. 2010), are psychostimulants (Hunt et al. 1974, Nakachi et al. 1995, Garris et al. 2003) and have significant abuse potential (Spyraki & Fibiger 1981, Phillips et al. 2003).
In the dorsal striatum of the rat, the DA terminal field exhibits domains of distinct fast and slow kinetics of DA release and clearance (Moquin & Michael 2009, Wang et al. 2010, Moquin & Michael 2011). We have thus far demonstrated that two drugs, raclopride and quinpirole, have domain-dependent actions on DA (Moquin & Michael 2009, Wang et al. 2010). The activity of the DA transporter (DAT) (Gulley & Zahniser 2003, Torres 2006, Schmitt & Reith 2010) appears to be domain-dependent as well, as we found the rate of extracellular DA clearance to be significantly faster in the fast compared to the slow domains (Moquin & Michael 2011). And, DAT reversal contributes to a DA autoinhibitory tone in the slow domains (Moquin & Michael 2009, Wang et al. 2010), which is surprising considering that DAT reversal is thought to require amphetamine-like drugs (Sulzer et al. 1993, Sulzer et al. 1995). The objective of the present study, therefore, was to test the hypothesis that the actions of nomifensine, a competitive DAT inhibitor (Hunt et al. 1974), might also be domain-dependent.
Materials and Methods
Carbon fiber electrodes
Borosilicate capillaries (0.4 mm ID, 0.6 mm OD, A-M systems Inc., Sequim, WA, USA), each containing a single carbon fiber (7-μm diameter, T650, Cytec Carbon Fibers LLC., Piedmont, SC,USA), were pulled to a fine tip using a vertical puller (Narishige, Los Angeles, CA, USA). The tip was sealed with epoxy (Spurr Epoxy, Polysciences Inc., Warrington, PA, USA), the exposed fiber was trimmed to a length of 200 μm, and a mercury drop was placed in the barrel for electrical contact to a hookup wire (Nichrome, Goodfellow, Oakdale, PA, USA).
Fast-scan cyclic voltammetry
Voltammetry was performed with an EI 400 (Ensman Instruments, Bloomington, IN) controlled by ‘CV Tar Heels v4.3’ software (courtesy of Dr. Michael Heien, University of Arizona, Tucson, AZ, USA). The reference electrode was Ag/AgCl. The waveform started at the rest potential (0 V vs. Ag/AgCl), ramped linearly (400 V/s) to +1.0 V, then to -0.5 V, and then to 0 V. Scans were repeated at 10 Hz. DA oxidation currents were recorded between 0.5 and 0.7 V on the initial ramp. DA was identified by inspection of background-subtracted voltammograms.
Electrode preparation and calibration
Electrodes were pretreated and calibrated in artificial cerebrospinal fluid (145 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 1.0 mM Mg2+, 152 mM Cl−, and 2.0 mM phosphate, pH 7.4). The pretreatment was a triangular potential waveform (0-2 V, 200 V/s for 3 s) (Feng et al. 1987, Wang et al. 2010). Pre- and post-calibration was performed in a flow cell with freshly prepared, nitrogen-purged dopamine HCl (Sigma Aldrich, St. Louis, MO, USA) standard solutions. In vivo DA concentrations were determined by post calibration results.
Drugs
Isoflurane (Aerrane, Baxter Healthcare, Deerfield, IL, USA) was delivered by means of a calibrated gas anesthesia machine (IsoTec, Harvard Apparatus, Holliston, MA, USA). Nomifensine maleate was used as received (Sigma Aldrich, St. Louis, MO, USA) and dissolved in phosphate buffered saline (155mM Na+, 155mM Cl−, 100mM phosphate, pH 7.4)
Animals
All procedures involving animals (male Sprague-Dawley rats, 250-350 g, Hilltop, Scottsdale, PA, USA) were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. Rats were intubated and anesthetized with isoflurane (2.5% by volume) and placed in a stereotaxic frame with the incisor bar raised to 5 mm above the interaural line(Pellegrino et al. 1979). Internal body temperature was monitored and maintained at 37°C by use of a heating blanket (Harvard Apparatus, Holliston, MA, USA). Holes were drilled through the skull for the reference, stimulating, and working electrodes. Electrical contact between brain tissue and a reference electrode was via a salt bridge. The stimulating electrode (bipolar stainless steel, MS303/a; Plastics One, Roanoke, VA, USA) was aimed at the medial forebrain bundle (MFB, 2.2 mm posterior to bregma, 1.6 mm lateral from bregma, and 7-8.5 mm below the cortical surface: the final vertical placement was adjusted to evoke DA release in the ipsilateral striatum) (Ewing et al. 1983, Kuhr et al. 1984, Stamford et al. 1988). The carbon fiber electrode was implanted into the dorsal striatum (2.5 mm anterior to bregma, 2.5 mm lateral from bregma, and 5 mm below the cortical surface: the final vertical placement was optimized as explained in the Results Section). Confirmation of electrode placements by histology was not considered necessary in this study because the dorsal striatum is a large brain structure in the rat and easily targeted. The optically isolated stimulus waveform (Neurolog 800, Digitimer, Heretfordshire, England) was a biphasic, constant-current, square wave (2 ms per pulse, 240 μA pulse height, and 60Hz frequency). The stimulus duration was 200 ms or 3 s (see Results section for detail on the stimulus duration).
Each rat received a series of pre-nomifensine stimuli, a single dose of nomifensine (20 mg/kg i.p), and a final post-nomifensine stimulus: the final stimulus was performed 30 min after nomifensine administration. The same electrodes, recording location, stimulating locations, stimulus parameters, etc., were used during the pre- and post-nomifensine responses. During this study, we compared pre- and post-nomifensine responses in the same group of animals to assure the effect of the drug was established at the same stimulating and recording electrodes. This approach, i.e. comparing pre- and post-drug responses is widely used (Wu et al. 2002, Venton et al. 2006, Moquin & Michael 2009, Wang et al. 2010) and is based on a number of early voltammetric studies that established the high stability of electrically evoked DA responses (e.g. (Ewing et al. 1983, Millar et al. 1985, Michael et al. 1987a, Michael et al. 1987b, Suaud-Chagny et al. 1995, Kulagina et al. 2001, Benoit-Marand et al. 2011).
According to Davidson et al (2000), prolonged exposure to nomifensine poisons carbon fiber electrodes. During this study, electrodes were exposed for only 30 min to a single dose of nomifensine. The poisoning effects noted by Davidson et al were not observed during this study.
Data analysis
The initial rate of evoked DA overflow was determined from the slope of the evoked responses during the first 200 ms of each electrical stimulus. The initial linear DA clearance rate was measured from the slope of the descending phase of the response after the end of the stimulus: linear segments of this phase were defined by at least three data points with an r2>0.96. Overshoot time was the length of time needed after the end of the stimulus for the evoked response to reach its maximum amplitude. The overshoot concentration was the amount by which the DA concentration continued to increase after the end of the stimulus. Statistical analyses were by the t-test and two-way ANOVA with a repeated measures design (SPSS software).
