Abstract
Background
We sought to define the frequency of falls in early PD and assess potential risk factors for falls in this population.
Methods
We analyzed the data from two randomized, placebo controlled trials (NET-PD FS1 and FS-TOO) of 413 individuals with early PD over 18 months of follow-up in FS1 and 12 months in FS-TOO. Falls were defined as any report of falls on the UPDRS or the adverse event log. We assessed the frequency of falls overall and by age. The relationship between prespecified fall risk markers and the probability of falling was assessed using logistic and multiple logistic regression. A hurdle Poisson model was used to jointly model the probability of remaining fall-free and the number of falls.
Results
During the follow-up period, 23% of participants fell, and 11% were habitual fallers. In a multiple logistic regression model, age, baseline UPDRS Falling score, and baseline PDQ-39 scores were associated with subsequent fall risk (P <0.001). Similarly, in a hurdle Poisson regression model, age, baseline UPDRS falling item, and baseline PDQ-39 were all significantly related to the probability of falling, but only UPDRS falling >0 was associated with the number of falls.
Conclusion
Falls are frequent and are associated with impaired quality of life, even in early PD. Current standard rating scales do not sufficiently explain future fall risk in the absence of a prior fall history. New assessment methods for falls and postural instability are required to better evaluate this important problem in clinical trials and clinical practice.
Keywords: Falls, Parkinson’s disease
Introduction
Falls are common in Parkinson disease (PD), with annual fall rates ranging from 46–68% in unselected PD populations [1–4], double the fall rate of the general elderly population. [5] Overall, about 25% of PD fallers sustain an injury during a fall [1, 2, 4], and worsening gait disorder and postural instability negatively impact health-related quality of life in PD. [6] Falls are therefore a critical factor in the management of PD, with significant impact on morbidity, nursing home placement, and health care costs. [7] Although several factors are associated with falls in PD, [1, 4] the most robust factor for future falls is a history of prior falls. [8] Clinicians are therefore limited in their ability to practice primary fall prevention, and are frequently restricted to trying to reduce the number of additional falls rather than preventing or delaying the conversion from non-faller to faller.
Examining populations of early PD can offer insight into which risk markers may be associated with this conversion and provide information that can guide primary fall prevention. It also offers the opportunity to examine fall risk in early PD, as falls from PD are typically considered a later phenomenon, increasing with the onset and progression of freezing and postural instability. We performed exploratory data analyses using the FS1 and FS-TOO datasets from the NINDS Exploratory Trials in Parkinson's Disease (NET-PD) consortium to evaluate the frequency of falls in early PD and to identify risk markers associated with the transition from non-falling to first or frequent falling.
Methods
Data were analyzed from two randomized, placebo controlled, Phase II futility clinical trials (FS1 [9] and FS-TOO [10]), which used the same design and were conducted within the same network of sites (NET-PD). These trials evaluated the futility of further study of four potentially disease modifying treatments and randomized patients to creatine, minocycline, CoQ-10, GPI-1815, or placebo in a total of 413 individuals with early PD, who were diagnosed within 5 years of study entry and did not require symptomatic therapy. Additional clinical characteristics have been reported elsewhere. [9, 10] Prior to subject enrollment of the initial clinical trials, all study activities were reviewed and approved by the coordinating IRB and site IRBs and informed consent was obtained for all participants.
To maximize the number of falls captured, a fall was defined either as a response on the UPDRS Falling item that was greater than 0 or a report of an adverse event coded as “Fall”. The Falling item asks about the participant’s level of falling (unrelated to freezing) during the past week and is scored ordinally with a range from 0 (none) to 4 (falls more than once daily). At every visit, participants were asked whether they had experienced an adverse event by asking “What unusual symptoms or medical problems have you experienced since the last visit?” Adverse events were centrally coded using WHO adverse reaction terminology. A habitual faller was defined as a participant who reported a fall at two or more visits at least one month apart. UPDRS scores were collected at baseline, 1, 3, 6, 9, 12 months (in both studies) and 18 months (in FS1 only).
