Abstract
While early events in the pathogenesis of acute lung injury (ALI) have been defined, little is known about mechanisms mediating resolution. To search for determinants of resolution, we exposed wild type (WT) mice to intratracheal lipopolysacaccharide (i.t. LPS) and assessed the response at intervals to day 10, when injury had resolved. Inducible nitric oxide synthase (iNOS) was significantly upregulated in the lung at day 4 after LPS. When iNOS−/− mice were exposed to i.t. LPS, early lung injury was attenuated, however recovery was markedly impaired compared to wild type (WT) mice. iNOS−/− mice had increased mortality and sustained increases in markers of lung injury. Adoptive transfer of WT (iNOS+/+) bone marrow-derived monocytes or direct adenoviral gene delivery of iNOS into injured iNOS−/− mice restored resolution of ALI. Irradiated bone marrow chimeras confirmed the protective effects of myeloid-derived iNOS, but not of epithelial iNOS. Alveolar macrophages exhibited sustained expression of co-signalling molecule CD86 in iNOS−/− mice compared to WT mice. Antibody-mediated blockade of CD86 in iNOS−/− mice improved survival and enhanced resolution of lung inflammation. Our findings show that monocyte-derived iNOS plays a pivotal role in mediating resolution of ALI by modulating lung immune responses, thus facilitating clearance of alveolar inflammation and promoting lung repair.
Introduction
Acute lung injury (ALI) remains an important clinical problem, affecting over 190,000 patients annually in the United States with an estimated 75,000 deaths per year (1). Despite extensive investigation of underlying mechanisms in ALI pathogenesis, therapy remains mainly supportive, with only lung protective ventilation offering survival benefits (2).
The pathogenesis of ALI is notable for the activation of inflammatory cells, including neutrophils and alveolar macrophages, with increased production of pro- and anti-inflammatory mediators. An acute exudative phase, lasting up to 5–7 days, is manifest pathologically as diffuse alveolar damage, with protein rich edema fluid, neutrophilic infiltration of the interstitium and airspaces, and variable amounts of hyaline membranes (3). After the acute phase of ALI, some patients progress to a fibro-proliferative phase (4–6) which has been correlated with an increased risk of death (7). Although it has long been recognized that ALI may evolve through these stages, investigation has largely been focused on defining and targeting early steps in the pathogenesis. Little attention has been devoted to identification of mechanisms responsible for resolution of lung injury.
Nitric oxide (NO) has been implicated in the pathophysiology of ALI in animals and humans (8–13). NO participates in the regulation of every organ system in normal and pathologic situations (14, 15). Although NO in the normal lung is produced by the constitutive isoforms of nitric oxide synthase (nNOS and eNOS), its production during inflammation is mainly due to the inducible isoform (iNOS) (16). In the lung the major cells expressing iNOS are alveolar macrophages, alveolar epithelial cells and inflammatory infiltrating cells (17).
ALI and sepsis are associated with increased iNOS-derived NO (18–20). Several groups noted that deletion or inhibition of iNOS in mice is protective from LPS-induced mortality (21, 22) indicating a pro-inflammatory role for iNOS. Others have found that iNOS deletion had no effect (23). NO can contribute to microvascluar injury, pulmonary edema and neutrophilic infiltration in mouse models of sepsis (22, 23), with variable effects on mortality. However NO can also have beneficial effects on the immune system (24), acting as an antimicrobial (25–28) or anti-inflammatory/ immunosuppressive agent (29–32), and can modulate signaling pathways including NF-κB (33–36).
In contrast to the effect on acute responses, little is known about the impact of iNOS in resolution of lung inflammation and injury. We examined the effects of iNOS deletion on resolution of lung injury in a mouse model of LPS-induced ALI. We found that iNOS−/− mice had a biphasic response, with reduced severity of lung injury on day 1 after intratracheal LPS (i.t. LPS), but subsequently with a marked delay in ALI resolution, indicating a previously undescribed role for iNOS in lung repair. The defect in resolution in iNOS−/− mice was abrogated by adoptive transfer of naïve CD11b+iNOS+/+ monocytes or direct adenoviral delivery of iNOS to the lung. Furthermore, epithelial-derived iNOS did not contribute significantly in promoting resolution of ALI. We found that macrophage-derived iNOS regulates CD86 expression, which actively participates in the regulation of lung inflammation. Monocyte-derived iNOS plays a pivotal role in resolution of lung inflammation and lung repair.
Methods
Animals
Male C57BL/6 wild type (WT) mice, 8–10 weeks old and C57BL/6 background iNOS−/− male mice and congenic CD45.1 male mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed at the Johns Hopkins University Asthma and Allergy Center, and experiments conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee.
Animal Preparation
Using our published model (37), mice were anesthetized with intraperitoneal (IP) ketamine/ acetylpromazine (150/13.5 mg/kg) before exposure of the trachea. Escherichia coli LPS (O55:B5 Sigma L2880), 3.75 μg/g mouse weight or sterile water (control) was then instilled intratracheally (total volume 35 μl) via a 20-gauge catheter. At 1, 4, 7 and 10 days after instillation, groups of mice were anesthetized with IP ketamine/acetylpromazine and euthanized by exsanguination from the inferior vena cava. The lungs were perfused with 1 ml of phosphate-buffered saline (PBS), then bronchoalveolar lavage (BAL) was performed on the right lung; the left lung was processed for histology. BAL samples were routinely cultured to assess for bacterial infection.
Macrophage cell lines
Alveolar macrophage immortalized cell line (MH-S; ATCC) were cultured in six-well plates until 80% confluence before experimental challenge. Peritoneal macrophages were lavaged from C57BL/6 wild-type (The Jackson Laboratory), six days after injection of 3 ml of sterile thioglycollate (Sigma-Aldrich). Cells adhered overnight in RPMI 1640 supplemented with 10% heat-inactivated low-LPS FBS and 1% penicillin-streptomycin/1% glutamine before use. Cell were harvested by gentle scraping and resuspended in a single cell suspension for flow cytometric staining.
Analysis of bronchoalveolar lavage (BAL)
BAL was obtained by cannulating the trachea with a 20-gauge catheter. The right lung was lavaged with two aliquots of 0.7 ml of PBS without calcium. BAL was centrifuged at 700 × g for 10 min at 4oC. The cell-free supernatants were stored at −80°C until further analysis. The cell pellet was diluted in PBS, and total cell number was counted with a hemocytometer after staining with Turk’s Stain Solution (MERCK, Tokyo, Japan). Cell differentials (300 cells per sample) were counted on cytocentrifuge preparation with Diff-Quik stain (Baxter Diagnostics, McGaw Park, IL). Total protein and albumin were measured in the cell-free supernatant using the bicinchonic acid assay (BCA) (Pierce, Rockford, IL) and mouse serum albumin enzyme-linked immunosorbent assay (ELISA) kit (alpha Diagnostic Intl. Inc, San Antonio, TX), respectively.
Measurement of Cytokines
The levels of tumor necrosis factor (TNF-α), interleukin IL-10, macrophage inflammatory protein 2 (MIP-2) and activated transforming growth factor (TGF-β1) were measured in BAL by ELISA (R&D System, Minneapolis, MN).
Lung Histology, Immunofluorescence and lung injury scoring
Lungs (n=5 per time point) were inflated to a pressure of 25 cmH2O using 1% low melting agarose (Invitrgen, Carsbad, CA) for histologic evaluation by hemotoxylin and eosin staining (38). iNOS expression was assessed as previously described (13). Left lungs were fixed in 10% formalin neutral buffer for 8–12 h, embedded in paraffin and sectioned. Briefly, for lung Immunofluorescence, frozen sections were fixed with 4% paraformaldehyde, permeabilized with Triton X 100 (Fisher) and subsequently were blocked with 10% donkey serum. Tissue sections were then incubated with FITC-conjugated iNOS mAb (BD Biosciences) for 1 hour and then washed three times with cold PBS. Coverslips were mounted using a Fluormount G (Southern Biotech) and analyzed using a fluorescent microscope.
For lung injury scoring two blinded investigators analyzed the samples and determined levels of lung injury according to a semi-quantitative scoring system outlined below. All lung fields (×20 magnification) were examined for each sample. Assessment of histological lung injury was performed using the following scoring: 1- normal; 2- focal (<50% lung section) interstitial congestion and inflammatory cell infiltration; 3- diffuse (>50% lung section) interstitial congestion and inflammatory cell infiltration; 4- focal (<50% lung section) consolidation and inflammatory cell infiltration; 5- diffuse (>50% lung section) consolidation and inflammatory cell infiltration. The mean score was used for comparison between groups.
