Abstract
Background
Most studies of indoor allergens have focused on the home environment. However, schools may be an important site of allergen exposure for children with asthma. We compared school allergen exposure to home exposure in a cohort of children with asthma. Correlations between settled dust and airborne allergen levels in classrooms were examined.
Methods
Settled dust and airborne samples from 12 inner-city schools were analyzed for indoor allergens using multiplex array technology (MARIA). School samples were linked to students with asthma enrolled in the School Inner-City Asthma Study (SICAS). Settled dust samples from students’ bedrooms were analyzed similarly.
Results
From schools, 229 settled dust and 197 airborne samples were obtained. From homes, 118 settled dust samples were obtained. Linear mixed regression models of log-transformed variables showed significantly higher settled dust levels of mouse, cat and dog allergens in schools than homes (545% higher for Mus m 1, estimated absolute difference 0.55 μg/g, p<0.0001; 198% higher for Fel d 1, estimated absolute difference 0.13 μg/g, p=0.0033; and 144% higher for Can f 1, estimated absolute difference 0.05 μg/g, p=0.0008). Airborne and settled dust Mus m 1 levels in classrooms were moderately correlated (r=0.48; p< 0.0001). There were undetectable to very low levels of cockroach and dust mite allergens in both homes and schools.
Conclusions
Mouse allergen levels in schools were substantial. In general, cat and dog allergen levels were low, but detectable, and were higher in schools. Aerosolization of mouse allergen in classrooms may be a significant exposure for students. Further studies are needed to evaluate the effect of indoor allergen exposure in schools on asthma morbidity in students with asthma.
Keywords: indoor allergens, asthma, inner city, urban, mouse, Mus m 1, Can f 1, Fel d 1, SICAS, school
INTRODUCTION
Overall asthma prevalence in the United States has increased by 12.3% from 2001 to 2009 among all age groups. The prevalence among those < 18 years is 9.6%, and is highest among poor children (13.5%) and among non-Hispanic black children (17.0%).1 Childhood asthma morbidity disproportionately affects minorities and low-income groups living in urban neighborhoods.1,2 Most studies evaluating modifiable exposures, such as indoor allergens, have focused on the home environment.3 The role of inner-city indoor allergen exposures in asthma development and morbidity is not well established. However, school environments may also be important, since children spend a large proportion of their day in school.
There are no published US studies thus far comparing indoor airborne and settled dust allergen levels in homes versus schools of asthmatic children. Our current study sought to assess the levels of indoor allergens in 12 urban elementary schools and compare them with levels of home indoor allergens for a subset of students with asthma. The primary objective of this study was to investigate the relation between settled dust and airborne allergen exposure in schools and in homes of children with asthma.
METHODS
As part of the School Inner-City Asthma Study (SICAS), 12 public elementary schools were selected from an inner-city, metropolitan area in the northeastern US from 2008–2010. Screening survey questionnaires were distributed to all the students attending these elementary schools to determine eligible asthmatics.4 Children with asthma attending these schools were recruited into SICAS based on established inclusion and exclusion criteria that were modeled from other studies.5,6
School/classroom settled and airborne dust samples were collected twice during the academic school year. The first school sample was obtained during the fall/winter season and a second school sample was obtained during the spring/summer season. One home settled dust sample was also collected. All samples were linked to the enrolled students and analyzed for indoor allergens. The study was approved by the institutional review board of Children’s Hospital Boston and the Brigham and Women’s Hospital. Written informed consent was obtained.
