Abstract
Objective
To compare equine synovial fluid (eSF) from post-injury and control joints for (1) cartilage boundary lubrication function, (2) putative boundary lubricant molecules hyaluronan (HA), proteoglycan-4 (PRG4), and surface-active phospholipids (SAPL), (3) relationships between lubrication function and composition, and (4) lubrication restoration by addition of HA.
Methods
eSF from normal (NL), acute injury (AI), and chronic injury (CI) joints were analyzed for boundary lubrication of normal articular cartilage as kinetic friction coefficient (μkinetic). eSF were also analyzed for HA, PRG4, and SAPL concentrations and HA molecular weight (MW) distribution. The effect of addition of HA, of different concentrations and MW, to AI- and NL-eSF samples on μkinetic was determined.
Results
The μkinetic of AI-eSF (0.036) was higher (+39%) than that of NL-eSF (0.026). Compared to NL-eSF, AI-eSF had a lower HA concentration (−30%) of lower MW forms, higher PRG4 concentration (+83%), and higher SAPL concentration (+144%). CI-eSF had μkinetic, HA, PRG4, and SAPL characteristics intermediate to that of AI-eSF and NL-eSF. Regression analysis revealed that μkinetic decreased with increasing HA concentration in eSF. The friction-reducing properties of HA alone improved with increasing concentration and MW. Addition of high-MW HA (4,000kDa) to AI-eSF reduced μkinetic to a value near that of NL-eSF.
Conclusion
In the acute post-injury stage, eSF exhibits poor boundary lubrication properties as indicated by a high μkinetic. HA of diminished concentration and MW may be the basis for this, and adding HA to deficient eSF restored lubrication function.
In synovial joints, articular cartilage bears load and slides relative to apposing tissue surfaces, with friction and wear reduced through a number of biophysical mechanisms including boundary lubrication (1, 2). Boundary lubrication of articular cartilage is mediated by synovial fluid (SF) components that reduce the interaction of articulating surfaces (3–5). Normal SF contains the molecules, hyaluronan (HA) (6), proteoglycan-4 (PRG4) (7, 38), and surface active phospholipids (SAPL) (8), implicated in contributing to the boundary lubrication of articular cartilage. Each of these molecules is present at high concentrations in synovial fluid (9–12) and has been localized at the surface of articular cartilage (8, 13, 14), as would be expected for a boundary lubricant. In this paper, the term PRG4 is used, as it is the name assigned by the Human Genome Organization Committee for proteins known as lubricin, superficial zone protein, and megakaryocyte stimulating factor (7, 38, 51).
Alteration of the friction-lowering function of SF may contribute to deterioration of articular cartilage in joint disease and after joint injury (15–19). However, the lubrication function of SF varied substantially in these studies, as did the biomechanical test methods and counter-face materials used in the lubrication tests. Lubricant solutions exhibit boundary-mode friction for cartilage-on-cartilage that is less than glass-on-rubber (15) and cartilage-on-glass (19, 20), and similar for glass-on-latex (16–18). After acute injury (21, 22), such as anterior cruciate ligament rupture, meniscal tear, or intra-articular fracture, synovial joints are pre-disposed to deterioration and premature osteoarthritis (OA). Such deterioration may involve reduction in functional boundary lubrication of articular cartilage due to alterations in the concentrations of SF lubricant molecules (18, 23, 24). After acute injury, the diminished lubrication properties of pathological SF have been associated with lower concentrations of PRG4 (18). In OA, the friction coefficient of SF tended to increase from normal when tested at a latex-glass interface (17), and the concentration and molecular weight (MW) distribution of HA are shifted to lower levels (25–28); however, the concentration and MW distribution of HA have not been associated directly with decreased lubrication. Additionally, the concentration of phospholipids was lower in acute injury compared to SF from uninjured joints, but higher in OA (9, 11). However, the contribution of SAPL to the boundary lubrication of articular cartilage has been controversial (34, 48, 49). It remains to be established if SF lubricant dysfunction occurs after different types of joint injury and whether such alterations relate to variations in the concentrations and quality of lubricant molecules.
Race horses are commonly afflicted with osteochondral fractures and OA of the carpal and metacarpophalangeal joints, and thus provide a natural model system for study of joint injury (29). Horses with joint injuries are often evaluated acutely for treatment of osteochondral chip fragments or slab fractures, and such joints exhibit signs of acute synovitis. In contrast, some horses are evaluated for more chronic joint damage and secondary osteoarthritic changes. The SF of such injured joints may be affected both in lubrication function and lubricant composition.
The objectives of this study were to determine, for equine synovial fluid (eSF) from acutely injured (AI), chronically injured (CI), and normal (NL) joints, (1) the coefficient of friction at a cartilage-cartilage interface in the boundary lubrication regime, (2) the concentrations and/or MW of HA, PRG4, and SAPL, (3) the relationships between lubrication function and composition, (4) the contribution of HA to cartilage-cartilage lubrication at different MW and concentrations, and (5) if addition of the deficient molecules to eSF could restore lubrication function.
Materials and Methods
Materials
Materials for lubrication testing were obtained as described previously (3) (additional details in Electronic Supplement). Hyaluronan (HA) was obtained as 6.4, 51, and 780 kDa forms from Lifecore Biomedical (Chaska, MN), ~800 kDa (SupArtz®, Smith and Nephew, Memphis, TN, MW range 620–1,170 kDa, polydispersity index 1.6 (30)), and 4,000 kDa (Healon®, Advanced Medical Optics, Santa Ana). Anti-lubricin rabbit polyclonal antibody, raised to amino acids 1356–1374 of human lubricin, was from Abcam (ab28484, Cambridge, MA).
Synovial Fluid Samples
Normal bovine SF (NL-bSF) was prepared as described previously (3) with n=5 pools, each from different adult animals.