Immunoblotting
In order to assess the relative abundance of NOS isoforms during acute and resolution phases of lung injury, we performed immunoblots of lung homogenates as described (39) to determine the expression of iNOS, eNOS and nNOS. Thirty-two μg of total protein per sample in 1.5% (w/v) SDS was resolved on 8% SDS-PAGE gels and transferred to nitrocellulose membranes (Bio-Rad. Hercules, CA), then incubated with antibodies to iNOS, total eNOS (BD Pharmingen, Cary, NC), phosphorylated eNOS (Cell Signaling. Danvers, MA) and nNOS (BD Pharmingen) and detected using ECL Plus (Amersham, Piscataway, NJ). Equivalence of samples was confirmed using β-actin (Sigma) as a loading control. Relative band intensities were determined by densitometry using UN-SCAN-IT imaging software (Bio-Medicine. Orem, UT).
Determination of nitrate/nitrite (NOx concentration) by Griess reaction
NO formation was quantified colorimetrically in BAL and lung homogenate with the Griess reaction (World Precision Instruments, Sarasota, FL) in duplicate wells following enzymatic conversion of nitrate to nitrite by nitrate reductase (World Precision Instruments, FL). Fifty μL of BAL sample and 50 μL of buffer were incubated with 100 μL of Griess reagent for 20 minutes at room temperature. Lung tissue was homogenized using a Polytron homogenizer in1 ml homogenization buffer containing 0.25 M sucrose, 1 mM EDTA,1 mM sodium azide, and protease inhibitors (Complete protease inhibitors; Roche). 100 μL of lung homogenate was incubated with 100 μL of Griess reagent for 20 minutes at room temperature. Absorbance was measured at 570 nm using a microreader, and NOx concentration assessed against a sodium nitrite standard curve (0 μM through 100 μM analyzed in duplicate).
Measurement of reactive oxygen species generation
Reactive oxygen species (ROS) were monitored by measurement of hydrogen peroxide generation, based on the oxidation of 2′,7′-dichlorodihydrofluoresein (H(2)DCF-DA) (Sigma-Aldrich) to a fluorescent 2′,7′-dichlorofluoresein (DCF). In brief, BAL cells were incubated with 100μM of the H(2)DCF-DA for 20 min at 37°C. After washing twice with FACS buffer and surface staining with CD11c, the intensity of DCF fluorescence was determined by using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA), with an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Phagocytic index
Alveolar macrophages (AM) were obtained from WT and iNOS−/− mice by bronchoalveolar lavage. AM (105/well in DMEM without FCS) were adhered for 30 min in wells of eight-well tissue culture-treated plastic slides (Lab-Tek). The cells were washed gently, and latex micro-beads labeled with FITC (1.7 mm in diameter; Polysciences), were added to the wells (8 × 106 beads/well). After 1 h of incubation at 37°C, the wells were washed gently with PBS, fixed with methanol at −20°C for 20 min, and washed extensively.
The cells were viewed by a blinded observer using a Nikon fluorescent microscope. In each well, the fraction of cells containing labeled beads and the phagocytic index were determined by counting at least 200 cells in random high-power fields. The phagocytic index (PI) was calculated as follows: PI = fraction of AM with beads X mean number of beads per positive AM. Three replicate wells were evaluated for each condition.
Bone marrow isolation, adoptive transfer and chimeras
iNOS−/− mice and CD45.1+ mice were used to harvest bone marrow cells, obtained from femurs and tibias by flushing with 50ml-PBS through a 26-gauge needle. After centrifuge, red blood cells were lysed, and remaining cells in RPMI-1640 medium were counted. CD11b+ cells were isolated from the bone marrow cells (BM) using magnetic bead separation with CD11b MicroBeads (Miltenyi Biotec. Auburn, CA). Magnetically retained CD11b+ cells were eluted and the purity was checked using a FACSCalibur flow cytometer (Becton Dickinson, NJ). The purity of CD11b+ bone marrow cell fractions was always greater than 99%. Purification of donor bone marrow (BM) cells with magnetic CD11b beads yielded isolated CD11b+ cells, approximately 40% monocytes and 60% neutrophils. Each single, purified cell suspension (25–30 × 106 cells in 200 μl PBS) was adoptively transferred to iNOS−/− mice (n=4–5) at the designated time after i.t. LPS via tail vein injection, and samples were harvested at days 2, 4 and 10 after i.t. LPS. Mice were allocated into the following groups: (i) WT without adoptive transfer, (ii) PBS 200 μl into iNOS−/− mice, (iii) WT BM into iNOS−/− mice, (iv) iNOS−/− BM into iNOS−/− mice.
Recipient mice, B6.SJL-Ptprca Pep3b/BoyJ (congenic CD45.1 WT) and iNOS−/− mice (6–7 weeks of age), were lethally irradiated with one exposure to 9 Gy (Gammacell 40; Atomic Energy of Canada, Ottawa, ON). Immediately after irradiation, 2×106 BM cells were injected via retroorbital injection. Mice were housed in microisolators for 8 weeks before ALI challenge and fed autoclaved food and water containing 5 mM sulfamethoxazole and 0.86 mM trimethoprim. Reconstitution was assessed by flow cytometry.
Flow Cytometry
Cells were first incubated with Fc Block-2.4G2(BD Pharmingen) antibody to block Fcγ III/II receptors before staining with a specific antibody. The following antibodies were purchased from BD Pharmingen (SanDiego, CA) and Biolegend: anti-Annexin V-PE, anti-7-AAD, anti-LY6G-FITC, anti-IA-IE-PE, anti-CD11b-APCe780, anti-CD45.1-PE, anti-CD45.2-PerCy5.5, anti-CD14-FITC, anti-CD86-APC, anti-CD80-PE and anti-F4/80-APC; relevant isotype antibodies were also purchased. Monocytes, alveolar macrophages and lymphocytes were gated with characteristic forward scatter/side scatter using a FACSAria instrument, and CellDiva for data acquisition (Becton Dickinson, San Jose, CA), and Flowjo for analysis (Tree Star Inc, San Carlos, CA).
Cell Sorting
WT and iNOS−/− mice were sacrificed four days after i.t. LPS. A 20 Ga was used to instill 1 ml dispase (Roche) followed by 0.5 ml of 1% liquid agarose, which was solidified with ice. The lungs were removed and incubated in DMEM with 100U DnaseI (Roche) per ml and separated into single cells and filtered through 70 μm (Becton Dickinson). CD11b+FSChi (monocytes) and CD326+CD11bnegative (lung epithelial cells) were isolated by fluorescence activated cells sorting (FACS) using a Becton Dickinson Aria FACS machine. After cytospin, cells were fixed with methanol, permeabilized with Triton X and stained with mouse iNOS-FITC (BD Biosciences) for 1 hour and analyzed by fluorescent microscopy.
Adenoviral iNOS gene construct and delivery
To generate an adenovirus expressing red-shifted GFP-tagged iNOS (NOS2-rsGFP), we obtained the vector pcDNA3.1neo-iNOS-rsGFP from Dr. N.T. Eissa (Baylor College of Medicine, Houston), sub-cloned the cDNA for the tagged iNOS and transferred iNOS-rsGFP into the shuttle vector, pAdCMV.DEST-V5 (InVitrogen). Replication defective virus was prepared by transfecting pAdCMV-DEST-NOS2-rsGFP into HEK293A cells. Extracts from cultures showing cytopathic effects were collected and purified using Adenopure Filter system (PureSyn). Control virus was constructed similarly. Viral titer is determined in HEK293A cells by counting GFP-positive cells after limiting dilution. Pharyngeal aspiration of 50 μl/mouse (109 IU/ml) of adenoviral stock was given at designated time points after injury.
In vivo Antibody-mediated blockade
iNOS−/− animals were given 250 μg/dose/mouse of intraperitoneal injections of anti-mouse CD86 monoloclonal antibody (GL-1; BioXcell) or isotype (rat IgG, Sigma-Aldrich) on days +2, +4, and +6 after i.t. LPS challenge. CD86 blockade was confirmed in BAL and lung cells by flow cytometry.
Statistical analysis
All values are reported as mean ± SEM. Parametric or nonparametric testing was performed as indicated. Results between groups were compared using the Mann-Whitney U test. Baseline and pre-and post-treatment data within a group were compared using repeated measure one-way analysis of variance (Fisher’s protected least significant difference test). The survival curve was established with Kaplan-Meier survival analysis. A p<0.05 was used as the cut-off point for significance.
