Abstract
The majority of the genome is noncoding and was believed to be nonfunctional. However, it is now appreciated that transcriptional control of protein coding genes resides within these noncoding regions. Thousands of genes encoding long intergenic noncoding RNAs (lincRNAs) have been recently identified throughout the genome, which positively or negatively regulate transcription of neighboring target genes. Both TMEVPG1 and its mouse orthologue encode lincRNAs and are positioned near the interferon gamma gene (IFNG). Here we show that transcription of both mouse and human TMEVPG1 genes is Th1 selective and dependent upon Stat4 and T-bet, transcription factors that drive the Th1 differentiation program. Ifng expression is partially restored in Stat4−/−Tbx21−/− cells through co-expression of T-bet and Tmevpg1 and Tmevpg1 expression contributes to but alone is not sufficient to drive Th1-dependent Ifng expression. Our results suggest that TMEVPG1 belongs to the general class of lincRNAs that positively regulate gene transcription.
Introduction
The immune system is directly responsible for protection against invading pathogens including bacteria and viruses. Production of IFN-γ by CD4+ Th1 cells, CD8+ T cells, natural killer (NK) and NKT cells dictates the ability to clear intracellular infections and promote tumor immunity. IFNG expression is the result of successful coordination of histone modifications, trans-activating factors and cis regulatory elements including distal conserved noncoding sequences (1–3). A naïve CD4+ T cell initiates a Th1 polarizing program upon antigenic stimulation in the presence of the cytokine IL-12 leading to the activation of the JAK/STAT pathway components STAT 1 and STAT 4 (4). These molecules promote transcription of the master Th1 transcription factor, T-bet (5–6) contributing to maintenance of IFNG locus permissivity as well as repression of the IL4 locus through covalent chromatin modifications (7). Understanding how the IFNG locus is regulated by noncoding elements of the genome, particularly in the context of Th1 cell differentiation, has been an area of extensive investigation.
Long intergenic noncoding RNAs (lincRNAs) are a new species of regulatory RNAs that exist throughout the genome but the complete understanding of their functions is incompletely understood (8). Initial findings indicate that thousands of lincRNAs exist in the vertebrate genome. In general, lincRNA genes are conserved across species, are positioned near their target protein coding genes and impact a variety of compartmentalized biological systems. For example, XIST is required for the process of X chromosome inactivation in females. With the assistance of six additional lincRNAs, XIST “paints” the inactive chromosome with repressive histone marks silencing transcription (9–11). HOTAIR and AIR regulate the HOXD and Igf2r loci, respectively, and are critical for development by modulating expression of these essential transcription factors. Recent evidence suggests that HOTAIR regulates expression of the HOXD locus by acting as a scaffold for histone modifying enzyme recruitment (12–15). LincRNA-p21 expression is enhanced by the potent tumor suppressor p53 and then subsequently represses transcription of genes downstream of p53 signaling (16). To date, few lincRNAs with enhancer function have been described (17–18). The function of lincRNAs in the immune system has not been actively investigated. A genome-wide screen identified a cluster of lincRNAs predicted to function in the immune system based upon the proximity to genes known to encode proteins with immune function (19). Additionally, independent noncoding RNA screens of activated CD8+ T cells and SARS-coronavirus infected cells have been conducted (19); however, the functional biology of these lincRNAs has not been determined.
TMEVPG1, a lincRNA transcript was initially identified in the context of Theiler’s virus infection whereby mice deficient in TMEVPG1 expression were unable to control the intracranial viral infection (20). Here we describe TMEVPG1 as a Th1 specific lincRNA that requires Stat4 and T-bet for active transcription and contributes to the transcription of the gene encoding IFN-γ.
Materials and Methods
Mice
BALB/c-J.Stat4−/−Tbx21−/−, DO11.10.Stat4−/−, DO11.10.Tbx21−/− and wildtype mice were obtained from Christopher L. Williams in the Boothby laboratory. The BALB/c-J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were bred in the Vanderbilt University animal facilities. Research using mice complied with all relevant institutional and federal guidelines and policies.