Equine SF samples were acquired by one of the authors (CWM) during arthroscopic surgery of adult horses (2–4 y.o.). Synovial fluid was aspirated from the injured carpal (n=14) or metacarpophalangeal (n=6) joint, as well as contralateral joints as controls (n=20). The eSF was classified as acute or chronic based on the estimated duration between joint injury and arthroscopic treatment, as well as arthroscopic observations. AI-eSF (n = 10) were from horses that presented for surgery within three weeks of clinical diagnosis, often with signs of moderate to severe synovitis. CI-eSF (n = 10) were from horses that presented for surgery more than three weeks after injury, where articular cartilage degeneration was often observed and synovitis was generally less severe. Figure 1A shows an arthroscopic view of a normal metacarpophalangeal joint; Figure 1B shows a view of the same location in a joint with an acute injury. Figure 1C shows a normal proximal intermediate carpal bone articular surface in the medial side of the antebrachiocarpal joint; Figure 1D shows a joint with a chronic fragment off the proximal intermediate carpal bone with erosion of articular cartilage on the surface, partial thickness erosion on the distal lateral radius (lower) and thickened synovial villi (black arrow), indicative of chronic synovitis (secondary osteoarthritis).
Figure 1.
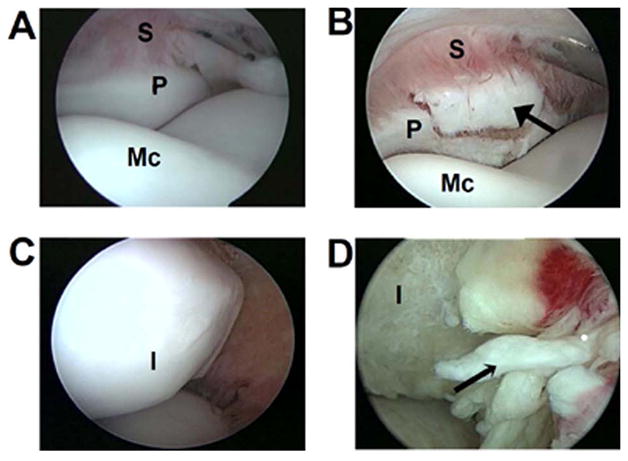
Arthroscopic views of a normal (A) and acutely (B) injured metacarpophalangeal joint with a fracture fragment (indicated by the solid arrow head) off the proximal dorsal aspect of the first phalanx (P) with synovium (S) and distal metacarpus (Mc) also shown; (C) normal and (D) chronic injury of the equine antebrachiocarpal joint with a fragment off the intermediate (I) carpal bone and full-thickness erosion of the articular cartilage, as well as chronic synovitis (thickened synovial villi indicated by narrow arrow head).
All eSF samples were clarified of cells and debris by centrifugation (3,000g, 30min) immediately after joint aspiration. The supernatants were then collected, stored at -20°C for up to two weeks, and then dispensed into aliquots and stored at −80°C before being accessed for the subsequent analysis, described below.
Experimental Design
Exp. 1: Variations in eSF
Biomechanical and biochemical analyses were performed to determine the effect of injury on eSF lubrication function and composition. Portions of NL-bSF (n=5), NL-eSF (n=20), AI-eSF (n=10), and CI-eSF (n=10) samples were analyzed by biomechanical lubrication tests for friction-lowering properties as indicated by kinetic steady-state (equilibrium) and static (start-up) coefficients of friction, μkinetic and μstatic, respectively. Other portions were analyzed by biochemical assays for the concentrations and/or MW of putative lubricant molecules, HA, PRG4, and SAPL. Univariate and multivariate regression analyses were performed to assess the relationship between friction coefficient and lubricant composition.
Exp. 2: Lubrication Properties of HA of Varying Size and Concentration
Portions of HA preparations were analyzed by biomechanical lubrication tests for friction lowering properties as indicated by μkinetic and μstatic. HA of MWs 6.4, 51, 780, and 4,000 kDa were analyzed at concentrations of 0.33, 1.1, and 3.3mg/ml, with n=6 for each MW and concentration combination.
Exp. 3: Restoration and Enhancement of Dysfunctional AI-eSF
Based on deficient friction-lowering properties and low HA values in Exp. 1, some samples of AI-eSF (n=7) and NL-eSF (n=9) were analyzed further. To portions of AI-eSF, HA in the form of SupArtz® (HA800, n=3) or Healon® (HA4000, n=4) was added such that the final concentration of exogenous HA in the eSF was 1.0 mg/ml, to restore HA concentrations to those similar to levels found within the normal range (0.3–1.3 mg/ml) reported for eSF (28, 31–33). Friction tests were then performed on AI-eSF and AI-eSF+HA samples. Similar experiments were carried out on NL-eSF with HA800 (n=5) or HA4000 (n=4). The addition of exogenous HA to all eSF resulted in a slight (10%) dilution of SF.
Lubrication Test
Portions of SF and HA samples were analyzed for μstatic and μkinetic in the boundary lubrication mode on articulating cartilage surfaces as described previously (3, 34). Intact articular surfaces were in the form of osteochondral cores and annuli harvested from the lateral and medial facets of the patellofemoral groove of adult bovine stifle joints, stored in phosphate buffered saline (PBS) supplemented with protease inhibitors (PIs) (2 mM Na-EDTA, 1 mM PMSF, 5 mM Benz-HCL, and 10 mM NEM) at −80°C. A total of 120 osteochondral fragments, in the form of 60 pairs of cores+annuli, from 14 stifle joints were used (details in Electronic Supplement). Test lubricants were also supplemented with PIs, resulting in a 3% dilution of the sample.