RESULTS
Resolution of lung injury is impaired in iNOS−/− mice
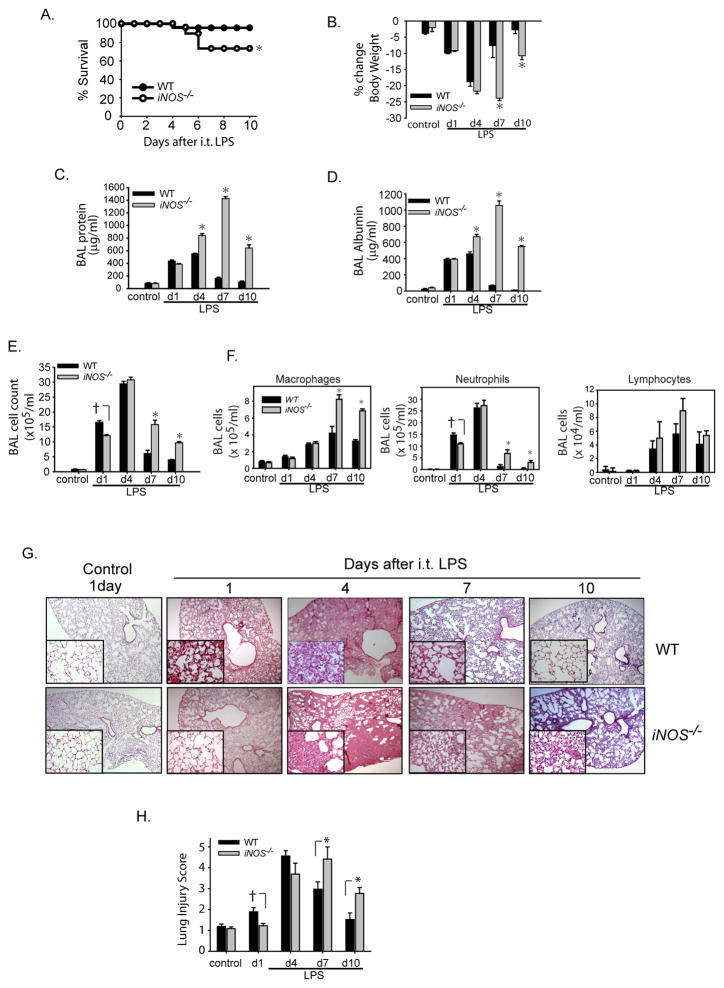
We examined the response of WT and iNOS−/− mice to i.t. LPS over 10 days. Survival was significantly reduced in iNOS−/− mice, evident after day 5 (Fig.1A). Surviving mice, both WT and iNOS−/−, appeared ill with lethargy, piloerection, and huddling behavior after i.t. LPS. These clinical signs resolved by day 5 to 6 in WT mice, but persisted in iNOS−/− mice. In addition, while both WT and iNOS−/− mice lost weight after i.t. LPS, WT mice regained baseline weight by day 10, while weight loss was sustained in iNOS−/− mice (Fig. 1B). BAL protein (Fig. 1C) and albumin (Fig. 1D) increased in both groups to more than 4–8 times baseline levels by day 1. In WT mice, BAL protein and albumin peaked at day 4, and returned to baseline by day 10. In iNOS−/− mice, BAL protein and albumin continued to increase out to day 7, and remained elevated at day 10 suggesting impaired resolution of alveolar-capillary injury. On day 1 after i.t. LPS, BAL total cell count was lower in iNOS−/− mice than in WT mice, while on days 7 and 10, it was higher in iNOS−/− mice (Fig. 1E). BAL macrophages increased in both groups, but remained higher in iNOS−/− mice on days 7 and 10 (Fig. 1F). Lymphocytes increased similarly in both WT and iNOS−/− mice at day 4 and remained elevated out to day 10 (Fig. 1F). BAL neutrophil numbers were significantly lower in iNOS−/− mice than in WT mice on day 1 after i.t. LPS (Fig. 1F), but the neutrophil number was increased in the iNOS−/− mice on day 7 and day 10. In WT mice, neutrophils were nearly undetectable in the alveolar compartment by day 10 after i.t. LPS. The pattern of histological changes in the lung was consistent with patterns for BAL protein and cells (Fig. 1G). Specifically, interstitial thickening and cellular infiltration were worse in WT mice at day 1 compared to iNOS−/− mice. However, at day 7 the histological changes were more pronounced in the iNOS−/− mice than in WT mice. By day 10, lung histology appeared near normal in the WT mice, yet histological changes persisted in the iNOS−/− mice. Blinded lung injury scoring confirmed a biphasic injury response in iNOS−/− mice; despite an attenuated early injury, iNOS−/− mice displayed a significant impairment in resolution of lung inflammation (Fig. 1H). These observations suggest an important role for iNOS in the resolution of lung injury.
Fig. 1. Resolution of lung injury is impaired in iNOS−/− mice.
WT and iNOS−/− mice (n= 8 per group per time point) were challenged with i.t. LPS. Survival (A) and (B) body weight relative to baseline were plotted at intervals after injury. Bronchoalveolar (BAL) protein (C), BAL albumin (D), BAL total cell counts (E) and BAL differential cell counts (F) were determined in WT and iNOS−/− mice after i.t. water (control) or i.t. LPS. (G) Histological sections were stained with H & E in WT and iNOS−/− mice. Original magnifications x20; x100 (insets). (H) Histopathological mean lung injury scores from x20 sections (n=4–6 animals per group per time point). Values expressed as mean ± SEM; *, p<0.05 log-rank test (mortality curves) and unpaired Student’s t test (for other injury parameters).
iNOS expression and source during ALI
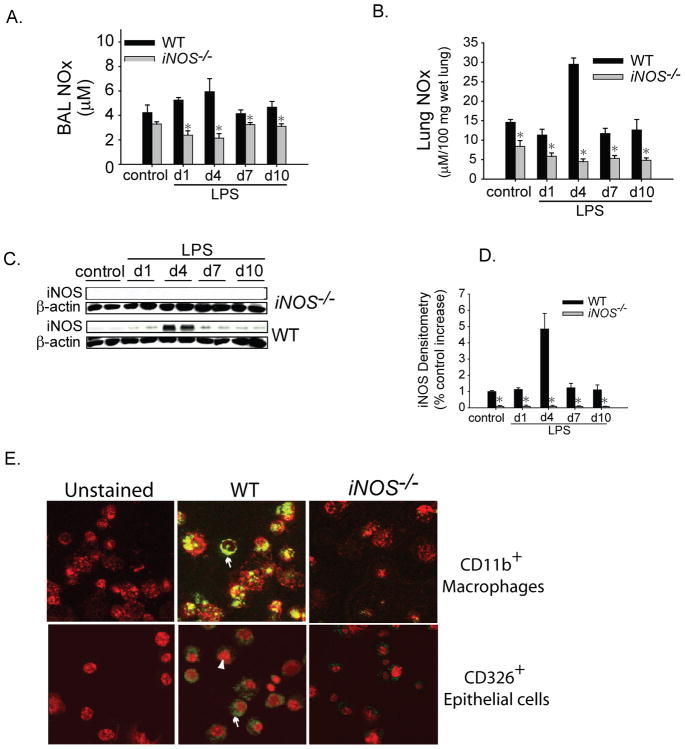
We sought to examine the patterns of NO generation in lung and BAL fluid using the Griess reaction, which measures total nitrate and nitrite (NOx). Both BAL (Fig. 2A) and lung NOx (Fig. 2B) were significantly reduced in the iNOS−/− mice compared to WT mice at all time points. In WT mice, NOx production peaked at day 4 after i.t. LPS in both BAL and lung homogenate. Protein immunoblots revealed that iNOS increased 5-fold in the lung of WT mice on day 4 after i.t. LPS, but was reduced to baseline levels by day 7 (Figs. 2C, D). iNOS protein was not detectable in iNOS−/− mice. The pattern of expression of total endothelial NOS (eNOS) and phosphorylated eNOS was not different between WT and iNOS−/− mice at any time point. (Supplementary Fig. 1A). Neuronal NOS (nNOS) was higher in iNOS−/− mice at day 4 after LPS and similar to WT mice at all other time points. iNOS labeling was evident by immunofluorescence in alveolar cells only from WT mice on day four after i.t. LPS. No iNOS labeling was detected in injured iNOS−/− mice (Supplementary Fig. 1B). To better define cellular sources for iNOS during lung inflammation, we harvested lungs from WT and iNOS−/− mice four days after i.t. LPS. Lungs were minced and enzymatically digested to obtain a single cell suspension. Lung cells were surface stained with markers for macrophages (CD11b) and epithelial cells (CD326) and subsequently sorted using a FACS Aria cytometer. WT CD11b+FSChi macrophages showed intense iNOS labeling by fluorescent microscopy while iNOS−/− macrophages showed no labeling. WT CD326+CD11bneg epithelial cells also labeled positive for iNOS, in contrast iNOS−/− epithelial cells showed no labeling (Fig. 2E). These data indicate that iNOS is upregulated in inflammatory macrophages and lung epithelial cells during LPS-induced lung inflammation.
Fig. 2. iNOS expression and source during ALI.
(A) BAL and (B) lung nitrate/nitrite (NOX) concentrations were measured in WT and iNOS−/− mice after i.t. water (control) or LPS at designated intervals. (C) iNOS immunoblotting of lung homogenates by SDS-PAGE at intervals after i.t. LPS in WT and iNOS−/− mice. β-actin used as loading control. (D) iNOS densitometry (iNOS/actin) was expressed as a % increase over the WT control. Values expressed as mean ± SEM; *, p<0.05 versus WT within same time point. (F) Lungs were harvested 4 days after i.t. LPS and enzymatically digested into a single cell suspension, surface stained for CD11b-PE-Texas red (red staining in top panels) and CD326. Cells were subsequently sorted (FACSAria) and placed in a cytospin for staining with iNOS-FITC (arrows) using fluorescent microscope. For lower panel, TO-PRO3 (Invitrogen) nuclear staining (arrowhead) was used.