Human and mouse lymphocyte culture conditions
Healthy human PBMC were isolated by Ficoll–Hypaque density centrifugation (GE Healthcare). CD4+ T cells were sorted by positive selection and stimulated with immobilized anti-CD3 (OKT3; ATCC, Manassas, VA) and soluble anti-CD28 under Th1 (IL-12 10 ng/ml) or Th2 (IL-4 10 ng/ml) polarizing conditions for three days followed by two days of culture with IL-2. Murine BALB/c-J, BALB/c-J.Stat4−/−Tbx21−/−, DO11.10.Stat4−/−, DO11.10.Tbx21−/− splenocytes (1×106 cells/ml) were stimulated with immobilized anti-CD3 (2C11; ATCC, Manassas, VA) or OVA323–339 peptide antigen (10 μg/ml) under Th1 (10 ng/ml IL-12 and 10 ug/ml anti IL–4 11B11; ATCC), Th2 (10 ng/ml IL-4 and 10 μg/ml anti–IFN-γ, R4-642; ATCC), Th17 (10 ng/ml IL-6, 10 ng/ml IL-23, 1 ng/ml TGFβ and 10 ug/ml anti-IFN-γ) or Th22 (5 ng/ml IL-1β, 10 ng/ml IL-6 and 5 ng/ml TNF-α) polarizing conditions for three days. CD4+ T cells were purified by positive selection (Miltenyi Biotec). Human or mouse cells were restimulated to generate effector cells by the addition of 50 ng/ml PMA and 1 μM ionomycin for 6 hrs or peptide antigen as described in the Results section.
RNA isolation and quantitative RT-PCR
Total RNA was isolated with Tri Reagent (Ambion, Inc.). cDNA was synthesized with the SSRIII kit (Invitrogen). Transcript levels were determined by SybrGreen quantitative RT-PCR (qPCR) using the following primer pairs: human TMEVPG1 forward 5′aaacgctggaggagaagtca 3′ and reverse 5′ttctcctccagcgttttacg 3′ and mouse Tmevpg1 forward 5′cctgaaaatcaccatgcaca 3′ and reverse 5′gttttcgggatgtcgtcaaa 3′. Human and murine message levels are expressed as the ratio to GAPDH (or Gapdh) transcript levels calculated directly from the Ct.
siRNA knockdown
Silencer® Select siRNA duplexes (Ambion, Inc.) were designed against the 5′ Tmevpg1 sequence: siRNA 1 sense 5′ GAGAAGAGCCUGAGAGAAATT 3′ and antisense 3′ TTCUCUUCUCGGACUCUCUUU 5′; siRNA 2 sense 5′ GCAGACUAAACUAGAUAGUTT 3′ and antisense 3′ TTCGUCUGAUUUGAUCUAUCA 5′. Ifng siRNA (siRNA ID: 158238) and non-specific scramble siRNA (sense 5′ caacugggacacauguguutt 3′ and antisense 3′ ttguugacccuguguacacaa 5′) were used as controls. siRNA duplexes (30 pmoles) were nucleofected into cells according to the manufacturer’s instructions (Lonza). After 4 hrs of recovery cells were cultured (5 ×105 cells/ml) under Th1 conditions for three days before restimulation. IFN-γ was measured in culture supernatants by ELISA (BD OptEIA).
Tmevpg1 sequencing and overexpression
Tmevpg1 cDNA clone AA162222 (Open Biosystems) was sequenced at the Vanderbilt Sequencing Facility. Full length Tmevpg1 was cloned into the pcDNA3.1/myc-His A overexpression vector (Invitrogen). CMV-Tmevpg1, CMV-Tbx21 or CMV-empty vectors were transfected (1 μg/106 cells) into polarized splenocytes using Amaxa Nucleofection (Lonzabio).
Statistical analysis
Statistical significance was determined by Student’s T test.