Samples were tested by preconditioning, 18% cartilage compression, 30 minute stress relaxation, and rotating at an effective sliding velocity of 0.3 mm/second, with pre-sliding durations (Tps, the duration the sample is stationary prior to rotation) of 120, 12, and 1.2 seconds (additional details in Electronic Supplement). Friction coefficients (μ) were calculated from the torque, τ, and equilibrium axial load measured immediately after the 30 minute stress relaxation period, with μstatic calculated from the peak |τ|, measured just after (within 10° of) the start of rotation, and μkinetic calculated from the |τ| averaged during steady-state sliding. Consistent with previous results (3, 34), μkinetic did not vary substantially with Tps, so μkinetic data are presented as the average at all Tps. Also consistent were μkinetic and μstatic for PBS (>0.20), so these results were not analyzed further.
Biochemical Analysis of Boundary Lubricants
Portions of SF samples were analyzed biochemically for the concentrations of HA, PRG4 and SAPL (details in Electronic Supplement). HA concentration in eSF samples was determined by an ELISA-like assay using HA binding protein (35). HA MW distribution (0.05 – 0.25, 0.25 – 0.5, 0.5 – 1, 1 – 2.5, and 2.5 – 7 MDa) in eSF samples was determined by horizontal electrophoresis through a 1% agarose gel (36, 37) and image processing. PRG4 concentration in eSF samples was quantified after Western Blot using anti-Lubricin antibody and purified Equine PRG4 standard (36). SAPL in eSF samples was measured by an assay that detects phospholipase-sensitive activity (9). To confirm the specificity of the spectrophotometric SAPL assay, absorption profiles of the assay product of SF samples, pooled for each group, were compared to that of SAPL standards.
Statistical Analysis
Data are presented as mean ± SEM. The effects of test lubricant on μstatic (with Tps as a repeated factor), μkinetic, and lubricant concentrations were assessed by ANOVA, followed by the Tukey post hoc test to determine which experimental group means differ from each other. Since an initial analysis assessing the carpal and metacarpophalangeal joints as factors did not show independent or interactive effects of joint location, all analysis was done without considering joint site as a factor. The dependencies of μkinetic and μstatic on the biochemical constituents (HA, PRG4, and SAPL) were analyzed by univariate regression as well as multivariate regression. Statistical analysis was performed using Systat 10.2 (Systat; Richmond, CA).
Results
Lubrication function of eSF
The boundary mode friction coefficients varied with test lubricant (p<0.05, Figure 2A) and Tps (p<0.001, Figure 2B). The lubricating abilities of equine and bovine SF on bovine cartilage were similar for normal SF from equine (NL-eSF, μkinetic = 0.026) and bovine (NL-bSF, μkinetic = 0.025, data not shown) joints (p=0.76). The μkinetic for eSF from acutely injured joints (AI-eSF, 0.036) was 39% higher than that of NL-eSF (p<0.05), and μkinetic for eSF from chronic injury joints (CI-eSF, 0.031) tended to be higher than that of NL-eSF (+20%, p=0.15).
Figure 2.
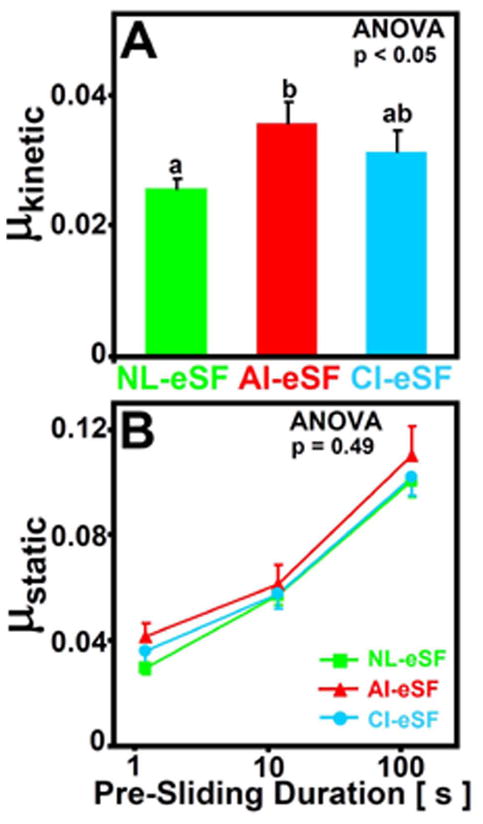
Effect of joint injury on the boundary lubrication of articular cartilage by eSF. (A) Kinetic friction coefficients and (B) static friction coefficients plotted on a semi-log scale for NL-eSF (n = 20), AI-eSF (n = 10) and CI-eSF (n = 10). Data are mean ± SEM, and differing letters indicate significant differences between groups at p<0.05.
While μstatic varied with Tps (p<0.001), it was not affected by joint injury (p=0.49) and did not show an interaction effect (p=0.76, Figure 2B). As Tps decreased from 120s to 1.2s, μstatic values approached μkinetic values (Figure 2A). Although μstatic values were not statistically different between groups, those at the shortest Tps of 1.2s were highest for AI-eSF (0.042) followed by that of CI-eSF (0.036) and that of NL-eSF and NL-bSF (both, 0.030), similar to the ordering of groups for μkinetic.
Biochemical analysis of eSF
Analysis of the concentrations of HA, PRG4 and SAPL in eSF revealed variations in lubricant molecule concentrations or MW with joint injury.
HA
The concentration of HA in eSF varied with joint injury (p<0.05, Figure 3A). Opposite to the higher friction coefficients for AI-eSF than that of NL-eSF, the HA concentration of AI-eSF (0.21 mg/ml) was 30% lower (p<0.05) than that of NL-eSF (0.30 mg/ml) and not significantly different for that of CI-eSF (p=0.40).
Figure 3.
