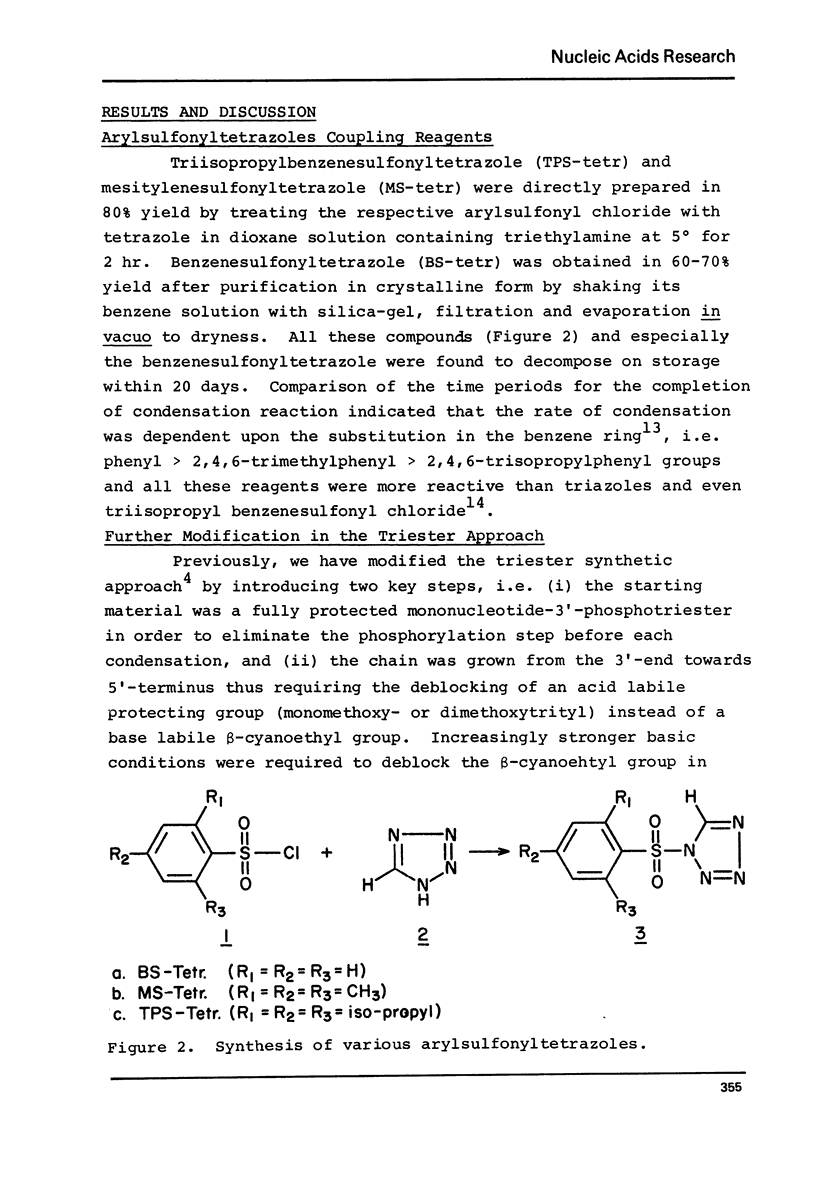
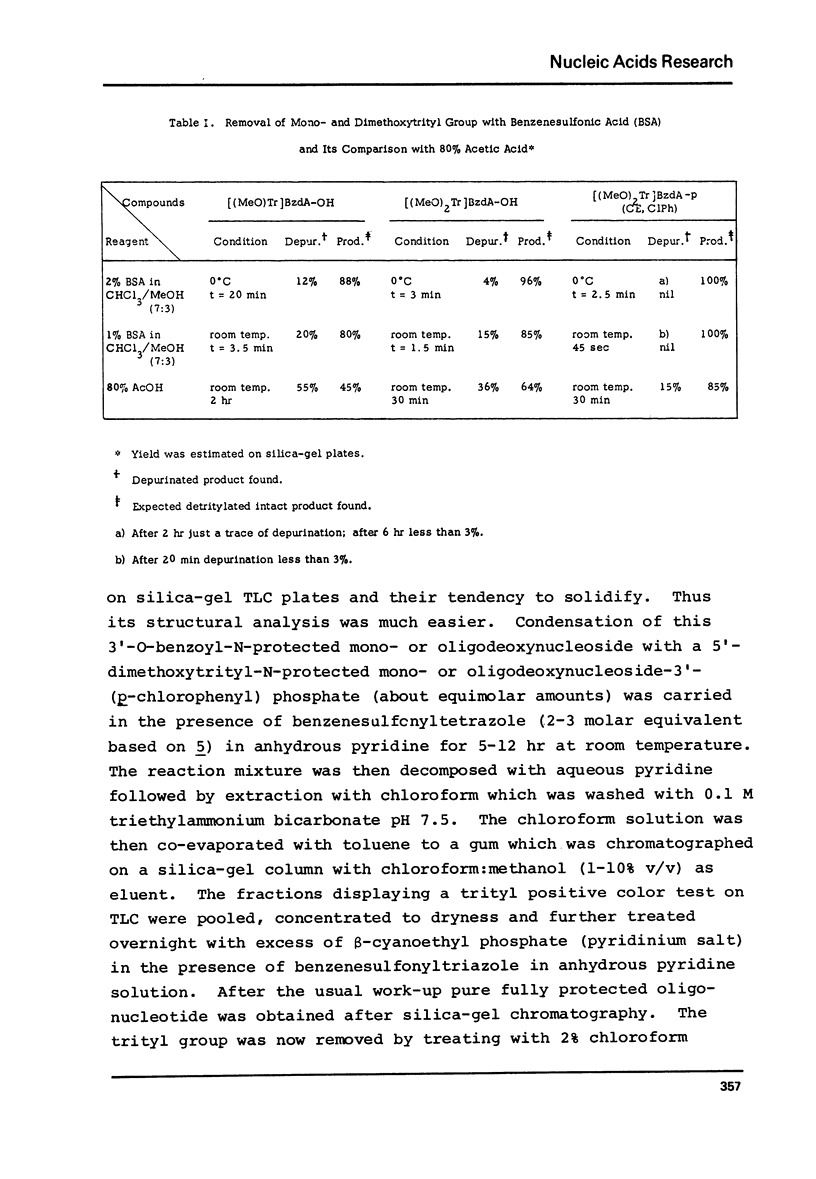
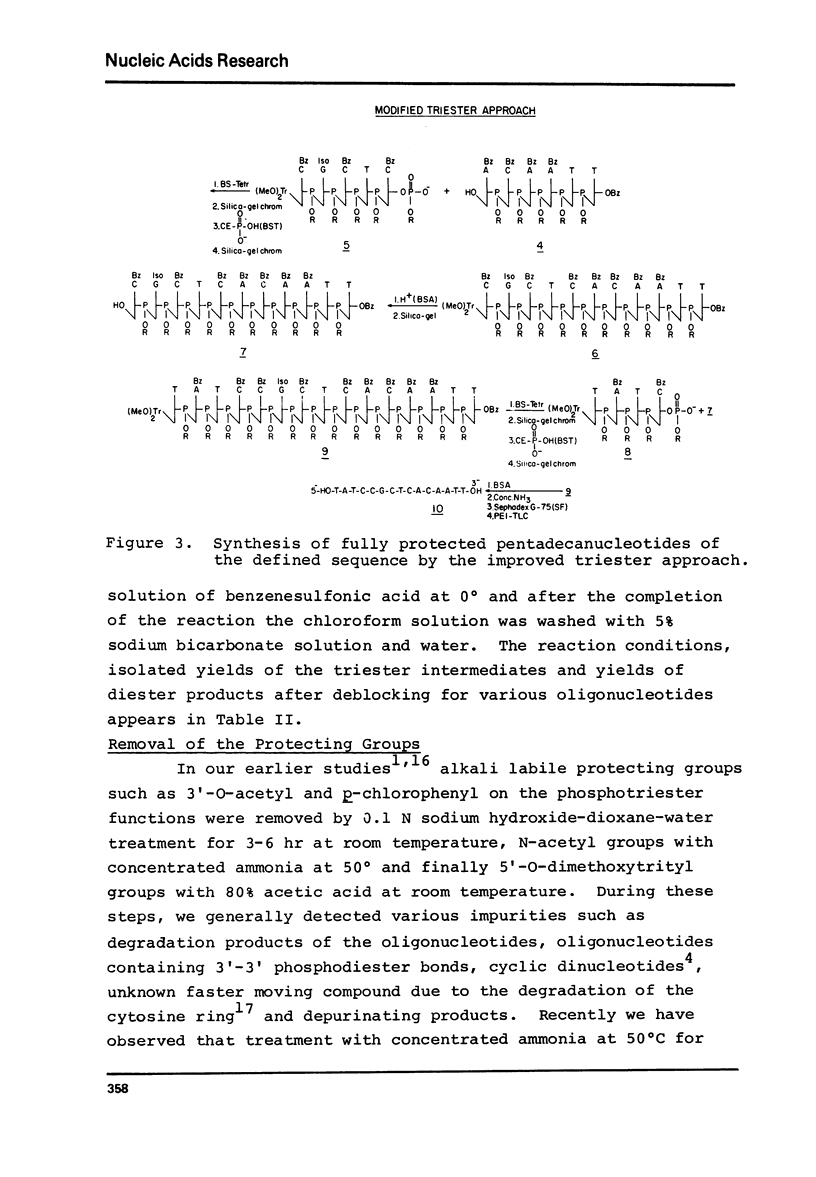
Abstract