Electron microscopy
Electron microscopy of carbon fiber probe tracks, including tissue processing and tracing the electrode track, was performed as previously described (Peters et al. 2004).
Results
Domain-dependent dynamics of evoked DA release and clearance
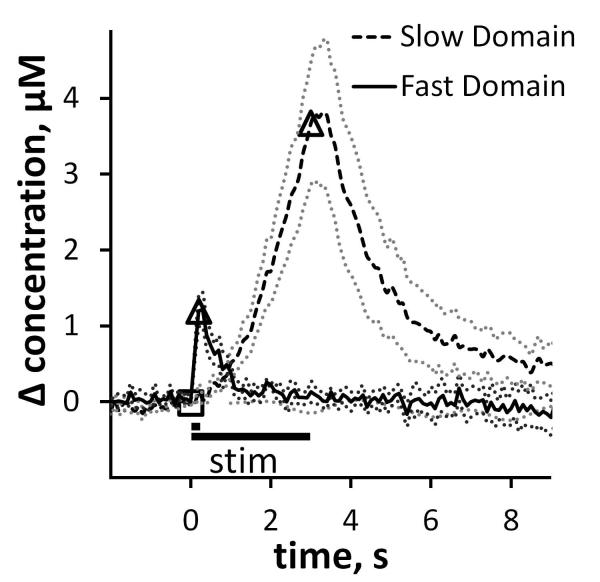
Evoked DA responses in the rat dorsal striatum are domain-dependent (Moquin & Michael 2009, Wang et al. 2010, Moquin & Michael 2011). Responses in fast domains (Fig 1 solid line) exhibit a) a rapid onset when the stimulus starts (DA is readily detected on the first FSCV measurement 100 ms after the stimulus begins), b) short term depression (less increase in DA during the 2nd 100 ms of the stimulus than during the first 100 ms), c) no delay in DA clearance after the stimulus ends (no “overshoot”), and d) rapid DA clearance. Responses in slow domains (Fig 1 dashed line) exhibit a) a slow onset when the stimulus starts (DA is non- or barely-detectable after the first 200 ms of the stimulus), b) short-term facilitation of the evoked response (the response rises more rapidly as the stimulus continues), c) a delay in clearance (overshoot) after the stimulus ends, and d) slow DA clearance.
Figure 1.
Fast and slow electrically evoked responses are recorded by fast scan cyclic voltammetry with carbon fiber microelectrodes in the dorsal striatum of isoflurane-anesthetized rats. In this and subsequent figures, the open symbols mark the beginning and end of the stimulus and the dotted lines show the SEM of the individual data points in each trace. These responses are the average (±SEM) of multiple individual responses (n= 6 fast and 8 slow) each recorded in a different rat with a different electrode. The fast and slow responses were obtained with identical procedures except for the stimulus duration (200 ms in the case of fast responses and 3 s in the case of slow: see text for full explanation).
The voltammetric responses in Fig 1 and subsequent figures are the average of a group of responses recorded in multiple animals. The dotted lines above and below the average responses show the SEM of each data point: because there are so many data points (10 s−1), individual error bars are omitted for clarity. The fast response in Fig 1 is the average (± SEM) of n=6 individual responses recorded with 6 different carbon fiber microelectrodes in 6 different rats. The slow response (Fig 1) is the average (± SEM) of n=8 responses recorded with 8 different carbon fiber microelectrodes in 8 different rats. So, Fig 1 contains data from 14 rats in total. Fig 1 establishes that the domain-dependent evoked responses are reproducible between subjects.
Slow domains are readily identified in all rats. However, recording from fast domains requires optimization of the placement of the voltammetric electrode (May & Wightman 1989a, Kawagoe et al. 1992, Garris et al. 1994, Garris et al. 2003, Venton et al. 2003). Optimization involves lowering the electrode in small increments (50-100 μm) and repeating the stimulus at each new recording site. To confine this study to the dorsal striatum, the electrodes were lowered by no more than 1 mm. If a fast domain was not identified by optimization, slow responses were collected: we did not attempt multiple or deeper electrode penetrations during this study. A fast site is identified in two ways. First, evoked DA release is observed upon the very first voltammetric scan, which is performed 100 ms after the stimulus begins. Second, the response exhibits short-term inhibition, i.e. the rate of evoked overflow during the second 100 ms of the stimulus is less than during the first 100 ms. These characteristics are completely and obviously different from those associated with slow responses wherein evoked DA release is delayed and exhibits short term facilitation. In this and prior studies we have adopted the practice of limiting the duration of the stimulus in fast domains to 200 ms. There are two reasons for this. First, beyond 200 ms the fast responses fade, i.e. the DA signal decreases even though the stimulus continues, due to the onset of autoinhibition induced by the evoked increase in extracellular DA: the fast responses, therefore, are highly transient (Moquin & Michael 2009). Second, if the stimulus continues beyond 200 ms, a slow response is observed. This implies that the dimensions of the fast domain is smaller than the length of the electrode such that the electrode is partially located in a fast domain and partially located in a slow domain (please also see Fig 8, below). We previously labeled this mix of fast and slow characteristics as a hybrid response (please see Moquin & Michael 2009 for additional details and examples of the hybrid response).
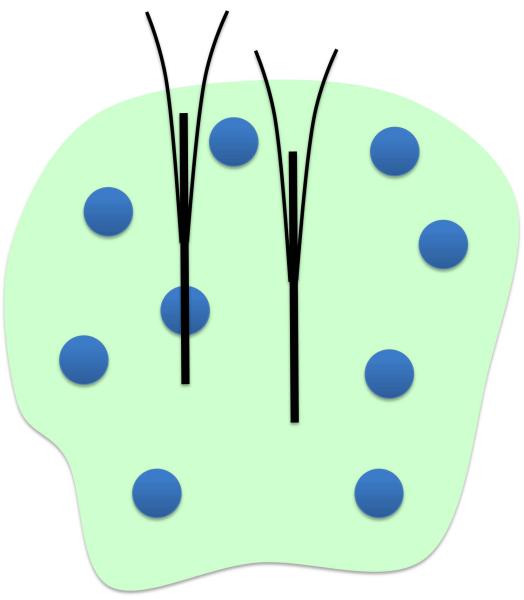
Figure 8.
Schematic of the proposed domain architecture depicting island-like fast domains (blue circles) on a slow background (pale green). One electrode is depicted as traversing a fast island while a second electrode is depicted within the slow domain. Lowering the second electrode to deeper recording sites would not result in detection of a fast domain.
