Abstract
Background
Despite better prognosis, there is a group of oropharyngeal squamous cell carcinoma (SCC) human papillomavirus (HPV)+ patients who experience treatment failure and succumb to distant metastasis.
Methods
Seventy-eight previously untreated patients nested in a concurrent chemoradiation protocol were reviewed to correlate patterns of local-regional tumor extent to distant metastasis. Biomarker assessment was: HPV in situ hybridization and epidermal growth factor receptor (EGFR) immunointensity.
Results
The 3-year disease-specific survival (DSS) for patients presenting with and without matted nodes was 69% and 94%, respectively (p = .003). Matted nodes were a poor prognostic factor independent of T classification, HPV, EGFR, and smoking status. For patients who were HPV+, 7 of 11 died of distant metastasis and 6 of 7 with distant metastasis had matted nodes.
Conclusion
Matted nodes are a novel marker of poor prognosis in oropharyngeal SCC independent of established prognostic factors. Matted nodes may identify patients at risk for the development of distant metastasis who could benefit from systemic therapy, whereas patients without matted nodes may be candidates for de-escalation of therapy.
Keywords: oropharyngeal squamous cell carcinoma, matted nodes, prognosis, biomarkers, lymph node metastasis
Oropharyngeal squamous cell carcinoma (SCC) is a disease related to smoking and alcohol consumption. More recently, it has been shown that high-risk human papillomavirus (HPV) is associated with and is a causative factor leading to oropharyngeal SCC.1 Patients who have HPV-positive cancers and do not have a history of tobacco or alcohol consumption have a better prognosis than patients who are HPV-negative with a history of tobacco and/or alcohol exposure.2-4
Despite better prognosis, there is still a group of patients who are HPV-positive who experience treatment failure and eventually succumb to their disease. Although most studies confirm excellent locoregional control rates with various treatment modalities,5 the incidence of treatment failures from distant metastasis in patients with oropharyngeal SCC has doubled over the past 20 years.6 Distant metastasis now accounts for approximately 45% of deaths in patients with oropharyngeal SCC.3,7 It would be beneficial to identify which subgroup of patients with oropharyngeal SCC is at risk for distant failure in this otherwise highly responsive group. Identifying these patients before therapy could have significant implications as treatment might be tailored in these patients to improve overall survival.
At the University of Michigan, the majority of patients with advanced-stage oropharyngeal SCC have been treated within a nested clinical trial, allowing uniformity when analyzing outcomes. We wanted to evaluate local-regional tumor extent as well as molecular markers in patients with oropharyngeal SCC that may be associated with distant metastasis. We hypothesized that there would be a correlation between certain patterns of local-regional tumor extent with distant metastasis.
PATIENTS AND METHODS
Study Population
This study was nested in a prospective phase II clinical trial (UMCC 02-021) designed to evaluate the toxicity and efficacy of weekly concomitant carboplatin, paclitaxel, and intensity-modulated radiation therapy for advanced-stage (III, IV) oropharyngeal SCC between 2003 and 2007. Patients were eligible if they had previously untreated, advanced-stage (III, IV), pathologically confirmed SCC of the oropharynx and had a pretreatment staging CT scan or CT/positron emission tomography (CT/PET) scan available for review within 4 weeks of starting treatment. Tumor staging was routinely performed by clinical examination and direct laryngoscopy in the operating room along with pretreatment staging CT or CT/PET correlation. Disease in all patients was staged based on the 2002 American Joint Committee on Cancer staging system. Patients were excluded if they had previous surgery or radiation therapy to the upper aerodigestive tract or neck.