Alveolar inflammatory milieu altered in iNOS−/− mice
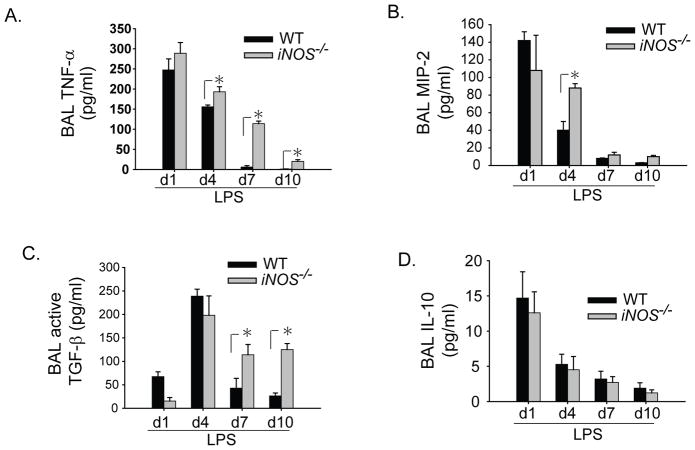
To begin examining potential mechanisms that might contribute to the difference in LPS response in iNOS−/− mice, we measured select BAL cytokines in the two groups. The pro-inflammatory cytokine TNF-α was elevated in both WT and iNOS−/− groups on day 1 after LPS (Fig. 3A), but was greater in the iNOS−/− mice on days 4, 7 and 10. The neutrophil chemokine MIP-2 was not different between WT and iNOS−/− mice on day 1 after i.t. LPS, however, was greater in the iNOS−/− mice on day 4 (Fig. 3B). Active TGF-β1 increased similarly in both groups on day 4 after i.t. LPS, but remained elevated on days 7 and 10 in the iNOS−/− mice (Fig. 3C). The anti-inflammatory cytokine IL-10 increased in both groups on day 1 after i.t. LPS, and was not different between the two groups at any time (Fig. 3D).
Fig. 3. Alveolar inflammatory milieu altered in iNOS−/− mice.
WT and iNOS−/− mice received i.t. LPS or sterile water (day 1 control). BAL pro-inflammatory (A) TNF-α, and (B) MIP-2; and anti-inflammatory (C) activated TGF-β1, and (D) IL-10 were measured by ELISA at designated intervals. Values expressed as mean ± SEM; *, p<0.05 compared to WT within same time point.
Alveolar neutrophil clearance impaired in iNOS−/− mice after ALI
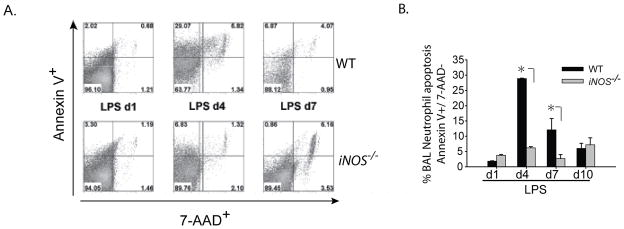
The persistent elevation of neutrophils in iNOS−/− mice on days 7 and 10 after i.t. LPS was striking, in contrast to WT animals in which alveolar neutrophils were nearly completely cleared. iNOS−/− mice displayed higher levels of neutrophil chemokine MIP-2 (Fig. 3B) by day 4 and had persistent disruption of their alveolar capillary membrane (elevated BAL albumin; Fig. 1D), suggesting roles for barrier disruption and chemokine gradients in neutrophil recruitment. We also evaluated neutrophil apoptosis and macrophage efferocytosis as potential contributors to the increase in alveolar neutrophils. To determine whether changes in apoptosis may contribute to the differences in neutrophil number, we harvested BAL cells at intervals after i.t. LPS, and assessed Annexin V and 7-AAD labeling of neutrophils by flow cytometry. On day 1 after LPS, apoptosis (Annexin V+, 7 AAD−) (Fig. 4A, B) was not different between groups. By day 4, apoptosis was significantly greater in neutrophils of WT mice compared to iNOS−/− mice, and remained elevated at day 7. By day 10, neutrophil apoptosis was similar between the two groups.
Fig. 4. Alveolar neutrophil clearance impaired in iNOS−/− mice after ALI.
(A) Neutrophil apoptosis (Annexin V+/7-AAD-) in the BAL from WT and iNOS−/− mice after i.t. LPS was assessed by flow cytometry. BAL neutrophils were gated by characteristic granulocyte Forward and Side scatter, and the population was further sub-gated for Gr-1+ neutrophils. (B) Average percentages for BAL neutrophils for annexin V+/7-AAD− in WT and iNOS−/− mice (n=4/group/time point). Values expressed as mean ± SEM; *, p<0.05 compared to WT within same time point.
We also examined alveolar macrophage phagocytosis using BAL macrophages harvested at intervals after i.t. LPS. Phagocytosis by alveolar macrophages from iNOS−/− mice was significantly lower than macrophages from WT mice on days 1 and 4 after i.t. LPS (Supplementary Fig. 1C). The phagocytic index was not different in the two groups on days 7 and 10 after i.t. LPS.
The absence of iNOS leads to sustained alveolar macrophage activation after LPS
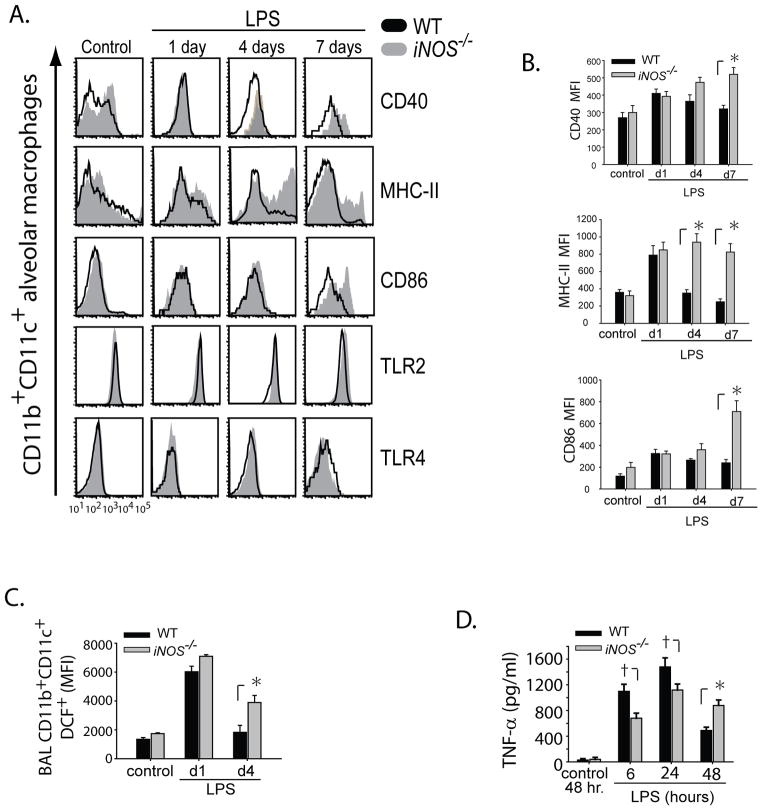
The marked delay in resolution of lung inflammation in the iNOS−/− mice, coupled with the persistent elevation of BAL TNF-α, led us to examine phenotypic differences between alveolar macrophages in WT and iNOS−/− mice. Using multi-parameter flow cytometry, we observed that CD11b+CD11c+ alveolar macrophages in WT and iNOS−/− mice exhibit a similar expression of accessory and co-stimulatory molecules CD40 and CD86 on day 1 after LPS. In contrast, by day 7 after i.t. LPS, iNOS−/− macrophages had sustained expression of CD40, CD86 and MHC-II (Fig. 5A). Cell surface expression of co-stimulatory molecule CD80 (not shown) and toll-like receptors (TLR2 and TLR4) was similar between the 2 groups at all intervals after i.t. LPS (Fig. 5A). The mean fluorescence intensity (MFI) of macrophage cell surface molecules was compared between WT and iNOS−/− mice (n=4 animals/ interval per group) (Fig. 5B), and reinforces the differences noted in the histograms (Fig. 5A).
Fig. 5. The absence of iNOS leads to sustained alveolar macrophage activation after LPS.
BAL was obtained from WT and iNOS−/− mice after i.t. water (control) or i.t. LPS at designated time points. (A) Shows multicolor flow cytometry histograms for BAL macrophages (CD11c+CD11b+) and their relative expression profiles for CD40, MHC-II, CD86, TLR2 and TLR4. (B) Mean Fluorescence Intensity (MFI) for cell surface BAL macrophage CD40, CD86 and MHC-II. (C) WT and iNOS−/− BAL macrophage (CD11c+) intracellular production of Reactive Oxygen species (ROS) as measured by fluorescent 2′,7′-dichlorofluoresein (DCF). (D) Thioglygollate-elicited macrophages were obtained at day 6 from WT and iNOS−/− mice, plated and stimulated with LPS (100 ng/ml). Cell supernatants were collected and used to measure TNF-α at designated intervals. Values expressed as mean ± SEM; *, p<0.05 compared to WT within same time point; † p<0.05 compared to iNOS−/− within same time point.