Data were pooled across studies, active treatment groups, and placebo groups in order to maximize the number of falls for the purposes of this exploratory analysis. All available follow-up time (out to 18 months) was used. (Exception: when testing for a potential study effect, the follow-up time was restricted to the common time period, 12 months.) Descriptive statistics were used to compare the frequency of falls by age group. To determine which baseline characteristics were associated with the probability of falling (at least once post-baseline), odds ratios were examined for each baseline variable of interest (step 1). Any variable significant at p<0.1 in step 1 was included as a covariate in a multiple logistic regression of the probability of falling (step 2). The final multiple logistic model included all variables significant at p<0.05 from step 2. This type of variable selection approach has been recommended when there are a large number of variables of interest and a small number of participants with the outcome of interest, as was the case with our dataset. [11] The independent variables assessed included demographic (age, gender) and baseline PD-related clinical measures , specifically the Geriatric Depression Scale score, UPDRS total score, Postural Instability–Gait Disorder score (average of falling, freezing, walking, postural instability, gait), Parkinson Disease Quality of Life (PDQ)-39 item 9 (fear of falling), PDQ-39 Summary score, Hoehn and Yahr score, Frontal Assessment Battery, and the Falling, Freezing, Walking, and Gait items of the UPDRS. Because a baseline UPDRS Postural Instability (PI) item score of 1 or higher was an exclusion criteria for both trials, the effect of this single item at baseline could not be examined. Baseline co-morbidities typically associated with increased fall risk in the general population were also examined, including the presence or absence of: musculoskeletal disorders, visual impairment, other neurological disorders, depression, and polypharmacy (defined as >4 medications). No patients reported a baseline medical history of dementia (defined as senile dementia, dementia NOS, or Alzheimer’s disease), so this co-morbidity could not be examined in this analysis.
To examine the baseline covariates associated with the total number of post-baseline falls, a Poisson regression model can be used. However, our dataset showed an excess number of ‘zero’ responses (80% of participants experienced no post-baseline falls), a situation in which Poisson regression may be inappropriate. To select an appropriate model for this situation, we considered the potential reasons for excess zeros. Excess zeros may occur because certain participants are resistant to falling and will never do so regardless of the length of observation (structural zeros). Alternatively, excess zeros may occur because a fall simply did not occur, for whatever reason, during the observation period (sampling zeros). Because all participants in the study are considered at-risk for falls given their PD diagnosis, we classified the excess zeros as ‘sampling zeros’; hence, a hurdle Poisson model [12] was considered appropriate for this situation. Hurdle Poisson regression modeling is a two-stage process that separately considers the factors associated with ‘fall’ or ‘no fall’ from the factors associated with the number of falls (when a fall has occurred). Two jointly fitted but separate regression models can explain the effects of covariates on this process: first, the probability of remaining fall-free, using a logit link; second, the number of falls (once the ‘hurdle’ of falling has been crossed) using a log link. The covariates that were significant at p<0.05 from the final multiple logistic regression were included in both regressions. The model was fitted using the maximum likelihood estimation method available in SAS procedure PROC NLMIXED.
Results
Of the 413 participants enrolled in FS1 and FS-TOO, 93 (23%) reported at least one fall at any time during the course of the study; 9 (2%) reported a fall at baseline, while 84 (20%) sustained a fall after baseline. Overall, 85 (21%) reported falling on the UPDRS (Falling >0) and 27 (7%) of participants reported a fall on the adverse event log. There were 66 participants (16%) who reported falling on the UPDRS (Falling>0) but not an adverse event, and 8 (2%) reported a fall on the adverse event log but responded as 0 for Falling at all visits. Forty-seven participants (11%) met the criteria for habitual fallers. Of all fallers, 20% sustained a fall within 2 years of diagnosis while 45% fell within 2–4 years since diagnosis.
