Abstract
Protein kinase Mzeta has been the subject of much recent interest, as it is the only molecule currently identified to maintain memory. Despite the wealth of studies investigating PKMζ in memory, questions remain about which types of memory PKMζ supports. Further, it is unclear how long the inhibitor of PKMz, ζ-pseudosubstrate inhibitory peptide (ZIP) remains in the brain after infusion. Here, we demonstrate that foreground context fear memory requires PKMζ activity in the amygdala. We also show that ZIP is fully cleared from the brain by 24h after infusion. These data contribute to a growing body of literature that demonstrates that PKMζ plays a key role in maintaining amygdala-dependent memory and provides new information about the degradation timecourse of the most commonly used inhibitor of PKMζ, ZIP.
Keywords: ZIP, PKMζ, context fear, fear conditioning, amygdala
1. Introduction
Protein kinase Mzeta (PKMζ) is an atypical isoform of protein kinase C (PKC) that has attracted intense interest in the past few years for its putative role in memory maintenance. PKMζ lacks the autoinhibitory domain that accompanies other PKC isoforms, allowing the molecule to remain constitutively active. The constant activity of PKMζ is believed to actively maintain the synaptic changes that occurred during memory formation, particularly the upregulation of AMPA receptors through the NSF-GluR2 pathway (Migues et al., 2010; Sacktor, 2010; Yao et al., 2008). Inhibition of PKMζ with zeta pseudosubstrate inhibitory peptide (ZIP) can disrupt established memories for a number of learning tasks (Hardt, Migues, Hastings, Wong, & Nader, 2009; Kwapis, Jarome, Lonergan, & Helmstetter, 2009; Pastalkova et al., 2006; Serrano et al., 2008; Shema et al., 2007), consistent with the idea that PKMζ supports memory maintenance.
Interestingly, it seems that not all forms of memory require PKMζ activity for maintenance. For example, “background” fear conditioning to the training context (acquired during a session in which a discrete conditional stimulus (CS), such as a white noise cue, is paired with an aversive unconditional stimulus (UCS), such as a shock) is not disrupted by PKMζ inhibition in the hippocampus, although hippocampal lesions disrupt this type of background context memory (Kwapis et al., 2009; Serrano et al., 2008). Hippocampal infusions of ZIP, however, are sufficient to disrupt object location memory (Hardt et al., 2009), and other spatial memories, like active avoidance memory and memory for the 8-arm radial arm maze (Pastalkova et al., 2006, Serrano et al., 2008). These studies demonstrate a clear need to assess the role of PKMζ in different forms of memory, particularly those that involve contextual, background, or procedural components.
More recent findings suggest that PKMζ inhibition may only temporarily disrupt memory expression of fear-potentiated startle, rather than permanently erasing the memory (Parsons & Davis, 2011). In this study, a test 10d after ZIP infusion had showed no effect of the drug on memory retention despite the same infusion producing profound memory deficits when animals were tested at both 1d and 10d after ZIP infusion. Another recent study, however, demonstrated a deficit in active avoidance responses when the memory was tested 7d after ZIP infusion (Gamiz & Gallo, 2011). Further, many of the previous studies testing the effects of ZIP on memory maintenance use a short injection-to-initial test interval, although it is unknown how long ZIP persists in brain tissue after infusion. It is possible that the initial test in these studies occurs while ZIP is still present in brain tissue and that memory expression or retrieval is impaired, rather than memory maintenance. In order to rule out the possibility that the peptide disrupts fear expression, and to clarify the results of previous studies using ZIP to inhibit PKMζ, it is important to know when the peptide is fully metabolized from brain tissue.
The objectives of this study were twofold. First, we wanted to determine whether PKMζ activity in the amygdala is required to maintain foreground context fear memory. In standard auditory fear conditioning, a discrete CS is paired with the UCS over the background information of the context. Although context conditioning occurs, the context-UCS association is weaker than the discrete CS-UCS association and is said to be a “background” association (Rescorla & Wagner, 1972). If no discrete CS is presented, however, the contextual information is the only cue the animal can use to predict the occurrence of the UCS. This shifts the context conditioning into a foreground association (Odling-Smee, 1975; 1978). Context-only training produces strong context fear memory that (like background context conditioning) depends on both the amygdala and hippocampus (Helmstetter, Parsons, & Gafford, 2008; Maren & Fanselow, 1997; Oliveira, Nobre, Brandão, Landeira-Fernandez, 2004; Phillips & LeDoux, 1992). While background context associations are known to require PKMζ in the amygdala for maintenance, it is currently unknown whether PKMζ maintains foreground context associations in a similar manner. It is possible that the relatively stronger foreground context association is resistant to disruption by ZIP in the amygdala, relying instead on other structures (such as the hippocampus) for maintenance.