We also sought to determine if CD11b+CD11c+ monocytes were functionally different between groups. Intracellular production of reactive oxygen species (ROS) as measured by DCF was elevated in both WT and iNOS−/− monocytes at day 1, but was significantly higher in iNOS−/− monocytes by day 4 (Fig. 5C). We also assessed thioglycollate-elicited peritoneal macrophages (1X 106 cells/well) from WT and iNOS−/− mice following stimulation with LPS (100 ng/ml). Supernatant TNF-α was higher in the LPS activated WT macrophages compared to the iNOS−/− macrophages at 6 and 24 hours after LPS. In contrast, by 48 hr, WT macrophages had a significant reduction in TNF-α secretion while iNOS−/− macrophages had persistently elevated levels of TNF-α, comparable to their 24 hr secretion profile (Fig. 5D). MIP-2 levels were similar in both groups at every interval (Supplementary Fig. 1D).
These findings indicate a role for iNOS in modulating alveolar macrophage phenotype and function.
Delivery of iNOS restores resolution of ALI in iNOS−/− mice
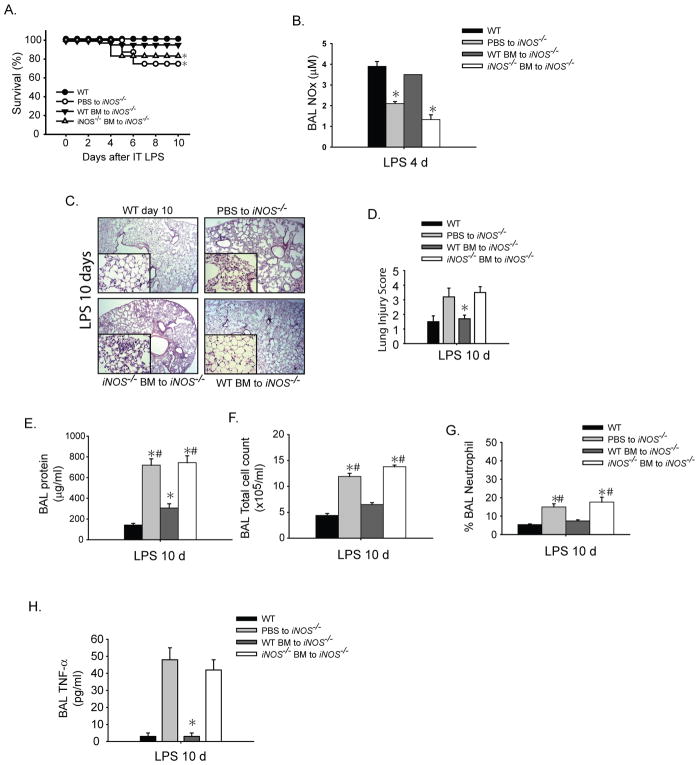
Our findings suggest that iNOS plays an important role in resolution of lung injury, and that alveolar macrophages are a potential cellular source of iNOS. To further assess a potential role for monocyte/macrophages in this response, we performed adoptive transfer of bone marrow-derived CD11b+ monocytes into iNOS−/− mice, and examined the effect on resolution of lung injury. iNOS−/− mice receiving tail vein injections of PBS or iNOS−/− BM-derived CD11b+ cells exhibited delayed resolution of lung injury and decreased survival. In contrast, iNOS−/− mice receiving WT iNOS+/+CD11b+ BM-derived cells delivered 24 hours after i.t. LPS had improved survival (Fig. 6A). Flow cytometry confirmed that following adoptive transfer, congenic donor bone marrow monocytes (CD45.1+) trafficked to the alveolar compartment and lung interstitium after LPS-induced ALI (Supplementary Fig. 2A). Donor CD45.1+F4/80+CD11b+ macrophages peaked in the alveolar and interstitial compartment at day 4 after adoptive transfer and injury (not shown).
Fig. 6. Adoptive transfer of WT monocytes restores resolution of ALI in iNOS−/− mice.
iNOS−/− mice were challenged with i.t. LPS and 24 hours after injury given PBS (sham) or bone marrow-derived CD11b+ monocytes isolated from iNOS+/+ or iNOS−/− mice by tail vein injection. (A) Survival curves for designated groups (n= 8–10 per group). (B) BAL NOx levels 4 days after i.t. LPS after designated transfers into iNOS−/− mice. Mean ± SEM; *; p<0.05 vs. Compared PBS to iNOS−/−, *#; p<0.05 vs. Compared to WT BM to iNOS−/− (C) Lung H–E sections and (D) histopathological lung injury scoring 10 days after i.t. LPS for designated groups (n=6 per group). (E) BAL protein, (F) BAL total cell counts, (G) BAL neutrophils (as a % of total BAL cells) and (H) BAL TNF-α levels 10 days after i.t. LPS are shown after designated transfers into injured iNOS−/− mice.
iNOS−/− mice receiving BM-derived iNOS+/+CD11b+ cells had higher NOx levels in the BAL (Fig. 6B) and lung homogenate on day 4 after adoptive transfer than did iNOS−/− mice receiving PBS or iNOS−/−CD11b+ cells (Supplementary Fig. 2B), confirming that adoptive transfer of WT CD11b+ cells led to increased local NO production in the alveolar space. iNOS−/− mice receiving iNOS+/+CD11b+ cells exhibited complete resolution of histological injury by day 10 (Figs.6C, D). BAL protein (Fig. 6E), total cell count (Fig. 6F) and BAL neutrophils (Fig. 6G) were elevated on day 10 in iNOS−/− mice receiving PBS or iNOS−/− CD11b+ cells, but were reduced nearly to WT levels following transfer of iNOS+/+CD11b+ cells. Mice receiving PBS or iNOS−/− BM cells had elevated BAL TNF-α levels out to day 10 after i.t. LPS, while transfer of BM iNOS+/+CD11b+ cells resulted in a reduction of BAL TNF-α to WT levels (Fig. 6H).
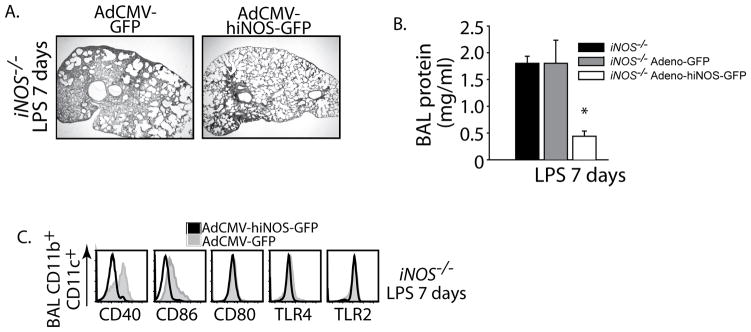
In order to test the potentially therapeutic effects of iNOS, we challenged iNOS−/− mice with i.t. LPS and after 48 hrs of established injury animals were assigned to 1 of 2 different treatment modalities: pharyngeal aspiration of a single dose of adenovirus encoding GFP driven by a CMV promoter (Ad5CMV-GFP) or an adenovirus encoding the human iNOS tagged to GFP (Ad5CMV-hiNOS-GFP). We measured outcomes at day 7 after i.t. LPS. Direct lung transgene delivery was confirmed by flow cytometry. Differential alveolar cell transduction of adenovirus was detected by GFP fluorescence (Supplementary Fig. 2C; macrophages>neutrophils>lymphocytes). In contrast, unstimulated animals had no transduction of alveolar macrophages (not shown), while lung parenchyma was effectively transduced in the absence of lung inflammation. Epithelial transduction increased when inflammation was present (Supplementary Fig. 2D). We found that in vivo transgene delivery of iNOS given as rescue therapy (48 hr after LPS) produced significant transduction of alveolar macrophages and drove resolution of lung inflammation in iNOS−/− mice. Furthermore, delivery of adeno-hiNOS-GFP down-regulated the expression of macrophage co-stimulatory molecules CD86 and CD40 (Fig. 7C). In contrast, adeno-GFP had no effect on these cellular and molecular targets, and did not alter the sustained expression of macrophage CD86 and CD40. In summary, direct delivery of iNOS to the air space rescued injured iNOS−/− mice and promoted resolution of ALI.
Fig. 7. Viral delivery of iNOS but not inhaled NO restores resolution of ALI in iNOS−/− mice.
iNOS−/− mice were challenged with i.t. LPS and 48 hours after injury received either a 50 μl pharyngeal aspiration of AdCMV-GFP or AdCMV-hiNOS-GFP (109 IU/ml). Outcomes measured 7 days after i.t. LPS. (A) Lung H–E sections and (B) BAL protein for designated treatments. (C) Flow cytometry histograms gated for iNOS−/− mice alveolar CD11b+CD11c+ macrophages and their relative expression of surface markers after pharyngeal aspiration of designated adenoviral transgene delivery.