School and home settled dust samples were obtained by trained research personnel using an Oreck XL (model BB870-AD) hand-held vacuum with a special dust collector (DACI lab, Johns Hopkins, Baltimore, MD) fitted into the inlet hose of the vacuum using a standardized protocol described in other published studies.6 School dust samples were collected from the desks, chairs and classroom floors of enrolled students. School cafeterias and gymnasiums linked to participating schoolchildren were also sampled. Vacuum sampling was performed for a total of 6 minutes per sample, 3 minutes on the floor and 3 minutes on the surfaces of desks and chairs and processed in a standard fashion.7 Bedroom dust samples were collected by subjects enrolled in the study using a standardized protocol utilized in previous studies.8 Surfaces of the mattress, bedding, and floor were vacuumed for at least 5 minutes, with particular attention to underneath and around the bed. Based on prior home-based studies, the bedroom was the home sampling location of choice for comparison in this school focused study.9
Classroom airborne dust samples were collected using a charged plate air sampler and established technique described by Platts-Mills et al.10,11 The air sampler was placed in a single classroom of each school for one week at least 2 feet away from any surface. Total quantities of airborne concentrations were converted to airborne concentrations in cubic meters using methods established by Platts-Mills et al.12
All dust samples were analyzed using a multiplex array for indoor allergens (MARIA) that measured nine allergens simultaneously: cockroach (Bla g 2), cat (Fel d 1), dog (Can f 1), mouse (Mus m 1), dust mite (Der p 1, Der f 1 and group 2), rat (Rat n 1) and Alternaria (Alt a 1) allergens. The lower limits of detection were 0.196 μg/g of dust for Bla g 2; 0.004 μg/g for Fel d 1, Alt a 1 and Rat n 1; 0.002 μg/g for Mus m 1; and 0.012 μg/g for Der f 1, Der p 1 and Can f 1. No significant levels of group 2, rat or Alternaria allergens were detected in the sample set, and results for these allergens are not shown in the data presentation.
Statistical Methods
Geometric means were calculated for dog, cat and mouse allergen in schools and homes. Allergen concentrations were log-transformed so the distributions were approximately normal for further analyses. T-tests were used to compare school versus home for dog, cat, and mouse allergens. The Wilcoxon rank sum test was used to compare school versus home for roach and dust mite allergens. Linear mixed regression models of log-transformed variables were done to account for correlation from repeated observations (subjects with measurements at both home and school). Sensitivity analyses were performed with removal of cafeterias from all schools and separately of School 5 and School 9 given the extraordinarily high levels of mouse and dog and cat allergen in these schools, respectively. Correlations between log-transformed airborne allergen levels and log-transformed settled dust allergen levels in classrooms were analyzed using the Pearson correlation. Airborne and settled dust values were matched by classroom and season of collection. Statistical analyses were completed using SAS 9.2 (SAS for Windows; SAS Institute Inc, Cary, North Carolina).
RESULTS
Table E1 summarizes the basic demographics of each of the 12 participating schools as reported by the schools. The 12 schools were located in the same northeastern inner-city and were built between the years 1904–1975.
Two-hundred twenty nine vacuum-collected settled dust samples were obtained from schools: 195 from classrooms (85.2%), 22 from cafeterias (9.6%), and 12 from gymnasiums (5.2%). One-hundred eighteen home settled dust samples were obtained. One-hundred ninety seven airborne dust samples were obtained from schools: 181 from classrooms (92%) and 16 from cafeterias (8%).
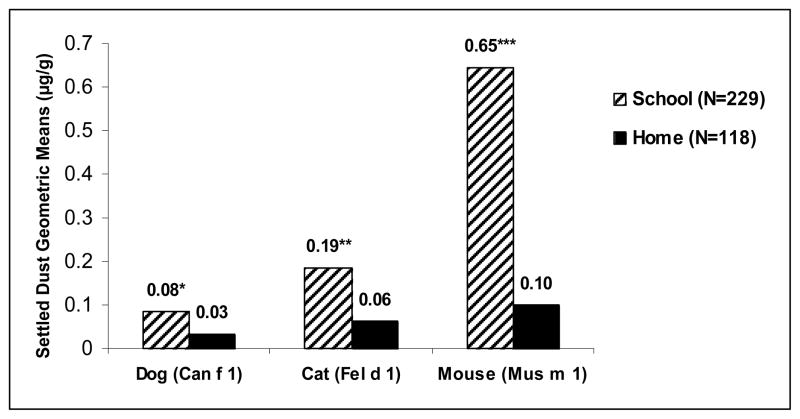
Table I shows geometric means and standard deviations for indoor animal allergens in schools and homes, whereas Table II shows geometric means, ranges and 25th to 75th percentiles for all detectable indoor allergens for both schools and homes. There were higher levels of dog, cat and mouse settled dust allergen in schools as compared to homes, though on average, for both schools and homes, the levels of dog and cat allergen were “trace” levels, not equivalent to those found in households with these pets (geometric means: Can f 1, 0.08 vs 0.03 μg/g; Fel d 1, 0.19 vs 0.06 μg/g; Mus m 1, 0.65 vs 0.10 μg/g) (see Figure 1). Exceptions were School 5 and School 9 (see Table I). School 5 had high levels of mouse allergen and School 9, despite no report of cat or dog in the school, had markedly high allergen levels that were reproducible in a second season of testing. T-tests on logged values showed significant differences between school and home settled dust levels for Can f 1 (p=0.0002), Fel d 1 (p=0.0013), Mus m 1 (p<0.0001).