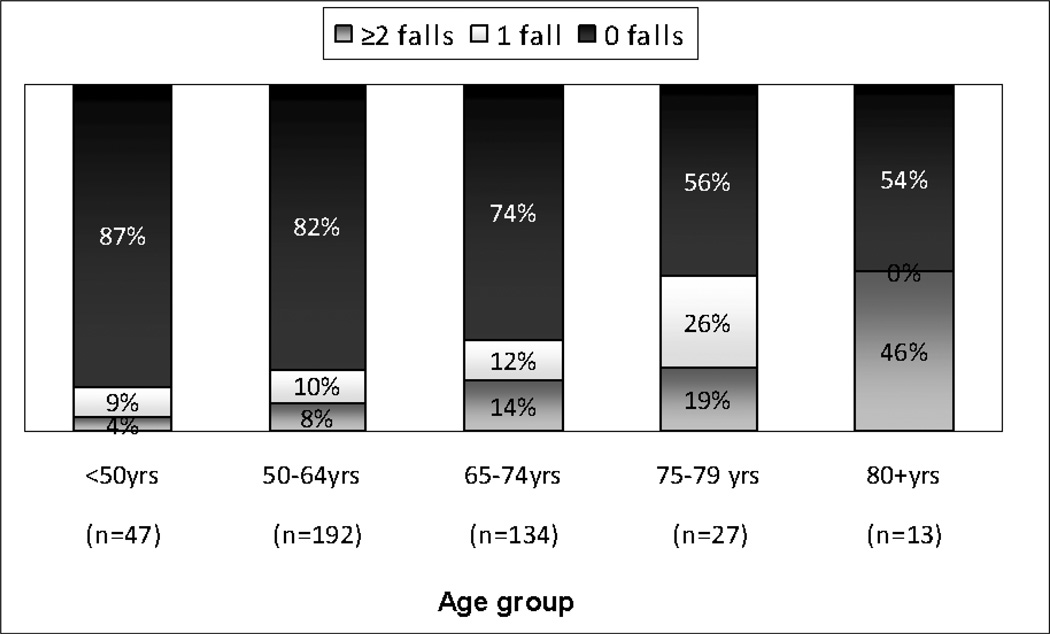
Fall frequency increased steadily with increasing age (Figure 1). In addition, habitual fallers accounted for a larger proportion of fallers as age increased. Although the fall frequency was lower in the younger age groups, 13% of individuals under age 50 and 18% of individuals between 50 and 64 years old fell during 12–18 months of follow-up.
Figure 1. Proportion and frequency of falls by age group.
“Falls” was defined by a score of greater than 0 on the UPDRS Falling at any visit or a report of the WHO term “Fall” on the adverse event log at any point in time during the course of the study. The UPDRS Falling score captures falls that occurred in the week preceding questionnaire administration.
The chi-square test was used to examine the potential effect of treatment group on the probability of falling post-baseline and a difference was not detected (χ25=5.7, p=0.33). The chi-square test was also used to examine the potential effect of study (FS-1 or FS-TOO). No difference was detected between studies on the probability of falling during the first 12 months of follow-up, the common time interval for both studies (χ21=0.17, p=0.68).
In univariable analyses of baseline variables, increasing age, increasing disease severity (as measured by UPDRS and several of its components), worsening depressive symptoms and worsened quality of life were significantly associated with subsequent falls during follow-up (Table 1). These and additional variables significant at p<0.1 in Step 1 were included in a multiple logistic regression model. During model building, both comorbidity of depression and geriatric depression score (GDS) had p-values <0.1 in the univariable tests but p-values >0.05 in the final multiple logistic model. To address concerns about collinearity, the model was re-run excluding one variable and then the other. The results did not change. Thus, neither of the depression variables provided additional information beyond that provided by other variables in the final model, and neither was included in the final model. Similarly, models were run including either the PIGD score or the individual significant components of the PIGD (Falling, Freezing, Walking, Gait) with and without total UPDRS score.
Table 1.
Univariable analyses of probability of falling (at least once post-baseline)
| Baseline Parameters | Odds Ratio | 95% CI | p-value* |
|---|---|---|---|
| Age (yrs) | 1.05 | (1.02, 1.07) | 0.0005 |
| Comorbidity of neurological disorder | 3.40 | (1.01, 11.41) | 0.052** |
| Comorbidity of depression | 1.68 | (0.97, 2.91) | 0.06 |
| Geriatric DepressionScale score | 1.11 | (1.02, 1.21) | 0.02 |
| UPDRS total | 1.07 | (1.04, 1.10) | <.0001 |
|
PIGD score (average of falling, freezing, walking, PI, gait) |
19.54 | (6.1,62.6) | <.0001 |
| UPDRS Falling>0 | 13.40 | (5.41,33.2) | <.0001 |
| UPDRS Freezing >0 | 1.58 | (0.30, 8.27) | 0.63** |
| UPDRS Walking>0 | 1.99 | (1.19,3.33) | 0.008 |
| UPDRS Gait>0 | 2.50 | (1.54,4.08) | 0.0002 |
| PDQ39 Fear of falling (treated as continuous) | 1.81 | (1.32,2.47) | 0.0002 |
| Disease Duration | 1.02 | (1.00,1.04) | 0.064 |
| PDQ-39 Summary score | 1.05 | (1.03,1.08) | <.0001 |
| Hoehn and Yahr score (treated as continuous) | 1.71 | (1.07,2.73) | 0.026 |
| Gender | 0.74 | (0.45, 1.20) | 0.22 |
| Comorbidity of Musculoskeletal disorders | 1.40 | (0.85,2.31) | 0.183 |
| Comorbidity of Vision disorders | 1.46 | (0.63, 3.42) | 0.38 |
| Polypharmacy (Use of >4 medications) | 1.18 | (0.72, 1.92) | 0.51 |
| Frontal Assessment Battery | 0.93 | (0.81, 1.07) | 0.32 |
Bolded items have p<0.1 and were included in multiple variable model.