Myeloid, but not epithelial-derived iNOS modulates resolution of lung injury
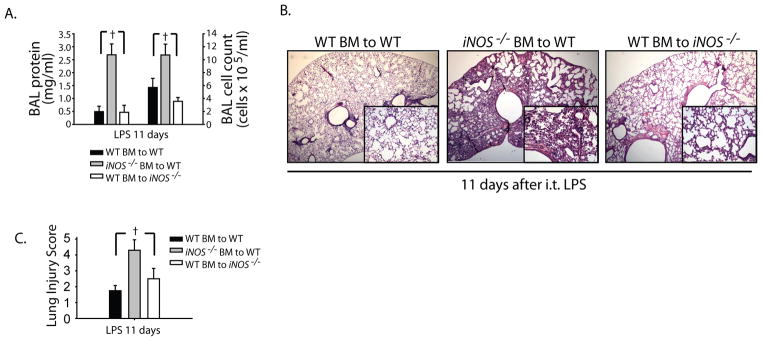
We have shown that iNOS is upregulated in inflammatory alveolar and lung epithelial cells during inflammation in WT animals. To evaluate the relative contributions of myeloid and epithelial-derived iNOS, we performed bone marrow irradiation chimeras between congenic WT (CD45.1) and iNOS−/− mice. Transfer of WT bone marrow (BM) into irradiated WT mice was used as controls and this group achieved complete resolution of i.t. LPS-induced lung injury at day 11. We noticed that in irradiated animals, the injury response was shifted after i.t. LPS evidenced by their peak weight loss around 6 days after LPS as compared to 4 days in non-irradiated animals (not shown). Similarly, WT BM transfer into irradiated iNOS−/− mice achieved near complete resolution of ALI, evidenced by reduction of BAL total cells and protein (Fig. 8A). These mice had myeloid iNOS but no epithelial-derived iNOS. In marked contrast, the transfer of iNOS−/− BM into irradiated WT mice, displayed sustained alveolar lung inflammation even 11 days after i.t. LPS with elevated BAL cell counts and protein, and persistent histological lung injury (Fig. 8A, B and C). This group had epithelial iNOS, but no myeloid iNOS.
Fig. 8. Myeloid, but not epithelial-derived iNOS modulates resolution of lung injury.
Bone marrow irradiated chimeras were generated between congenic WT (CD45.1) and iNOS−/− mice. WT bone marrow (BM) to WT mice were used as irradiated controls. After 8 weeks post radiation, mice were challenged with i.t. LPS and followed out to 11 days. (A) BAL protein and total cell counts were measured in designated groups. (B) Lung H–E sections are shown for designated groups 11 days after i.t. LPS; original magnifications x20; x100 (insets). (C) Lung Injury scoring was performed from 20X lung histopathological sections.
These results are consistent with our adoptive transfer experiments using bone marrow-derived monocytes, and indicate that epithelial-derived iNOS does not play an important role in the resolution of experimental lung injury after LPS.
iNOS regulates CD86 expression to regulate lung inflammation
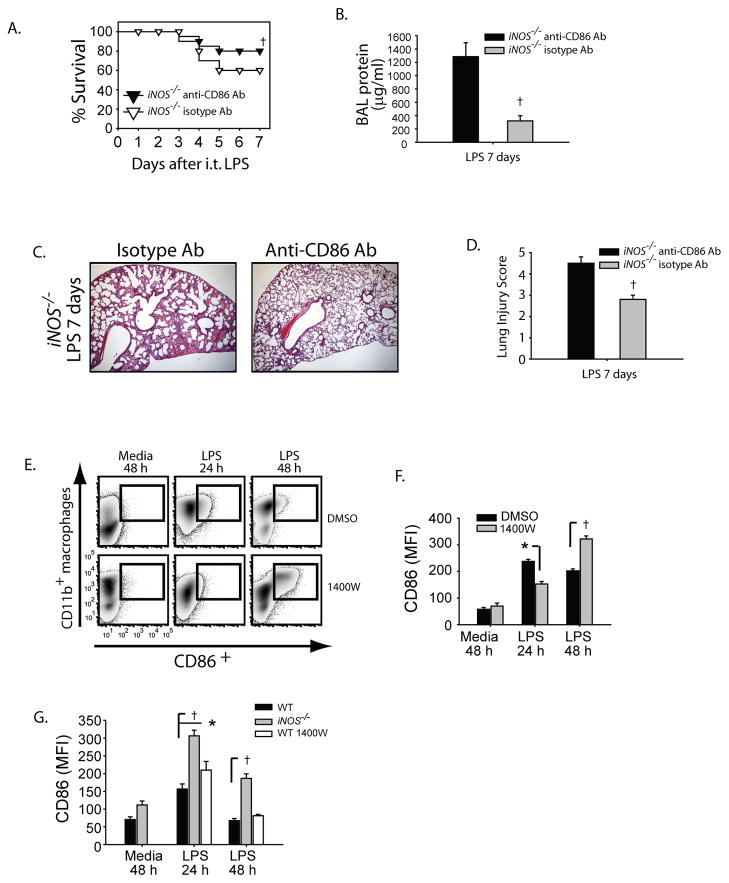
We sought to determine if sustained CD86 expression in macrophages contributed to persistent lung inflammation seen in injured iNOS−/− mice. To avoid attenuating the initial inflammatory response to i.t. LPS and thus altering the primary injury, we administered intraperitoneal (i.p.) injections of monoclonal anti-CD86 (250 μg/mouse/dose; BioXcell) or isotype antibody (Rat IgG, Sigma-Aldrich) two days after LPS exposure and again on days +4 and +6. Seven days after i.t. LPS, survival was improved by 33% in the anti-CD86 treated iNOS−/− mice (Fig. 9A, 80% vs 60% in isotype-treated group). BAL protein (Fig. 9B) and total cell counts (not shown) were markedly attenuated in the anti-CD86 group. Lung histology (Fig. 9C) showed enhanced resolution of lung inflammation in iNOS−/− mice that received anti-CD86 compared to the isotype-treated mice (Fig. 9C, D). We confirmed blockade of CD86 of mononuclear cells in the lung by flow cytometry (not shown).
Fig. 9. iNOS regulates CD86 expression to regulate lung inflammation.
iNOS−/− mice were challenged with i.t. LPS and 48 hours after injury received either isotype Ab (Rat IgG) or anti-mouse CD86 (clone GL-1) on days +2, +4 and +6 after ALI. Survival (A) and BAL protein were evaluated in iNOS−/− mice after i.t. LPS. (C) Histological sections were stained with H & E (Original magnifications x20). (D) Histopathological mean lung injury scores from x20 sections (n=4–6 animals per group per group). The alveolar macrophage cell line (MH-S) was challenged with media or LPS (100 ng/ml) in the presence of DMSO or specific iNOS inhibitor 1400W (25 mM) at designated intervals. (E) Flow cytometry density plots show the relative expression of surface CD86 at designated intervals after LPS. (F) Mean fluorescence intensity (MFI) for CD86 expression was measured in MH-S cells and resident peritoneal macrophages (G) and compared between groups at intervals. Values expressed as mean ± SEM; † *, p<0.05 log-rank test (mortality curves) and unpaired Student’s t test (for other injury parameters).
To confirm that iNOS modulates macrophage CD86 expression, we challenged an alveolar macrophage cell line (MH-S) with LPS in the presence or absence of a specific iNOS inhibitor (1400W) and at 24 and 48hr analyzed cell surface expression for CD86 by flow cytometry. Density plots showed that while CD86 expression on the cell surface of CD11b+ macrophages was attenuated 24 hours after LPS in the iNOS inhibited (1400W) cells, CD86 expression was higher in the same group when analyzed at 48 hr (Fig. 9E, F). To evaluate the ex-vivo macrophage response, we isolated and plated naive peritoneal macrophages from WT and iNOS−/− mice, then stimulated them with LPS. A group of WT macrophages were treated with 1400W. Cells at intervals were subsequently surface stained for CD86 expression. iNOS−/− and WT macrophages treated with 1400W had higher expression of CD86 at 24 hr as compared to WT macrophages (Fig. 9G). After 48 hr of LPS stimulation iNOS−/− macrophages had sustained CD86 expression while the WT and WT 1400W-treated cells returned back to baseline expression. Our findings support iNOS as a key regulator of macrophage CD86 expression during lung inflammation.
Discussion
Nitric oxide has been implicated in the pathogenesis of lung inflammation and injury, principally as having a deleterious role in early injury. The role of iNOS as a pro-inflammatory mediator in the lung has been described in numerous animal models, including ovalbumin-induced airway inflammation, carrageenan-induced pleuritis (40), ventilator-induced lung injury (41), direct infectious or LPS-induced ALI (12, 42, 43) and sepsis or indirect LPS-induced ALI (10, 19, 21, 22). Consistent with reports of the pro-inflammatory role of iNOS (44), we found in our established model of intratracheal LPS-induced lung injury that the absence of iNOS attenuated early injury. In marked contrast, the absence of iNOS significantly delayed resolution of lung inflammation. Our findings reinforce the need to distinguish early from late responses when seeking a role for a specific mediator or pathway during an inflammatory response.