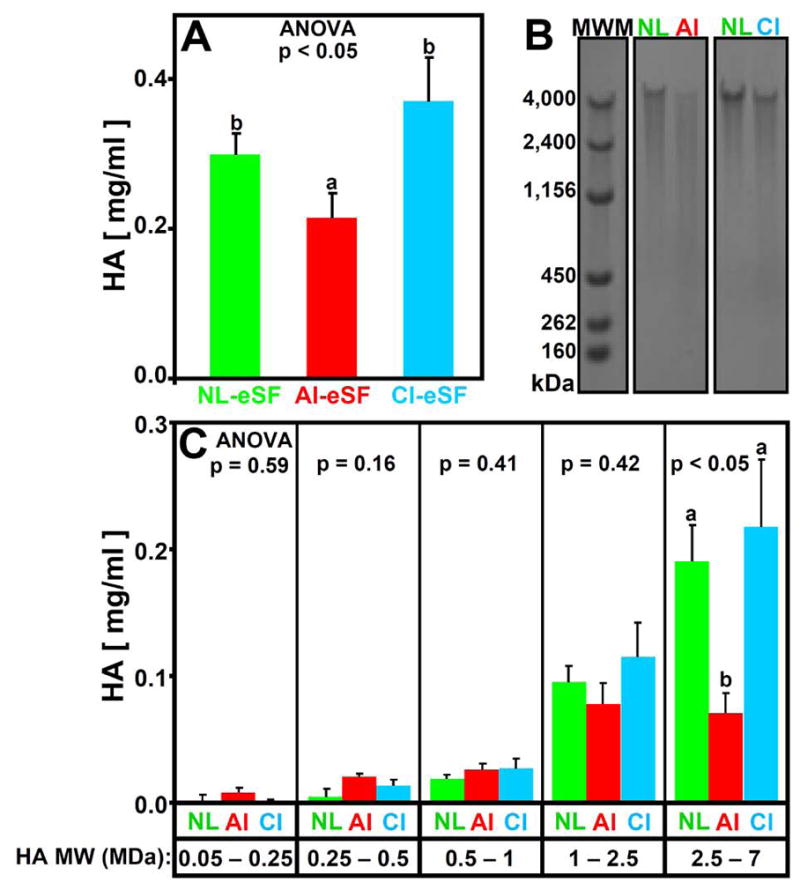
Effect of joint injury on hyaluronan (HA) (A) concentration (mg/ml) and (B, C) HA molecular weight (MW) distribution in NL-eSF (n = 19–20), AI-eSF (n = 10) and CI-eSF (n = 10). (B) Electrophoretic separation of typical samples. (C) Concentration of HA in 0.05 – 0.25, 0.25 – 0.5, 0.5 – 1, 1 – 2.5, and 2.5 – 7 MDa ranges. Data are mean ± SEM, and differing letters indicate significant differences between groups at p<0.05.
The MW distribution of HA in eSF varied between NL-, AI-, and CI-eSF (p<0.05), being shifted with injury to lower MW forms (Figure 3B and C). Relative to NL-eSF, AI-eSF had HA concentrations that were similar in the lower MW ranges of 0.05 – 2.5 MDa (p=0.16 – 0.59), and markedly lower in the highest MW range, 2.5 – 7 MDa (−63%, p<0.05). CI-eSF was similar to NL-eSF in HA concentrations at all HA MW ranges (p=0.48 – 0.99).
PRG4
The concentration of PRG4 in eSF varied with joint injury (p=0.01). Western Blot analysis of individual NL-, AI-, and CI-eSF samples identified a specific immunoreactive band (Figure 4A and Electronic Supplement Figure S1). PRG4 concentrations were highest for AI-eSF (104 μg/ml, p<0.05 vs NL-eSF), then CI-eSF (95 μg/ml, p=0.066 vs NL-eSF), and then NL-eSF (57 μg/ml) (Figure 4B).
Figure 4.
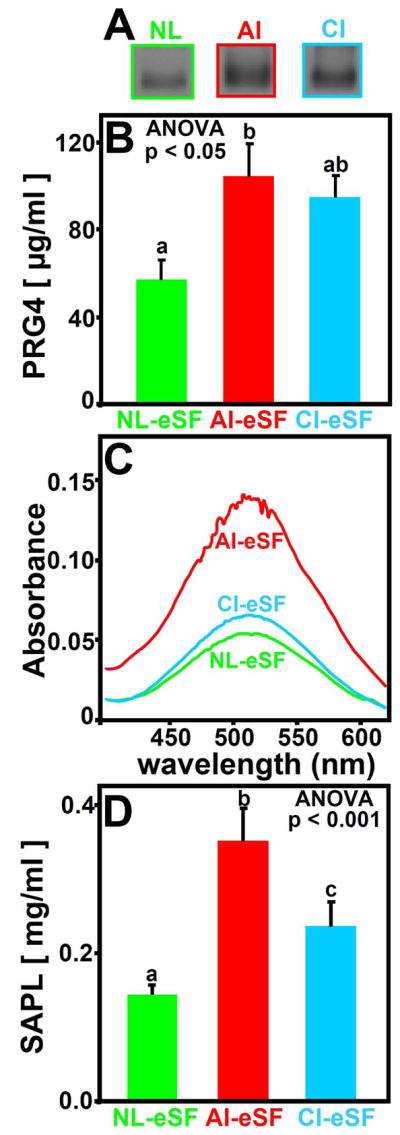
Effect of joint injury on PRG4 (A, B) and SAPL (C, D) in NL-eSF (n=19–20), AI-eSF (n=10), and CI-eSF (n=10) (A) Representative PRG4 Western blot probed with an antibody to Lubricin/PRG4; (B, D) concentrations of PRG4 (μg/ml) and SAPL (mg/ml), respectively; and (C) spectrophotometric absorption profiles for the reaction products of SAPL from pooled NL-eSF, CI-eSF, and AI-eSF. Data are mean ± SEM, and differing letters indicate significant differences between groups at p<0.05 or p<0.001.