Event rate for post-baseline falls = 20% (n=84).
PIGD: Postural Instability and Gait Disorder
Based on Pearson’s Chi-square test (binary outcomes) or likelihood ratio Chi-square test (for continuous and ordinal outcomes).
Based on Fisher’s exact test due to sparse cell counts.
In the final multiple logistic model, age, baseline PDQ-39 score and baseline Falling >0 were significantly associated with post-baseline falls (Table 2). Residual diagnostics identified a single outlier of age 33, baseline UPDRS Falling=0, PDQ-39 score=5.5, and UPDRS score=15. Removal of the outlier resulted in an improved Hosmer Lemeshow Goodness-of-Fit test (χ28=7.4, p=0.49 without the outlier versus χ28=13.4, p=0.09 with the outlier), but did not change the point estimates of the odds ratios or their p-values. Results are reported with the outlier included. The ROC is 0.73 which is considered acceptable discrimination (Hosmer Lemeshow). To further examine the association between PDQ-39 and post-baseline falls, we examined the PDQ-39 subscores in a multiple logistic model (the PDQ-39 summary score was excluded). The ADL section was the only statistically significant subscore (p=0.0018), so the original model with the PDQ-39 summary score (rather than the subscores) was retained as the final model.
Table 2.
Multiple logistic regression of probability of falling (at least once post-baseline)
| Baseline Parameters | Adjusted Odds Ratio | 95% Confidence Limits |
p-value |
|---|---|---|---|
| Age (yrs) | 1.05 | (1.02, 1.08) | <.001 |
| UPDRS Falling>0 | 7.1 | (2.7, 18.8) | <.0001 |
| PDQ-39 Summary score | 1.05 | (1.02, 1.07) | <.0001 |
Model was fit with 412 complete observations (1 deleted due to missing values).
Event rate for post-baseline falls = 20% (n=84).
Table 3 shows the results of the hurdle Poisson model regression for number of falls post-baseline. The odds of remaining fall-free decreased with increasing age (OR=0.95, 95%CI = [0.93, 0.98]) and lower PD-related quality of life (OR=0.96, 95%CI = [0.93,0.98] for PDQ-39); however, after experiencing a fall, there was no significant effect of age (RR = 1.02, 95% CI = [1.00,1.04]) or PDQ-39 (RR=1.00, 95% CI = [0.98, 1.01]) on the number of falls. The odds of remaining fall-free post-baseline were also decreased in those reporting falls at baseline (UPDRS Falling > 0, OR=0.14); in addition, after experiencing a fall, those reporting falls at baseline (UPDRS Falling > 0) have a higher risk of further falls (RR = 2.92, 95% CI = [1.64, 4.20]). The AIC/BIC score for the hurdle Poisson model (AIC=571.9 BIC = 603.9) suggests that this model is a significant improvement over a standard Poisson model (AIC=634.3, BIC=650.4) for the number of falls post-baseline (fall count).
Table 3.
Hurdle Poisson model for number of falls post-baseline
| Baseline Variables | Risk Ratio (RR) |
Standard Error |
p-value | 95% CI | |
|---|---|---|---|---|---|
|
Number of falls (Poisson part) |
Age (yrs) | 1.02 | 0.01 | 0.09 | (1.00, 1.04) |
| UPDRS Falling>0 | 2.92 | 0.65 | <.0001 | (1.64,4.20) | |
| PDQ-39 Summary score | 1.00 | 0.01 | 0.65 | (0.98, 1.01) | |
|
Odds Ratio (OR) |
Standard Error |
p-value | 95% CI | ||
|
Probability of remaining fall-free (Hurdle part) |
Age (yrs) | 0.95 | 0.01 | <.001 | (0.93, 0.98) |
| UPDRS Falling>0 | 0.14 | 0.07 | <.0001 | (0.00, 0.28) | |
| PDQ-39 Summary score | 0.96 | 0.01 | 0.0001 | (0.93, 0.98) |
Model was fit with 412 complete observations (1 deleted due to missing values).