Our second goal was to identify the timecourse of ZIP degradation following its infusion into the amygdala. It is currently unknown how long the peptide remains in brain tissue following intracranial infusion, even though this information is vital to interpreting previous studies using ZIP to inhibit PKMζ activity. In order to determine whether behavioral effects observed following ZIP injections represent disruption of the memory or could potentially result from the peptide disrupting memory expression or retrieval, it is important to know how long the peptide remains in the brain after infusion. To this end, we injected a biotinylated version of ZIP into brain tissue and used immunohistochemistry to determine when the peptide is fully cleared from the tissue after infusion.
2. Materials and Methods
2.1. Subjects and Surgery
Subjects were 50 adult male Long-Evans rats obtained from Harlan (Madison, WI) weighing approximately 350g. Animals were housed individually in shoebox cages with free access to water and rat chow. The colony room was maintained under a 14:10h light/dark cycle (lights on at 7:00 a.m.) and all behavioral tests were conducted during the light portion of this cycle. All procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Milwaukee.
Subjects were adapted to handling for three consecutive days before surgery. Immediately before surgery, each rat was anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (1.5 mg/rat) followed by a second IP injection of ketamine hydrochloride (100 mg/kg). Animals were then prepared with bilateral stainless steel 26-gauge cannulae (Plastics One, Roanoke, VA) aimed at the basolateral nucleus of the amygdala (BLA) using stereotaxic coordinates (2.8 mm posterior, ±5.0 mm lateral, 7.2 mm ventral) relative to Bregma (Paxinos & Watson, 1998). Cannulae were secured to the skull with stainless steel screws, superglue, and dental acrylic. Following surgery, the incision site was swabbed with a lidocaine and prilocaine solution (2.5%/2.5%) to minimize discomfort during the recovery period. Stainless steel obdurators remained in the cannulae when rats were not being injected to prevent occlusion. Each rat was given a recovery period of at least seven days before behavioral testing.
2.2. Apparatus
Fear conditioning training was conducted in a set of four Plexiglas and stainless steel chambers housed within sound-attenuating boxes. The floor was composed of stainless steel rods spaced 1.5 cm apart through which footshocks were delivered. Each chamber was illuminated by an overhead 7.5-W bulb and was connected to its own shock generator-scrambler (Grason-Stadler, West Concord, MA). Ventilation fans provided constant background noise (approximately 60dB). Chambers were cleaned with a solution of 5% ammonium hydroxide between animals.
2.3. Drug Preparation and Infusion
All subjects were exposed to the restraint and injection procedure for the three days preceding training or infusion. Each rat was transported to the laboratory, wrapped in a towel, and gently restrained by hand for several minutes while the infusion pump was activated to allow rats to habituate to its noise. The obdurators were temporarily removed at this time and the area was gently swabbed to clean and remove debris from the cannulae. Immediately following restraint handling, rats were returned to their homecages.
Drugs were prepared fresh on the day of infusion. In Experiment 1, myristoylated ZIP (myr-SIYRGARRWRKL-OH, Invitrogen, Carlsbad, CA) was dissolved in sterile saline (pH ~7.0, Teknova, Hollister, CA) to create a final concentration of 10nmol/µl. This concentration was chosen based on its effectiveness in past studies in our lab (Kwapis et al., 2009) and in other labs (Parsons & Davis, 2011; Pastalkova et al., 2006; Serrano et al., 2008). In Experiment 2, biotinylated ZIP (bioZIP; Myr-SIYRRGARRWRKL-Ahx-(K-Ahx-Biotin)-CONH2; Abgent, San Diego, CA) was similarly diluted in sterile saline to 10nmol/µl. BioZIP was only infused into one hemisphere; the opposite hemisphere was infused with saline for comparison.
All rats received an infusion volume of 0.5µl into the basolateral amygdala over a period of 60s. The injection cannulae, which were cut to extend approximately 0.5–0.7 mm beyond the guide cannulae, remained in place for an additional 90s after the infusion to ensure proper diffusion. Previous work with these parameters indicates that they produce coverage throughout the amygdala (Parsons, Gafford, & Helmstetter, 2006; Kwapis et al., 2009). Directly after infusions, rats were returned to their homecages.
2.4. Behavioral Procedures
2.4.1. Experiment 1
After adaptation to transportation and restraint handling, animals were trained with unsignaled (context-only) fear conditioning (n=22) in which a 2-minute baseline period was followed by 5 unsignaled shocks (1mA; 1s) presented with a 60s ITI. Animals were removed from the conditioning chambers 2 minutes after the final shock, for a total session length of 8 minutes. 1d or 7d later, animals were infused with either ZIP (1d, n=5; 7d, n=6) or saline (1d, n=6; 7d, n=5) into the BLA. A context test was given 2h post-infusion to test the animals’ context fear memory. For the context test, animals were transported to the laboratory, where they were exposed to the training context for 8 min. A second context test was conducted 24h later to determine whether any effects of ZIP were permanent or transient.