SAPL
The SAPL concentration in eSF also varied with joint injury (p<0.001, Figure 4D). Compared to that of NL-eSF, SAPL concentration was markedly higher in AI-eSF (+144%, p<0.001, Figure 4D) and elevated in CI-eSF (+64%, p<0.05). Absorption profiles for the reaction products of SF treated with phospholipase C were similar for all pooled SF samples (Figure 4C), supporting the fidelity of the assay.
Regression analysis indicated certain correlations between friction coefficients and eSF lubricant molecule concentrations. Univariate regression showed a significant negative correlation between μkinetic and HA (slope = −0.031 ml/mg, r2 = 0.195, p<0.01) as well as positive correlations between μkinetic and SAPL (slope = +0.045 ml/mg, r2 = 0.277, p<0.005) and between μkinetic and PRG4 (slope = +0.003 ml/mg, r2 = 0.124, p<0.05). Similar correlation trends were also observed for μstatic (data not shown). Multivariate regression revealed the independent relationship of μkinetic to HA (mg/ml), PRG4 (mg/ml), and SAPL (mg/ml), μkinetic = −0.019*HA + 0.032*PRG4 + 0.029*SAPL + 0.029 with r2 = 0.342, p=0.001 (p<0.01 for HA; p<0.05 for PRG4; and p<0.001 for SAPL).
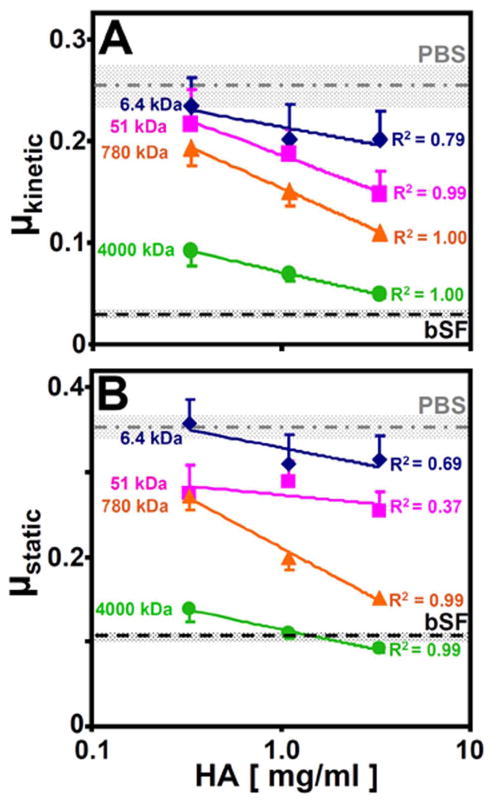
Lubrication properties of HA
The friction-reducing properties of HA solutions for articular cartilage depended on both HA concentration and MW (Figure 5). μkinetic decreased with increasing HA concentration (p<0.001) and HA MW (p<0.001, Figure 5A). At 0.33mg/ml HA, μkinetic of 6.4kDa and 51kDa HA were similar to that of PBS (0.255) (p=0.98 and 0.85, respectively), while μkinetic for 4,000 kDa HA was significantly reduced from that of PBS (−64%, p<0.01). Similar trends were observed at 1.1 and 3.3mg/ml HA. For 4,000kDa HA increasing from 0.33 to 3.3mg/ml, μkinetic attained low values, not significantly different than that of bovine SF (p=0.47, Figure 5A). μstatic increased with Tps (p<0.001) and decreased with increasing HA concentration (p<0.001) and HA MW (p<0.001), with trends similar to those for μkinetic at all Tps. Results for μstatic at Tps of 120s are shown in Figure 5B, and similar trends were found for Tps of 1.2 and 12s (data not shown).
Figure 5.
Dependence of (A) kinetic (μkinetic) and (B) static (μstatic) friction coefficients on the MW (kDa) and concentration (mg/ml) of HA. n = 6; data are mean ± SEM.
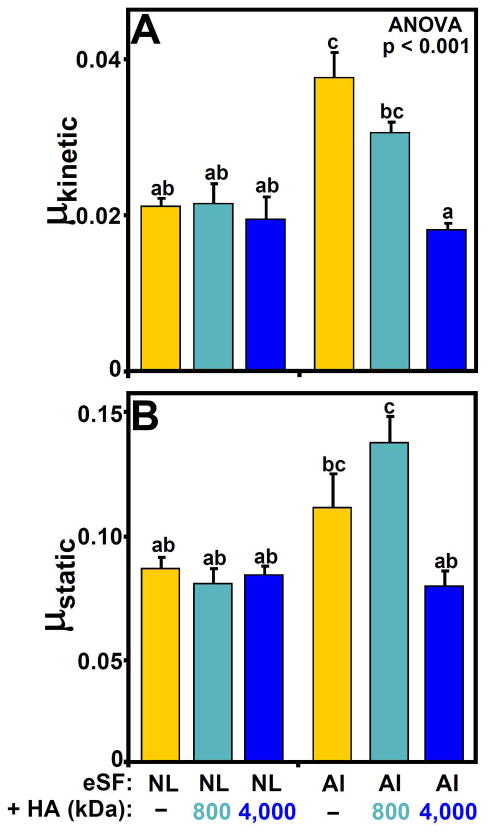
Restoration of dysfunctional AI-eSF
The boundary lubrication function of eSF from AI horses was normalized by addition of high MW HA. For both μkinetic and μstatic (at Tps = 120s), the experimental groups that were similarly low in friction coefficient (denoted by an “a” in Figure 6A and Figure 6B, respectively) included NL-eSF alone, NL-eSF with added HA800, NL-eSF with added HA4000, as well as AI-eSF with added HA4000. In contrast, AI-eSF alone (for μkinetic, Figure 6A) and AI-eSF with added HA800 (for μstatic, Figure 6B) had the highest friction coefficients.
Figure 6.