Table I.
School and Home Settled Dust and Air Levels of Can f 1, Fel d 1 and Mus m 1 Allergen
| DUST μg/g Geometric Mean (GSD) |
AIR ng/m3 Geometric Mean (GSD) |
|||||||
|---|---|---|---|---|---|---|---|---|
| SCHOOL* | N | Can f 1 | Fel d 1 | Mus m 1 | N | Can f 1 | Fel d 1 | Mus m 1 |
| 1 | 44 | 0.05 (4.90) | 0.10 (8.99) | 0.76 (5.03) | 40 | 1.00 (2.69) | 2.34 (3.70) | 1.50 (3.08) |
| 2 | 23 | 0.03 (3.07) | 0.11 (6.09) | 0.47 (6.00) | 17 | 0.67 (2.12) | 1.72 (3.18) | 1.57 (3.22) |
| 3 | 35 | 0.07 (3.50) | 0.13 (5.82) | 1.84 (4.53) | 36 | 1.50 (2.72) | 2.35 (3.78) | 2.68 (4.01) |
| 4 | 16 | 0.09 (4.49) | 0.13 (7.14) | 0.14 (6.01) | 13 | 0.71 (1.76) | 0.92 (2.17) | 0.64 (4.71) |
| 5 | 14 | 0.03 (4.02) | 0.08 (6.48) | 33.77 (4.27) | 10 | 2.85 (3.20) | 7.43 (3.31) | 39.91 (3.20) |
| 6 | 28 | 0.09 (4.40) | 0.20 (7.06) | 0.15 (4.42) | 24 | 0.67 (3.04) | 0.47 (3.59) | 0.39 (4.14) |
| 7 | 9 | 0.04 (6.41) | 0.03 (8.27) | 2.94 (4.75) | 9 | 4.91 (1.72) | 3.30 (1.73) | 4.09 (1.55) |
| 8 | 4 | 0.03 (5.37) | 0.08 (35.24) | 8.23 (2.16) | 2 | 0.87 (2.11) | 2.09 (1.54) | 6.03 (1.46) |
| 9 | 16 | 20.76 (2.52) | 203.64 (1.20) | 0.03 (18.60) | 13 | 1.32 (2.49) | 2.75 (2.30) | 1.09 (1.74) |
| 10 | 18 | 0.10 (14.59) | 0.38 (41.90) | 0.54 (28.93) | 14 | 1.83 (2.02) | 2.33 (1.85) | 4.43 (1.86) |
| 11 | 4 | 0.05 (4.82) | 0.10 (9.97) | 0.38 (20.89) | 4 | 0.92 (4.77) | 6.43 (1.66) | 4.26 (2.40) |
| 12 | 18 | 0.03 (2.98) | 0.03 (5.25) | 0.70 (8.33) | 15 | 1.11 (2.63) | 0.90 (2.64) | 1.34 (2.24) |
| OVERALL§ | 229 | 0.08 (8.71) | 0.19 (18.00) | 0.65 (11.77) | 197 | 1.17 (2.85) | 1.82 (3.68) | 1.80 (4.55) |
| HOME** | 118 | 0.03 (7.93) | 0.06 (20.84) | 0.10 (12.73) |
School samples collected from the classrooms, cafeterias and gymnasiums linked to enrolled students with asthma
Home samples collected from the bedrooms of enrolled students with asthma. An extra home settled dust sample was obtained by 2 students.
5 missing classroom dust samples and 19 missing classroom airborne samples.
GSD, geometric standard deviation
Table II.