2.4.2. Experiment 2
After recovering from surgery, animals were handled for three consecutive days prior to injection as previously described. On the fourth day of handling, all animals received an intra-BLA injection of bioZIP into one hemisphere and vehicle into the opposite hemisphere (0.5µl each). Animals were then sacrificed every 2–3 hours post-infusion (n=2/timepoint; 0h, 2h, 5h, 8h, 11h, 14h, 17h, 20h, 22h, 24h, 26h, 29h, 3d, and 1wk post-infusion) and the brain tissue was analyzed to assess the presence of the peptide.
2.5. Histology and Histochemistry
After behavioral testing was complete, all animals were killed by an overdose of isoflurane and transcardially perfused with saline followed by a 10% buffered formalin solution. Heads were removed and submerged in buffered formalin for at least 24 hours. Brains were then removed and soaked in a 30% sucrose formalin solution for a minimum of 24 hours. For cannulae placement verification (Experiment 1), frozen 40 µm sections were collected throughout the amygdala, mounted on slides, and stained with cresyl violet. Injection sites were determined with the aid of a rat brain atlas (Paxinos & Watson, 1998). Animals with injection sites outside the basolateral subdivision of the amygdala were not included in the analyses.
For Experiment 2, histochemistry was performed following tissue fixation in order to determine the timecourse of ZIP degradation by visualizing the biotin label attached to the peptide. Tissue fixation was performed in the same manner as in Experiment 1, except that the brains were removed immediately following perfusion and were soaked directly in formalin for the first night followed by 30% sucrose formalin the following day. After at least 24h in sucrose formalin, brains were rinsed three times for ten minutes with 0.1 M PBS and frozen 40 µm coronal sections were collected throughout the amygdala in 24-well plates filled with 500 µl of 0.1 M PBS. Slices were washed on a titer plate with gentle agitation with 1% Triton in PBS (three times, ten minutes each) and incubated with the VECTASTAIN Elite ABC reaction (Vector Labs, Burlingame, CA) for one hour at room temperature. Slices were then rinsed twice with 0.05 M Tris-HCl (pH 7.4) for ten minutes and incubated with diaminobenzidine (DAB) reaction for ten minutes (0.04% DAB in 0.05 M Tris-HCl buffer). To develop the stain, 1% H2O2 was added to each well and the development was stopped by rinsing each well with 0.05 M Tris-HCl buffer (five times, five minutes each). Slices were then mounted on slides and allowed to dry for four hours before being imaged with a microscope (Olympus, Center Valley, PA). The slices were then washed for five minutes in 70%, 95%, and 100% EtOH solutions and Xylene to clear the tissue before coverslipping. Cleared slices were imaged with a computerized image processing system (MCID; Imaging Research, Inc., St. Catherine’s, ON, Canada).
2.6. Data Analysis
The main behavioral dependent variable for Experiment 1 was the amount of time the rats spent engaged in freezing behavior. Freezing was defined as the absence of all bodily movement except that which is required for respiration. All other behavior was scored as general activity. A computer based digital video observation system (FreezeScan 1.0, CleverSys. Inc., Reston, VA, USA) continuously scored each rat as freezing or active throughout each session. Freezing during all behavioral sessions was analyzed as a percentage of each minute. Behavioral data were analyzed using two-way ANOVAs (factors: drug and injection timepoint) and Students t-tests (to test the drug effects for each timepoint separately. In all analyses, an α value of 0.05 was required for significance.
For Experiment 2, optical density measurements were assessed to determine the timecourse of ZIP degradation. Densitometry software (NIH ImageJ) was used to obtain optical density measurements for the side of the brain injected with biotinylated ZIP and stained with the ABC reagent. For each brain slice, after clearing the tissue with Xylene, the optical density on the drug-injected hemisphere was normalized to the background staining, as taken from a parallel position on the vehicle-injected hemisphere. At least three slices were analyzed for each animal. A 60 × 60 pixel box was anchored to the bottom of the cannula tract on the drug-injected hemisphere to determine the optical density of the stain. The sampling box was then dragged to an identical position on the vehicle-infused hemisphere to get an accurate sample of the background for each slice. The background value was then subtracted from the optical density value of the stained hemisphere to account for any variance in the background staining between slices. Linear contrast analysis and planned comparisons were conducted on these values to determine whether the drug degradation occurred in a linear fashion and to determine whether the drug was fully degraded by 24h post-injection, as anticipated (Pastalkova et al., 2006).