Dependence of (A) kinetic (μkinetic) friction and (B) static (μstatic) friction (at Tps = 120s) of NL- and AI-eSF before and after the addition of exogenous HA (+HA) of average MW 800 or 4,000 kDa. n = 3 – 9; Data are mean ± SEM, n = 3 – 9, and differing letters indicate significant differences between groups at p<0.05 or p<0.001.
Discussion
The results of this study indicate that SF lubrication function and composition are altered coordinately after acute joint injury to race horses, and that supplementation in vitro of the abnormal SF with high-MW HA restores boundary lubrication function. In the acute stage, the boundary lubrication function of SF is reduced, as indicated by a friction coefficient that is higher than normal (μkinetic =0.036 vs 0.026, Figure 2). This alteration in lubrication function may be due to the diminished concentration and MW of HA in AI-eSF (Figure 3), despite the elevated concentrations of PRG4 and SAPL in AI-eSF over NL-eSF (Figure 4). The role for HA appears particularly important since the friction-lowering properties of HA were size- and concentration-dependent (Figure 5). Also, the addition of HA4000 to AI-eSF restored boundary lubrication function, lowering μkinetic by ~30% to a level indistinguishable from NL-eSF (Figure 6). In the later chronic stage after injury, the boundary lubrication function of SF appears to be partially recovered, possibly due to restoration and normalization of HA, PRG4, and SAPL concentrations (Figures 3 and 4). Articular cartilage may be particularly vulnerable when boundary lubrication is deficient in the acute stage after injury. During this time, addition of lubricant molecules to SF may restore its lubrication function.
A direct comparison of the boundary lubricating ability and lubricant composition of pathological SF to its normal counterpart is important for understanding the molecular basis for altered lubrication function. Both pathological and normal contralateral SF could be obtained fresh in a sufficient quantity and controlled manner for the experiments performed here. The carpal and metacarpophalangeal joints of the race horses from which SF samples were aspirated are weight-bearing and loaded actively, providing a useful model system to study joint injury. Although equine cartilage was not studied as a substrate for the boundary lubrication tests of eSF, normal eSF tested on adult bovine tissue substrates had a lubricating ability similar to that of bovine SF. Finally, although lubricant molecules may differ between species in concentration and quality, understanding the composition–function relationship of putative lubricant molecules in eSF provides insight into the molecular basis for SF and articular cartilage alteration with injury, and possible sequelae.
The analysis of the boundary lubricating ability of eSF after injury is consistent with and extends previous studies on SF from humans and animal injury models that used non-cartilage substrate friction test systems. At a latex-glass interface, boundary lubrication function of AI-SF was also reduced for that aspirated from humans with knee joint synovitis (17) and from rabbits after ACL and PCL transection (18). Thus, such SF from injured joints reduces surface interactions between articulating substrates at both cartilage-cartilage and non-cartilage interfaces. The relatively normal lubricating ability of CI-eSF and OA human SF (17) was also observed for SF from patients with degenerative joint disease (16), where friction coefficients similar to those measured here (~0.024) for NL-eSF and NL-bSF were measured for a latex-glass interface, consistent with the ability of SF from joints with certain chronic pathologies to reduce the surface interaction between articulating surfaces similar to that of normal SF. Friction coefficients for PBS at a cartilage-cartilage interface in the present study (~0.26) were ~3x that (~0.09) for saline controls at a latex-glass interface (16–18). The different absolute friction values between previous and current studies may be attributable to differences in the test configurations, substrate materials, and test protocols. Examining the lubricating ability of pathological SF on physiological test substrates mimics certain aspects of naturally articulating surfaces and allows for molecular interactions that occur during physiological articulation (40), expanding on findings for the boundary lubricating ability of SF at a non-cartilage interface. The differences in the effect of injury on SF static and kinetic friction properties, as well as the effect of added HA, remain to be clarified, and may involve detailed surface interactions or other modes of lubrication. Deficient lubrication by AI-eSF also leads to an altered mechanobiologic environment for cartilage, with elevated shear strains observed during tibio-femoral human cartilage articulation in the presence of AI-eSF (39) further supports the phenomenon of impaired boundary lubrication function of SF after joint injury.
The alterations in SF lubricant composition with injury and disease observed in this study are in general agreement with previous studies on HA concentration and MW distribution in SF. The concentration of HA in eSF from clinically normal horses has been reported to average 0.33 – 1.3 mg/ml (28, 31–33), while that from horses with various arthritides is somewhat less, 0.18 – 0.3 mg/ml HA in post-traumatic arthritis (28, 41) and 0.18 – 0.74 mg/ml with more chronic injury conditions (27, 28, 41). Consistent with the current findings, the HA concentration in SF from acutely injured joints from horses (32) and humans (42, 43) were lower than that in control SF. The altered HA concentrations observed in the present studies indicate local changes afflicting a specific joint, rather than a systemic change. Thus, although lowered HA concentrations with injury are evident, the wide variability of reported HA values suggests possible effects of sample source, analysis method, species, particular joint and state of health, injury, or disease.
The present results extend knowledge of the altered MW distribution of HA in SF with joint diseases. Previous studies of SF have generally described the predominant HA MW range rather than quantitative distributions, with that from patients with rheumatoid arthritis and other joints diseases being 0.3 – 5 MDa compared to normal SF of 2 – 10 MDa (9, 25, 26), consistent with the AI-eSF findings of the present study. Previously, HA MW in normal eSF (2 – 3 MDa) was not found to differ significantly from that of SF from horses with established arthritis (1.5 – 3 MDa) (28), consistent with CI-eSF. Thus, the present studies provide new, quantitative information about the effect of traumatic injury on HA MW distribution in SF.