Results
Selective expression of TMEVPG1 and its murine orthologue under Th1 polarizing conditions
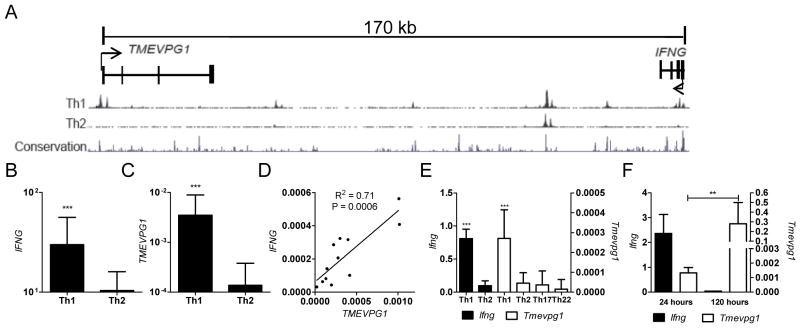
Utilizing the UCSC genome browser configuration we identified a gene, AK124066, also named TMEVPG1, which is predicted to transcribe a spliced, noncoding mRNA transcript and is positioned approximately 170 kb from the IFNG coding region (Fig. 1A). The 33 kb long TMEVPG1 gene is comprised of four exons and encodes an mRNA of 1791 bp in length. TMEVPG1, similar to IFNG, possesses multiple Th1 specific DNAse I hypersensitivity sites at its promoter as well as epigenetic histone marks, H3K9 acetylation and H3K4 mono- and tri-methylation, that are known to be associated with active transcription. Both TMEVPG1 and its mouse ortholog are located on the opposing strand to IFNG. The transcriptional start site of mouse Tmevpg1 is positioned 117 kb from the Ifng transcriptional start site and is spliced into a mature transcript 918 bp in length. The promoter region and first intron of Tmevpg1 also exhibit considerable sequence conservation with human TMEVPG1. These data are consistent with the possibility that TMEVPG1 encodes a lincRNA transcript selectively expressed in Th1 cells relative to Th2 cells.
FIGURE 1.
TMEVPG1 is selectively expressed in Th1 cells and positively correlates with IFNG expression in human and mouse. A, Relative genomic positions of TMEVPG1 and IFNG on human chromosome 12. Th1/Th2 DNAse I hypersensitivity sites and mammalian conservation peaks are also shown. Arrowheads indicate the orientation of transcription. B and C, Human CD4+ T cells (n=12) were cultured under Th1 or Th2 polarizing conditions for three days. B, IFNG and C, TMEVPG1 transcript levels relative to GAPDH were determined by SybrGreen qPCR. D, Relationship between IFNG and TMEVPG1 transcript levels from whole blood samples (PAXgene collection tubes) were determined by linear regression analysis (n=12). E, Ifng and Tmevpg1 transcript levels relative to Gapdh in Th1, Th2, Th17 or Th22 polarized cultures. F, Tmevpg1 and Ifng transcript levels relative to Gapdh in CD4+ effector Th1 cells after restimulation with PMA and ionomycin. Results are expressed as the mean ± SD of three independent experiments.**p ≤ 0.01 and ***p ≤ 0.001
To address this possibility, human CD4+ T cells were stimulated in vitro under Th1 or Th2 polarizing conditions for three days, followed by two days of additional culture with IL-2 before restimulation with PMA and ionomycin. Transcript levels of IFNG, TMEVPG1, and GAPDH were determined by qPCR. Transcript levels of IFNG and TMEVPG1 were substantially greater in Th1 cultures compared to Th2 cultures (Fig. 1B and 1C). We also determined transcript levels of IFNG and TMEVPG1 in PBMCs from healthy human control subjects and compared transcript levels by linear regression. We found a positive correlation between IFNG and TMEVPG1 transcript levels relative to GAPDH (Fig. 1D). Taken together these results demonstrate that, like IFNG, TMEVPG1 transcript levels are selectively expressed in Th1 cultures relative to Th2 cultures. The linear regression analysis further indicates a strong association between IFNG and TMEVPG1 transcript levels in PBMCs.
To assess whether murine Tmevpg1 was also preferentially expressed in polarized Th1 cells, we measured Tmevpg1 transcript levels in total splenocytes cultured under Th1 or Th2 polarizing conditions. RNA was isolated after three days in culture at the peak of Ifng expression during primary stimulation. Tmevpg1 and Ifng message levels were measured by qPCR. Consistent with our results in human lymphocytes, Tmevpg1 transcript levels were significantly greater in Th1 cells than in Th2 cells (Fig. 1E). Tmevpg1 transcript levels were also analyzed in Th17 and Th22 polarized cells to confirm Th1 specificity (Fig 1E). We also restimulated Th1 effector cells with PMA and ionomycin and followed Tmevpg1 transcript levels over time. IL-2 was added to these cultures to sustain viability. Restimulation resulted in a marked increase in Tmevpg1 transcript levels that was sustained over several days (Fig. 1F). In contrast to what we observed in Th1 effector cultures, Tmevpg1 transcript levels were undetectable in effector CD8+ T cells polarized under Th1 culture conditions, which produced a significant amount of IFN-γ (1.7 fold Gapdh transcript levels measured by qPCR). We conclude from these experiments that Th1 selective Tmevpg1 expression is conserved between murine and human lymphocytes but is not expressed by CD8+ T cells under these culture conditions. Further, restimulation of Th1 cells results in greater Tmevpg1 expression levels than observed in primary Th1 cultures implicating a role for Tmevpg1 in effector Th1 cells.