Domain-dependent effects of nomifensine: slow domains
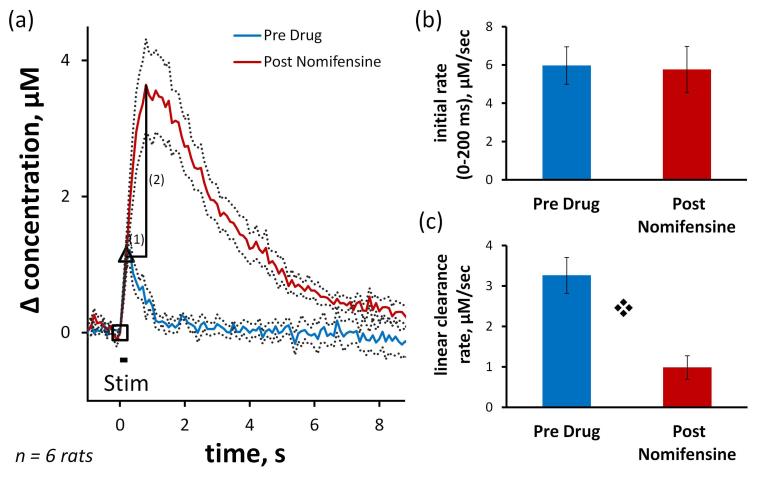
Nomifensine has multiple effects on slow responses (Fig 2a: this figure includes the slow pre-drug response from Fig 1 for comparison). The post-nomifensine response (Fig 2a) is the average (±SEM) of the recordings in the same group of 8 rats. Nomifensine significantly (p<0.005) increased the initial rate of evoked overflow during the first 200 ms of the stimulus (Fig 2b), eliminated the tendency of the signal to rise more rapidly as the stimulus continued (Fig 2a: the rising phase of the post-nomifensine response is nearly linear rather than curved upwards), and significantly increased the amplitude and duration of the signal overshoot (see Fig 6). Nomifensine did not significantly affect the slope of the initial linear segment of the DA clearance profile (Fig 2c) but slowed the nonlinear segment after the DA signal fell below a DA concentration near 4 μM (see Fig 2a inset for a comparison of DA clearance starting at a concentration of 4 μM).
Figure 2.
Nomifensine (20 mg/kg i.p.) affects evoked responses in slow domains. Fig 2a) Average (±SEM, n=8) of individual responses. Inset: Nomifensine slows the nonlinear segment of DA clearance below 4 μM. Fig 2b) Nomifensine significantly increases the rate of evoked overflow during the first 200 ms of the stimulus (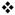 p<0.005, paired t-test). Fig 2c) Nomifensine has no effect on the rate of linear DA clearance during the descending phase of the response. Comparison of the clearance profiles during the final 4 μM of each response (inset) shows that nomifensine slowed the nonlinear phase of clearance in the slow domain.
p<0.005, paired t-test). Fig 2c) Nomifensine has no effect on the rate of linear DA clearance during the descending phase of the response. Comparison of the clearance profiles during the final 4 μM of each response (inset) shows that nomifensine slowed the nonlinear phase of clearance in the slow domain.
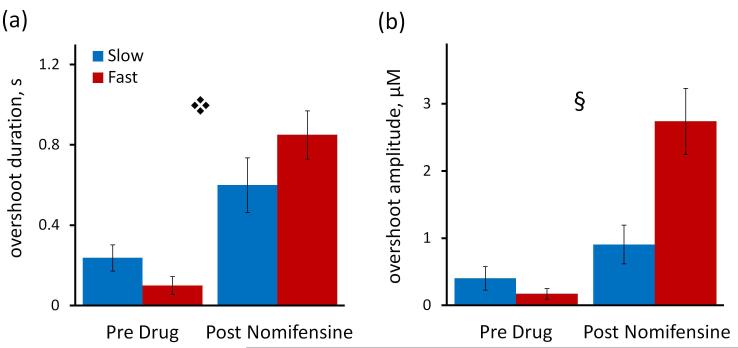
Figure 6.
Nomifensine affects signal overshoot, a measure of the delay at the end of the stimulus (see Fig 4a for guidelines). Fig 6a) Nomifensine significantly increased the overshoot duration to 0.60 sec in the slow domains and to 0.85 sec in the fast domain (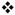 2-way ANOVA with repeated measures: treatment F (1,12) = 64.152, p < 0.000005, interactions F (1,12) = 7.783, p < 0.02). Fig 6 b) Nomifensine significantly increased the overshoot amplitude in fast but not slow domains (§ two way ANOVA with repeated measures: domains (fast and slow) F(1,12) = 5.264, p < 0.05, treatment F(1,12) = 51.659, p < 0.000002, interactions F(1,12) = 23.166, p < 0.0005).
2-way ANOVA with repeated measures: treatment F (1,12) = 64.152, p < 0.000005, interactions F (1,12) = 7.783, p < 0.02). Fig 6 b) Nomifensine significantly increased the overshoot amplitude in fast but not slow domains (§ two way ANOVA with repeated measures: domains (fast and slow) F(1,12) = 5.264, p < 0.05, treatment F(1,12) = 51.659, p < 0.000002, interactions F(1,12) = 23.166, p < 0.0005).
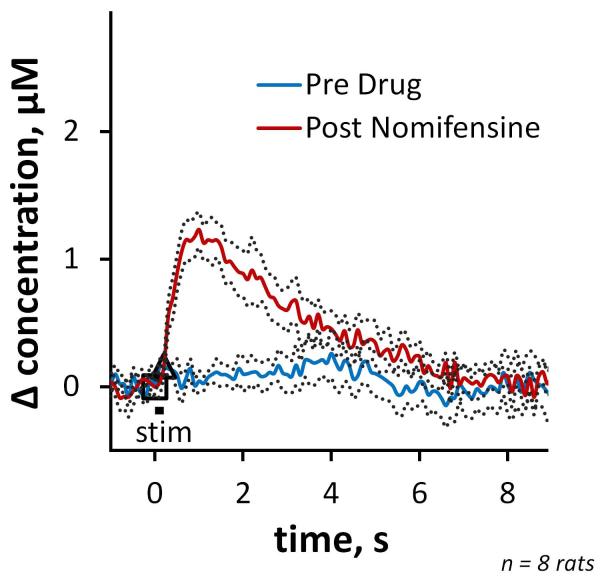
In the pre-drug condition, 200-ms stimuli evoked little-or-no detectable response in slow domains (Fig 3 blue). However, responses were clearly detected after nomifensine administration (Fig 3red). The onset of these responses was delayed with respect to the start of the stimulus: in 5 of 8 cases, the onset of the response occurred after the end of the stimulus.
Figure 3.
Pre- and post-nomifensine responses (average ±SEM, n=8) recorded in slow domains using a 200 ms stimulus duration.