Allergen Levels in Schools and Homes of Students with Asthma
| Allergen | Location | Allergen Levels, μg/g
|
||
|---|---|---|---|---|
| Range | Geometric Mean | 25th to 75th percentile | ||
| Can f 1 | School* | 0.01–99.42 | 0.08 | 0.01–0.26 |
| Home** | 0.01–74.48 | 0.03 | 0.01–0.03 | |
|
| ||||
| Fel d 1 | School | 0.004–285.78 | 0.19 | 0.02–0.65 |
| Home | 0.004–392.34 | 0.06 | 0.004–0.20 | |
|
| ||||
| Mus m 1 | School | 0.001–544.37 | 0.65 | 0.15–3.32 |
| Home | 0.002–82.56 | 0.10 | 0.02–0.54 | |
|
| ||||
| Bla g 2 | School | 0.20–0.29 | 0.20 | 0.20–0.20 |
| Home | 0.20–0.90 | 0.20 | 0.20–0.20 | |
|
| ||||
| Der f 1 | School | 0.01–1.64 | 0.04 | 0.01–0.10 |
| Home | 0.01–11.77 | 0.08 | 0.01–0.24 | |
|
| ||||
| Der p 1 | School | 0.01–0.78 | 0.01 | 0.01–0.01 |
| Home | 0.01–11.01 | 0.02 | 0.01–0.01 | |
School samples collected from the classrooms, cafeterias and gymnasiums linked to enrolled students with asthma
Home samples collected from the bedrooms of enrolled students with asthma
Figure 1.
Settled dust geometric mean levels of Can f 1, Fel d 1 and Mus m 1 in all samples (school vs home). T-tests were performed on logged values, *p=0.0002, **p=0.0013, ***p<0.0001.
In general dust mite levels were very low in both homes and schools (see Tables II and supplemental E2). There were higher levels of dust mite settled dust allergens in homes as compared to schools (geometric means: Der f 1, 0.08 vs 0.04 μg/g; Der p 1, 0.02 vs 0.01 μg/g) but no difference between school and home for cockroach settled dust allergen (geometric mean: Bla g 2, 0.20 vs 0.20 μg/g) (see supplemental Table E2). Wilcoxon rank sum tests on logged values showed significant differences between school and home settled dust Der f 1 (p=0.0004) and Der p 1 (p=0.0023) but not for Bla g 2 (p=0.16).
Linear mixed regression models of log-transformed variables, accounting for correlation from repeated observations, showed significantly higher settled dust levels of allergens in schools than homes (545% higher for mouse (Mus m 1) with an estimated absolute difference of 0.55 μg/g, p<0.0001; 198% higher for cat (Fel d 1) with an estimated absolute difference of 0.13 μg/g, p=0.0033; and 144% higher for dog (Can f 1) with an estimated absolute difference of 0.05 μg/g, p=0.0008). When cafeteria samples (N=22) were removed from analyses, the mixed model analyses still showed significantly higher settled dust levels of allergens in schools than homes (485% higher for Mus m 1 with an estimated absolute difference of 0.49 μg/g, p<0.0001; 284% higher for Fel d 1 with an estimated absolute difference of 0.17 μg/g, p=0.0003; and 180% higher for Can f 1 with an estimated absolute difference of 0.06 μg/g, p=0.0001).
Sensitivity analyses were performed without School 5 and School 9, separately (see Table I). For School 5, mixed models showed significantly higher school settled dust allergen levels than home for Mus m1, Can f 1 and Fel d 1 (398% higher for Mus m1 with an estimated absolute difference of 0.40 μg/g, p<0.0001; 158% higher for Can f 1 with an estimated absolute difference of 0.05 μg/g, p=0.0005; 214% higher for Fel d 1 with an estimated absolute difference of 0.13 μg/g, p=0.0026). For School 9, mixed models showed significantly higher school settled dust allergen levels than home for Mus m 1 and Can f 1 only (704% higher for Mus m 1 with an estimated absolute difference of 0.70 μg/g, p<0.0001 and 65% higher for Can f 1 with an estimated absolute difference of 0.02 μg/g, p=0.03). There was no significant difference between school and home for Fel d 1 settled dust allergen (77% increase in Fel d 1 with an estimated absolute difference of 0.05 μg/g, p=0.08).
On a baseline survey questionnaire administered only to asthmatic students enrolled in SICAS, 16% and 10% of 136 students answered YES to having a dog in the past 12 months or currently, respectively, and none of School 9 students (total N=9) answered YES to either question; 24% and 21% of all students answered YES to having a cat in the past 12 months or currently, respectively, and 11% of School 9 students answered YES to both questions. On the survey questionnaire, screening all students with and without asthma, 24% of 2,011 students (N=485) answered YES to having a cat or dog as a pet in their home; 42% of 43 students attending School 9 answered YES to this question, similar to School 5 (44% of 36 students) and School 7 (40% of 65 students). Thirty-two percent of 1,926 students (N=620) answered YES to having seen mice or cockroaches in their home in the past year.