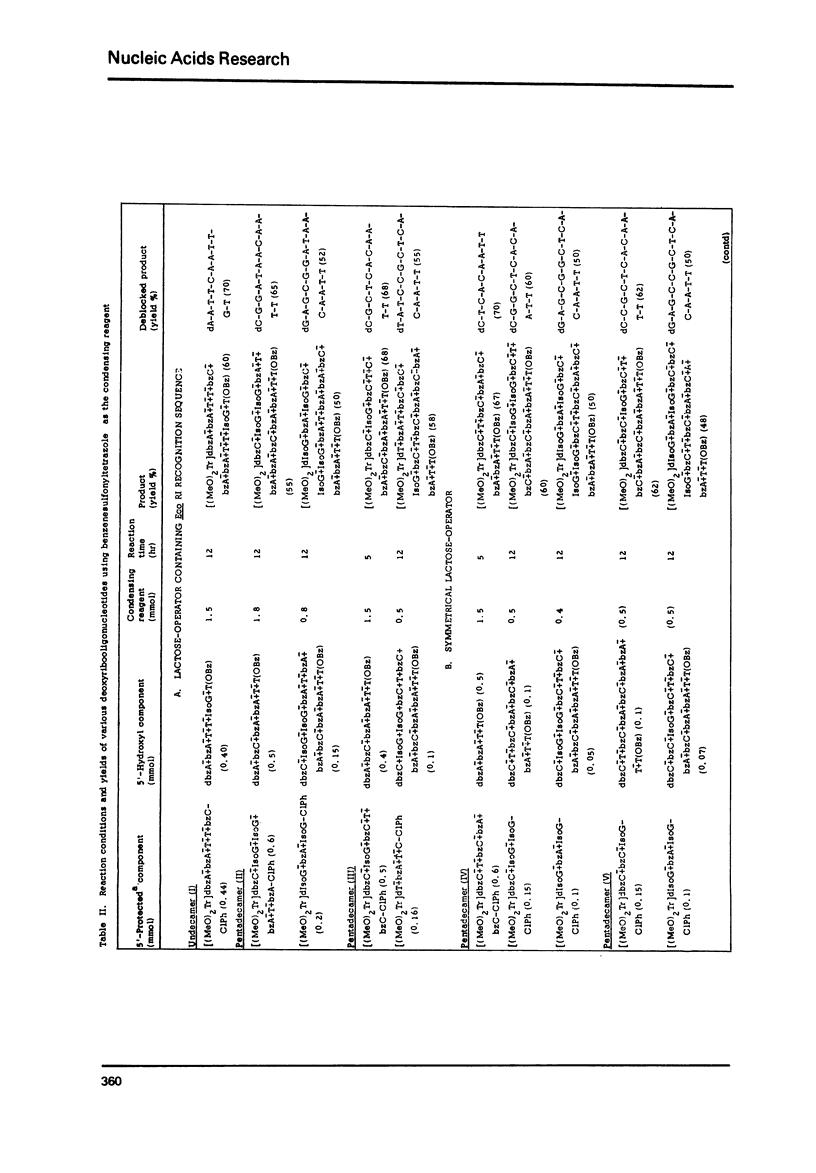
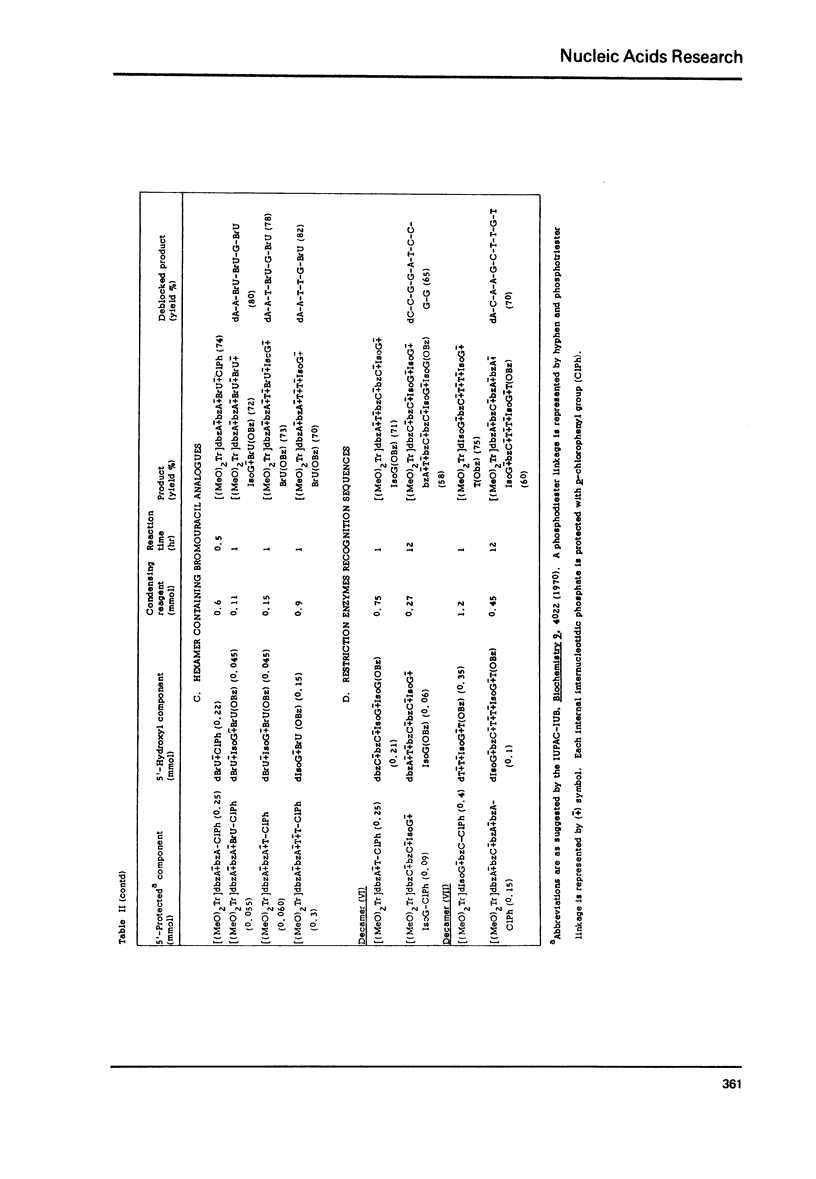
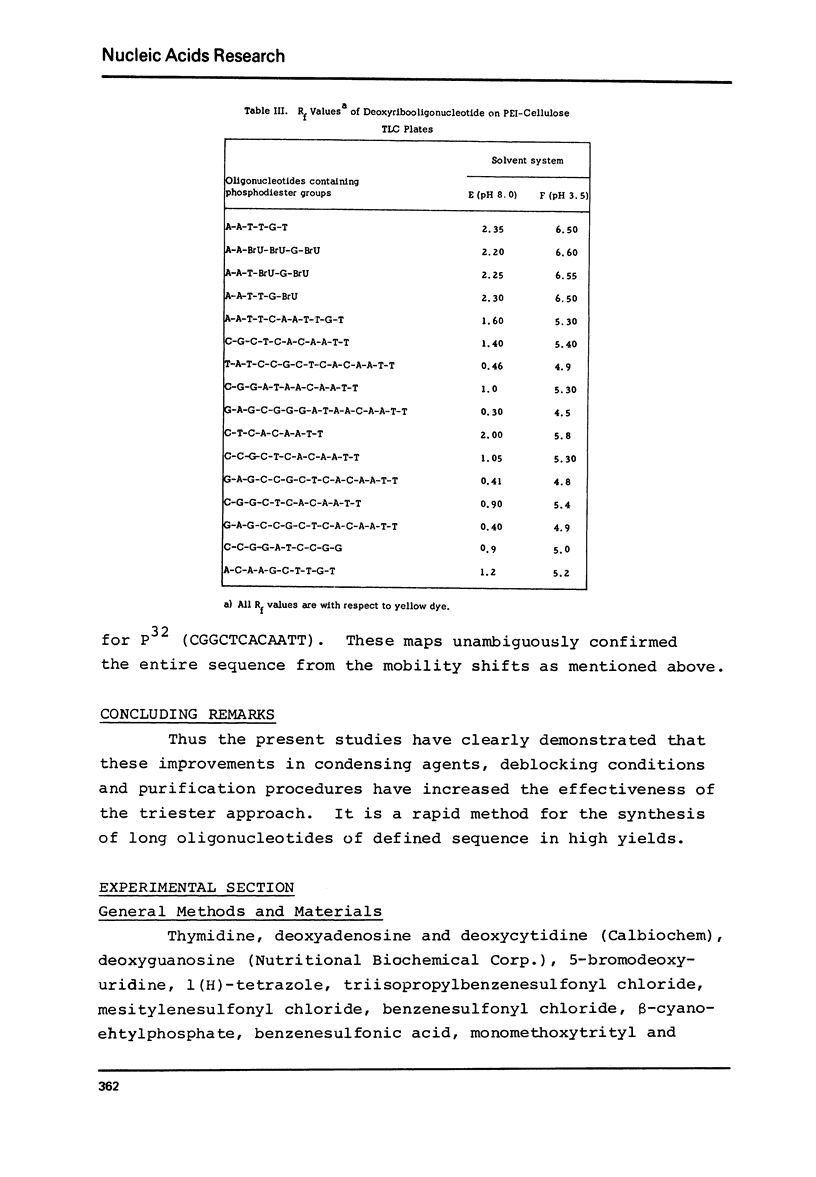
The modified triester approach has been further improved and refined to the synthesis of defined sequences of deoxyribo-oligonucleotides. Improvements include arylsulfonyltetrazoles as faster and milder condensing agents, benzenesulfonic acid to avoid depurination during deblocking of trityl protecting groups and improved chromatographic procedures for purification of triester intermediates and purification of the final product containing 3'-5' phosphodiester linkages.