Domain-dependent effects of nomifensine: fast domains
Nomifensine has multiple effects on fast responses (Fig 4a: this figure includes the fast response from Fig 1 for comparison), however these are distinct from the effects observed in slow domains. The post-nomifensine response is the average (± SEM) of the recordings in the same group of 6 rats. Nomifensine did not affect the initial response rate during the 200 ms stimulus (Fig 4a and 4b), dramatically increased the duration and amplitude of the stimulus overshoot (see also Fig 6), significantly (p<0.0005) decreased the slope of the initial linear segment of the clearance profile (Fig 4a and 4c), and also slowed the nonlinear segment of the DA clearance profile.
Figure 4.
Nomifensine affects evoked responses in fast domains. Fig 4a) Average (±SEM, n=6) of individual responses. The guidelines show how the duration (1) and amplitude (2) of the overshoot are defined (see Fig 6). Fig 4b) Nomifensine does not affect the initial rate of evoked overflow during the first 200 ms of the stimulus. Fig 4c) Nomifensine significantly decreases the slope of the linear segment of DA clearance ( p < 0.0005, paired t-test).
p < 0.0005, paired t-test).
Domain-dependent effects of nomifensine: comparisons
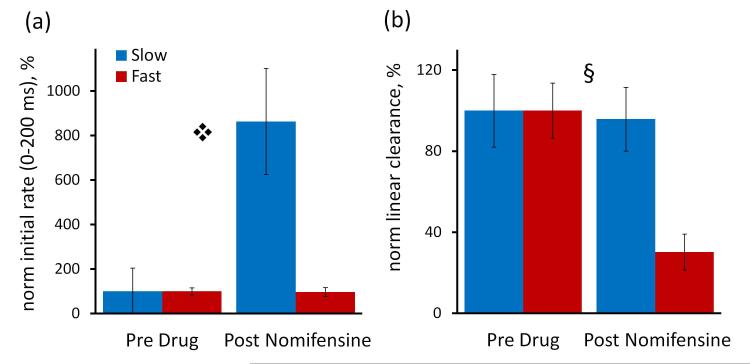
To emphasize and clarify the domain-dependent actions of nomifensine, we report the initial response rates (0-200 ms) and linear clearance rates normalized with respect to their pre-nomifensine values (Fig 5). Nomifensine significantly increased the normalized initial response rate in slow but not fast domains (Fig 5a). According to 2-way ANOVA (details provided in the Fig 5 legend), the drug treatment (pre- and post-nomifensine, p<0.002) and interactions (p<0.002) are significant. Nomifensine significantly slowed the normalized rate of linear DA clearance in fast but not slow domains (Fig 5b): the drug treatment (p<0.000002) and interactions (p<0.000005) are significant.
Figure 5.
The normalized effects of nomifensine are domain-dependent. The initial rates of overflow (Fig 5a) and linear clearance (Fig 5b) are normalized with respect to their pre-nomifensine values. Fig 5a) Nomifensine significantly increased the normalized initial rate of overflow in slow but not fast domains (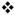 , 2-way ANOVA with repeated measures: drug treatment (pre- and post-nomifensine) F(1,12) = 14.517, p < 0.002, interactions F(1,12) = 14.771, p < 0.002). Fig 5b) Nomifensine significantly decreased the rate of linear DA clearance in fast but not slow domains (§ 2-way ANOVA with repeated measures: treatment F(1,12) = 79.332, p < 0.000002, interactions F(1,12) = 62.172, p < 0.000005)
, 2-way ANOVA with repeated measures: drug treatment (pre- and post-nomifensine) F(1,12) = 14.517, p < 0.002, interactions F(1,12) = 14.771, p < 0.002). Fig 5b) Nomifensine significantly decreased the rate of linear DA clearance in fast but not slow domains (§ 2-way ANOVA with repeated measures: treatment F(1,12) = 79.332, p < 0.000002, interactions F(1,12) = 62.172, p < 0.000005)
Nomifensine significantly affected the duration and amplitude of the overshoot after the end of the stimulus (measured according to the guidelines in Fig 4a). Nomifensine significantly increased the overshoot duration (p<0.000005) but to a greater extent in fast domains (Fig 6a). Nomifensine significantly increased the overshoot amplitude (p<0.00002) but to a greater extent in fast domains (Fig 6b: 2-way ANOVA details are in the figure legend).
In both fast and slow domains, nomifensine slowed the rate of non-linear clearance, as expected given that nomifensine is a competitive DAT inhibitor (Wightman et al. 1988, Peters & Michael 2000, Wu et al. 2001b, Michael et al. 2005).
Electron Microscopy
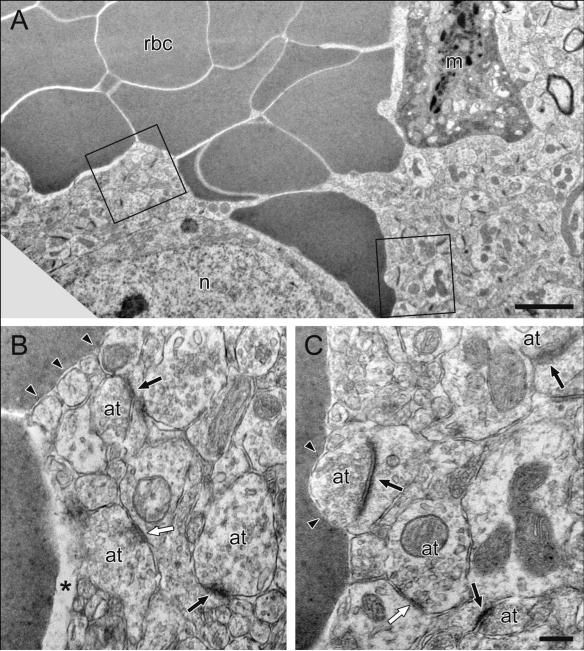
Electron microscopy (Fig 7) exposes the ultrastructural details of the tissue architecture in the vicinity of the electrode track. Key features of the image are explained in the figure legend. This image was obtained, as previously described (Peters et al. 2004) by starting above the recording site, where the track formed by the glass barrel of the electrode is obvious, and following the track ventrally until it exhibits dimensions commensurate with the diameter of the carbon fiber. In this image, the track is visualized as an approximately round spot of red blood cells that apparently filled the void created when the electrode was explanted from the tissue. Because these red blood cells are outside vessels, they were not removed during the perfusion. The track has a well-defined boundary where the red blood cells meet the tissue. Numerous identifiable elements are present in close proximity to this boundary (see Fig 7 legend for details), including axon terminals forming symmetric or asymmetric synaptic junctions. Numerous synaptic junctions with normal morphology are present less than 1 μm from the track boundary.
Figure 7.