Event rate for post-baseline falls = 20% (n=84).
There was no association detected between fallers and a change in clinical diagnosis from idiopathic PD to another condition during the course of the trial (Fisher’s Exact Test, p=0.43). Only 3% of fallers were considered to have an alternative diagnosis at the end of the study.
Discussion
Our analysis supports that even for early PD, prior fall history is strongly associated with subsequent falls and falls are associated with impaired quality of life. Both the multiple logistic regression and the hurdle Poisson regression models showed similar findings, and the hurdle Poisson model allows further understanding of impact of the covariates on ‘the odds of remaining fall free’ and ‘the risk of further falls’, within an unified framework. We demonstrated that the hurdle Poisson regression model had a superior fit to a Poisson regression model for data having ‘excess zeros’ (most people did not fall). Given these data, the probability of future falls over the course of 1 year is best explained by older age, having a UPDRS fall item >0, and worse PDQ-39 summary score at baseline. Our analysis extends prior work by demonstrating that prior fall history is more strongly associated with subsequent fall risk, even in early untreated PD, than other reported fall risk factors including disease severity, freezing, gait dysfunction, comorbid conditions impacting fall risk, or polypharmacy. Age remains significantly associated with falls, even among individuals of similar PD disease severity, and needs to be accounted for in subsequent research related to falls.
The observed association between quality of life and fall risk is consistent with other studies that have found correlations between falls, functional mobility, and balance impairment with decreased quality of life in PD [6, 13]. Fear of falling has been associated with increased fall risk. [14] However, although we observed an independent association with fear of falling as measured by its item on the PDQ-39, the probability of falling was better explained by other patient characteristics. Most likely this result represents related but unmeasured factors contributing to both increased disability and increased fall risk.
Our results suggest that even early PD individuals are at-risk for falls and therefore may benefit from the aggressive multidisciplinary fall risk modification (including physical and occupational therapy, medication adjustment, and home safety evaluations) currently in standard use to reduce falls in other populations. Our results also highlight the need for novel therapies to reduce falls in PD. While the UPDRS serves as an excellent measure of disease severity, individual motor examination items do not appear to explain the odds of falling after adjusting for other covariates, suggesting the need for developing more accurate, valid measures to assess fall risk in this population [15]. The inconsistencies between fall capture on the UPDRS and fall capture on the adverse event logs also highlight the limitations of asking about falls retrospectively, although some inconsistencies are expected since the UPDRS only asks about falls occurring over the past week. Prospective fall detection by fall diary has been shown to be more accurate than retrospective recall, [16] but relying on patient diaries increases clinical trial burden and may be impractical for routine clinical use.
Gait dysfunction, freezing, and postural instability are all considered important PD-specific risk factors for falls. Because the presence of postural instability at baseline was an exclusion criteria for the clinical trials we analyzed, we cannot comment on the impact of postural instability on fall risk in early PD; however, our current standard measures for freezing and gait dysfunction did not explain the probability of falling after adjusting for other covariates. Also, a fall proportion of 23% despite the absence of baseline postural instability suggests that fall risk in early PD may be related to factors other than postural instability or that current clinical measures of postural instability are not sensitive enough. Other factors not measured in this trial (for example, orthostatic hypotension) may also be important in defining fall risk in PD.
One of our original aims was to describe the incidence of falls over the course of the study and compare this to the general population. This aim could not be achieved, since the questions used to capture falls in various databases were too different to make direct comparisons. Although population differences and data collection methods preclude direct statistical comparison, our age-adjusted rates for falls remain high compared to the rates described in the general population, especially for younger age groups. [17] For example, our fall rate for people with PD under age 65 was 167 per 1000, while the National Health Interview Survey reported rate is 28.25.
Another limitation of our study is the method used to capture falls, which may underestimate their frequency. When the UPDRS is administered as designed, falls are only reported if they occur in the past week. Similarly, only noteworthy falls would have been recorded as an adverse event. Despite this possible underestimation, falls are disturbingly common even early in the course of PD. Finally, because of the short follow-up period in the original trials (12–18 months), we were not able to examine meaningful associations between changes in potential risk factors and the impact on subsequent fall frequency over time. These could be examined in a longer study.
