Abstract
Purpose
This phase II component of a multi-institutional phase II/III randomized trial assessed the feasibility and preliminary efficacy of acupuncture-like transcutaneous electrical nerve stimulation (ALTENS) in reducing radiation-induced xerostomia.
Methods
Head and neck cancer patients who were 3–24 months from completing radiotherapy ± chemotherapy (RT±C) and experiencing xerostomia symptoms with basal whole saliva production ≥0.1 ml/min and without recurrence were eligible. Patients received twice weekly ALTENS sessions (24 over 12 weeks) using a Codetron™ unit. The primary objective assessed the feasibility of ALTENS treatment. A patient was considered compliant if 19/24 ALTENS were delivered, with a targeted 85% compliance rate. Secondary objectives measured treatment-related toxicities and ALTENS effect on overall radiation-induced xerostomia burden using the University of Michigan Xerostomia-Related Quality of Life Scale (XeQOLS).
Results
Of 48 accrued patients, 47 were evaluable. Median age was 60 years; 84% were male, 70% completed RT±C for > 12 months and 21% had received prior pilocarpine. All ALTENS sessions were completed in 34 patients, but 9 and 1 completed 20–23 and 19 sessions respectively, representing a 94% total compliance rate. 6-month XeQOLS scores were available for 35 patients; 30 (86%) achieved a positive treatment response with a mean reduction of 35.9% (SD 36.1). Five patients developed grade 1–2 gastrointestinal toxicity and one had grade 1 pain event.
Conclusions
ALTENS treatment for radiation-induced xerostomia can be uniformly delivered in a cooperative multicenter setting and has possible beneficial treatment response. Given these results, the phase III component of this study was initiated.
Keywords: head and neck cancer, xerostomia, radiation, acupuncture, ALTENS
INTRODUCTION
Xerostomia remains a common complication of radiation treatment for head and neck cancers, despite the introduction of newer radiation treatment techniques such as intensity-modulated radiation therapy (IMRT). In one prospective study, over 30% of patients treated with IMRT still reported RTOG grade 2 or higher xerostomia symptoms at six months after completion of treatment 1. The presence of xerostomia continues to adversely affect patients’ quality of life and, as such, the optimum management of this complication is under active investigation. Treatment with saliva substitutes and stimulation of salivary flow by pharmacological methods provides some symptomatic relief but no long lasting effect once active treatment is discontinued. Moreover, the administration of pharmacological methods is sometimes limited by associated adverse cholinergic side effects rendering treatments intolerable for the patient 2.
A single institutional phase II trial conducted at McMaster University (Hamilton, Ontario, Canada) suggested that non-invasive acupuncture-like transcutaneous nerve stimulation (ALTENS) on selected sets of acupuncture points increases whole salivary production and reduces radiation-induced xerostomia symptoms, with the benefits sustained for more than 6 months to one year 3. Patients reported an improvement in tongue dryness, speech, swallowing and overall comfort of the mouth. Consistency of saliva and oral mucous was also improved and associated with an improvement in taste. These positive results support the hypothesis that ALTENS treatment of selected acupuncture points based on traditional Chinese medicine principles may be effective in managing radiation-induced xerostomia symptoms. Ideally, a phase III placebo-controlled trial should be undertaken to further evaluate this hypothesis. However, the use of sham ALTENS treatment as a placebo is not possible since ALTENS treatment necessitates the induction of a strong, but subnoxious sensation. The use of low intensity stimulation, or stimulating locations that are not acupuncture points, may still induce an increase in endorphin release that may indirectly affect salivary function. Therefore, a randomized trial that compares ALTENS treatment with the current standard pharmaceutical intervention, that is oral pilocarpine, was considered acceptable to evaluate the comparative effectiveness and adverse effects of ALTENS treatment for radiation-induced xerostomia.
Since there was no data regarding the feasibility of ALTENS delivery in a multicenter setting, a phase II trial component aimed to evaluate this feasibility issue was deemed necessary prior to the start of the phase III component. This report summarizes the phase II results of this ongoing trial assessing the feasibility and potential utility of ALTENS in reducing radiation-induced xerostomia in a cooperative group setting.
METHODS
This study was coordinated by the Radiation Therapy Oncology Group (RTOG) and performed with the approval of the institutional review board for human research at each participating institution.
The primary objective of this phase II study was to evaluate the feasibility of ALTENS treatments in a cooperative group setting. The secondary objectives were to determine ALTENS toxicities, and to assess preliminary efficacy of ALTENS by assessing xerostomia-related quality of life.
Patient Eligibility
Patients were eligible if they had a histologically confirmed diagnosis of head and neck cancer and had received radical radiation therapy, standard or IMRT, plus or minus chemotherapy, 3 months or more but less than 2 years from the time of study enrollment; no evidence of disease recurrence based on clinical examination, nasolaryngopharyngeal scope evaluation and imaging studies 8 weeks prior to enrollment; residual salivary function with unstimulated (basal) whole saliva production (WSP) equal or greater than 0.1 ml/min (having refrained from eating or drinking oral fluid for 2 hours prior to assessment); and had provided informed written consent. Use of pilocarpine or cevimeline was discontinued at least 2 weeks prior to enrollment. Patients were excluded if they were females who were pregnant, or had unstable cardiac disease, a pacemaker, or a Zubrod performance status greater than 2.