The in vitro finding that addition of high-MW HA to AI-eSF restores lubricant function suggests that intra-articular supplementation may modulate and restore, to some degree, the boundary lubrication function of SF after injury. Although the concentration of exogenous HA that was added to AI-eSF was higher than the average measured levels in NL-eSF samples, the increase in concentration by the addition of HA (to 1 mg/ml) is within the concentration range of HA in normal eSF reported previously and lower than that of normal human SF. The absence of an apparent effect of addition to NL-eSF of HA800 or HA4000 on friction coefficients of articulating cartilage may be due to a saturating effect of HA in the presence of PRG4 in normal SF (34) or a small effect size. Further analysis of the dose-dependent effects of exogenous HA on the lubricating ability of HA-deficient SF would help to elucidate whether lower concentrations of added high MW HA would also enhance SF lubrication function. HA supplementation is a common clinical treatment for people with osteoarthritis, and postulated to have disease modifying and chondroprotective effects (44). Previous studies have focused on clinical outcomes of HA supplementation and biological mediators rather than direct effects of HA, or other putative lubricant molecules, on SF lubricant function. Since HA has a relatively short half-life, a single bolus addition of HA may not provide long lasting enhancement of the lubricant function.
The effects of joint injuries and disease on the concentration of PRG4 in SF appear somewhat variable. The elevation in PRG4 concentration determined here is consistent with the initial transient elevation in biosynthesis and secretion as a response to cartilage injury (45) in vitro. The majority of PRG4 secreted by chondrocytes is released into the synovial fluid rather than retained with the matrix (38). While some previous studies of injury in animal models (18, 23, 46) and humans (47) have found a decrease in PRG4 concentration, other studies of acute injury and OA in humans have found an increase (19, 52). These variable PRG4 concentrations may be due to a number of factors including the type of injury, the duration of injury before the SF was analyzed, the sample source, and the analysis method. Previous studies included soft tissue and joint destabilization injuries, while the current study included osteochondral damage. The particular type of injury may affect both the regulated level of PRG4 secretion, as well as pathways of efflux from the joint, both of which affect PRG4 concentration in SF.
The injury-mediated increase in phospholipid concentration appears consistent, although the lubrication consequences are somewhat controversial. With acute and chronic injury phospholipid concentration increased, from 0.1 mg/ml in normal human SF to 0.2–0.3 mg/ml for human OA SF and 0.5 mg/ml for SF from total knee arthroplasties (9). However, the contribution of SAPL to the boundary lubrication of articular cartilage has been conflicting (34, 48, 49). The results of the present study, elevation of both SAPL and friction coefficients, argue against the role of phospholipid as a boundary protectant of the articular cartilage surface after acute injury. Here also, more detailed analysis of the distribution of phospholipids may clarify their role (11, 12). The increased SAPL concentration after injury may be due to the release of cellular debris, in proportion to the degree of synovitis.
The current studies provide further evidence that molecular features of lubricant molecules can affect their functional quality in certain states of post-traumatic joint injury, and such features may be important considerations for therapeutic interventions. The approach of analyzing both the concentration and structure of lubricant molecules present in injury SF, together with testing their role independently and as a supplement in cases of deficiency, can be used to screen putative lubricant therapies. Such analyses may also clarify the interaction of chemical factors. The results also suggest that the natural time course of altered lubrication is important. At particular stages following injury, biological, physical, and surgical treatments to modulate lubrication of HA, as well as PRG4, may help to protect the articular cartilage and joint from damage. Surgical removal or fixation of an osteochondral fragment may be needed to restore the joint environment. Also, deficiencies in lubrication may occur not only after injury occurring naturally, but also following arthroscopic procedures including cartilage repair (50).
Supplementary Material
Supplement Figure S1: Representative PRG4 Western blot probed with an antibody to Lubricin/PRG4. Full length gel from Figure 4A.
Acknowledgments
This work was supported in part by the National Institutes of Health and the National Science Foundation, and by a grant to the University of California, San Diego, in support of Dr. Robert Sah, from the Howard Hughes Medical Institute through the HHMI Professors Program. Additional individual support was received from an NSF Graduate Research Fellowship (JMA) and UCSD Chancellor’s Research Scholarship (LAS). We thank Nicholas S. Gastelum for assistance with preliminary studies.
References
- 1.Swanson SAV. Friction, wear, and lubrication. In: Freeman MAR, editor. Adult Articular Cartilage. 2. Tunbridge Wells, England: Pitman Medical; 1979. pp. 415–60. [Google Scholar]
- 2.Ateshian GA, Mow VC. Friction, lubrication, and wear of articular cartilage and diarthrodial joints. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. 3. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 447–94. [Google Scholar]
- 3.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Gleghorn JP, Bonassar LJ. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech. 2008;41:1910–8. doi: 10.1016/j.jbiomech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 6.Ogston AG, Stanier JE. The physiological function of hyaluronic acid in synovial fluid: viscous, elastic and lubricant properties. J Phys. 1953;119:244–52. doi: 10.1113/jphysiol.1953.sp004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225:195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz IM, Hills BA. Surface-active phospholipids as the lubricating component of lubricin. Br J Rheumatol. 1998;37:21–6. doi: 10.1093/rheumatology/37.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Mazzucco D, Scott R, Spector M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: correlation with flow properties. Biomaterials. 2004;25:4433–45. doi: 10.1016/j.biomaterials.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.McIlwraith CW, Billinghurst RC, Frisbie DD. Current and future diagnostic means to better characterize osteoarthritis in the horse - Routine synovial fluid analysis and synovial fluid and serum markers. AAEP Proceedings. 2001;47:171–91. [Google Scholar]