Tmevpg1 is regulated by Th1 transcription factors
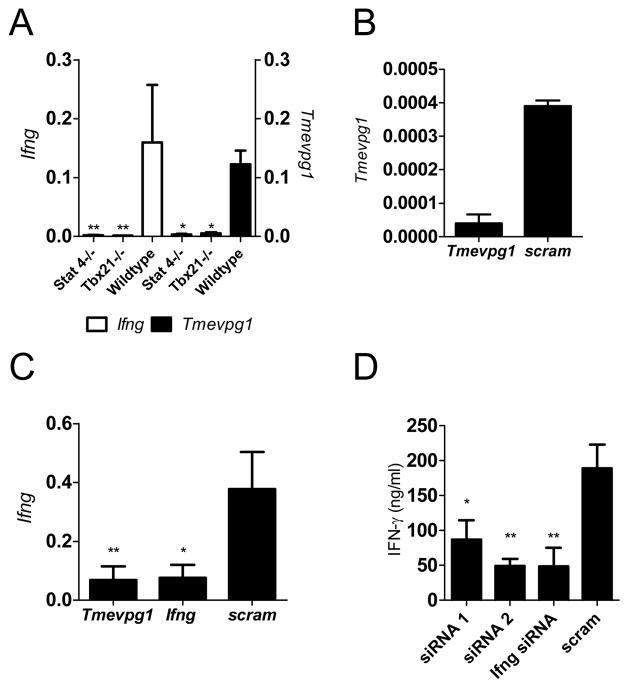
Th1 selective expression of IFN-γ in our model system is dependent upon the transcription factors Stat4 and T-bet. Because Tmevpg1 also displays selective Th1 expression we determined if Tmevpg1 expression was also dependent upon Stat4 and T-bet. To do so, we isolated splenocytes from DO11.10.Stat4−/− and DO11.10.Tbx21 −/− (T-bet knockout) transgenic mice and stimulated the cells in vitro with OVA323–339 peptide and IL-12. After three days, CD4+ T cells were purified and restimulated with OVA323–339 peptide for 48 hrs. As expected, Ifng transcript levels were substantially diminished in Th1 cells in the absence of Stat4 or T-bet compared to the DO11.10 wildtype control mice (Fig. 2A). Tmevpg1 transcript levels were also substantially reduced in T cells deficient in either Stat4 or T-bet (Fig. 2A).
FIGURE 2.
Tmevpg1 induction is dependent upon Stat4 and T-bet transcription factors. A, Ifng and Tmevpg1 expression in Th1 cells from DO11.10.Stat4−/−, DO11.10.Tbx21−/− (T-bet knockout) and wildtype TCR transgenic mice relative to Gapdh. B, C and D, Tmevpg1-specific, Ifng-specific, or scrambled siRNA duplexes were introduced into Th1 polarized CD4+ T cells by nucleofection. B, Tmevpg1, C, Ifng, and D, IFN-γlevels (measured by ELISA) were determined after restimulation with PMA and ionomycin. Results are expressed as the mean ± SD of three independent experiments. *p ≤ 0.05 and **p ≤ 0.01
Influence of Tmevpg1 on Ifng transcription
LincRNAs have been clustered into two functionally distinct categories: repressors and enhancers of transcription of protein coding genes. We aimed to determine the function of Tmevpg1 in Th1 cells via siRNA-mediated knockdown of Tmevpg1. Nucleofection of Tmevpg1-specific siRNA duplexes 1 and 2 resulted in a reduction of Tmevpg1 (Fig. 2B) and Ifng (Fig. 2C) transcript levels relative to the scramble siRNA transfected polarized splenocytes. Knockdown of Tmevpg1 by siRNA duplex 1 or siRNA duplex 2 resulted in a two-fold or four-fold reduction in IFN-γ protein, respectively, relative to transfection with a non-specific scrambled siRNA (Fig. 2D). Knockdown with Ifng siRNA resulted in a similar decrease in IFN-γ protein concentrations. Nucleofection with both Tmevpg1 siRNA duplexes 1 and 2 caused a comparable reduction in Tmevpg1 transcript levels while siRNA knockdown of Ifng did not affect transcript levels of Tmevpg1. Our conclusion is that Tmevpg1 plays a role in Ifng expression by Th1 cells.