ALTENS Treatments
ALTENS treatments were administered with a Codetron™ (model 902-C, EHM Rehabilitation Technologies Ltd., Ontario, Canada) TENS units and Karaya electrode pads. This particular TENS device allows user to change stimulation parameters including frequency, wave form and intensity of stimulation. It also differs from conventional TENS units that it embeds a random circuit that enables random switching among six electrodes to prevent brain habituation to continuous stimulation 4. A pre-selected group of acupuncture points including live electrodes on bilateral SP6, ST36, LI4, and the ground electrode on CV24, was stimulated, Figure 1 and Photo 1. Locations of the acupuncture points were based on previous literature, Table 1 3,5. Square pulses of 250 millisecond duration were delivered in trains with a 4 Hz repetition rate. One acupuncture point was stimulated for 10 seconds at a time. CV24, as the site for the ground electrode, was stimulated throughout the whole treatment session. Intensity of stimulation (between level 3 to 6 on the machine) was adjusted to produce a deep strong, mild aching sensation at each acupuncture point. Random switching among electrodes was employed. ALTENS treatment was commenced within 14 days after study registration. All patients received twice weekly ALTENS sessions (20 minutes stimulation per session) for a total of 24 sessions given in 12 weeks, and no further sessions were allowed after 12 weeks. A maximum of 2 weeks without treatment was allowed and all outstanding sessions were administered in the remainder of the 12 week period, not to exceed 3 sessions per week. All treatment sessions were delivered at RTOG participating academic and community-based institutions.
Figure 1.
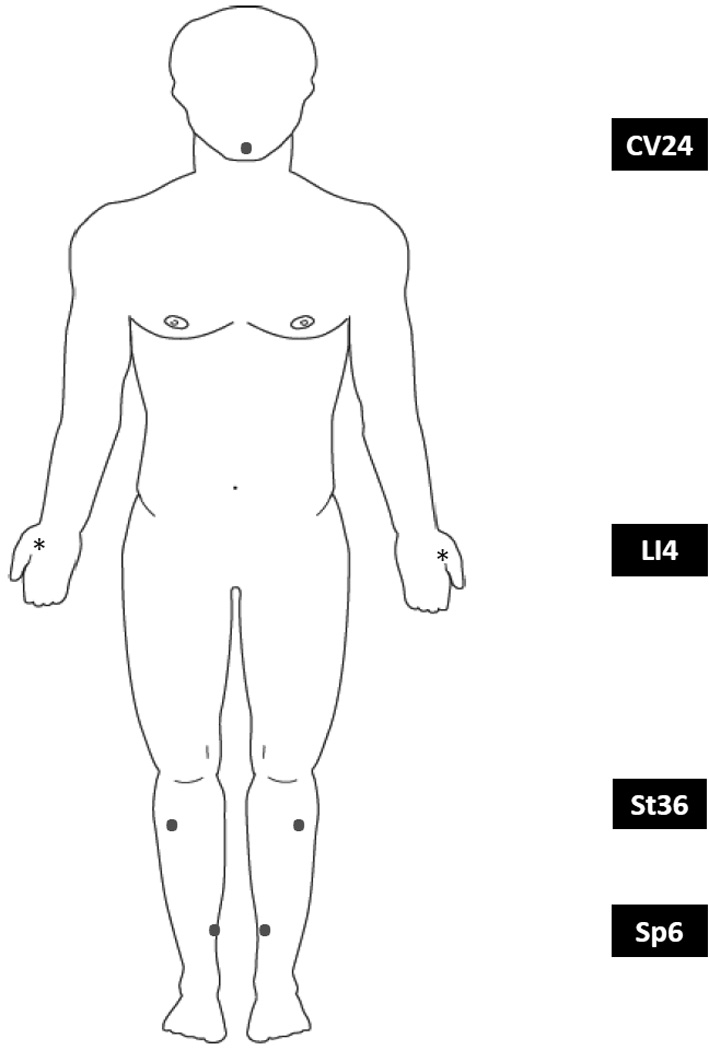
Acupunture points used in this study.
Photo 1.