Pretreatment Imaging
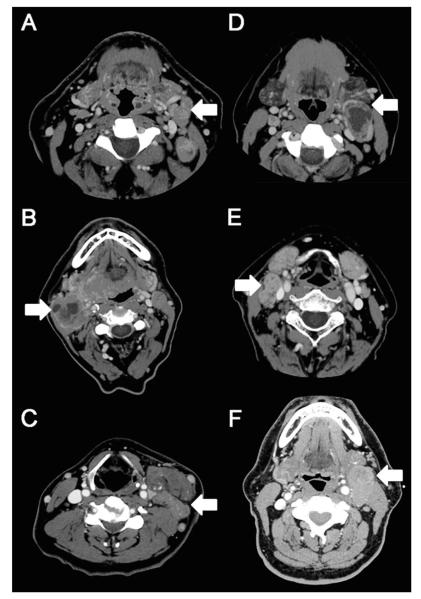
Pretreatment staging CT or CT/PET scans within 4 weeks of starting therapy were reviewed by a neuroradiologist (M. I.) without knowledge of patient outcome. A number of local-regional patterns were tabulated, including primary tumor site and size, distance of the primary tumor from the midline, and encasement of the carotid artery by the primary tumor. The size (largest 2 dimensions) and distribution (level I–V) of each pathologic lymph node was recorded for each level of the neck. N3 nodal disease was defined clinically as a lymph node or group of lymph nodes greater than 6 cm. Matted nodes were 1 of the nodal characteristics that were recorded and was defined as 3 nodes abutting one another with loss of intervening fat plane that is replaced with evidence of extracapsular spread (Figure 1). Extracapsular spread (ECS) was defined with imaging as loss of the sharp plane between the capsule of the lymph node and the surrounding fat.
FIGURE 1.
CT scans demonstrating examples of matted nodes (A–C) and non-matted nodes (D–F). (A–C) Shows examples of patients with matted nodes demonstrated by the corresponding arrows. (A) Shows 3 matted nodes in level II of the left neck (arrow). (B) Shows 2 nodes with cystic degeneration and loss of intervening fat plane in level II of the right neck (arrow). There is a third node with loss of intervening fat plane on more inferior images not demonstrated on this radiograph. (C) Shows 3 matted nodes in level III of the left neck (arrow). (D–F) Shows examples of patients with nonmatted nodes demonstrated by arrows. (D) Shows a single large cystic node in level II of the left neck (arrow). (E) Shows 2 nodes with loss of intervening fat plane in level II/III of the right neck (arrow). There are no lymph nodes seen at other levels. (F) Shows a single large node in level II of the left neck (arrow).
Population Characteristics
There were 87 consecutive patients who met the criteria and were enrolled in this clinical trial. Nine patients were excluded from this study because pretreatment imaging was unavailable for review or they had excisional lymph node biopsies before referral and staging CT scan. Seventy-eight previously untreated patients were identified, and baseline characteristics are shown in Table 1. There were 70 male patients, and the average age was 58.5 years. Matted nodes were observed in 21% (16 of 78) of the population. The frequency of involved subsites were 47% base of tongue (37 of 78), 51% tonsil (40 of 78), and 1% posterior pharyngeal wall (1 of 78). There were 37% (29 of 78) who had T4 tumors and 86% (67 of 78) who were stage IV. Tobacco status was defined categorically as never, prior (quit greater than 6 months before diagnosis), or current use of cigarettes, cigars, pipe, chewing tobacco, snuff, or snus. There were 19 patients who were never tobacco users, 40 patients were prior tobacco users, and 19 patients were current tobacco users.
Table 1.
Baseline characteristics stratified by the presence of matted nodes.
| Characteristics | No. of patients with matted nodes (16) |
No. of patients with nonmatted nodes (62) |
p value* |
|---|---|---|---|
| Age, mean (SD) | 58.4 (9.2) | 58.5 (8.4) | .97 |
| Subsite | .83 | ||
| BOT | 44% (7) | 48% (30) | |
| Tonsil | 56% (9) | 50% (31) | |
| Postpharyngeal wall | 0% (0) | 2% (1) | |
| Overall stage | .11 | ||
| III | 0% (0) | 18% (11) | |
| IV | 100% (16) | 82% (51) | |
| T classification | .002 | ||
| T1 | 0% (0) | 10% (6) | |
| T2 | 25% (4) | 39% (24) | |
| T3 | 0% (0) | 24% (15) | |
| T4 | 75% (12) | 27% (17) | |
| N classification | .02† | ||
| N0 | 0% (0) | 11% (7) | |
| N1 | 0% (0) | 13% (8) | |
| N2a | 0% (0) | 10% (6) | |
| N2b | 38% (6) | 37% (23) | |
| N2c | 31% (5) | 22% (13) | |
| N3 | 31% (5) | 8% (5) | |
| HPV status | .05 | ||
| Positive | 63% (10) | 82% (51) | |
| Negative | 31% (5) | 11% (7) | |
| Missing | 6% (1) | 7% (4) | |
| EGFR status | .1 | ||
| Positive | 31% (5) | 58% (36) | |
| Negative | 38% (6) | 27% (17) | |
| Missing | 31% (5) | 15% (9) | |
| Tobacco status | .18 | ||
| Never | 12% (2) | 27% (17) | |
| Prior | 44% (7) | 53% (33) | |
| Current | 44% (7) | 20% (12) |
Abbreviations: BOT, base of tongue; HPV, human papillomavirus; EGFR, epidermal growth factor receptor.