In this model, lung injury progresses and peaked on day 4 after LPS. At that time we observed an induction of iNOS and NOx in the alveolar space, after which resolution of lung inflammation proceeded. As described by others (45), we believe early pro-inflammatory events are important for induction of repair mechanisms. In the absence of iNOS repair was significantly delayed. Similarly, we recently described a pivotal role for alveolar Regulatory T cells (Tregs) in resolution of experimental lung injury, which peaked on days 4–7 after ALI (37). Potential interactions between iNOS and Tregs in this response are the subject of ongoing investigation.
The role of iNOS in inflammation and infection can vary depending on the setting, the organ involved, the cell type and/or the stage of the inflammatory response, ultimately enhancing inflammation or abrogating it (46). Kristof found that iNOS−/− mice displayed attenuated ALI in a murine model of septic shock (10). Similarly, Shanley found that exogenous NO increased inflammation and vascular leak and that the absence of iNOS was associated with an attenuated acute inflammatory response to intratracheal endotoxin (24). However, a few reports indicate a more complex role for iNOS in injury responses (sequestration, adhesion and activation of neutrophils in the lung endothelium) (47). For example, iNOS deficient mice are resistant to LPS-induced hypotension, but suffered as much LPS-induced liver damage as wild type counterparts (22).
We observed a delay in epithelial-endothelial barrier repair in the iNOS−/− mice compared to WT as evidenced by the persistently elevated alveolar protein and albumin. Both iNOS expression and the production of TGF-β play critical roles in vascular function during inflammatory responses, however, an imbalance can lead to pathology (48). In WT mice, active alveolar TGF-β peaked at day 4, concurrent with maximal iNOS expression, and then fell to baseline levels on subsequent days. In contrast, iNOS−/− mice had persistently elevated levels of TGF-β, perhaps reflecting a negative feedback loop between iNOS and TGF-β (49). Although TGF-β can act as a potent anti-inflammatory and reparative mediator, uncontrolled and sustained elevation of TGF-β can lead to increases in alveolar epithelial permeability (50) and fibrotic complications (51). This is supported by Hochberg, who noted increased fibrotic complications with markedly elevated kidney TGF-β levels after unilateral ureteral obstruction in iNOS−/− mice, as compared to WT mice (52). Furthermore, although similar levels of alveolar collagen were present in WT and iNOS−/− mice by day 4, the latter group had markedly elevated collagen levels while WT mice had returned back to baseline by day 7 (unpublished observations), supporting a role for iNOS in the fibroproliferative response after ALI.
Apoptosis of inflammatory cells is fundamental to resolution of inflammation, and failure to clear inflammatory cells leads to excessive tissue damage and sustained injury (45, 53). In contrast to the complete alveolar neutrophil clearance in WT mice, alveolar neutrophilia persisted in iNOS−/− mice. Several potential factors may contribute to the sustained elevation of alveolar neutrophils in the absence of iNOS. MIP-2, a potent neutrophil chemokine, was higher by day 4 in the BAL in iNOS−/− mice, and along with the persistently damaged alveolar-endothelial barrier seen in later stages in iNOS−/− mice, likely contributed to the elevated number of neutrophils. In addition, neutrophil apoptosis and efferocytosis were both impaired in iNOS−/− mice; NO can induce apoptotic neutrophil death (54). We cannot exclude other factors contributing to persistent alveolar neutrophilia in iNOS−/− mice, for instance production of neutrophil chemokine KC and/or LIX by a persistently injured alveolar epithelium or enhanced Th17 responses, which can be involved in LPS-induced lung inflammation (55).
Alveolar macrophages (AM) play a critical role in the pathogenesis of ALI (56, 57). We observed similar number, phenotype and function of alveolar macrophages in WT and iNOS−/− mice during early lung injury. In marked contrast, during the resolution stage (>4 days after i.t. LPS), iNOS−/− animals had increased alveolar macrophage numbers with significant differences in phenotype and function compared to WT macrophages. Macrophage co-signaling molecules (CD86, CD40) and MHC-II were sustained at higher levels in the absence of iNOS, indicating a persistently activated macrophage phenotype. Co-signaling molecules are cell surface glycoproteins, usually expressed in macrophages and other antigen presenting cells (APCs), which direct, modulate and fine-tune T-cell responses (58, 59). Emerging data suggests that co-signaling molecules may regulate innate immune responses, best described in the context of antigen-specific models. For instance, genetic deletion of the co-signaling molecule CD40 in mice decreased susceptibility to direct injury with LPS and polymicrobial sepsis (60, 61). Additionally, co-signaling molecules (CD40 and CD80/86) regulate inflammation in a model of polymicrobial sepsis, and humans with septic shock have higher monocyte expression of CD40 and CD80 compared to patients with sepsis or healthy controls (62). Consistent with our findings, Shi et al described that in a model of autoimmune myasthenia gravis, iNOS−/−macrophages had a higher expression pattern of CD40 and MHC-II compared to WT, and sustained production of pro-inflammatory TNF-α and reactive oxygen species (ROS) (32).
During lung inflammation iNOS has been reported to originate from inflammatory cells (neutrophils, macrophages) or epithelial cells (63). Although we cannot exclude a potential role for epithelial-derived iNOS during resolution of lung inflammation, our adoptive transfer experiments support a central role for macrophage-derived iNOS in this model. Bone marrow-derived CD11b+ monocytes trafficked to the inflamed lung and alveolar compartments following adoptive transfer. CD11b+iNOS+/+ but not CD11b+iNOS−/− bone-marrow derived monocytes restored resolution of ALI, even when delivered 24 hours after established injury, suggesting a potential rescue therapy. Similarly, delivery of iNOS into the lung via adenovirus restored resolution. Importantly, adenoviral iNOS was readily taken up by macrophages in the inflamed alveolus, while transduction of the resident alveolar macrophage in the uninjured lung was minimal. In contrast, the lung epithelium was readily transduced in normal and inflamed conditions. To dissect the role of cellular iNOS in driving resolution of experimental lung inflammation, we created irradiated chimeras which showed the myeloid-derived iNOS was sufficient to restore the normal lung repair. Lung epithelial- iNOS had no effect in mediating resolution of ALI. Although bone marrow chimeras between WT and iNOS −/− have been previously described in murine models of sepsis-induced (64, 65), these studies identified that myeloid-derived iNOS contributed to pulmonary oxidant stress and microvascular leak, while parenchymal-derived iNOS had no apparent effect. Our results appeared to contradict these reports, although there are two main differences. First, our model of ALI is direct with a significant epithelial injury component followed by an influx of inflammatory cells; other investigators report results from indirect models of ALI where there is no or modest alveolitis. Second, the models reported focused on early events after ALI (hours); we have focused on later stages, during resolution and repair of ALI. Although inhaled NO has been tested in patients with ALI and transient improvements have been shown in physiological parameters such as oxygenation and pulmonary vascular resistance, four multicenter, randomized, placebo-controlledtrials have failed to show a therapeutic roleof inhaled NO in patients with acute respiratory failure (66–68). We find that upregulation of iNOS and NOx is tightly regulated following LPS-induced lung injury, so that location, cellular source, timing and duration of increased NO may be critical to its overall effect, and therefore may not be adequately reproduced through continuous inhaled delivery of NO. Further definition of differences between inhaled NO and upregulation of cell-based iNOS/NO may provide important mechanistic insights that could be leveraged for design of therapy.
Although iNOS-derived NO has a diverse range of immunological effects, we found the sustained expression of alveolar macrophage co-signalling molecule CD86 in the absence of iNOS to be a prominent feature. We considered several factors regarding the timing of CD86 antibody-mediated blockade. First, we wanted to intervene when ALI was fully established and thus prove that our intervention can be used as rescue therapy, directly relevant to strategies for therapy in most patients with ALI. Second, we sought to avoid attenuating the early inflammatory response with pre-treatment or early intervention, as the focus of this line of investigation is on mechanisms of resolution. Our laboratory is pursuing subcellular mechanisms of iNOS-mediated CD86 regulation. Sustained CD86 expression could also be explained by enhanced antigen presenting cell survival and responsiveness to TLR agonist seen in the absence of iNOS, as recently described (69). Sustained macrophage CD86 expression in conjunction with high MHC-II expression observed in iNOS−/− mice, could potentially lead to enhanced co-stimulation of Th1 responses from CD4+ cells, which were abundant by day 4–7 after alveolar inflammation and contribute to persistent lung immunopathology. In addition to the persistent CD86 expression in iNOS−/− monocytes, we also observed sustained upregulation of CD40 expression. Its regulation by iNOS and its role in the regulation of lung inflammation will need further evaluation. We were unable to use antibody-mediated blockade as available murine antibodies are agonistic for CD40. We cannot exclude a synergistic role for sustained macrophage CD40 expression in iNOS-mediated modulation of lung inflammation, although blockade of CD86 was sufficient to restore resolution of ALI.