- 11.Rabinowitz JL, Gregg JR, Nixon JE. Lipid composition of the tissues of human knee joints. II. Synovial fluid in trauma. Clin Orthop Rel Res. 1984:292–8. [PubMed] [Google Scholar]
- 12.Sarma AV, Powell GL, LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res. 2001;19:671–6. doi: 10.1016/S0736-0266(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 13.Zea-Aragon Z, Ohtsuki K, Ohnishi M, Ohno S. Immunohistochemical study of the upper surface layer in rat mandibular condylar cartilage. Histol Histopathol. 2004;19:29–36. doi: 10.14670/HH-19.29. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–20. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 15.Reimann I. Pathological human synovial fluids. Viscosity and boundary lubricating properties. Clin Orthop Rel Res. 1976;119:237–41. [PubMed] [Google Scholar]
- 16.Davis WHJ, Lee SL, Sokoloff L. Boundary lubricating ability of synovial fluid in degenerative joint disease. Arthritis Rheum. 1978;21:754–60. doi: 10.1002/art.1780210703. [DOI] [PubMed] [Google Scholar]
- 17.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–64. [PubMed] [Google Scholar]
- 18.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–55. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 19.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Friction coefficient and superficial zone protein are increased in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680–7. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009;27:771–7. doi: 10.1002/jor.20798. [DOI] [PubMed] [Google Scholar]
- 21.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–8. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 22.Wilder FV, Hall BJ, Barrett JP, Jr, Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage. 2002;10:611–6. doi: 10.1053/joca.2002.0795. [DOI] [PubMed] [Google Scholar]
- 23.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231–7. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–9. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl LB, Dahl IM, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817–22. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–76. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- 27.Fuller CJ, Barr AR, Sharif M, Dieppe PA. Cross-sectional comparison of synovial fluid biochemical markers in equine osteoarthritis and the correlation of these markers with articular cartilage damage. Osteoarthritis Cartilage. 2001;9:49–55. doi: 10.1053/joca.2000.0349. [DOI] [PubMed] [Google Scholar]
- 28.Tulamo RM, Heiskanen T, Salonen M. Concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with diseased joints. Am J Vet Res. 1994;55:710–5. [PubMed] [Google Scholar]
- 29.McIlwraith CW. Use of synovial fluid and serum biomarkers in equine bone and joint disease: a review. Equine Vet J. 2005;37:473–82. doi: 10.2746/042516405774480102. [DOI] [PubMed] [Google Scholar]
- 30.Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24:629–42. doi: 10.2165/00002512-200724080-00002. [DOI] [PubMed] [Google Scholar]
- 31.Rowley G, Antonas KN, Hilbert BJ. Quantitation of hyaluronic acid in equine synovia. Am J Vet Res. 1982;43:1096–9. [PubMed] [Google Scholar]
- 32.Saari H, Konttinen YT, Tulamo RM, Antti-Poika I, Honkanen V. Concentration and degree of polymerization of hyaluronate in equine synovial fluid. Am J Vet Res. 1989;50:2060–3. [PubMed] [Google Scholar]
- 33.Palmer JL, Bertone AL, McClain H. Assessment of glycosaminoglycan concentration in equine synovial fluid as a marker of joint disease. Can J Vet Res. 1995;59:205–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–91. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 35.Fosang AJ, Hey NJ, Carney SL, Hardingham TE. An ELISA plate-based assay for hyaluronan using biotinylated proteoglycan G1 domain (HA-binding region) Matrix. 1990;10:306–13. doi: 10.1016/s0934-8832(11)80186-1. [DOI] [PubMed] [Google Scholar]
- 36.Blewis ME, Lao BJ, Schumacher BL, Bugbee WD, Sah RL, Firestein GS. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A. 2010;16:1329–37. doi: 10.1089/ten.tea.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 1994;219:278–87. doi: 10.1006/abio.1994.1267. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–52. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 39.Wong BL, Kim SHC, Antonacci JM, McIlwraith CW, Sah RL. Cartilage shear dynamics during tibio-femoral articulation: effect of acute joint injury & tribosupplementation on synovial fluid lubrication. Osteoarthritis Cartilage. 2010;18:464–71. doi: 10.1016/j.joca.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benz M, Chen N, Jay G, Israelachvili J. Static forces, structure and flow properties of complex fluids in highly confined geometries. Ann Biomed Eng. 2005;33:39–51. doi: 10.1007/s10439-005-8961-z. [DOI] [PubMed] [Google Scholar]
- 41.Tulamo RM, Houttu J, Tupamaki A, Salonen M. Hyaluronate and large molecular weight proteoglycans in synovial fluid from horses with various arthritides. Am J Vet Res. 1996;57:932–7. [PubMed] [Google Scholar]
- 42.Asari A, Miyauchi S, Sekiguchi T, Machida A, Kuriyama S, Miyazaki K, et al. Hyaluronan, cartilage destruction and hydrarthrosis in traumatic arthritis. Osteoarthritis Cartilage. 1994;2:79–89. doi: 10.1016/s1063-4584(05)80058-5. [DOI] [PubMed] [Google Scholar]
- 43.Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta. 1997;266:117–28. doi: 10.1016/s0009-8981(97)00122-8. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216–24. doi: 10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Jones AR, Chen S, Chai DH, Stevens AL, Gleghorn JP, Bonassar LJ, et al. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum. 2009;60:133–42. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 46.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60:2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozturk HE, Stoffel K, Jones CF, Stachowiak GW. The effect of surface-active phospholipids on the lubrication of osteoarthritic sheep knee joints: Friction. Tribology Letters. 2004;16:283–9. [Google Scholar]
- 49.Jay GD, Cha D-J. The effect of phospholipase digestion upon the boundary lubricating activity of synovial fluid. J Rheumatol. 1999;26:2454–7. [PubMed] [Google Scholar]
- 50.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–88. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 51.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90:291–7. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 52.Ballard BL*, Antonacci JM*, Temple-Wong MM, Hui AY, Schumacher BL, Bugbee WD, Schwartz AK, Girard PJ, Sah RL. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg. 2011 doi: 10.2106/JBJS.K.00046. (in press) * contributed equally to the preparation of this article. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure S1: Representative PRG4 Western blot probed with an antibody to Lubricin/PRG4. Full length gel from Figure 4A.