The p value tests association with matted node category for each variable. Fisher exact test is used for dichotomous variables. Likelihood ratio chi-square test with Monte Carlo estimation of errors is used for variables with more than 2 levels. Missing values are excluded.
We expect this difference to be present since matted nodes are defined by characteristics present only in N2b or worse patients.
Treatment Protocol
Patients were treated with radiation 5 days per week. The prescribed doses were 70 gray (Gy) at 2.0 Gy per fraction to gross disease and 59 to 63 Gy at 1.7 to 1.8 Gy per fraction to low-risk and high-risk subclinical regions, respectively, delivered concomitantly according to published methods.8,9 Chemotherapy consisted of weekly carboplatin (area under the curve 1) intravenous over 30 minutes and paclitaxel 30 mg/m2 intravenous over 1 hour. Hydration and antiemetics were administered according to the standard of care.
Tissue Microarray and Immunostaining
A tissue microarray (TMA) was constructed from pretreatment biopsies of the primary tumor for 73 of the 78 patients. Five patients did not have adequate tissue samples available for analysis. Tissue microarray slides were processed by a previously described method.2 HPV status was determined with in situ hybridization by a previously described method using SensiGen LLC of Ann Arbor, Michigan.10 Eighty-four percent of the patients (61 of 73) were HPV positive.
Epidermal growth factor receptor (EGFR) intensity was scored as follows: 1, equal to no staining; 2, low intensity; 3, moderate; and 4, high intensity. Scores from 3 cores, 0.6 mm in diameter, were averaged from each patient. EGFR-negative was defined as an averaged score of less than 2.5 and EGFR-positive was defined as an averaged score of 2.5 or greater. There were 41 patients who were EGFR positive and 23 patients who were EGFR negative. The pathologist was blinded to the clinical outcome.
Statistical Analysis
Outcomes of interest were overall survival (OS), disease-specific survival (DSS), and pattern of recurrence (local, regional, or distant). Survival estimates were defined from the date of diagnosis. An OS event was defined as death from any cause; a DSS event was defined as death from cancer, deaths from other causes were censored at the date of death.
Bivariate associations between matted nodes and other clinical variables were tested with exact tests (ie, Fisher exact test, chi-square test with Monte Carlo estimates for error terms). Survival estimates were computed using the Kaplan–Meier method. Univariate associations with survival were tested with the log-rank test and multivariate Cox models were used to test the association of matted nodes with decreased survival after controlling for clinical variables including age, T classification, tobacco status, HPV status, and EGFR expression.
All patients provided written informed consent to participate in the University of Michigan Specialized Program of Research Excellence (SPORE) program and the prospective clinical trial, which were approved by the Institutional Review Board for human experimentation of the University of Michigan.
RESULTS
The 3-year OS and DSS for the entire cohort were 84% and 89%, respectively, with a median follow-up of 51 and 49 months. The 3-year DSS for patients who presented with matted nodes was 69% and for patients who had no evidence of matted nodes it was 94% (Figure 2; p = .0001).
FIGURE 2.