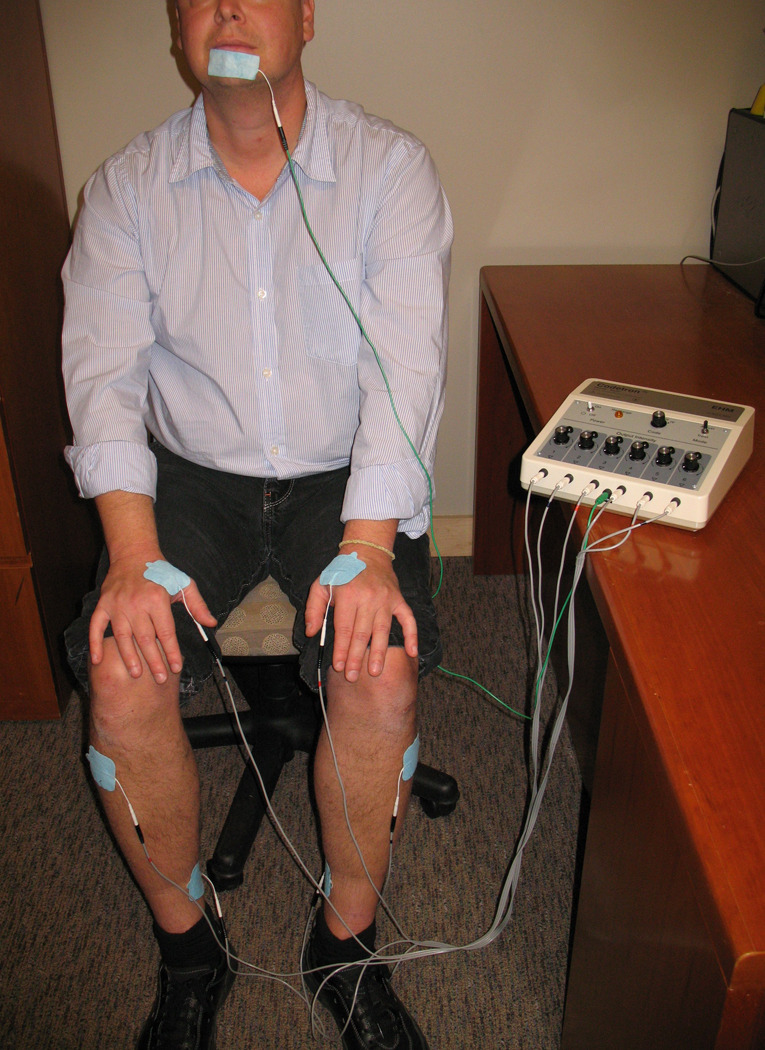
Table 1.
The locations of the acupuncture points utilized:
|
LI4 • He Gu • Large Intestine 4 Location: On the dorsum of the hand, approximately at the midpoint of the second metacarpal bone, in the belly of the first interosseus dorsalis muscle. |
|
SP6 • San Yin Jiao • Spleen 6 Location: On the medial leg, 4 finger breadth superior to the tip of medial malleolus, on the posterior border of the tibia. |
|
ST36 • Zu San Li • Stomach 36 Location: On the leg, one finger breadth lateral to the tibia's anterior crest, 4 finger breadth inferior to the depression to the lateral side of the patella. |
|
CV24 • Cheng Jiang • Conception Vessel 24 On the chin, in the depression in the center of the mentolabial groove, below the middle of the lower lip. |
Staff who administered the ALTENS at each institution for this study received training in ALTENS treatment delivery at the biannual RTOG meetings prior to study recruitment. Slides of training materials and a training video were posted on the RTOG website for easy access online. For each enrolled patient, electrode pad positions on the acupuncture points were recorded using digital photographs that were sent electronically to the principal investigator (R. Wong) for rapid approval before the third treatment session.
Quality of life and Patient-reported Xerostomia
Preliminary efficacy of ALTENS treatment was assessed using the self-reported University of Michigan Xerostomia-Related Quality of Life Scale (XeQOLS). This scale contains 15 items covering four major domains of oral health-related quality of life including physical (4 items), personal/psychological (4 items), social functioning (3 items) and pain/discomfort issues (4 items) 6. Each item is scored on a 5 point (0–4) Likert-like scale with higher scores indicating increased xerostomia burden. An overall score is the average of the average scores of each domain and can range from 0 to 4. High reproducibility and sensitivity in using this scale were validated in previous trials, and strong correlation with salivary flow was demonstrated 7, 8, 9, 10, 11. The XeQOLS was administered at baseline (study registration) and 6 months after study enrollment.
Toxicity Assessment
Acute adverse events (AEs) were assessed per the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [NCI-CTCAE v3], (MedDRA version 6) at each treatment session and follow up assessment. The incidence of the worst grade toxicity sustained up to 90 days from treatment initiation was recorded as an acute toxicity event. In the event of a grade 3 skin reaction occurring at a acupuncture point (except CV24), the point would not be treated until reaction became grade 1. If CV24 developed grade 3 skin reaction, ALTENS was held until the reaction became grade 1.
Statistics
The primary objective of the phase II component of this study was to evaluate the feasibility of ALTENS treatments in a cooperative group setting. Feasibility of ALTENS treatment was determined by patient compliance. A patient was considered compliant if 19 out of 24 treatment sessions were successfully delivered within the 12 week period. Fleming’s two-stage design was used to evaluate treatment compliance to allow early evaluation 12. Using a successful target compliance rate of 80%, statistical power of 0.87 and a type I error rate of 0.13, it was determined that 13 patients were needed for the first stage analysis. If there were less than 9 compliant patients, ALTENS treatment would be deemed not feasible. If all 13 patients were compliant, treatment would be immediately accepted as feasible. If there were between 9 to 12 compliant patients, a second stage analysis with 39 evaluable patients would be required. If 31 or more patients were compliant in the second stage analysis, the treatment would be deemed feasible. Forty-five patients were targeted to allow for an 8% ineligibility/lost rate.