3. Results
3.1. Experiment 1: Intra-amygdala ZIP and Foreground Context Memory
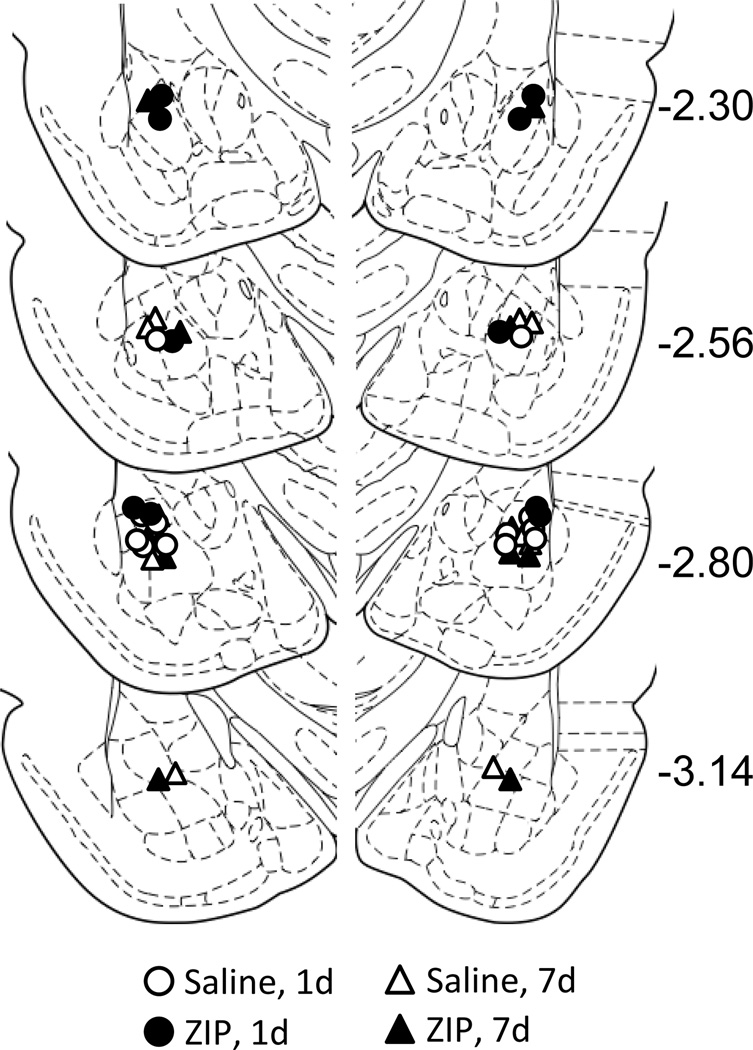
Most of the cannulae placements were in or just above the BLA. Four animals were excluded from analysis due to misplaced cannulae (one from the 1d injection group, and three from the 7d injection group). The cannulae placements for the remaining 22 animals were deemed acceptable and were included in subsequent analyses (Figure 1).
Figure 1.
Location of cannulae placements for animals infused with either saline (white symbols) or ZIP (black symbols). Animals were infused either 1d (circles) or 7d (triangles) after training with context-only fear conditioning.
The goal of our first experiment was to determine whether the maintenance of foreground context memory requires PKMζ activity in the BLA. To this end, we trained animals with context-only fear conditioning and infused ZIP or saline into the BLA one or seven days later (Figure 2A). All animals showed normal acquisition of context-only fear conditioning before drug infusion (data not shown). Low freezing was observed during the two-minute baseline period (all groups, M < 1%), indicating that the animals did not respond to the training context before the UCS was presented. Importantly, no differences in mean postshock freezing behavior were observed at the end of the training session (F(3,18) = 0.854, p = 0.482), indicating that the animals showed equivalent freezing levels prior to drug infusion.