Electron microscopic images of a carbon fiber track in the rat dorsal striatum. Fig 7A) At lower magnification, the track appears as an approximately round spot filled with red blood cells (rbc) that apparently fill the void formed when the electrode is explanted. Also visible are a single reactive monocyte (m) and the cell body of a neuron (n). The regions of interest outlined by boxes are shown at higher magnification in Fig 7B, Fig 7C. Blood cells are directly apposed to neuronal structures (arrowheads) or separated from them by a slightly larger space (asterisk in B). The morphological appearance of these neuronal structures is normal. Multiple axon terminals (at) form symmetric (white arrows) or asymmetric synapses (black arrows) onto dendritic shafts or spines, respectively. Scale bar in Fig 7A corresponds to 2 μm for Fig 7A and the scale bar in Fig 7C corresponds to 0.25 μm for Fig 7B and Fig 7C.
Discussion
This study reinforces the presence of distinct fast and slow DA kinetic domains in the rat dorsal striatum (Fig 1) and demonstrates that the actions of nomifensine on evoked DA responses are domain-dependent. Although several previous studies have examined nomifensine’s actions on evoked DA (Jones et al. 1995, Garris et al. 2003, Robinson & Wightman 2004, Borland et al. 2005, Benoit-Marand et al. 2011), none addressed the domain-dependency. As discussed in detail below, the impact of nomifensine on evoked DA responses cannot be explained solely by its ability to increase the effective KM of DA uptake, which points to an additional action of nomifensine on DA. Based on the findings of this study, we propose that additional action involves an interaction with restricted DA diffusion processes in the extracellular space.
Detection of DA domains
In our experience, slow domains are found throughout the dorsal striatum (see Materials and Methods for coordinates) but it is necessary to optimize the electrode placement in order to identify fast domains. Optimization to find DA “hot spots” is a common procedure (May & Wightman 1989a, Kawagoe et al. 1992, Garris et al. 1994, Garris et al. 2003, Venton et al. 2003), but relatively little attention has been devoted to the “cold spots”, which have been viewed as non-DAergic sites (Venton et al. 2003). Our recent reports, however, demonstrated that the cold spots are slow DAergic domains wherein DA terminals are autoinhibited (Moquin & Michael 2009, Wang et al. 2010). Although evoked DA release in the striatum is generally described as heterogeneous (Wightman et al. 1988, May & Wightman 1989b, Kawagoe et al. 1992, Zahniser et al. 1999, Venton et al. 2003), the fast and slow responses are reproducible across subjects (Fig 1).
Our findings provide some preliminary indication of the architecture of the domains. The schematic in Fig 8 depicts fast domains as “islands” on a slow background, with one microelectrode traversing a fast island and a second contained entirely within the slow “sea”. The idea that fast domains are smaller than the length of the electrodes (200 μm) rests on the observation of hybrid responses, consisting of both fast and slow components, when the stimulus extends beyond 200 ms(Moquin and Michael 2009). The spacing between the fast domains appears to be more than the length of the electrode (200 μm), since many recording sites produce only the slow response.
A model for evaluating evoked responses
Evoked DA responses are often evaluated with a model that combines the rates of DA release and clearance (May et al. 1988, Wightman et al. 1988, Kennedy et al. 1992, Wu et al. 2001a, Michael et al. 2005):
| Equation 1 |
where [DA] is the extracellular DA concentration, t is time, f is the stimulus frequency, [DA]p is the concentration of DA released per stimulus pulse, and Vmax and KM are the maximal rate and Michaelis constant, respectively, of DA uptake. So, f · [DA]P is the rate of evoked DA release and is the rate of DA uptake.
According to this model (see Supplementary Information), the difference between the rate of evoked release and DA uptake determines the rising phase of the evoked response, whereas uptake alone determines the descending phase of the response. If the DA concentration sufficiently exceeds KM, then the descending phase of the response is predicted to exhibit an initial linear segment, reflecting the zero-order kinetics of saturated transporters (V = Vmax), followed by a nonlinear segment when transporters are no longer saturated (V ≈ k[DA], where k = Vmax/KM) (Peters & Michael 2000, Wu et al. 2001b).
Measured responses sometimes exhibit delays at the beginning and end of the stimulus. These delays are not described by Equation 1 and are usually attributed to diffusion across a gap between the electrode and DA terminals (Kristensen et al. 1987, Engstrom et al. 1988, Garris et al. 1994, Jones et al. 1995). Such a gap might be caused by the use of a Nafion film or damage to the tissue adjacent to the electrode. The delays can be removed with a deconvolution algorithm. Once the responses are deconvoluted, the model can be used to determine “intrinsic” values [DA]p, Vmax, and KM (Engstrom et al. 1988, Wightman et al. 1988, May & Wightman 1989a, Kawagoe et al. 1992, Garris et al. 1994, Wu et al. 2001b, Venton et al. 2002, Garris et al. 2003). Without deconvolution, the slopes of the responses can be used to estimate “apparent” kinetic values, which are likely to include diffusion contributions since diffusion acts on local DA concentrations (Rice & Cragg 2008).
Evaluating slow responses
The rising phase of slow responses exhibits an obvious delay when the stimulus starts (the onset is delayed, begins slowly, and speeds up as the stimulus continues). But, rather than diffusion, this delay is due to autoinhibition: the delay is eliminated by the D2 antagonist, raclopride, and enhanced by the D2 agonist, quinpirole (Moquin & Michael 2009). Because the delay is not due to diffusion, the deconvolution algorithm cannot be used, so we analyzed these responses for apparent kinetic parameters (we have not yet attempted to elaborate on Equation 1 to include autoinhibition: however, see (Montague et al. 2004)). Qualitatively, Equation 1 predicts that a competitive uptake inhibitor, which increases the effective KM (Miller & Tanner 2008), is expected to a) increase the slope of the rising phase of the response, b) have no effect on the rate of the initial linear segment of the DA clearance profile, and c) increase the concentration at which the clearance kinetics transition from zero- to first-order (Peters & Michael 2000, Wu et al. 2001b). Nomifensine produces all of these predicted effects in slow domains (Fig 2).
Two aspects of the post-nomifensine response in slow domains deserve further consideration. First, nomifensine eliminated the response delay at start of the stimulus. This reinforces the conclusion that the delay is not diffusional because DA terminals must be present very near the electrode in order to detect DA release so quickly (100 ms) after the stimulus begins. Because nomifensine targets DAT, this observation also reveals the presence of functional DATs very near to the electrode, and in the dorsal striatum only DA terminals express this protein (Rocha et al. 1998). The detection by EM of axon terminals in close proximity to the electrode track (Fig 7) also supports this conclusion. An alternative possibility is that nomifensine eliminated the onset delay by triggering the desensitization of autoreceptors (Katz et al. 2010): at present, we consider this mechanism unlikely because a) uptake inhibition is expected to increase the rate overflow and b) in our hands the D2 agonist quinpirole further suppressed evoked release, which is not the expected consequence of desensitization (Moquin & Michael 2009). Second, even though nomifensine decreased the onset delay, it increased the response overshoot at the end of the stimulus (Fig 6). The asymmetry of nomifensine’s impact on the response delays (i.e. decreasing the delay at the start of the stimulus and increasing the delay at the end of the stimulus) is very surprising and has not been commented on before in the literature. As mentioned above, delays are usually attributed to a diffusion gap, but a diffusion gap causes symmetrical delays, i.e. a larger gap will increase the delay at both the start and end of the stimulus (see Supplementary Information). As discussed below, the asymmetry of the delays observed in this study require careful consideration.