Secondary objectives were to assess treatment-related toxicities and to evaluate the preliminary efficacy of ALTENS as measured by the XeQOLS 6. In terms of patient-reported xerostomia, a positive treatment response was defined as a reduction of at least 20% from baseline to 6 months in the XeQOLS. Four and 10 patients in the first and second stage analysis, respectively, had to show positive treatment response to proceed to the phase III component of the study.
RESULTS
Patient Characteristics
From September 2008 to October 2009, nine RTOG member institutions recruited 48 patients into the phase II component of this study. Forty-seven patients were evaluable as one patient was excluded for registration errors. Patient characteristics are described in Table 2. Median age was 60 years (range: 24–80). Eighty-four percent (40) were male. The majority (87%) had grade 0 Zubrod performance status. Seventy percent completed radiation +/− chemotherapy treatment at least 12 months prior to study registration. Eighty-one percent of patients had prior chemotherapy and 21% had history of prior pilocarpine use.
Table 2.
Patients’ Pretreatment Characteristics
| ALTENS (n=47) |
||
|---|---|---|
| Age (yrs) | ||
| Median | 60 | |
| Q1 – Q3 | 56–64 | |
| Min – Max | 24–80 | |
| Baseline Xerostomia Related Quality of Life (XeQOLS‡) | ||
| Median | 1.4 | |
| Q1 – Q3 | 0.9 – 2.1 | |
| Min – Max | 0.3 – 3.2 | |
| n | % | |
| Zubrod Performance Status | ||
| 0 | 41 | 87 |
| 1 | 6 | 13 |
| Gender | ||
| Male | 40 | 84 |
| Female | 7 | 16 |
| Country of Residence | ||
| United States | 36 | 77 |
| Canada | 11 | 23 |
| Prior Chemotherapy | ||
| No | 9 | 19 |
| Yes | 38 | 81 |
| Time since RT+/™Chemo Treatment | ||
| 3–6 months | 5 | 11 |
| 6–12 months | 9 | 19 |
| > 12 months | 33 | 70 |
| Prior Use of Pilocarpine | ||
| No | 37 | 79 |
| Yes | 10 | 21 |
XeQOLS scores range from 0 – 4. Higher scores indicate increased xerostomia burden.
ALTENS Feasibility
In the first stage feasibility analysis, 12 out of 13 patients (92%) were compliant with treatment. Of these, 10 patients completed all 24 treatment sessions, one patient completed 23 sessions and another completed 22 sessions. The patient who was not compliant completed 10 ALTENS sessions and had to discontinue for lack of transportation. The high compliance rates of the first stage analysis allowed for the implementation of second stage phase II recruitment.
For the second stage feasibility analysis, all 47 patients recruited were included. Forty-four of these 47 patients (94%) were successful in complying with ALTENS treatments. Of these, 34 patients (72%) completed all treatment sessions. There were 3 patients who missed only 1 session, 5 patients 2 sessions, 1patient 4 sessions, and 1 who missed 5 sessions. Reasons for non-compliance with ALTENS treatment among the 3 patients included the lack of transportation for 1 patient (described in the first stage analysis) and device malfunction for the other 2 patients. Taken in totality, the two-stage analysis results demonstrate the feasibility of ALTENS treatment delivery in a multicenter cooperative group setting.
Patient-reported Xerostomia
At the time of this report, XeQOLS scores at 6 months from registration were available for 35 out of 47 patients to evaluate ALTENS treatment response. Positive treatment response was achieved in 30 patients (86%). The median percent of XeQOLS scores reduction, indicating an improvement of symptoms, was 42.9%: results ranged from a 100% reduction to a 111% increase. The mean reduction was 35.9% (standard deviation = 36.1), Table 4. These results met the required response rate to proceed with the phase III component of this study. Of the 30 XeQOLS responders, 28 were compliant, completing at least 19 ALTENS sessions and 2 were not compliant.
Table 4.
Xerostomia Related Quality of Life Scale (XeQOLS) Change Scores
| ALTENS (N=47) |
|
|---|---|
| Not Evaluated at 6 months | 12 (26%) |
| Evaluated at 6 months | 35 (74%) |
| Treatment Response* | |
| Yes | 30 (86%) |
| No | 5 (14%) |
| Percent Change in XeQOLS Score‡ | |
| Mean (Std. Dev.) | −35.9 (36.1) |
| Minimum | −100 |
| 1st Quartile | −58 |
| Median | −42.9 |
| 3rd Quartile | −21.7 |
| Maximum | 111 |
Percent change scores 6 months after initiation of treatment from baseline score.
At least 20% reduction (percent change ≤-20)
Negative values indicate lower XeQOLS score and reduced xerostomia burden.
Acute Toxicity
There were no significant acute side effects associated with ALTENS treatment. Acute adverse events (NCI-CTCAE v3) reported as definitely, probably, or possibly related to ALTENS treatment included grade 1 and 2 gastrointestinal symptoms in 5 patients and one grade 1 pain event, Table 5.
Table 5.