In summary, falls are common in early untreated PD, with fall history, age and quality of life scores significantly associated with the odds of falling in this population. Further work is needed to develop optimal assessments for fall risk in PD.
Acknowledgements
The authors thank the NET-PD investigators, coordinators, and participants for their work in the original trials. The original trials were funded by the NINDS under the NET-PD consortium grant NIH 3U01NS044427.
This data analysis was not sponsored.
Author Roles:
Dr. Voss participated in conceiving, organizing and executing the project; designed and reviewed the analysis, and wrote the first draft of the manuscript.
Dr. Elm participated in organizing and executing the project; designed and executed the analysis, and assisted in writing the first draft of the manuscript.
Ms. Wielinksi participated in conceiving, and executing the project; assisted in designing and reviewed the analysis, and provided review and critique of the manuscript.
Dr. Aminoff participated in executing the project, reviewing the data analysis, and reviewed and critiqued the manuscript.
Dr. Bandyopadhyay participated in executing the project, designing and performing the data analysis, and provided review and critique of the manuscript.
Dr. Chou participated in executing the project, reviewing the data analysis, and reviewed and critiqued the manuscript.
Dr. Sudarsky participated in executing the project, reviewing the data analysis, and reviewed and critiqued the manuscript.
Dr. Tilley participated in conceiving, organizing, and executing the project; assisted in designing, executing, and reviewed the analysis, and provided review and critique of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Voss reports no disclosures related to the content of the manuscript.
Dr. Elm (elmj@musc.edu) reports no disclosures related to the content of the manuscript.
Ms. Wielinski (catherine.wielinski@parknicollet.com) reports no disclosures related to the content of the manuscript.
Dr. Aminoff (aminoffm@neurology.ucsf.edu) reports no disclosures related to the content of the manuscript.
Dr. Bandyopadhyay (dbandyop@umn.edu) reports no disclosures related to the content of the manuscript.
Dr. Chou (klchou@med.umich.edu) reports no disclosures related to the content of the manuscript.
Dr. Sudarsky (lsudarsky@partners.org) reports no disclosures related to the content of the manuscript.
Dr. Tilley reports no disclosures (barbara.c.tilley@uth.tmc.edu) related to the content of the manuscript.
References
- 1.Pickering RM, Grimbergen YAM, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson's disease. Movement Disorders. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 2.Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. Journal of Neurology. 2005;252:1310–1315. doi: 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- 3.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson's disease: characteristics of fallers and non-fallers. Age & Ageing. 2001;30:47–52. doi: 10.1093/ageing/30.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE, et al. Predictors of deterioration in health-related quality of life in Parkinson's disease: results from the DATATOP trial. Movement Disorders. 2008;23:653–659. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Williams CS. Falls, Injuries Due to Falls, and the Risk of Admission to a Nursing Home. The New England Journal of Medicine. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 8.Thurman DJ, Stevens JA, Rao JK. Practice Parameter: Assessing patients in a neurology practice for risk of falls: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:473–479. doi: 10.1212/01.wnl.0000299085.18976.20. [DOI] [PubMed] [Google Scholar]
- 9.The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 10.The NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68:20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 11.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edition. Wiley Interscience; 2000. [Google Scholar]
- 12.Mullahy J. Specification and Testing of Some Modified Count Data Models. Journal of Econometrics. 1986;33:341–365. [Google Scholar]
- 13.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? Journal of Neurology, Neurosurgery, and Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak M, Pang M. Fear of falling is independently associated with recurrent falls in patients with Parkinson's disease: a 1-year prospective study. Journal of Neurology. 2009;256:1689–1695. doi: 10.1007/s00415-009-5184-5. [DOI] [PubMed] [Google Scholar]
- 15.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie L, Byles J, D'Este C. Validation of self-reported fall events in intervention studies. Clinical rehabilitation. 2006;20:331–339. doi: 10.1191/0269215506cr947oa. [DOI] [PubMed] [Google Scholar]
- 17.Adams PF, Barnes PM, Vickerie JL. Summary health statistics for the U.S. population: National Health Interview Survey, 2007. National Center for Health Statistics. Vital Health Stat. 2008;10(238) [PubMed] [Google Scholar]