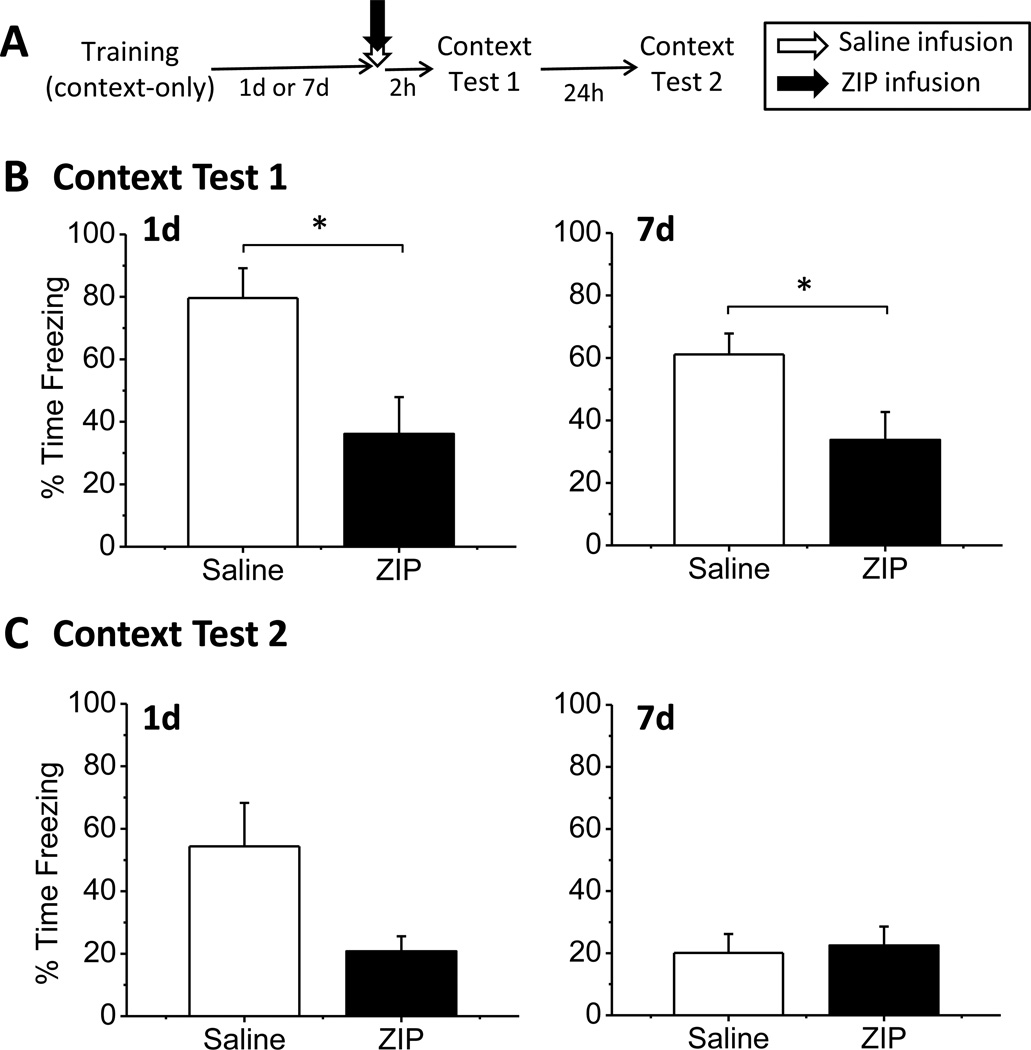
Figure 2.
ZIP infusion in the amygdala disrupts foreground context fear memory. (A) The experimental timeline. (B) ZIP injected into the BLA both 1d (left panel) and 7d (right panel) disrupted context fear. Graphs show mean percent time freezing during the entire 8-min context test. (C) Average freezing during context test 2, 24h after context test 1. No significant differences were observed for either the 1d post-training (left) or 7d post-training (right) groups. ZIP animals showed no evidence of memory recovery.
One or seven days after training, animals were infused with ZIP or saline into the BLA. Two hours after the drug infusion, animals were given a context test to determine whether context memory maintenance was disrupted (Figure 2B). ZIP infusion into the BLA disrupted context memory both one day and seven days after training. Analysis of mean freezing behavior during the context test revealed a main effect of drug (F(1,18) = 14.020, p = 0.001), but no main effect of injection time (F(1,18) = 1.203, p = 0.287) and no significant drug × time interaction (F(1,18) = 0.729, p = 0.404). The lack of a significant interaction indicates that ZIP infusion disrupted fear similarly at both one and seven days after training. Student’s t-tests comparing each injection timepoint separately revealed that ZIP significantly impaired freezing at both one day post-training (t(9) = 2.899, p = 0.018) and at seven days post-training (t(9) = 2.372, p = 0.042).
Animals were retested 24h after the initial test to determine whether the effects of ZIP on context fear were permanent or transient (Figure 2C). The saline groups both showed reduced freezing relative to the initial test, likely the result of extinction that occurred during the first test, when the animals were exposed to the training context without shock. While the ZIP groups were no longer significantly different from control animals for either the one day (t(9) = 2.105, p = 0.065) or one week (t(9) = −0.273, p = 0.791) infusion timepoints, ZIP animals did not show any evidence of memory recovery. Instead, ZIP animals remained freezing at low levels that were similar to the freezing levels observed on the initial test. This indicates that the memory deficit observed in ZIP animals was relatively long-lasting. Thus, our first experiment demonstrates that ZIP infusion into the BLA is able to disrupt the maintenance of both one- and seven-day-old foreground context fear memories.
3.2. Experiment 2: Timecourse of ZIP Degradation
Experiment 2 was designed to assess the timecourse of ZIP degradation following local microinjection of the peptide into the amygdala. The findings of past studies on PKMζ are based on the assumption that ZIP is fully degraded by the time that long-term tests are conducted, by approximately 24–48h after infusion. One recent study suggests that ZIP may temporarily disrupt memory expression, rather than permanently disrupting memory maintenance (Parsons & Davis, 2011). The actual timecourse of ZIP degradation, however, has not yet been assessed. To determine whether ZIP effects observed in past studies and in the current set of experiments may be due to memory expression effects, rather than disruption of memory retention, it is important to definitively determine how long ZIP is present in the brain following infusion. We injected a biotinylated version of ZIP (bioZIP) into one hemisphere of the BLA and vehicle into the opposite BLA and sacrificed animals every 2–3 hours post-infusion. The brain tissue was then exposed to the ABC reaction to detect the presence of bioZIP to determine the timepoint at which ZIP is fully degraded from the brain after local microinfusion.