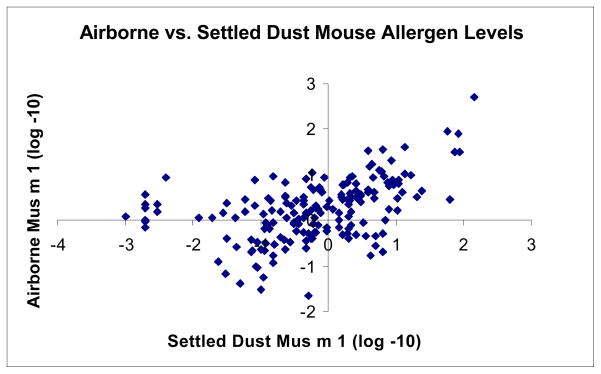
The geometric mean classroom mouse settled dust allergen concentration was 0.64 ug/g, and the geometric mean airborne concentration was 1.80 ng/m3. Airborne and settled dust mouse allergen levels in classrooms were moderately correlated (r=0.48; p<0.0001; see Figure 2). Settled dust and airborne cat and dog allergen levels in classrooms were not significantly correlated.
Figure 2.
Scatter plot of levels of airborne Mus m 1 and settled dust Mus m 1 (N=180); r=0.48, P < 0.0001.
DISCUSSION
Most indoor allergen exposure studies have focused on the home environment because homes are often considered the main exposure site. However, non-residential indoor environments such as schools and day care facilities are increasingly becoming recognized as important sites of exposure as children spend a large part of their childhood and adolescent years in these environments.13 SICAS is the first inner-city school based study in the United States to compare indoor allergen levels of school classrooms, gymnasiums, and cafeterias connected to students with asthma with their home allergen levels. Airborne and settled dust samples were obtained from schools and homes for comparison.
In keeping with previous studies, mouse allergen settled dust levels were significantly elevated in inner-city homes with lower levels of cat and dog allergen levels.14,15 Mouse allergen (Mus m 1) plays an important role in asthma morbidity.15 Mouse allergen sensitization in a New York City birth cohort was significantly associated with asthma and high levels of air pollution.16 It is quite prevalent in inner-city homes with a log-fold higher concentration in inner-city homes than in suburban homes.17 Matsui et al. reported more days of asthma symptoms and rescue medication use and a greater risk of asthma-related health care use in inner-city Baltimore preschool children exposed to >0.5 μg/g of Mus m 1 in bedroom settled dust.18 Our bedroom levels were lower at a mean level of 0.1 μg/g. However, our school Mus m 1 settled dust levels were similar at a mean level of 0.65 μg/g.
The striking findings of this study are the higher levels of mouse, cat and dog allergens found in schools as compared to homes, with Mus m 1 being the most elevated (see Figure 1). This is a particularly important finding for students with asthma attending these inner-city schools. In contrast to pet allergens, it is unlikely that mouse allergen is being brought into schools on clothing. Furthermore, on the screening survey questionnaire, only 32% of all students answered YES to having seen mice or cockroaches in their home in the past year. The highest levels of mouse allergen are often found in cafeterias, kitchens, or rooms where food is present. When cafeteria samples were removed from analyses, mouse allergen levels remained significantly higher in schools than in homes. Despite removal of school 5 from sensitivity analyses, mouse allergen levels continued to be significantly higher in schools than homes. Thus, classrooms are an important site of exposure.
The baseline survey questionnaire administered to students with asthma enrolled in SICAS showed that very few (less than 25%) were exposed to cat or dog at home currently or in the past 12 months. Kitch et al. showed that cat and dog ownership is less common among those living in poverty areas or in households with low family income.19 This suggests that inner-city students with asthma are exposed to animal allergens in schools and not necessarily at home. Previous school-based studies performed in Sweden have shown higher levels of cat and dog allergen in schools and little to no dust mite and cockroach allergens present.20,21 In contrast, a school-based study in Norway demonstrated that dust from schools contained no animal allergens.22 There were no school pets or guide dogs among the 12 schools. The primary source of dog and cat allergen in schools, therefore, is believed to be from the clothing of other students and staff with pets.23 Threshold levels of cat, Fel d 1, and dog, Can f 1, settled dust allergens have been reported for asthma symptoms in sensitized individuals (8.0 μg/g and 10.0 μg/g, respectively). Our overall school settled dust levels for cat and dog allergens were very low compared to these numbers. School 9 was the only school with very high levels of both cat and dog allergen in all settled dust samples obtained (see Table I). Further investigation of this school did not reveal any other source for these animal allergens.