Analysis of the slope of the rising and descending phases of the slow responses yields a set of apparent DA kinetic parameters (Fig 2b and 2c). The uniformity of the slow domain responses implies an absence of concentration gradients during these measurements, so the apparent values are probably not greatly affected by diffusion. In that case, the apparent rate of DA clearance reflects the activity of transport, which primarily occurs via the DAT (Moron et al. 2002, Torres 2006) with possible contributions from other transporters (Wu et al. 1998, Moron et al. 2002, Larsen et al. 2011). However, our experiments were not designed to resolve the contribution of individual transporters. The apparent rates must be viewed as semi-quantitative because EM analysis (Fig 7) shows that the electrodes are partially blocked, in this instance by a reactive monocyte (an immune cell) near the electrode track: such blockage of the electrode surface leads to underestimation of DA concentrations and rates. Assuming that the blockage is constant over the short duration of these experiments, proportional comparisons of the apparent clearance rates are justified (Fig 5).
Evaluating fast responses
In fast domains, the evoked responses (pre-nomifensine) exhibit no delay when the stimulus starts or stops. The responses exhibit all the main features predicted by Equation 1 (see Supplementary Information): a) the signal rises without delay when the stimulus begins, b) the signal slows down as the stimulus continues (because the rate of DA clearance increases as the DA concentration rises), and c) the DA clearance profile exhibits an initial linear segment (zero-order clearance) followed by a non-linear segment (the expected conversion to first-order clearance). This supports the view that the fast responses are affected by neither autoinhibition nor diffusion gaps.
Some features of the post-nomifensine fast responses are also consistent with the kinetic model (Equation 1). The most obvious is the overall decrease in the rate of DA clearance after the end of the stimulus (see Supplementary Information), since nomifensine is a DAT inhibitor. On the other hand, nomifensine had no effect on the initial rate of evoked release (Equation 1 predicts an increase) and dramatically increased the duration and amplitude of the overshoot (Equation 1 does not predict overshoot). Thus, as in slow domains, nomifensine has a highly asymmetric effect on the response delays not predicted by the kinetic model.
It is important to emphasize that we cannot use the deconvolution algorithm to remove the overshoot from the post-nomifensine fast responses. The delay in these responses is highly asymmetric, whereas the deconvolution was developed to correct symmetric delays arising from diffusion gaps (see Supplementary Information). In prior studies, diffusion gaps were attributed to Nafion films, which were not used in this study, or tissue damage. Although tissue damage is a concern when implanting devices into brain tissue (Jaquins-Gerstl & Michael 2009, Jaquins-Gerstl et al. 2011), EM shows no evidence of a damage-related diffusion gaps in our studies (Fig 7). Moreover, the pre-nomifensine response is not delayed, so there is no evidence for diffusion gaps in the fast recording sites.
It is necessary to consider whether the proposed island-like geometry of the fast domains (Fig 8) contributes to the overshoot. For example, it is often mentioned (Nicholson 1995, Borland et al. 2005) that uptake inhibition provides DA with more time to diffuse further from its source. However, if molecules are produced within a small source, the rapid outward diffusion into an ever-expanding volume leads to rapid dilution at increasing distances from the source: this makes overshoots less likely, not more likely. For example, Rice and Cragg calculated the diffusion and uptake of DA after release from a single vesicle (Rice & Cragg 2008). Their calculations show that even in the absence of uptake (see their Fig 1) DA in the striatum reaches its peak extracellular DA concentration within a few milliseconds of the quantal event, whereas the overshoot duration observed during our study reached as long as 850 ms (Fig 6).
It is also known that DAT inhibitors can activate DA release from intraneuronal storage pools (Ewing et al. 1983, Venton et al. 2006). It is possible that activation of storage pools could contribute to the post-nomifensine response observed during this study. However, additional release would not by itself lead to asymmetry of the response delays, which are the focus of the remainder of this discussion.
A role for restricted diffusion in the effects of nomifensine
As explained above, the existing model of DA kinetics (Equation 1), even when adjusted for a diffusion gap (Supplementary Information), cannot explain nomifensine’s prominent impact on the asymmetry of the evoked responses. Thus, it appears that DA uptake is somehow coupled to extracellular DA diffusion in a manner not yet fully understood. We now present a new explanation that invokes restricted DA diffusion in the extracellular space.
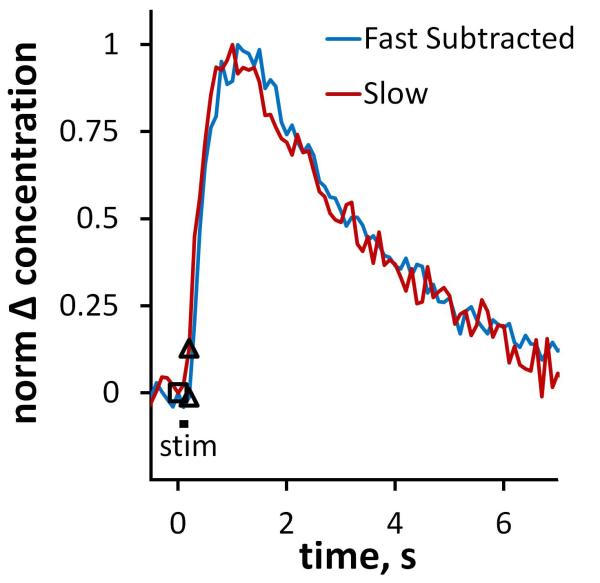
The overshoot in the post-nomifensine fast response is remarkable in that its duration of 850 ms is 4-times longer than the stimulus itself (Figs 4 and 6). A similar overshoot occurs in slow domains when the stimulus is likewise limited to 200 ms (see Fig 3 and Fig 9). The overshoots show that DAT inhibition allows DA to continue diffusing to the recording electrode long after DA terminals stop releasing DA. But, the large amplitude of the post-nomifensine responses shows that DA does not suffer substantial dilution during this process, so the diffusion occurs over a short distance. This combination, long diffusion time combined with little dilution, is highly suggestive of a restricted diffusion process.
Figure 9.