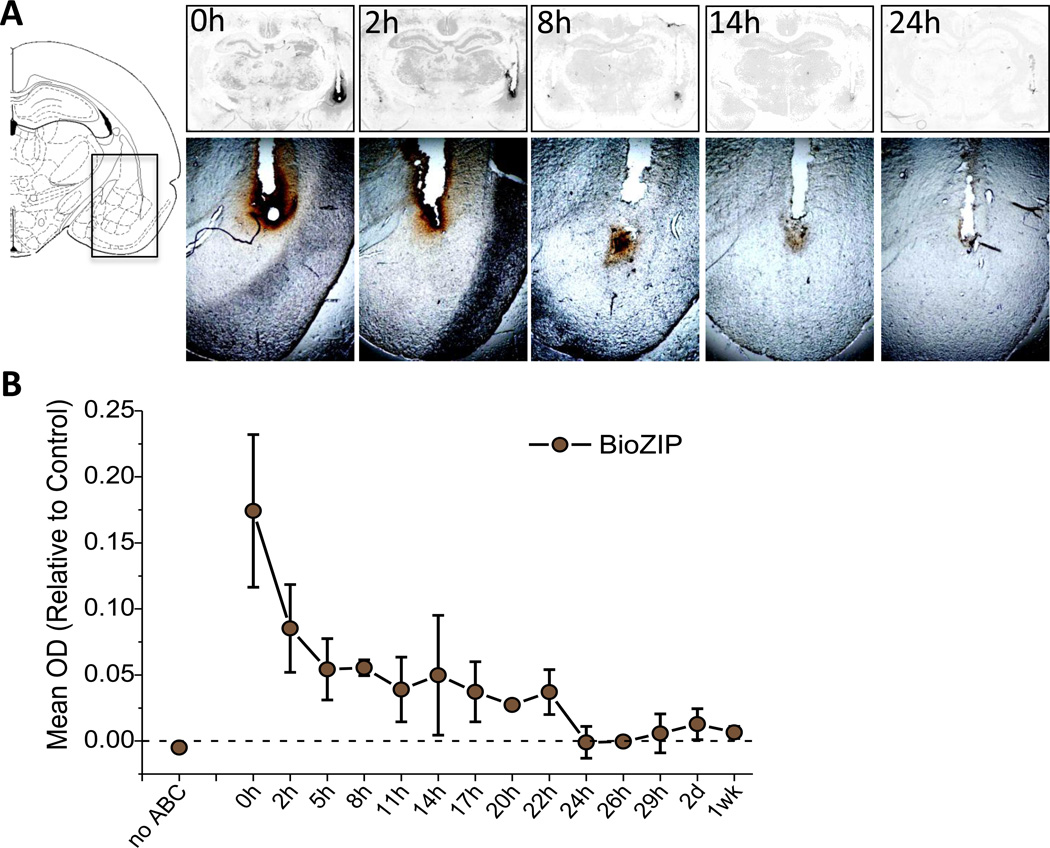
BioZIP was consistently detected in brain tissue from animals sacrificed within the first 22h after drug infusion, however, no bioZIP was detected in tissue taken from animals sacrificed 24h or more post-infusion. Figure 1A shows representative slices from 0h, 2h, 8h, 14h, and 24h post-infusion. Qualitatively the biotin label is darkest at 1h and 2h and fades as the time post-infusion increases until it disappears at 24h. Further, BioZIP was detected throughout the amygdala region, indicating that our injection parameters produce appropriate coverage of the BLA. Figure 1B displays the quantification for all animals in this study, in which the optical density (OD) of the stained hemisphere is normalized to background, taken from the control hemisphere. Consistent with the pattern observed in Figure 1A, the OD intensity is highest immediately after infusion and decreases over time. A one-way ANOVA demonstrated that there was a significant effect of time on OD (F(13, 14) = 3.24, p = 0.019). Further, these data fit a linear contrast analysis (F(13,1) = 28.096, p < 0.001), indicating that the degradation of ZIP occurs in a linear fashion, decreasing in intensity as the time after infusion increases. Planned comparisons demonstrated that OD measurements from animals sacrificed 24h post-infusion and later are significantly lower than the OD from animals sacrificed before 24h post-infusion (t(14) = 4.063, p = 0.001), consistent with the observation that no BioZIP was detected 24h post-infusion or later (Figure 1A). These data indicate that by 24h post-infusion, ZIP is fully degraded from the brain.