Adverse Events Reported as Definitely, Probably, or Possibly Related to Treatment
| ALTENS (N=47) Grade |
|||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Gastrointestinal | 2 | 3 | 0 | 0 | 0 |
| Pain | 1 | 0 | 0 | 0 | 0 |
| Worst non-hematologic | 3 | 3 | 0 | 0 | 0 |
| (6%) | (6%) | (0%) | (0%) | (0%) | |
| Worst overall | 3 | 3 | 0 | 0 | 0 |
| (6%) | (6%) | (0%) | (0%) | (0%) | |
Adverse events were graded with CTCAE version 3.0
DISCUSSION
Needle acupuncture has been shown to have promising positive effects on radiation-induced xerostomia in several non-randomized studies and one small randomized controlled study 13–18. However, the general reluctance of patients to have needle insertions and the requirement of trained expertise for treatment delivery may render needle acupuncture difficult to offer at conventional clinics 19. Non-invasive alternatives to needle acupuncture have been explored using transcutaneous nerve stimulators with selected treatment parameters, such as the acupuncture-like transcutaneous nerve stimulator (ALTENS). A-delta and C nerve fibres that are involved in needle acupuncture treatment with the typical ‘De qi’ response (experienced as a mild ache by the subject) can be activated by ALTENS using high intensity and low frequency stimulation 20, 21, 22. The use of electrode pads that are 3 to 4 cm in size also make the precise localization of acupuncture points (usually 0.5 to 2 cm in size) less important. Without needle insertion, the risk of deep tissue injury is eliminated. Thus, ALTENS treatments can be administered with minimal training in operating the device and with knowledge of simple anatomical information to local relevant acupuncture points. Studies have shown that ALTENS gives comparable treatment results to needle acupuncture treatment for multiple clinical indications, such as musculoskeletal pain disorders 23,24, 25. The Codetron™ units utilized in this study have an additional advantage in having a unique randomization circuitry that minimizes brain habituation to the continuous electrical stimulation and may improve treatment effectiveness 4. A randomized study comparing Codetron® to electroacupuncture (needle acupuncture with electrical stimulation) in chronic musculoskeletal pain also demonstrated that Codetron™ exerts comparable or better results than electroacupuncture 26.
Acupuncture points utilized in this study were selected based on Traditional Chinese Medicine principles and on the positive results of the single institutional phase II trial mentioned above 3. In Traditional Chinese acupuncture practice, selection of acupuncture points for treating a disease largely depends on the particular meridian/s involved in the disease process, the energetic nature and the traditionally identified functions of individual acupuncture points. The trajectories of the Large Intestine, Stomach, Spleen and Conception meridians pass through the mandible area where major salivary glands are located, and the oral cavity where minor salivary glands are located. Acupuncture points along these four meridians have been used in previous positive studies for xersotomia treatment 13–18. For example, the Large Intestine meridian was commonly utilized in these reported studies. In one study, LI2, an acupuncture points on the radial side of the distal phalanx of the index finger, used together with three auricular points, was shown to produce sustained relief of xerostomia symptoms 17. In this study protocol, an acupuncture point from each of these four meridians was chosen for stimulation. LI4 on the Large Intestine meridian, is commonly used for parotitis treatment and is postulated to have a direct effect on the parotid glands. ST36 on the Stomach meridian, was selected for its traditionally recognized “tonification” effect that improves overall energy of the meridian and promotes its flow through all meridians. Since Stomach and Large intestine meridians are in the same energetic group, YangMing, ST36 stimulation can also potentiate the treatment effect of LI4. On the Spleen meridian, SP6 is an acupuncture point situated on the confluence of three Ying meridians that, when stimulated, promotes body fluid, including salivary production. CV24 on the Conception meridian, stimulates salivary flow and is commonly indicated for treating xerostomia caused by various pathological conditions 5.
Although the underlying mechanisms by which acupuncture treatment may improve salivary function are still unclear, recent evidence suggests that acupuncture treatment may work through differential stimulation of the autonomic nervous system 27. Activation of the parasympathetic component increases overall volume of salivary production and deactivation of sympathetic component stimulation reduces salivary viscosity 28. Parasympathetic stimulation also enhances the release of specific neuropeptides including calcitonin gene-related peptide (C-GRP), a potent vasodilator that also promotes salivary secretion 29, 30, 31, 32. A brain functional magnetic resonance study also demonstrated that needling LI2, compared to a sham acupuncture point, can activate the insular and the operculum that overlap brain areas known to be involved in gustation and salivation 33. ALTENS treatment may act via similar mechanisms to acupuncture in alleviating symptoms due to radiation-induced xerostomia.
The use of electrical stimulation may have additional advantages. In two clinical studies of patients with xerostomia resulting from various causes, there was significant improvement in salivary production and symptoms associated with xerostomia after electrical stimulation using a device applied to the tongue and hard palate 34, 35. Laboratory studies have shown that electrical stimulation of the parasympathetic nerve to the parotid and submandibular glands in rats causes a mitogenic response as indicated by an increase in tritiated thymidine uptake of the glands 36. This implies the regeneration of salivary gland acinar cells may be possible after initial interventions and may explain the long-term benefits of acupuncture and ALTENS treatments demonstrated in several clinical studies 3,37, 38. Other mechanisms include direct stimulation of minor salivary glands that may not have suffered as much radiation damage to increase salivary output.