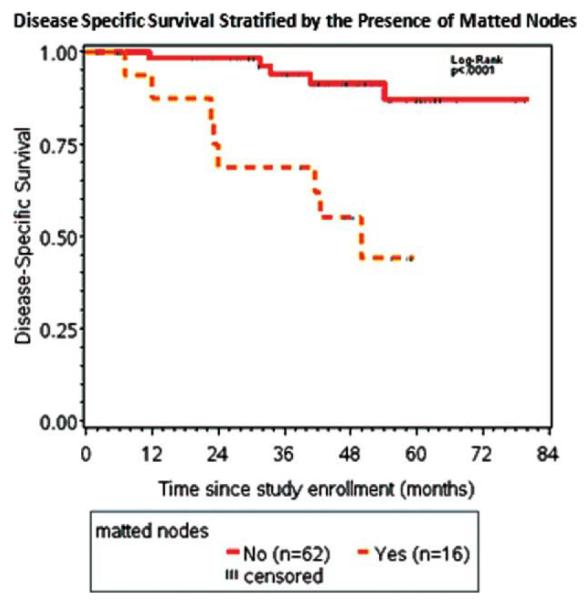
Kaplan–Meier survival curves of the entire cohort stratified by the presence of matted nodes. The 3-year disease-specific survival (DSS) for matted nodes patients was 69% and for patients with no evidence of matted nodes was 94% (p = .0001). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
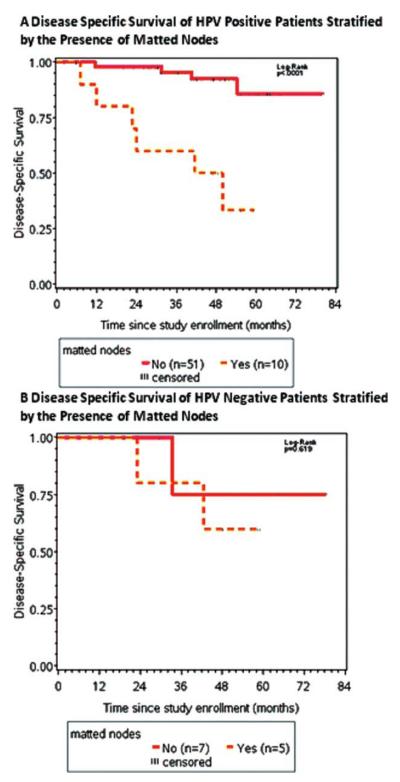
In the HPV-positive population, matted nodes were a prognostic factor for poor outcome (Figure 3A; p = .0004). There were 61 patients who were HPV positive, of which 10 had matted nodes. Of the 10 of 61 patients who were HPV positive and had matted nodes, 6 of 10 (60%) died of their disease. Of the 51 of 61 patients who were HPV positive and had no evidence of matted nodes, 5 of 51 (9.8%) died of their disease.
FIGURE 3.
(A) Kaplan-Meier survival curves of patients with human papillomavirus (HPV)-positive stratified by matted nodes. For the patients who were HPV-positive with matted nodes, all of 6 deaths were due to distant metastasis. (B) Kaplan-Meier survival curves of patients who were HPV-negative stratified by matted nodes (p = .62). There was limited power given the sample size of this cohort. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the HPV-negative population, there was no significant difference in DSS when stratifying by matted nodes (Figure 3B; p = .62). There were 12 patients who were HPV negative, of which 5 had matted nodes. Of the 5 of 12 patients who were HPV negative and had matted nodes, 2 of 5 (40.0%) died of their disease. Of the 7 of 12 patients who were HPV negative and had no evidence of matted nodes, 1 of 7 (14.3%) died of their disease. Given the observed differences in survival, a powered analysis of a larger cohort of patients who are HPV negative is warranted.
Matted nodes were not a surrogate marker for N3 neck disease. There were 10 patients with N3 disease, of which 5 had matted nodes. Of the patients with N3 disease and matted nodes, 60% (3 of 5) died of their disease compared with 20% (1 of 5) of patients with N3 disease and no evidence of matted nodes. In patients with N2b disease, there were 29 patients, of which 6 had matted nodes. Of the patients with N2b with matted nodes, 3 of 6 (50%) died of their disease, and 3 of 23 (13%) with no evidence of matted nodes died of their disease. In the N2c group, there were 18 patients, of which 5 have matted nodes. Of the patients with matted nodes, 4 of 5 (80%) died of their disease and 3 of 13 (23%) with no evidence of matted nodes died of their disease.