The “pure overshoots” observed in fast and slow domains post-nomifensine in response to a 200 ms stimulus. The two responses are normalized to their maximum amplitudes. The blue line was obtained from the fast domain by subtracting the pre-drug response from the post-nomifensine response. The red line is from Fig 3.
In this restricted diffusion scenario, DA molecules are released near the electrode but are restricted from diffusing to the electrode and thus go undetected. There are several ways in which diffusion can be restricted but an obvious candidate in brain tissue is the tortuosity of the extracellular space (Nicholson 1995, Nicholson & Sykova 1998, Sykova & Nicholson 2008). Because the extracellular space is tortuous, it is possible that some DA molecules are released into confined spaces connected to the surroundings by narrow passageways or bottlenecks. If diffusion through the bottlenecks is slow, DA could be taken up faster than it can diffuse to the surrounding. In this scenario, then, a DAT inhibitor would promote the escape, and thus detection, of DA with the long-duration overshoot reflecting the rate of DA diffusion through the bottlenecks.
This restricted diffusion scenario explains the asymmetric impact of nomifensine on the response delays as not all DA molecules are restricted from diffusing to the electrode. The rapid onset of the fast response is presumably due to non-restricted molecules, whereas the overshoot is due to restricted molecules. The restricted diffusion concept also explains the asymmetry of nomifensine’s actions on the slow responses. According to this new explanation of events, the decreased delay in the onset of the response is attributable, in the normal way, to a decreased rate of uptake (according to Equation 1, less uptake during the stimulus should increase the response rate), while the overshoot derives from overcoming restricted diffusion.
The literature offers precedent for this restricted diffusion concept. It was previously invoked by Floresco et al. (Floresco et al. 2003) to explain the effects of uptake inhibition on the detection of DA by microdialysis. Floresco et al. suggested that DA molecules are restricted to synaptic clefts until the DAT is inhibited. This idea, however, is at odds with the concept that DA molecules escape rapidly from striatal synapses (Garris et al. 1994), given their nanometer dimensions (see, for example, Fig 7). Despite this caveat, it is interesting to note that the restricted diffusion model appears to be consistent with DA measurements by both voltammetry and microdialysis.
Other mechanisms of restricted diffusion are known. For example, diffusion can be restricted if molecules interact with stationary binding sites (see section 8.4 of (Crank 1956)). In this case, molecules would not ‘break through’ to the electrode until the binding sites were completely occupied, which could be a consequence of uptake inhibition. Further investigation will be required to explain the precise mechanism by which DA diffusion is restricted but our results clearly suggest that DAT interacts with a complex diffusion process to maintain control of extracellular DA concentrations. Nomifensine’s actions cannot be explained solely on the basis of an increase the KM of DA uptake.
Conclusion
This study reinforces the existence of distinct DA dynamical domains in the dorsal portion of the rat striatum and identifies the domain-dependent actions of nomifensine. The asymmetrical effects of nomifensine on the domain-selective responses suggest that DAT interacts with complex DA diffusion processes in the striatum. The restricted diffusion model is interesting in light of our previous reports that local differences in basal DA concentrations cause, via autoinhibition, the domain-dependent DA dynamics (Moquin & Michael 2009, Wang et al. 2010). These local differences in DA concentration, while detected by voltammetry, are difficult to understand unless the local DA concentrations are somehow prevented from freely mixing. Thus, the present study not only reinforces the existence of the domains but also contributes to explaining them.
Supplementary Material
Acknowledgments
The authors are grateful to Prof. Stephen G. Weber, Department of Chemistry, University of Pittsburgh, for helpful discussions of restricted diffusion. This work was financially supported by the NIH (Grant MH 075989).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- PBS
phosphate buffered saline
Footnotes
There are no conflicts of interest.
References
- Benoit-Marand M, Ballion B, Borrelli E, Boraud T, Gonon F. Inhibition of dopamine uptake by D(2) antagonists: an in vivo study. Journal of Neurochemistry. 2011;116:449–458. doi: 10.1111/j.1471-4159.2010.07125.x. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi GY, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. Journal of Neuroscience Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Crank J. The mathematics of diffusion. Clarendon Press; Oxford: 1956. [Google Scholar]
- de la Fuente-Fernandez R, Schulzer M, Kuramoto L, et al. Age-Specific Progression of Nigrostriatal Dysfunction in Parkinson’s Disease. Annals of Neurology. 2011;69:803–810. doi: 10.1002/ana.22284. [DOI] [PubMed] [Google Scholar]
- Engstrom RC, Wightman RM, Kristensen EW. Diffusional Distortion in the Monitoring of Dynamic Events. Analytical Chemistry. 1988;60:652–656. [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221:169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Feng JX, Brazell M, Renner K, Kasser R, Adams RN. Electrochemical pretreatment of carbon fibers for in vivo electrochemistry: Effects on sensitivity and response time. Analytical Chemistry. 1987;59:1863–1867. doi: 10.1021/ac00141a028. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. Journal of Neuroscience. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald MD, Aminoff MJ. Therapies for Dopaminergic-Induced Dyskinesias in Parkinson Disease. Annals of Neurology. 2011;69:919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. European Journal of Pharmacology. 2003;479:139–152. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. Journal of Neuroscience. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Kannengi Mh, Raynaud JP. Nomifensine: a new potent inhibitor of dopamine uptake into synaptosomes from rat-brain corpus striatum. Journal of Pharmacy and Pharmacology. 1974;26:370–371. doi: 10.1111/j.2042-7158.1974.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, Michael AC. Comparison of the brain penetration injury associated with microdialysis and voltammetry. Journal of Neuroscience Methods. 2009;183:127–135. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, Shu Z, Zhang J, Liu YS, Weber SG, Michael AC. Effect of Dexamethasone on Gliosis, Ischemia, and Dopamine Extraction during Microdialysis Sampling in Brain Tissue. Analytical Chemistry. 2011;83:7662–7667. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 1995;274:396–403. [PubMed] [Google Scholar]
- Katz NS, Guiard BP, El Mansari M, Blier P. Effects of acute and sustained administration of the catecholamine reuptake inhibitor nomifensine on the firing activity of monoaminergic neurons. Journal of Psychopharmacology. 2010;24:1223–1235. doi: 10.1177/0269881109348178. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience. 1992;51:55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. Journal of Neurochemistry. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Son Y-D, Kim H-K, Lee S-Y, Cho S-E, Kim Y-B, Cho Z-H. Antipsychotic-Associated Mental Side Effects and Their Relationship to Dopamine D(2) Receptor Occupancy in Striatal Subdivisions A High-Resolution PET Study With (11)C Raclopride. Journal of Clinical Psychopharmacology. 2011;31:507–511. doi: 10.1097/JCP.0b013e318222353a. [DOI] [PubMed] [Google Scholar]