Levels of cockroach (Bla g 2) and dust mite (Der f 1, Der p 1 and Group 2) were undetectable to very low in dust samples from both schools and homes, although mite allergen levels were higher in homes than schools. In contrast, school-based studies performed in southeast Texas and Manchester, England found detectable levels of cockroach allergen in all schools.24,25 The mean concentrations of Der f 1 and Der p 1 did not exceed 10 μg/g and the mean concentration of Bla g 2 did not exceed 8 U/g (equal to 0.32 μg/g), levels which have been associated with asthma symptoms (Table III).26,27 The relative absence of dust mites and cockroach is likely due to the long, dry, and very cold winters in the city in which our study took place. Both dust mites and cockroach require high humidity and warmth to survive. Other studies have also revealed low levels of dust mite allergen in Northeastern cities.27,28 It has been shown that dust mite exposure and sensitivity are highest in the South and Northwest. Additionally, none of the participating schools had wall to wall carpeting or upholstered furnishings in classrooms, a usual reservoir for dust mites. The finding of low cockroach levels, however, in our inner-city school and home samples is curious. There have been a number of studies published to date showing a high prevalence of cockroach allergen in urban schools and homes in cities such as New Orleans, New York, Baltimore and Boston.19,29–31 It may be that cockroach allergen levels vary by location even within a city, by race/ethnicity, and by gradation in poverty levels.
Classroom settled dust and airborne mouse allergen levels were significantly correlated (see Figure 2). To our knowledge, this is the first published correlation between settled dust and airborne mouse allergen levels in inner-city classrooms. This is consistent with previous findings of mouse allergen in the homes of inner-city children with asthma.17 The overall airborne concentration of classroom Mus m 1, 1.8 ng/m3, is comparable to levels seen in a mouse research and production facility.32 Interestingly, settled dust and airborne concentrations of both dog and cat allergens were not correlated (r=0.08, p=0.29 and r=0.14, p=0.07, respectively). Bollinger et al. also showed no correlation between airborne and settled dust cat allergen levels.33 This suggests that settled dust levels of dog and cat allergen are not predictive of airborne allergen levels and exposure. This is especially interesting since dog, cat and mouse allergens are all found in the particle size fraction from 2–20 μm.34 This brings up the question of whether Mus m 1 aerosolizes more easily than dog and cat allergen and if so, what the chemical and physical factors of Mus m 1 allergen are that would allow for this in addition to surface characteristics, such as carpeting vs no carpeting.
The strength of our study includes the measurement of settled dust concentrations of multiple indoor allergens in both the home and school environments of inner-city students with asthma as well as measurement of airborne levels in schools. We were able to obtain multiple settled dust and airborne samples from each school, including cafeterias and gyms, during the school year. We did not collect airborne dust samples from the homes of students with asthma, and therefore, could not make correlations between settled dust and airborne allergen concentrations in the homes or compare airborne allergen levels between schools and homes. Home settled dust samples were collected only once during the year. We realize that it would be ideal to have multiple home samples, matching the classroom samples. However, the home sample served as a marker of baseline chronic home-specific exposure after hours, primarily during sleep, and has been considered the most relevant in other urban inner-city studies, focused on the home.6,35 The possibility of selection bias also exists since we only sampled the homes of students with asthma enrolled in SICAS. We acknowledge that there may be potential limitations in subject-collected settled dust bedroom samples. However, our methods are modeled after rigorously established methods of subject-collected standards that demonstrated excellent correlations with staff-collected dust collections.8,36
CONCLUSION
In summary, we found higher levels of mouse, cat and dog settled dust allergen in schools versus homes of students with asthma, with mouse being the highest. Mouse allergen levels in schools were substantial. In general, cat and dog allergen levels were detectably low but still higher in schools. Although very low, dust mite settled dust allergen levels were higher in homes than schools. Levels of cockroach settled dust allergen was virtually undetectable in both schools and homes. There was a significant correlation between classroom settled dust and airborne mouse allergen concentrations, suggesting that settled dust mouse allergen aerosolizes and may be a significant exposure for students. These are very important findings and indicate that pest reduction and allergen abatement strategies should also focus on the school environment. Exposure and sensitization to indoor allergens are strong risk factors for allergic respiratory diseases, namely asthma.37,38 Further studies are needed to evaluate the role of school indoor allergen exposure and its effect on asthma morbidity in students with asthma.