Distant metastasis was more common in patients with matted nodes, whereas patients with no evidence of matted nodes were more likely to develop a local recurrence (Table 2). There were 16 patients with matted nodes, 10 of these patients died (62%). Of the patients with matted nodes who died, the cause of death was lung metastasis in 8 of 10 patients. In the HPV-positive cohort, 7 of 61 patients died of distant metastasis and 6 of the 7 patients that died of distant metastasis had matted nodes.
Table 2.
Cause of death stratified by matted nodes.
| Deceased patients |
T classification |
N classification |
M classification |
Overall stage |
HPV status |
EGFR status |
Smoking status |
Cause of death |
|---|---|---|---|---|---|---|---|---|
| Matted nodes | ||||||||
| 4 | 2b | 0 | 4 | Negative | Positive | Current | Pulmonary embolism | |
| 4 | 2b | 0 | 4 | Negative | Positive | Current | Lung metastasis | |
| 4 | 3 | 0 | 4 | Positive | Positive | Previous | Lung metastasis | |
| 4 | 2c | 0 | 4 | Positive | Positive | Previous | Lung metastasis | |
| 4 | 2c | 0 | 4 | Positive | Negative | Current | Lung metastasis | |
| 2 | 2c | 0 | 4 | Unknown | Unknown | Previous | Severe dementia, LTF | |
| 4 | 3 | 0 | 4 | Positive | Positive | Previous | Lung metastasis | |
| 4 | 2b | 0 | 4 | Positive | Unknown | Never | Lung metastasis | |
| 4 | 2c | 0 | 4 | Positive | Unknown | Never | Lung metastasis | |
| 4 | 3 | 0 | 4 | Negative | Positive | Current | Lung metastasis | |
| Nonmatted nodes | ||||||||
| 2 | 2b | 0 | 4 | Positive | Positive | Previous | Unknown, NED | |
| 4 | 0 | 0 | 4 | Negative | Positive | Current | GI adenocarcinoma | |
| 2 | 2c | 0 | 4 | Positive | Positive | Never | Unknown, NED | |
| 3 | 2c | 0 | 4 | Positive | Positive | Current | Local recurrence | |
| 4 | 2c | 0 | 4 | Negative | Positive | Current | Local recurrence | |
| 4 | 2b | 0 | 4 | Positive | Positive | Never | Unknown, LTF | |
| 2 | 2a | 0 | 4 | Positive | Positive | Previous | Local recurrence | |
| 2 | 2b | 0 | 4 | Positive | Negative | Previous | Bone metastasis | |
| 3 | 0 | 0 | 3 | Positive | Positive | Current | Local recurrence | |
| 4 | 2c | 0 | 4 | Negative | Positive | Current | MI, NED | |
| 1 | 3 | 0 | 4 | Positive | Positive | Previous | Local recurrence |
Abbreviations: HPV, human papillomavirus; EGFR, epidermal growth factor receptor; LTF, lost to follow-up; NED, no evidence of disease; GI, gastrointestinal; MI, myocardial infarction.
Previous smoker is defined as tobacco use in the last 6 months.
The patients with the best prognosis were the HPV positive nonsmokers. There were 16 never smokers who were HPV positive, 2 patients died of their disease in this cohort. Both patients had matted nodes and died of distant metastasis.
Matted nodes were an independent prognostic factor of poor outcome among the current or previous tobacco-use population (p = .03). There were 59 patients who were current or previous tobacco users; 14 of them had matted nodes. Of the 14 of 59 patients who were previous or current tobacco users and had matted nodes, 6 of 14 (42.9%) died of their disease. Of the 45 of 59 patients who were previous or current tobacco users and had no evidence of matted nodes, 6 of 45 (13.3%) died of their disease. Interestingly, 14 of 16 patients who had matted nodes were previous or current tobacco users.