A multi-center setting, including both academic and non-academic cancer centers, was selected in conducting this investigation to ensure that the study results reflect how well the interventions being examined work in a conventional clinical environment. Since there were no previous RTOG trials using ALTENS, and no published data regarding the feasibility of ALTENS treatment in a multi-center setting, a phase II component was added to the designed phase III randomized trial to demonstrate the feasibility of ALTENS treatment and a potential positive treatment response. The high percentage of patients complying with protocol therapy clearly demonstrates that ALTENS is feasible in a conventional treatment setting. The rapid patient recruitment rate, averaging 6 patients per month, also reflects the ease of implementation of this device and the high level of patient’s acceptance of this treatment modality. The device malfunction that led to the non-compliance of two patients was found to be users’ errors. All devices were rechecked by the manufacturer and were shown to have functioned properly. The subjects must have had evidence of residual salivary function and have completed radical radiation treatment longer than 3 months, but no longer than 2 years, prior to entering the study. These criteria were included with the anticipation that patients with some residual functional salivary tissue would have a higher chance of response. This was also the study population examined in previous trials 3. Although some reports have suggested a slight recovery of salivary function after radical radiation to the salivary glands, most studies demonstrated irreversible salivary dysfunction. Progressive salivary acinar necrosis and gland atrophy with associated salivary functional decline have been shown to occur until 6 to 8 months after radiation therapy 39, 40. Early intervention may best be delivered during this period of functional decline to arrest deterioration of salivary function and to induce regeneration of salivary gland tissue.
In this phase II study, response to ALTENS treatments was assessed using the University of Michigan XeQOLS at 6 months from randomization. From the study results, it was intriguing to observe that 30/35 (86%) of patients reported a positive response with a mean reduction in XeQOLS scores of 35.9%. The facts that this endpoint was assessed at almost three months after ALTENS treatment completion and that 89% of patients recruited had developed xerostomia for 6 or more months after radiation therapy suggest a sustained response, compared to the 3 to 5 hours duration of action of pilocarpine. This may also imply the occurrence of salivary tissue regeneration. These improved XeQOLS scores may, however, also be explained by the possible natural recovery of salivary function after parotid sparing radiation techniques as suggested in a previous study 41. The randomized phase III component of this study will help to define the full efficacy of ALTENS treatment in alleviating radiation-induced xerostomia. The 'dose-response ' relationship, i.e. the optimal number of treatment sessions and duration of each session, has not been established. The utilization of 24 sessions given twice weekly over 12 weeks was largely based on the phase II trial at McMaster University mentioned above. The results of this study contribute to the dose-response data that are needed to better define optimum ALTENS treatment sessions.
ALTENS appears to be well tolerated without significant toxicities. There were only five patients who reported grade 1 and 2 gastrointestinal toxicities and one with grade 1 facial pain (CTCAE v3) in this phase II component of RTOG 0537. Interestingly, increased dry mouth is the only gastrointestinal toxicity that was reported by the five patients during ALTENS delivery, and that none of these patients reported subsequent worsening of their XeQOLS scores in follow-up. The reasons for this transient complaint of increased mouth dryness are unclear although anxiety during treatment may be a contributing factor. There was one patient who reported worsened XeQOLS by 111% at 6 months follow-up. This patient finished his radiation therapy plus chemotherapy 3 to 6 months before having ALTENS treatment and had no prior oral pilocarpine treatment. The reasons for the worsening of this patient’s xerostomia symptoms, again are unclear. Further follow-up assessments of this patient and the availability of more patients’ data in the phase III component of the RTOG0537 trial may help to identify possible contributing factors.
Given the feasibility of delivering ALTENS treatment in a multicenter setting and positive patient-reported treatment responses, upon the approval of the United States National Cancer Institute, the phase III component of RTOG 0537 initiated enrollment of patients in August of 2010. One hundred and forty-four eligible patients will be accrued and stratified according to previous pilocarpine usage and time interval after radical radiation. Patients will be randomized to oral pilocarpine, 5 mg three times a day for 12 weeks, or ALTENS treatments (administered with the Codetron™) two times weekly for 12 weeks. The primary efficacy endpoint is the XeQOLS score at nine months from randomization. Secondary endpoints include basal and stimulated (citric acid-primed) whole salivary production as measured by sialometry and treatment toxicity profiles.
Conclusions
The results of this phase II component of RTOG 0537 demonstrate the feasibility of delivering quality-assured ALTENS treatments for radiation-induced xerostomia in a cooperative multicenter setting, and show that ALTENS treatments may reduce patient-reported xerostomia symptoms. With the approval of the United States National Cancer Institute, the phase III component of RTOG 0537, comparing the efficacy of ALTENS versus pilocarpine in reducing radiation-induced xerostomia symptoms, was opened for patient accrual in August, 2010.
Table 3.