4. Discussion
The current study had two major findings. First, we demonstrated that inhibiting PKMζ in the amygdala with ZIP was sufficient to disrupt both one-day and one-week-old foreground context fear memories. This indicates that foreground context associations require PKMζ activity for maintenance up to at least one week after acquisition. Our second major finding was that ZIP degrades in a linear fashion from brain tissue and is fully eliminated by 24h after infusion. Together, these results provide new information about PKMζ’s role in maintaining context fear and demonstrate, for the first time, the timecourse of ZIP degradation from the brain.
The first objective of this study was to determine whether PKMζ activity in the amygdala is necessary to maintain foreground context fear memory. Recent studies have suggested that some memories, specifically contextual, procedural, and background associations, do not require PKMζ activity for maintenance in some structures (Kwapis et al., 2009; Serrano et al., 2008). Here, we demonstrate that foreground, context-only fear conditioning, requires PKMζ activity in the BLA for maintenance. Infusion of ZIP into the amygdala either one or seven days after acquisition disrupted context fear, indicating that PKMζ activity in the amygdala maintains foreground context fear for at least one week after training. Foreground context fear, like background contextual fear, therefore requires PKMζ activity in the amygdala.
In our behavioral study, we chose to use saline, rather than a scrambled peptide control, for comparison to ZIP. While an inactive peptide control would provide the ideal comparison, previous results from our lab (Kwapis et al., 2009) and others (Volk et al., 2011) suggest that scrambled ZIP (myr-RLYRKRIWRSAGR-OH) is able to inhibit both PKMζ activity and memory maintenance and therefore serves as a poor control. We instead chose to compare ZIP-injected animals to vehicle-injected controls, which showed robust memory typical of animals trained with context-only fear conditioning.
Our second goal was to determine how long ZIP remains in the brain after infusion. Using a biotinylated version of the peptide, we were able to visualize ZIP’s degradation timecourse. We found that ZIP degraded in a linear fashion and was fully eliminated from brain tissue by 24h post-infusion. This is the first demonstration of the timecourse of ZIP degradation, an important piece of information for interpreting the results of long-term memory tests following ZIP infusion. This information indicates that any test 24h post-infusion or later occurs in the absence of the peptide. Behavioral deficits observed after the 24h post-infusion timepoint, therefore, cannot be explained as a disruption of memory expression resulting from residual peptide lingering in the infused brain structure.
Although it is clear that the biotinylated peptide is fully cleared from the brain by 24h post-infusion, the data need to be interpreted cautiously. First, the addition of a biotin label onto the C-terminus of the peptide might have affected the peptide’s rate of degradation. Although it was necessary to include this label in order to visualize the peptide, it is possible that the addition of biotin (with a molecular weight of 224.31 g/mol) affected the stability of ZIP or prevented the peptide from being able to cross the cell membrane, preventing it from entering the cell and being degraded in the same manner as normal ZIP. Further, it is possible that the biotin label was detached from the peptide after infusion, so that the assay for biotinylation was detecting detached biotin molecules, rather than the peptide itself. While these concerns are certainly possible, we believe that they are unlikely for a number of reasons. First, biotin-labeled peptides are commonly used to assess peptide spread and stability (e.g. Blum & Dash, 2004; Dash, Mach, Moody, & Moore, 2004) and studies have shown that peptide biotinylation does not prevent the peptide from being incorporated into cell bodies (Blum & Dash, 2004). Second, research has demonstrated that after incorporation into a peptide, the biotinlysine bond is cleaved only after the rest of the peptide is degraded (Koivusalo, Elorriaga, Kaziro, & Ochoa, 1963; Craft, Goss, Chandramouli, & Wood, 1985), so it is unlikely that the biotin molecule simply detached from the peptide. These results, therefore, provide initial information on the timecourse of ZIP degradation that is crucial for interpreting the results of many studies using ZIP to inhibit PKMζ.
Based on our BioZIP timecourse, in the current study, our initial testing timepoint (two hours after drug infusion) occured while residual ZIP remains in the brain tissue. A follow-up test conducted the following day (26–28h post-infusion) demonstrated that the deficits observed in the first test were persistent. This second test occurred after ZIP was fully eliminated from amygdala tissue, so the low freezing levels cannot be explained by residual ZIP disrupting freezing expression. It is important to note that Parsons and Davis (2011) observed lasting disruption of memory if an initial test was conducted shortly (one or two days) after ZIP infusion but failed to observe an effect if the initial test was conducted long after infusion (10 or 15 days later). Gamiz and Gallo (2011), on the other hand, observed disruption of an active avoidance response when the memory was tested 7d after ZIP infusion without a previous test. Here, our tests occurred both 2 and 26 hours after infusion; it is unclear whether we would observe a memory deficit at the 26 hour test if separate groups of rats were tested at each timepoint. Indeed, we chose the two hour post-infusion timepoint to maximize the likelihood that we would observe a ZIP-induced deficit in order to determine whether PKMζ plays any role in maintaining foreground context fear in the amygdala. It seems, however, that the effects observed for tests conducted one or two days after infusion (Parsons & Davis, 2011) or seven days after infusion (Gamiz & Gallo, 2011) cannot be explained by residual ZIP disrupting memory retrieval, as our results demonstrate that the peptide (injected at the same volume and concentration as Parsons & Davis, 2011) is fully removed by 24h post-infusion.