Matted nodes were also a poor independent prognostic factor in the EGFR-positive population (Figure 4; p = .002). There were 41 patients who were EGFR-positive, 5 of them had matted nodes. Of the 5 of 41 patients who were EGFR positive and had matted nodes, 3 of 5 (60%) died of their disease. Of the 36 of 41 patients who were EGFR positive and had no evidence of matted nodes, 3 of 36 (8%) died of their disease. There were 24 patients in the EGFR-negative population and there was no significant difference in DSS when stratifying by matted nodes.
FIGURE 4.
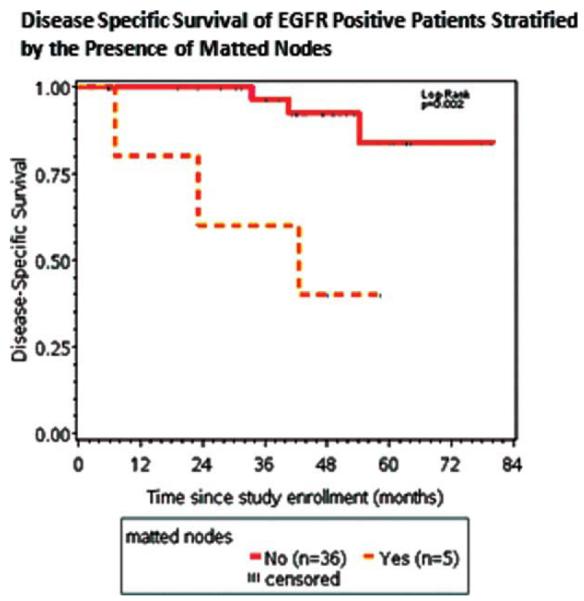
Kaplan–Meier survival curves of the epidermal growth factor receptor (EGFR)-positive cohort stratified by the presence of matted nodes. The 3-year disease-specific survival (DSS) was 60% for EGFR-positive patients with matted nodes and 96% for EGFR-positive patients with no evidence of matted nodes. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A multivariate analysis was performed and matted nodes remained an independent predictor of poor prognosis after controlling for T classification, HPV status, EGFR expression, and tobacco status (DSS; hazard ratio = 9.46; 95% confidence interval, 1.52–59.06; p = .016).
DISCUSSION
In this cohort, matted nodes are a novel marker of poor prognosis in oropharyngeal SCC and are independent of other established prognostic factors. Furthermore, matted nodes can identify patients before treatment who are at high risk for failure due to distant metastasis, particularly in the HPV-positive population.
The epidemiology of oropharyngeal SCC has been redefined over the last decade. HPV, smoking, alcohol, and cannabis have all been identified as etiologic agents for the development of oropharyngeal SCC. As research has proceeded, a complex interplay between HPV infection, amount of smoking behavior, and bulk of disease has been described.3 In the current study, the finding of matted nodes shows promise for quantifying important versus less important “bulk of disease.” Our findings suggest that matted nodes may be an important predictor of survival that is independent of HPV, EGFR, and smoking status. Matted nodes were more prevalent in the HPV-negative cohort (5 of 12) than the HPV-positive cohort (10 of 61) and were associated with distant metastasis in both groups. This correlation advances the determination of risk factors for treatment failure in oropharyngeal SCC and contributes to identifying patients who are at risk for distant metastasis and may require more systemic treatment.
Our patient population was derived from a prospective cohort of patients treated in a uniform prospective chemotherapy and intensity-modulated radiation therapy protocol. It should be noted that our protocol incorporated concomitant carboplatin and paclitaxel with radiotherapy, which could be inferior to concurrent cisplatin in patients with matted lymph nodes. Such high-dose regimens, perhaps with induction chemotherapy, may be more efficacious in patients with poor prognostic clinical features.11
Given the well-established survival benefit in the HPV-positive cohort, the Radiation Therapy and Oncology Group is beginning trials to de-escalate therapy in these patients to minimize treatment-related effects. The finding of matted nodes may help to identify patients who are likely to fail concomitant chemotherapy and radiation therapy and might be considered for exclusion or stratification in such deescalation trials. Conversely, the 3-year DSS in the HPV-positive cohort with no evidence of matted nodes was 90%. This subgroup has an excellent prognosis and consideration of de-escalated treatment would seem to be warranted.