ALTENS Treatment Compliance
| ALTENS (N=47) |
||
|---|---|---|
| n | % | |
| Treatment completed per protocol* | 44 | 94 |
| Completed all 24 sessions | 34 | 74 |
| Completed 23 sessions | 3 | 6 |
| Completed 22 sessions | 5 | 11 |
| Completed 20 sessions | 1 | 2 |
| Completed 19 sessions | 1 | 2 |
| Treatment not completed per protocol | 3 | 6 |
| Completed 13 sessions (device malfunction) | 1 | 2 |
| Completed 10 sessions (no transportation) | 1 | 2 |
| Completed 2 sessions (device malfunction) | 1 | 2 |
Patients completing at least 19 out of 24 sessions were compliant.
Acknowledgments
This work was supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the National Cancer Institute.
Footnotes
Presented at the 52nd Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 31st - November 4th, 2010, San Diego, CA. and at the Radiation Therapy Oncology Group Semiannual Meeting, January 13th–16th, 2011.
This contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The authors have no disclosure, financially and non-financially, relating to the products described in this manuscript and to the conduction of this study.
References
- 1.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74(1):1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan D. Xerostomia: diagnosis and management. Oncology (Williston Park) 1996;10(3 Suppl):7–11. [PubMed] [Google Scholar]
- 3.Wong RK, Jones GW, Sagar SM, Babjak AF, Whelan T. A Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57(2):472–480. doi: 10.1016/s0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 4.Pomeranz B, Niznik G. Codetron, a new electrotherapy device overcomes the habituation problems of conventional TENS devices. Amer J Electromedicine. 1987:22–26. (First Quarter) [Google Scholar]
- 5.Cheng X, editor. Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1987. [Google Scholar]
- 6.Henson BS, Inglehart MR, Eisbruch A, Ship JA. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncol. 2001;37(1):84–93. doi: 10.1016/s1368-8375(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 7.Eisbruch A, Marsh LH, Martel MK, et al. Comprehensive irradiation of head and neck cancer using conformal multisegmental fields: assessment of target coverage and noninvolved tissue sparing. Int J Radiat Oncol Biol Phys. 1998;41(3):559–568. doi: 10.1016/s0360-3016(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 8.Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36(2):469–480. doi: 10.1016/s0360-3016(96)00264-7. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 10.Henson BS, Eisbruch A, D'Hondt E, Ship JA. Two-year longitudinal study of parotid salivary flow rates in head and neck cancer patients receiving unilateral neck parotid-sparing radiotherapy treatment. Oral Oncol. 1999;35(3):234–241. doi: 10.1016/s1368-8375(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 11.Ship JA, Eisbruch A, D'Hondt E, Jones RE. Parotid sparing study in head and neck cancer patients receiving bilateral radiation therapy: one-year results. J Dent Res. 1997;76(3):807–813. doi: 10.1177/00220345970760031401. [DOI] [PubMed] [Google Scholar]
- 12.Kramar A, Potvin D, Hill C. Multistage designs for phase II clinical trials: statistical issues in cancer research. Br J Cancer. 1996;74(8):1317–1320. doi: 10.1038/bjc.1996.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH, Chung WK, Kang W, Choi SM, Cho CK, Son CG. Manual acupuncture improved quality of life in cancer patients with radiation-induced xerostomia. J Altern Complement Med. 2008;14(5):523–526. doi: 10.1089/acm.2007.0793. [DOI] [PubMed] [Google Scholar]
- 14.Garcia MK, Chiang JS, Cohen L, et al. Acupuncture for radiation-induced xerostomia in patients with cancer: a pilot study. Head Neck. 2009;31(10):1360–1368. doi: 10.1002/hed.21110. [DOI] [PubMed] [Google Scholar]
- 15.Simcock R, Fallowfield L, Jenkins V. Group acupuncture to relieve radiation induced xerostomia: a feasibility study. Acupunct Med. 2009;27(3):109–113. doi: 10.1136/aim.2009.000935. [DOI] [PubMed] [Google Scholar]
- 16.Kahn ST, Johnstone PA. Management of xerostomia related to radiotherapy for head and neck cancer. Oncology (Williston Park) 2005;19(14):1827–1832. [PubMed] [Google Scholar]
- 17.Johnstone PA, Peng YP, May BC, Inouye WS, Niemtzow RC. Acupuncture for pilocarpine-resistant xerostomia following radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2001;50(2):353–357. doi: 10.1016/s0360-3016(00)01530-3. [DOI] [PubMed] [Google Scholar]
- 18.Pfister DG, Cassileth BR, Deng GE, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J Clin Oncol. 2010;28(15):2565–2570. doi: 10.1200/JCO.2009.26.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditto B, Gilchrist PT, Holly CD. Fear-related predictors of vasovagal symptoms during blood donation: it's in the blood. J Behav Med. 2011 Jul 13; doi: 10.1007/s10865-011-9366-0. [DOI] [PubMed] [Google Scholar]