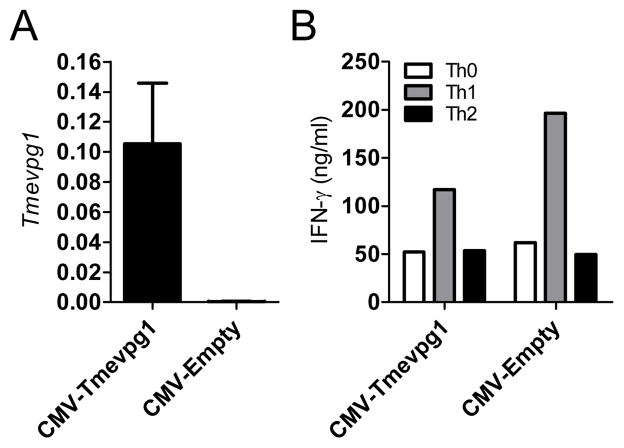
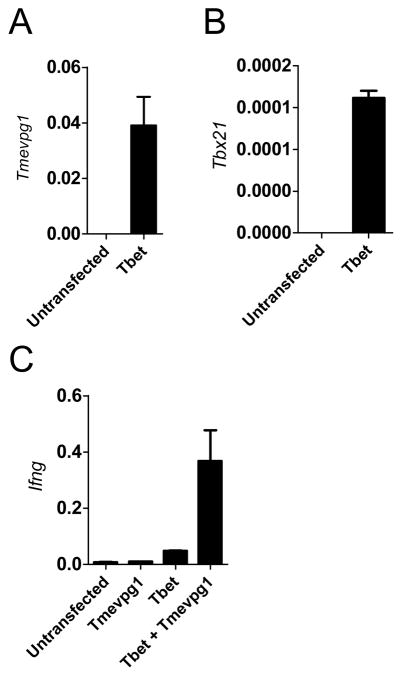
Based upon the results from the siRNA knockdown experiments, we determined if overexpression of Tmevpg1 was sufficient to induce Ifng transcription. Full length Tmevpg1 was cloned into a CMV expression plasmid. Total BALB-c/J splenocytes were stimulated with anti-CD3 under non-polarizing conditions (Th0) or under Th1 or Th2 polarizing conditions for three days. CD4+ T cells were isolated and CMV-Tmevpg1 or CMV-empty vectors were then transfected (1 μg of plasmid/106 cells). After a period of rest, cells were restimulated with PMA and ionomycin. Nucleofection of CMV-Tmevpg1 into primary Th1 cells resulted in an increased expression of Tmevpg1 compared to the empty vector control (Fig. 3A). Ectopic expression of Tmevpg1 in primary CD4+ T cells resulted in no significant increase in IFN-γ protein in Th0, Th1, or Th2 cells relative to transfection with an empty vector control (Fig. 3B). We further examined whether Tmevpg1 was sufficient to restore Ifng transcript expression in the absence of Stat4 and T-bet. CMV-Tmevpg1, CMV-Tbx21 or CMV-empty vector plasmids were transfected into Th1 polarized BALB/c-J.Stat4−/−Tbx21 −/− splenocytes. After a period of rest, cultures were restimulated with PMA and ionomycin. Tmevpg1, Tbx21 and Ifng transcript levels were determined by qPCR. We observed Tmevpg1 expression to be restored by ectopic expression of T-bet alone in the BALBc/J.Stat4−/−Tbx21 −/− cells (Fig 4A). CMV-Tmevpg1 and CMV-Tbx21 co-transfection resulted in a substantial increase in Ifng transcript levels relative to transfection of CMV-Tmevpg1 or CMV-Tbx21 alone (Fig. 4C). We conclude from these experiments that overexpression of Tmevpg1 in trans alone is not sufficient to induce increased Ifng transcription in Th0, Th1, or Th2 cells or in Stat4−/−Tbx21 −/− cells except in the presence of T-bet.
FIGURE 3.