Supplementary Material
Acknowledgments
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Funding Sources: NIH Grants: R01 AI 073964 and R01 AI 073964-02S1 (PI-Wanda Phipatanakul).
References
- 1.control cfd. Vital Signs: Asthma Prevalence, Disease Characteristics, and Self-Management Education --- United States, 2001–2009, 2011.
- 2.Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199–1206. doi: 10.1016/j.jaci.2009.04.030. quiz 1207–1198. [DOI] [PubMed] [Google Scholar]
- 3.Carlsten C, Ferguson A, Dimich-Ward H, Chan H, DyBuncio A, Rousseau R, Becker A, Chan-Yeung M. Association between endotoxin and mite allergen exposure with asthma and specific sensitization at age 7 in high-risk children. Pediatr Allergy Immunol. 2011;22:320–326. doi: 10.1111/j.1399-3038.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Redline S, Gruchalla RS, Wolf RL, Yawn BP, Cartar L, Gan V, Nelson P, Wollan P. Development and validation of school-based asthma and allergy screening questionnaires in a 4-city study. Ann Allergy Asthma Immunol. 2004;93:36–48. doi: 10.1016/S1081-1206(10)61445-7. [DOI] [PubMed] [Google Scholar]
- 5.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, Rao D, Permaul P, Gaffin JM, Rogers CA, Muilenberg ML, Gold DR. The school inner-city asthma study: design, methods, and lessons learned. J Asthma. 2011;48:1007–1014. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff-Mortimer K, Wedner HJ, Crain E, Eggleston P, Evans R, Kattan M, Kercsmar C, Leickly F, Malveaux F, Smartt E, Weiss K. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Smedje G, Norbäck D, Edling C. Asthma among secondary schoolchildren in relation to the school environment. Clin Exp Allergy. 1997;27:1270–1278. [PubMed] [Google Scholar]
- 8.Arbes SJ, Sever M, Vaughn B, Mehta J, Lynch JT, Mitchell H, Hoppin JA, Spencer HL, Sandler DP, Zeldin DC. Feasibility of using subject-collected dust samples in epidemiologic and clinical studies of indoor allergens. Environ Health Perspect. 2005;113:665–669. doi: 10.1289/ehp.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, Mortimer KM, Mitchell H, Ownby D, Slavin R, Malveaux F. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 10.Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TA. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin Exp Allergy. 2003;33:986–991. doi: 10.1046/j.1365-2222.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 11.Platts-Mills J, Custis N, Kenney A, Tsay A, Chapman M, Feldman S, Platts-Mills T. The effects of cage design on airborne allergens and endotoxin in animal rooms: high-volume measurements with an ion-charging device. Contemp Top Lab Anim Sci. 2005;44:12–16. [PubMed] [Google Scholar]
- 12.Platts-Mills JA, Custis NJ, Woodfolk JA, Platts-Mills TA. Airborne endotoxin in homes with domestic animals: implications for cat-specific tolerance. J Allergy Clin Immunol. 2005;116:384–389. doi: 10.1016/j.jaci.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Abramson SL, Turner-Henson A, Anderson L, Hemstreet MP, Bartholomew LK, Joseph CL, Tang S, Tyrrell S, Clark NM, Ownby D. Allergens in school settings: results of environmental assessments in 3 city school systems. J Sch Health. 2006;76:246–249. doi: 10.1111/j.1746-1561.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 15.Pongracic JA, Visness CM, Gruchalla RS, Evans R, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101:35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 16.Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, Sjodin A, Needham L, Perera FP, Whyatt RM. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatr Allergy Immunol. 2010;21:260–267. doi: 10.1111/j.1399-3038.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, Eggleston PA. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, Diette GB. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 19.Kitch BT, Chew G, Burge HA, Muilenberg ML, Weiss ST, Platts-Mills TA, O’Connor G, Gold DR. Socioeconomic predictors of high allergen levels in homes in the greater Boston area. Environ Health Perspect. 2000;108:301–307. doi: 10.1289/ehp.00108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munir AK, Einarsson R, Schou C, Dreborg SK. Allergens in school dust. I. The amount of the major cat (Fel d I) and dog (Can f I) allergens in dust from Swedish schools is high enough to probably cause perennial symptoms in most children with asthma who are sensitized to cat and dog. J Allergy Clin Immunol. 1993;91:1067–1074. doi: 10.1016/0091-6749(93)90221-z. [DOI] [PubMed] [Google Scholar]