In conclusion, our data suggest that foreground context fear memory maintenance requires PKMζ activity in the BLA. Further, we have shown that ZIP degrades in a linear fashion and is removed from brain tissue by 24h after infusion. These data contribute to a growing body of literature that demonstrates that PKMζ plays an important (though not fully defined) role in memory maintenance.
Figure 3.
ZIP is fully eliminated by 24h after infusion. Representative images taken from slices taken 0h, 2h, 8h, 14h, and 24h post-infusion reveal a gradual reduction in the peptide over time, with no staining observed 24h post-infusion or later. (A) Left panel: schematic illustrates the location of the infusion site; Top panel: cleared images used for quantification; Bottom panel: precleared slices imaged at 2x magnification to demonstrate the gradual decrease in biotin stain (brown) over time. (B) Quantification of biotin stain in cleared slices. The optical density (OD) of the drug-infused hemisphere was normalized to the background, as taken from a parallel position on the vehicle-injected hemisphere. No ABC = control slice not exposed to ABC reagent. BioZIP = biotinylated ζ-pseudosubstrate inhibitory peptide.
Highlights.
We used ZIP to inhibit PKMζ in the amygdala 1 or 7d after “foreground” context fear conditioning
We also used biotinylated ZIP to measure the degradation time course of ZIP
We found that PKMζ is required to maintain context fear conditioning memory
ZIP is fully degraded from brain tissue by 24h after intracranial infusion
Acknowledgements
This research was supported by the National Institutes of Mental Health (NIMH) grants R01MH069558 to Fred J. Helmstetter and NIMH grant F31MH090685 to Janine L. Kwapis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blum S, Dash PK. A cell-permeable phospholipase Cγ1-binding peptide transduces neurons and impairs long-term spatial memory. Learning & Memory. 2004;11:239–243. doi: 10.1101/lm.74104. [DOI] [PubMed] [Google Scholar]
- Craft DV, Goss NH, Chandramouli N, Wood HG. Purification of biotinidase from human plasma and its activity on biotinyl peptides. Biochemistry. 1985;24:2471–2476. doi: 10.1021/bi00331a012. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moody MR, Moore AN. Performance in long-term memory tasks is augmented by a phosphorylated growth factor receptor fragment. Journal of Neuroscience Research. 2004;77:205–216. doi: 10.1002/jnr.20174. [DOI] [PubMed] [Google Scholar]
- Gamiz F, Gallo M. Intra-amygdala ZIP injections impair the memory of learned active avoidance responses and attenuate conditioned taste aversion acquisition in rats. Learning & Memory. 2011;18:529–533. doi: 10.1101/lm.2253311. [DOI] [PubMed] [Google Scholar]
- Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMz maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2009;2010:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed plasticity, and fear conditioning. Neurobiology of Learning and Memory. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, Elorriaga C, Kaziro Y, Ochoa S. Bacterial biotinidase. The Journal of Biological Chemistry. 1963;238:1038–1042. [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behavioral Neuroscience. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficis in contextual fear conditioning in rats. Neurobiology of Learning and Memory. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMζ maintains memories by regulating GliR2-dependent AMPA receptor trafficking. Nature Neuroscience. 2010;13:630–636. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. The role of background stimuli during pavlovian conditioning. The Quarterly Journal of Experimental Psychology. 1975;27:201–209. doi: 10.1080/14640747508400480. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. The overshadowing of background stimuli: Some effects of varying amounts of training and UCS intensity. The Quarterly Journal of Experimental Psychology. 1978;30:737–746. doi: 10.1080/14640747808400698. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Nobre MJ, Brandão ML, Landeira-Fernandez J. Role of amygdala in conditioned and unconditioned fear generated in the periaqueductal gray. Neuroreport. 15:2281–2285. doi: 10.1097/00001756-200410050-00028. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. The Journal of Neuroscience. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMz inhibition in the amygdala. Nature Neuroscience. 2011;14:295–296. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nature Reviews Neuroscience. 2010;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Fenton AA. PKMz maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biology. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Bachman J, Johnson RC, Yu Y, Huganir RL. Insights into synaptic plasticity and memory maintenance from the protein kinase Cζ knockout mouse; Poster presented at the annual meeting of the Society for Neuroscience; Washington, D.C.. 2011. Nov, [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Sacktor TC. PKMζ maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. The Journal of Neuroscience. 2008;18:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]