Full text
PDF



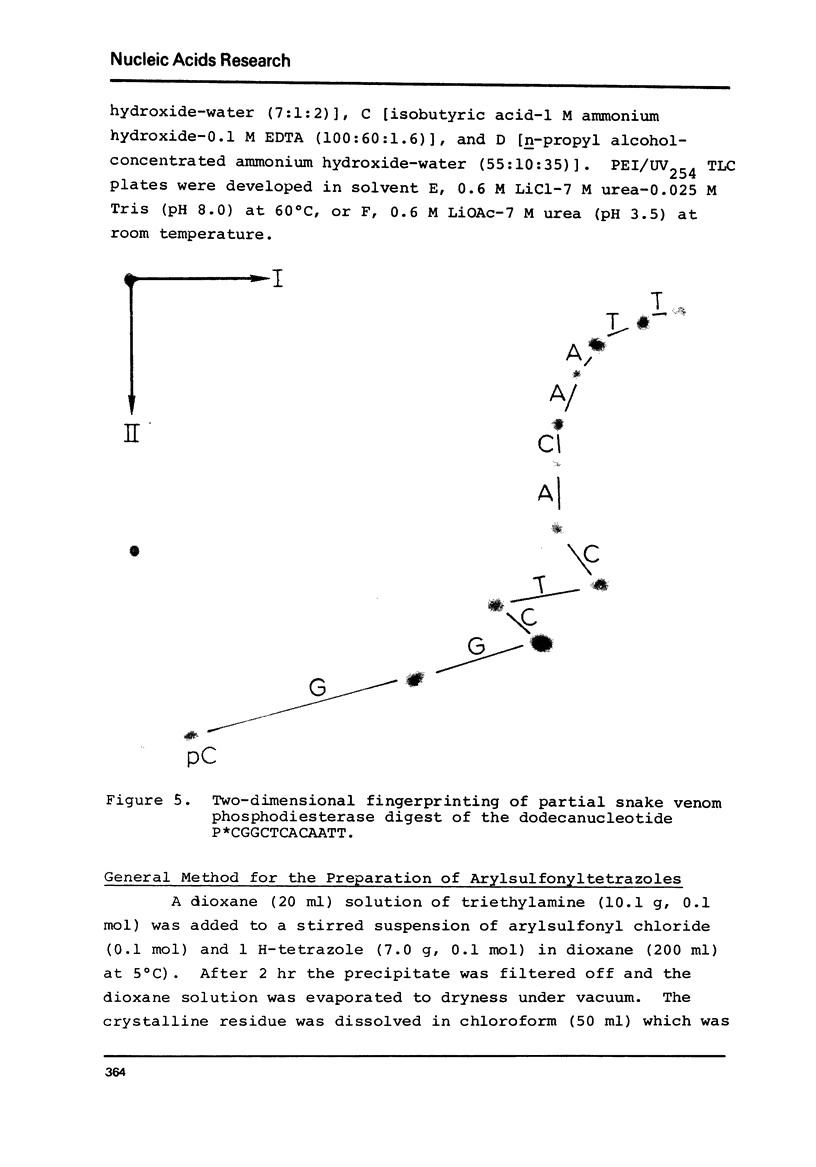







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Rizk I. Synthese von Oligodesoxynucleotiden über Phosphorsäure-triester. Chem Ber. 1969;102(7):2362–2377. doi: 10.1002/cber.19691020724. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Narang S. A., Bahl C. P., Marians K. J., Wu R. Chemical synthesis and sequence studies of deoxyribooligonucleotides which constitute the duplex sequence of the lactose operator of Escherichia coli. J Biol Chem. 1975 Jun 25;250(12):4592–4600. [PubMed] [Google Scholar]
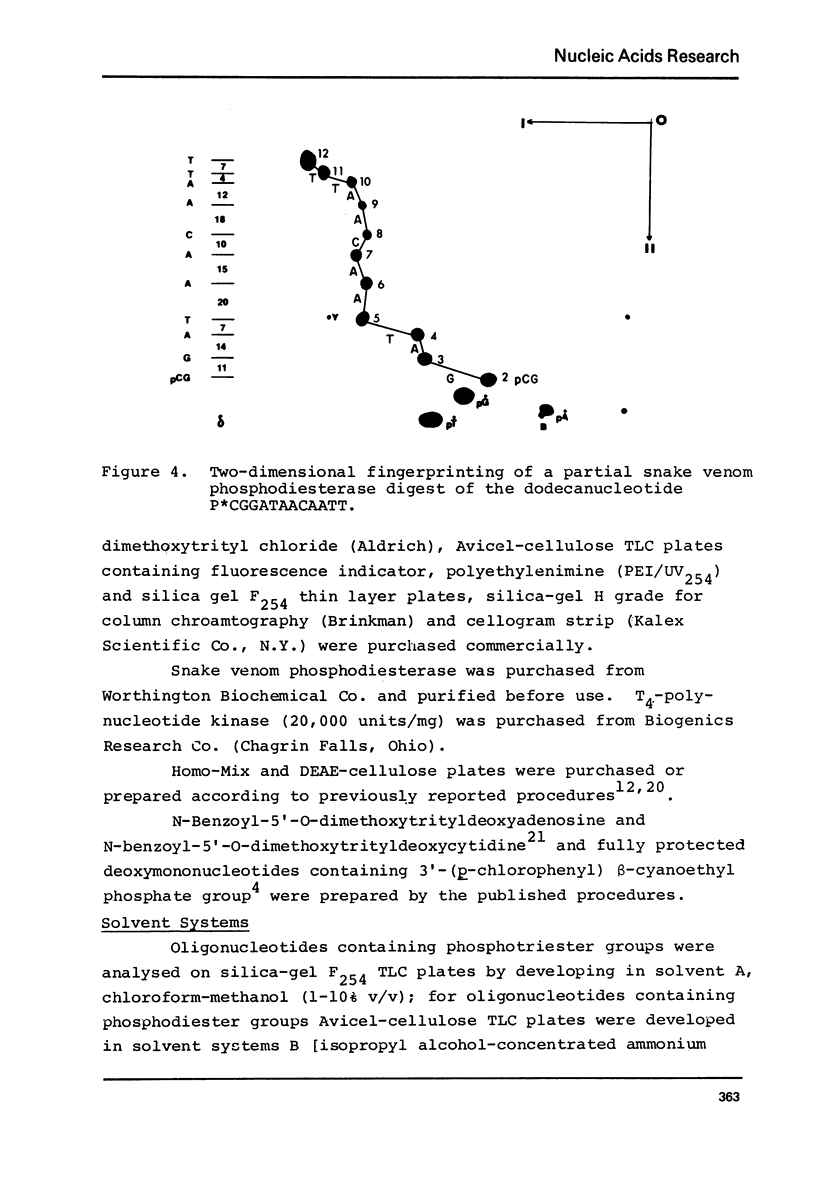
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians K. J., Wu R., Stawinski J., Hozumi T., Narang S. A. Cloned synthetic lac operator DNA is biologically active. Nature. 1976 Oct 28;263(5580):744–748. doi: 10.1038/263744a0. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Griffin B. E. 5 s RNA conformation. Studies of its partial T 1 ribonuclease digestion by gel electrophoresis and two-dimensional thin-layer chromatography. J Mol Biol. 1972 Dec 30;72(3):633–643. doi: 10.1016/0022-2836(72)90181-7. [DOI] [PubMed] [Google Scholar]
- Neilson T., Werstink E. S. Synthesis of the anticodon loop of Escherichia coli methionine transfer ribonucleic acid. J Am Chem Soc. 1974 Apr 3;96(7):2295–2297. doi: 10.1021/ja00814a074. [DOI] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Randerath K. Ion-exchange thin-layer chromatography. 18. Detection of purine derivatives in the nanogram range by phosphorescence at 77 degrees K. Anal Biochem. 1967 Dec;21(3):480–485. doi: 10.1016/0003-2697(67)90327-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]