- 21.Perzanowski MS, Rönmark E, Nold B, Lundbäck B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103:1018–1024. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 22.Dotterud LK, Van TD, Kvammen B, Dybendal T, Elsayed S, Falk ES. Allergen content in dust from homes and schools in northern Norway in relation to sensitization and allergy symptoms in schoolchildren. Clin Exp Allergy. 1997;27:252–261. [PubMed] [Google Scholar]
- 23.Berge M, Munir AK, Dreborg S. Concentrations of cat (Fel d1), dog (Can f1) and mite (Der f1 and Der p1) allergens in the clothing and school environment of Swedish schoolchildren with and without pets at home. Pediatr Allergy Immunol. 1998;9:25–30. doi: 10.1111/j.1399-3038.1998.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 24.Tortolero SR, Bartholomew LK, Tyrrell S, Abramson SL, Sockrider MM, Markham CM, Whitehead LW, Parcel GS. Environmental allergens and irritants in schools: a focus on asthma. J Sch Health. 2002;72:33–38. doi: 10.1111/j.1746-1561.2002.tb06509.x. [DOI] [PubMed] [Google Scholar]
- 25.Custovic A, Green R, Taggart SC, Smith A, Pickering CA, Chapman MD, Woodcock A. Domestic allergens in public places. II: Dog (Can f1) and cockroach (Bla g 2) allergens in dust and mite, cat, dog and cockroach allergens in the air in public buildings. Clin Exp Allergy. 1996;26:1246–1252. [PubMed] [Google Scholar]
- 26.Dust mite allergens and asthma--a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 28.Gruchalla RS, Pongracic J, Plaut M, Evans R, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Rabito FA, Carlson J, Holt EW, Iqbal S, James MA. Cockroach exposure independent of sensitization status and association with hospitalizations for asthma in inner-city children. Ann Allergy Asthma Immunol. 2011;106:103–109. doi: 10.1016/j.anai.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, Mellins RB, Hoepner L, Andrews H, Lopez-Pintado S, Quinn JW, Perera FP, Miller RL, Jacobson JS, Perzanowski MS. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128:284–292.e287. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amr S, Bollinger ME, Myers M, Hamilton RG, Weiss SR, Rossman M, Osborne L, Timmins S, Kimes DS, Levine ER, Blaisdell CJ. Environmental allergens and asthma in urban elementary schools. Ann Allergy Asthma Immunol. 2003;90:34–40. doi: 10.1016/S1081-1206(10)63611-3. [DOI] [PubMed] [Google Scholar]
- 32.Curtin-Brosnan J, Paigen B, Hagberg KA, Langley S, O’Neil EA, Krevans M, Eggleston PA, Matsui EC. Occupational mouse allergen exposure among non-mouse handlers. J Occup Environ Hyg. 2010;7:726–734. doi: 10.1080/15459624.2010.530906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollinger ME, Wood RA, Chen P, Eggleston PA. Measurement of cat allergen levels in the home by use of an amplified ELISA. J Allergy Clin Immunol. 1998;101:124–125. doi: 10.1016/S0091-6749(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 34.Erwin EA, Woodfolk JA, Custis N, Platts-Mills TA. Animal danders. Immunol Allergy Clin North Am. 2003;23:469–481. doi: 10.1016/s0889-8561(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 35.Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, Malindzak GS, Enright P, Evans R, Morgan W, Stout JW. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110:939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss ST, O’Connor GT, DeMolles D, Platts-Mills T, Sparrow D. Indoor allergens and longitudinal FEV1 decline in older adults: the Normative Aging Study. J Allergy Clin Immunol. 1998;101:720–725. doi: 10.1016/S0091-6749(98)70300-8. [DOI] [PubMed] [Google Scholar]
- 37.Platts-Mills TA, Blumenthal K, Perzanowski M, Woodfolk JA. Determinants of clinical allergic disease. The relevance of indoor allergens to the increase in asthma. Am J Respir Crit Care Med. 2000;162:S128–133. doi: 10.1164/ajrccm.162.supplement_2.ras-15. [DOI] [PubMed] [Google Scholar]
- 38.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9:128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.