Tmevpg1 is necessary but not sufficient for IFN-γ expression by Th1 cells. CMV-Tmevpg1 or CMV-empty expression plasmids were introduced into polarized cultures by nucleofection and cultures were restimulated with PMA and ionomycin. A, Tmevpg1 transcript levels are expressed relative to Gapdh. B, IFN-γ levels in Th0, Th1, and Th2 polarized cultures were determined by ELISA. Results represent the mean of at least three separate experiments
FIGURE 4.
Cooperative action of T-bet and Tmevpg1 restores Ifng expression. CMV-Tmevpg1, CMV-Tbx21 and/or CMV-empty vector plasmids were transfected into Th1 polarized BALB-c/J.Stat4−/−Tbx21−/− cells. Cells were restimulated with PMA and ionomycin. A, Tmevpg1 B, Tbx21 and C, Ifng transcript levels are expressed relative to Gapdh. Results represent the mean of at least three independent experiments.
Discussion
Presently, lincRNAs segregate into two functional categories that either repress or enhance the transcription of protein coding genes. To summarize our results, Tmevpg1 and its human ortholog, are expressed selectively in Th1 cells relative to Th2, Th17 and Th22 cells and expression is dependent upon the Th1 specific transcription factors, Stat4 and T-bet. Our results also demonstrate that Tmevpg1 influences Ifng transcription in response to the Th1 differentiation program. In contrast, ectopic expression of Tmevpg1 does not increase Ifng transcript levels in Th0, Th1, or Th2 cells, however, Tmevpg1 is able to partially restore Ifng expression when T-bet is also overexpressed. One possible interpretation is that Tmevpg1 must be expressed from its endogenous locus, or in cis, to stimulate Ifng transcription. A second possible interpretation, which our data favor, is that Tmevpg1 must act in concert with T-bet, or other critical trans-activating factors, to influence Ifng transcription. Other studies of enhancer lincRNAs are consistent with our results and these lincRNAs also fail to stimulate transcription of protein coding genes in trans or require additional transactivation factors to drive their transcription (17).
A general emerging model is that cell-type specific transcription factors bind to lincRNA promoters to drive their transcription. The lincRNAs bind to ubiquitous proteins required to establish the epigenetic code and by mechanisms that are incompletely understood direct these proteins to their target protein-coding genes (18, 20). This model does not rule out the possibility that these cell-type specific transcription factors also target protein-coding genes. Our results demonstrate that one lincRNA, Tmevpg1, contributes to Ifng expression as part of the Th1 differentiation program. We predict that additional lincRNAs play critical roles in developmental programs required to establish the different functions of the immune system.
Acknowledgments
This work was supported by US NIH Grants AI044924 (TMA) and AI077528 (MRB). The Vanderbilt University Medical Center Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404)
We thank J. W. Thomas for discussion and comments. We thank M.A. Henderson for laboratory assistance and management.
Abbreviations used
- lincRNA
long intergenic noncoding RNA
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Wilson C, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 2.Aune TM, Collins PL, Chang S. Epigenetics and T helper 1 differentiation. Immunology. 2009;126:299–305. doi: 10.1111/j.1365-2567.2008.03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins PL, Chang S, Henderson M, Soutto M, Davis GM, McLoed AG, Townsend MJ, Glimcher LH, Mortlock DP, Aune TM. Distal Regions of the Human IFNG Locus Direct Cell Type-Specific Expression. J Immunol. 2010;185:1492–501. doi: 10.4049/jimmunol.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 5.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh T, Watford W, Schones D, Peng W, Sun H, Paul W, O’Shea J, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 7.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–79. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemson CM, McNeil JA, Willard HF, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996;132:259–75. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–3. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 11.Kay GF, Penny GD, Patel D, Ashworth A, Brockdorff N, Rastan S. Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell. 1993;72:171–82. doi: 10.1016/0092-8674(93)90658-d. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–9. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sleutels F, Zwart R, Barlow D. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–3. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 16.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–19. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, Wysocka J, Lei M, Dekker J, Helms JA, Chang HY. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, Fan L, Koziol MJ, Gnirke A, Nusbaum C, Rinn JL, Lander ES, Regev A. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol. 2010;28:503–10. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigneau S, Rohrlich PS, Brahic M, Bureau JF. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol. 2003;77:5632–8. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oswalkd-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, Jenkins CA, Judson MA, Drake WP. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77:3740–3748. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]