- Kristensen EW, Kuhr WG, Wightman RM. Temporal Characterization of Perfluorinated Ion Exchange Coated Microvoltammetric Electrodes for in vivo Use. Analytical Chemistry. 1987;59:1752–1757. doi: 10.1021/ac00141a003. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Ewing AG, Caudill WL, Wightman RM. Monitoring the stimulated release of dopamine with in vivo voltammetry. I: Characterization of the response observed in the caudate nucleus of the rat. Journal of Neurochemistry. 1984;43:560–569. doi: 10.1111/j.1471-4159.1984.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102:121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Larsen MB, Sonders MS, Mortensen OV, Larson GA, Zahniser NR, Amara SG. Dopamine Transport by the Serotonin Transporter: A Mechanistically Distinct Mode of Substrate Translocation. Journal of Neuroscience. 2011;31:6605–6615. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos MA, Han K-A, Heien ML, Ewing AG. Using in Vivo Electrochemistry To Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter. Acs Chemical Neuroscience. 2010;1:74–83. doi: 10.1021/cn900017w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LJ, Kuhr WG, Wightman RM. Differentiation of dopamine overflow and uptake processes in the extracellular fluid of the rat caudate nucleus with fast-scan in vivo voltammetry. Journal of Neurochemistry. 1988;51:1060–1069. doi: 10.1111/j.1471-4159.1988.tb03069.x. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Effects of D2 Antagonists on Frequency Dependent Stimulated Dopamine Overflow in Nucleus Accumbens and Caudate Putamen. Journal of Neurochemistry. 1989a;53:898–906. doi: 10.1111/j.1471-4159.1989.tb11789.x. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Heterogeneity of Stimulated Dopamine Overflow Within Rat Striatum as Observed With in vivo Voltammetry. Brain Research. 1989b;487:311–320. doi: 10.1016/0006-8993(89)90835-4. [DOI] [PubMed] [Google Scholar]
- Michael AC, Borland LM, Mitala JJ, Willoughby BM, Motzko CM. Theory for the impact of basal turnover on dopamine clearance kinetics in the rat striatum after medial forebrain bundle stimulation and pressure ejection. Journal of Neurochemistry. 2005;94:1202–1211. doi: 10.1111/j.1471-4159.2005.03265.x. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB. Dynamics of the recovery of releasable dopamine following electrical stimulation of the medial forebrain bundle. Neuroscience Letters. 1987a;76:81–86. doi: 10.1016/0304-3940(87)90196-0. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB. Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Research. 1987b;421:325–335. doi: 10.1016/0006-8993(87)91302-3. [DOI] [PubMed] [Google Scholar]
- Millar J, Stamford JA, Kruk ZL, Wightman RM. Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. European Journal of Pharmacology. 1985;109:341–348. doi: 10.1016/0014-2999(85)90394-2. [DOI] [PubMed] [Google Scholar]
- Miller A, Tanner J. Essentials of Chemical Biology: Structure and Dynamics of Biological Macromolecules. John Wiley & Sons Ltd.; Chichester, West Sussex, UK: 2008. [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PEM, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. Dynamic gain control of dopamine delivery in freely moving animals. Journal of Neuroscience. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. Journal of Neurochemistry. 2009;110:1491–1501. doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. An inverse correlation between the apparent rate of dopamine clearance and tonic autoinhibition in subdomains of the rat striatum: a possible role of transporter-mediated dopamine efflux. Journal of Neurochemistry. 2011;117:133–142. doi: 10.1111/j.1471-4159.2011.07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. Journal of Neuroscience. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi N, Kiuchi Y, Inagaki M, Inazu M, Yamazaki Y, Oguchi K. Effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviours in rats. European Journal of Pharmacology. 1995;281:195–203. doi: 10.1016/0014-2999(95)00246-h. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophysical Journal. 1995;68:1699–1715. doi: 10.1016/S0006-3495(95)80348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends in Neurosciences. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Movement Disorders. 2008;23:S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. Plenum Press; New York, NY: 1979. [Google Scholar]
- Peters JL, Michael AC. Changes in the kinetics of dopamine release and uptake have differential effects on the spatial distribution of extracellular dopamine concentration in rat striatum. Journal of Neurochemistry. 2000;74:1563–1573. doi: 10.1046/j.1471-4159.2000.0741563.x. [DOI] [PubMed] [Google Scholar]
- Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. Journal of Neuroscience Methods. 2004;137:9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien M, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. Journal of Neurochemistry. 2011;117:937–948. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: Rethinking dopamine transmission in the nigrostriatal pathway. Brain Research Reviews. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. Journal of Neurochemistry. 2004;90:894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nature Neuroscience. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. Journal of Neuroscience. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, et al. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlochtermeier L, Stoy M, Schlagenhauf F, et al. Childhood methylphenidate treatment of ADHD and response to affective stimuli. European Neuropsychopharmacology. 2011;21:646–654. doi: 10.1016/j.euroneuro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith MEA. Regulation of the dopamine transporter Aspects relevant to psychostimulant drugs of abuse. Addiction Reviews 2. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC. Intravenous self-administration of nomifensine in rats: Implications for abuse potential in humans. Science. 1981;212:1167–1168. doi: 10.1126/science.7195072. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Stimulated limbic and striatal dopamine release measured by fast cyclic voltammetry: anatomical, electrochemical and pharmacological characterisation. Brain Research. 1988;454:282–288. doi: 10.1016/0006-8993(88)90828-1. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Dugast C, Chergui K, Msghina M, Gonon F. Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. Journal of Neurochemistry. 1995;65:2603–2611. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine Redistributes Dopamine from Synaptic Vesicles to the Cytosol and Promotes Reverse Transport. Journal of Neuroscience. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S. Amphetamine and Other Weak Bases Act to Promote Reverse Transport of Dopamine in Ventral Midbrain Neurons. Journal of Neurochemistry. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiological Reviews. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE. The dopamine transporter proteome. Journal of Neurochemistry. 2006;97:3–10. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PEM, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. Journal of Neuroscience. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Analytical Chemistry. 2002;74:539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PEM, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. Journal of Neurochemistry. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Wang YX, Moquin KF, Michael AC. Evidence for coupling between steady-state and dynamic extracellular dopamine concentrations in the rat striatum. Journal of Neurochemistry. 2010;114:150–159. doi: 10.1111/j.1471-4159.2010.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. Journal of Neuroscience. 2001a;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: An in vivo voltammetric study. Journal of Neuroscience. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. Journal of Neuroscience Methods. 2001b;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen JW, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake(2)) and evidence for the expression of the transporter in the brain. Journal of Biological Chemistry. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: Regulation by extracellular dopamine concentration and dopamine transporter inhibitors. Journal of Pharmacology and Experimental Therapeutics. 1999;289:266–277. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.