ECS and tumor burden are also considered negative prognostic factors in oropharyngeal SCC.3,12 Matted nodes are distinct from ECS and N3 neck disease, conveying a worse prognosis independent of these conventional clinical predictors. ECS was defined radiographically in this study because pathologic confirmation was not possible because all patients were managed nonsurgically. When Carvalho et al13 reviewed CT scans and then correlated their pathologic specimens in patients with head and neck SCC, they found that a CT scan was only able to detect 10 of 16 patients who had ECS in their pathologic specimens. Therefore, it is possible that we have overestimated the prevalence of ECS based on radiologic findings. The incidence of ECS with imaging in our study was 74% (58 of 78). As a comparison to recently published surgical cohorts, Weinstein et al14 and Rich et al15 reported an incidence of ECS in pathologic specimens of 41% and 85%, respectively. Of course, these comparisons are difficult because ECS is a continuum ranging by location and degrees of microscopic through macroscopic spread.
While we cannot explain the mechanism for the survival difference in patients with N3 disease with and without matted nodes, this is most likely due to differences in tumor biology. Histologic confirmation of cancer and/or ECS in the imaged lymph nodes was not possible in this study because these patients were managed nonsurgically. Thus, there is the possibility that some of the imaged lymph nodes could have been inflammatory in nature or associated with a significant immunologic response in the nodes. The biologic behavior of cancer in patients with matted nodes may reflect a propensity to spread into multiple regional lymph nodes as well as distant sites. Whether this is due to differences in inherent metastatic characteristics or alterations in host immunity remains conjecture. It is possible that there are molecular differences between the primary and the matted lymph nodes. Confirmatory studies are needed when pathologic tissue is available to delineate the biologic differences of these tumors.
In this cohort, multiple marker analysis is limited due to sample size. Nevertheless, multivariable analysis did suggest that, despite the wide confidence interval, matted nodes were associated with a poor prognosis controlling for T classification, HPV, and EGFR status, as well as tobacco use.
Matted nodes are associated with poor prognosis in oropharyngeal SCC independent of other known clinical and molecular markers. Matted nodes may identify a group of patients who frequently develop distant metastasis and who may benefit from systemic therapy, whereas patients in low-risk populations with no evidence of matted nodes may benefit from de-escalation of therapy.
Acknowledgments
Contract grant sponsor: P50 CA97248 National Institutes of Health (NIH) National Cancer Institute (NCI) NIDCR Specialized Program of Research Excellence (SPORE), SPORE in Head and Neck Cancer - The Molecular Basis of Head and Neck Cancer Therapy, PI: Gregory Wolf, MD.
Footnotes
This work was presented at the American Head and Neck Society Research Workshop, Arlington, Virginia, October 2010.
REFERENCES
- 1.Marur S, D’souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Logan HL. Human papillomavirus and head and neck cancer. Am J Clin Oncol. 2009 doi: 10.1097/COC.0b013e31818b8fee. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 6.Taneja C, Allen H, Koness RJ, Radie-Keane K, Wanebo HJ. Changing patterns of failure of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2002;128:324–327. doi: 10.1001/archotol.128.3.324. [DOI] [PubMed] [Google Scholar]
- 7.Daly ME, Le QT, Maxim PG, et al. Intensity-modulated radio-therapy in the treatment of oropharyngeal cancer: clinical out-comes and patterns of failure. Int J Radiat Oncol Biol Phys. 2010;76:1339–1346. doi: 10.1016/j.ijrobp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radio-therapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102:7683–7688. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 12.Klozar J, Kratochvil V, Salakova M, et al. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S75–S82. doi: 10.1007/s00405-007-0557-9. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho P, Baldwin D, Carter R, Parsons C. Accuracy of CT in detecting squamous carcinoma metastases in cervical lymph nodes. Clin Radiol. 1991;44:79–81. doi: 10.1016/s0009-9260(05)80500-8. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein G, O’Malley B, Cohen M, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–1085. doi: 10.1001/archoto.2010.191. [DOI] [PubMed] [Google Scholar]
- 15.Rich JT, Milov S, Lewis JS, Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]