- 20.Howson DC. Peripheral neural excitability. Implications for transcutaneous electrical nerve stimulation. Phys Ther. 1978;58(12):1467–1473. doi: 10.1093/ptj/58.12.1467. [DOI] [PubMed] [Google Scholar]
- 21.Wang KM, Yao SM, Xian YL, Hou ZL. A study on the receptive field of acupoints and the relationship between characteristics of needling sensation and groups of afferent fibres. Sci Sin B. 1985;28(9):963–971. [PubMed] [Google Scholar]
- 22.Johnson MI. The analgesic effects and clinical use of acupuncture-like TENS (AL-TENS) Phys Ther Rev. 1998;3(2):73–93. [Google Scholar]
- 23.Fox EJ, Melzack R. Transcutaneous electrical stimulation and acupuncture: comparison of treatment for low-back pain. Pain. 1976;2(2):141–148. [PubMed] [Google Scholar]
- 24.Laitinen J. Acupuncture and transcutaneous electric stimulation in the treatment of chronic sacrolumbalgia and ischialgia. Am J Chin Med (Gard City N Y) 1976;4(2):169–175. doi: 10.1142/s0192415x76000214. [DOI] [PubMed] [Google Scholar]
- 25.Gadsby JG, Flowerdew MW. Transcutaneous electrical nerve stimulation and acupuncture-like transcutaneous electrical nerve stimulation for chronic low back pain. Cochrane Database Syst Rev. 2000;2(2):CD000210. doi: 10.1002/14651858.CD000210. [DOI] [PubMed] [Google Scholar]
- 26.Cheng R, Pomeranz B. Electrotherapy of chronic musculoskeletal pain: Comparison of electroacupuncture and acupuncture-like transcutaneous electrical nerve stimulation. Clin J Pain. 1986;2:143–149. [Google Scholar]
- 27.Sakatani K, Kitagawa T, Aoyama N, Sasaki M. Effects of acupuncture on autonomic nervous function and prefrontal cortex activity. Adv Exp Med Biol. 2010;662:455–460. doi: 10.1007/978-1-4419-1241-1_65. [DOI] [PubMed] [Google Scholar]
- 28.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. Sensory stimulation (acupuncture) increases the release of calcitonin gene-related peptide in the saliva of xerostomia sufferers. Neuropeptides. 1999;33(3):244–250. doi: 10.1054/npep.1999.0759. [DOI] [PubMed] [Google Scholar]
- 30.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. Sensory stimulation (acupuncture) increases the release of vasoactive intestinal polypeptide in the saliva of xerostomia sufferers. Neuropeptides. 1998;32(6):543–548. doi: 10.1016/s0143-4179(98)90083-x. [DOI] [PubMed] [Google Scholar]
- 31.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. The influence of sensory stimulation (acupuncture) on the release of neuropeptides in the saliva of healthy subjects. Life Sci. 1998;63(8):659–674. doi: 10.1016/s0024-3205(98)00317-8. [DOI] [PubMed] [Google Scholar]
- 32.Blom M, Lundeberg T, Dawidson I, Angmar-Mansson B. Effects on local blood flux of acupuncture stimulation used to treat xerostomia in patients suffering from Sjogren's syndrome. J Oral Rehabil. 1993;20(5):541–548. doi: 10.1111/j.1365-2842.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 33.Deng G, Hou BL, Holodny AI, Cassileth BR. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI-2 acupuncture point: a randomized controlled study. BMC Complement Altern Med. 2008;7(8):37. doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talal N, Quinn JH, Daniels TE. The clinical effects of electrostimulation on salivary function of Sjogren's syndrome patients. A placebo controlled study. Rheumatol Int. 1992;12(2):43–45. doi: 10.1007/BF00300975. [DOI] [PubMed] [Google Scholar]
- 35.Weiss WW, Jr, Brenman HS, Katz P, Bennett JA. Use of an electronic stimulator for the treatment of dry mouth. J Oral Maxillofac Surg. 1986;44(11):845–850. doi: 10.1016/0278-2391(86)90219-3. [DOI] [PubMed] [Google Scholar]
- 36.Schneyer CA, Humphreys-Beher MG, Hall HD, Jirakulsomchok D. Mitogenic activity of rat salivary glands after electrical stimulation of parasympathetic nerves. Am J Physiol. 1993;264(5 Pt 1):G935–G938. doi: 10.1152/ajpgi.1993.264.5.G935. [DOI] [PubMed] [Google Scholar]
- 37.Johnstone PA, Niemtzow RC, Riffenburgh RH. Acupuncture for xerostomia: clinical update. Cancer. 2002;94(4):1151–1156. [PubMed] [Google Scholar]
- 38.Blom M, Lundeberg T. Long-term follow-up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Dis. 2000;6(1):15–24. doi: 10.1111/j.1601-0825.2000.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 39.Frank RM, Herdly J, Philippe E. Postradiotherapy dental dystrophies and salivary glands. Actual Odontostomatol (Paris) 1965;19(70):129–155. [PubMed] [Google Scholar]
- 40.Busuttil A. Irradiation-induced changes in human salivary glands. Clin Otolaryngol Allied Sci. 1977;2(3):199–206. doi: 10.1111/j.1365-2273.1977.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 41.Clavel S, Nguyen DH, Fortin B, et al. Simultaneous Integrated Boost Using Intensity-Modulated Radiotherapy Compared with Conventional Radiotherapy in Patients Treated with Concurrent Carboplatin and 5-Fluorouracil for Locally Advanced Oropharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.10.061. Epub. [DOI] [PubMed] [Google Scholar]