Acute lung injury continues to be a major clinical problem, with significant annual morbidity and mortality, for which therapy to date is largely supportive. Here we demonstrate a critical role for macrophage-derived iNOS in promoting resolution of lung injury. iNOS has differential effects depending on the stage of injury, with a predominant early pro-inflammatory effect and a reparative, anti-inflammatory late phase effect. The late effects are at least partially mediated by alveolar macrophage activation and modulation of CD86 expression, but may occur via effects on other pathways including Regulatory T Cells (Tregs), lipid mediators and Th17 responses among others. The surprising identification of iNOS as a determinant of resolution of lung injury provides new insights into resolution of inflammation, and may create opportunities for development of new approaches to therapy.
Supplementary Material
Acknowledgments
Work supported by NHLBI HL089346 and the Johns Hopkins Bayview Scholars Program (LSK) and by K99HL103973 (FRD).
Mark Soloski, PhD and Joe Chrest for their assistance in the Johns Hopkins Bayview Flow Cytometry Core. James Watkins and Andre Robinson for expert assistance with tissue processing for histologic studies. Tony Eissa (Baylor College of Medicine) for kindly providing hiNOS-GFP vector.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 5.Anderson WR, Thielen K. Correlative study of adult respiratory distress syndrome by light, scanning, and transmission electron microscopy. Ultrastruct Pathol. 1992;16:615–628. doi: 10.3109/01913129209023751. [DOI] [PubMed] [Google Scholar]
- 6.Meduri GU, Eltorky M, Winer-Muram HT. The fibroproliferative phase of late adult respiratory distress syndrome. Semin Respir Infect. 1995;10:154–175. [PubMed] [Google Scholar]
- 7.Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107:196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 8.Baron RM, I, Carvajal M, Liu X, Okabe RO, Fredenburgh LE, Macias AA, Chen YH, Ejima K, Layne MD, Perrella MA. Reduction of nitric oxide synthase 2 expression by distamycin A improves survival from endotoxemia. J Immunol. 2004;173:4147–4153. doi: 10.4049/jimmunol.173.6.4147. [DOI] [PubMed] [Google Scholar]
- 9.Haddad IY, Crow JP, Hu P, Ye Y, Beckman J, Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol. 1994;267:L242–249. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- 10.Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 11.Kooy NW, Royall JA, Ye YZ, Kelly DR, Beckman JS. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L198–209. doi: 10.1152/ajplung.00136.2003. [DOI] [PubMed] [Google Scholar]
- 13.Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1164–1172. doi: 10.1152/ajplung.00248.2005. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 15.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 16.van der Vliet A, Cross CE. Oxidants, nitrosants, and the lung. Am J Med. 2000;109:398–421. doi: 10.1016/s0002-9343(00)00479-4. [DOI] [PubMed] [Google Scholar]
- 17.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 19.Razavi HM, Werhun R, Scott JA, Weicker S, Wangle F, McCormack DG, Mehta S. Effects of inhaled nitric oxide in a mouse model of sepsis-induced acute lung injury. Crit Care Med. 2002;30:868–873. doi: 10.1097/00003246-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Wong JM, Billiar TR. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv Pharmacol. 1995;34:155–170. doi: 10.1016/s1054-3589(08)61084-4. [DOI] [PubMed] [Google Scholar]
- 21.Wei XQ, I, Charles G, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 22.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 23.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 25.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 27.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 28.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Rollinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 29.Spiecker M, Darius H, Kaboth K, Hubner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63:732–739. [PubMed] [Google Scholar]
- 30.Bobe P, Benihoud K, Grandjon D, Opolon P, Pritchard LL, Huchet R. Nitric oxide mediation of active immunosuppression associated with graft-versus-host reaction. Blood. 1999;94:1028–1037. [PubMed] [Google Scholar]
- 31.Kahn DA, Archer DC, Gold DP, Kelly CJ. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J Exp Med. 2001;193:1261–1268. doi: 10.1084/jem.193.11.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi FD, Flodstrom M, Kim SH, Pakala S, Cleary M, Ljunggren HG, Sarvetnick N. Control of the autoimmune response by type 2 nitric oxide synthase. J Immunol. 2001;167:3000–3006. doi: 10.4049/jimmunol.167.5.3000. [DOI] [PubMed] [Google Scholar]
- 33.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. Faseb J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 34.Vodovotz Y, Chesler L, Chong H, Kim SJ, Simpson JT, DeGraff W, Cox GW, Roberts AB, Wink DA, Barcellos-Hoff MH. Regulation of transforming growth factor beta1 by nitric oxide. Cancer Res. 1999;59:2142–2149. [PubMed] [Google Scholar]
- 35.Obermeier F, Gross V, Scholmerich J, Falk W. Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide. J Leukoc Biol. 1999;66:829–836. doi: 10.1002/jlb.66.5.829. [DOI] [PubMed] [Google Scholar]
- 36.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 37.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halbower AC, Mason RJ, Abman SH, Tuder RM. Agarose infiltration improves morphology of cryostat sections of lung. Lab Invest. 1994;71:149–153. [PubMed] [Google Scholar]
- 39.King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De LF, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162:1859–1866. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:470–479. doi: 10.1164/rccm.200411-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanley TP, Zhao B, Macariola DR, Denenberg A, Salzman AL, Ward PA. Role of nitric oxide in acute lung inflammation: lessons learned from the inducible nitric oxide synthase knockout mouse. Crit Care Med. 2002;30:1960–1968. doi: 10.1097/00003246-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 46.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C, Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. Faseb J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 48.Berg DT, Gupta A, Richardson MA, O’Brien LA, Calnek D, Grinnell BW. Negative regulation of inducible nitric-oxide synthase expression mediated through transforming growth factor-beta-dependent modulation of transcription factor TCF11. J Biol Chem. 2007;282:36837–36844. doi: 10.1074/jbc.M706909200. [DOI] [PubMed] [Google Scholar]
- 49.Jun CD, Choi BM, Kim SU, Lee SY, Kim HM, Chung HT. Down-regulation of transforming growth factor-beta gene expression by antisense oligodeoxynucleotides increases recombinant interferon-gamma-induced nitric oxide synthesis in murine peritoneal macrophages. Immunology. 1995;85:114–119. [PMC free article] [PubMed] [Google Scholar]
- 50.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 52.Hochberg D, Johnson CW, Chen J, Cohen D, Stern J, Vaughan ED, Jr, Poppas D, Felsen D. Interstitial fibrosis of unilateral ureteral obstruction is exacerbated in kidneys of mice lacking the gene for inducible nitric oxide synthase. Lab Invest. 2000;80:1721–1728. doi: 10.1038/labinvest.3780182. [DOI] [PubMed] [Google Scholar]
- 53.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 54.Fortenberry JD, Owens ML, Brown MR, Atkinson D, Brown LA. Exogenous nitric oxide enhances neutrophil cell death and DNA fragmentation. Am J Respir Cell Mol Biol. 1998;18:421–428. doi: 10.1165/ajrcmb.18.3.2875. [DOI] [PubMed] [Google Scholar]
- 55.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 56.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med. 2005;171:806–813. doi: 10.1164/rccm.200407-953OC. [DOI] [PubMed] [Google Scholar]
- 57.Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, Christman JW. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26:572–578. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- 58.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 59.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, Kojima K, Shimokata K, Hasegawa Y. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:808–815. doi: 10.1165/rcmb.2003-0197OC. [DOI] [PubMed] [Google Scholar]
- 61.Gold JA, Parsey M, Hoshino Y, Hoshino S, Nolan A, Yee H, Tse DB, Weiden MD. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect Immun. 2003;71:3521–3528. doi: 10.1128/IAI.71.6.3521-3528.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med. 2008;177:301–308. doi: 10.1164/rccm.200703-515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoyt JC, Ballering J, Numanami H, Hayden JM, Robbins RA. Doxycycline modulates nitric oxide production in murine lung epithelial cells. J Immunol. 2006;176:567–572. doi: 10.4049/jimmunol.176.1.567. [DOI] [PubMed] [Google Scholar]
- 64.Razavi HM, Wangle F, Weicker S, Rohan M, Law C, McCormack DG, Mehta S. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004;170:227–233. doi: 10.1164/rccm.200306-846OC. [DOI] [PubMed] [Google Scholar]
- 65.Wangle F, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634–1639. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 66.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Jr, Kelly KM, Smith TC, Small RJ. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. Jama. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 67.Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K, Jr, Hyers TM, Papadakos P. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911–919. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 69.Giordano D, Li C, Suthar MS, Draves KE, Ma DY, Gale M, Jr, Clark EA. Nitric oxide controls an inflammatory-like Ly6C(hi)PDCA1+ DC subset that regulates Th1 immune responses. J Leukoc Biol. 89:443–455. doi: 10.1189/jlb.0610329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.