Abstract
GM-CSF is a potent pro-inflammatory cytokine that plays a pathogenic role in the CNS inflammatory disease, EAE. As IL-27 ameliorates EAE, we hypothesised that IL-27 suppresses GM-CSF expression by T cells. We found that IL-27 suppressed GM-CSF expression in CD4+ and CD8+ T cells in splenocyte and purified T cell cultures. IL-27 suppressed GM-CSF in Th1, but not Th17 cells. IL-27 also suppressed GM-CSF expression by human T cells in non-polarised and Th1 but not Th17 polarised PBMC cultures. In vivo, IL-27p28 deficiency resulted in increased GM-CSF expression by CNS infiltrating T cells during Toxoplasma gondii infection. While in vitro suppression of GM-CSF by IL-27 was independent of IL-2 suppression, IL-10 up-regulation or SOCS3 signalling, we observed that IL-27-driven suppression of GM-CSF was STAT1 dependent. Our findings demonstrate that IL-27 is a robust negative regulator of GM-CSF expression in T cells which likely inhibits T cell pathogenicity in CNS inflammation.
Introduction
Granulocyte-macrophage colony stimulating factor (GM-CSF) is a pro-inflammatory haematopoietic growth factor, produced by many cell types including T cells (1). GM-CSF is important in many cellular processes including dendritic cell activation, granulocyte survival and enhancement of macrophage/microglial function (2–4).
Previous studies in EAE have demonstrated an essential pathogenic role for T cell-derived GM-CSF in CNS inflammation (4–8). Studies using Toxoplasma gondii infection in mice, which can also cause CNS inflammation, reported a detrimental role for GM-CSF by increasing parasitic burden in peritoneal macrophages (9). Taken together, these studies suggest that therapeutic targeting of GM-CSF may be beneficial in CNS inflammatory disease. However regulation of GM-CSF expression, particularly by T cells, is not well understood and is a critical gap in our knowledge of GM-CSF biology.
Interleukin 27 (IL-27) is a heterodimeric member of the IL-12 cytokine family (10) that is produced mainly by activated APCs (10). IL-27 was initially shown to promote Th1 differentiation (11), as well as to inhibit the differentiation of naïve CD4+ T cells to other helper subsets (11, 12). However, reports have demonstrated an insensitivity of committed Th17 cells to IL-27 in vitro (12, 13). IL-27 also influences production of a range of cytokines, including IL-2 and IL-10 (14–17). IL-27 also suppresses EAE, in part via Th17 cell inhibition and IL-10 up-regulation (12, 18, 19). Furthermore, IL-27R deficient mice also exhibit enhanced CNS inflammation when chronically infected with T. gondii, further demonstrating that IL-27 regulates CNS inflammation (20).
Given the suppressive effect of IL-27 on CNS inflammation (16, 18–20) and the pathogenic role of GM-CSF in CNS inflammation, we hypothesised that IL-27 negatively regulates GM-CSF. Here we show that IL-27 suppressed GM-CSF production by CD4+ and CD8+ T cells under non-polarising and Th1 conditions, but not Th17 conditions, in both murine and human cultures. This suppression was mediated by JAK2/Tyk2 and STAT1 signalling. IL-27p28-deficient mice exhibited elevated proportions of GM-CSF-producing T cells during T. gondii infection in vivo. Taken together, these findings demonstrate that IL-27 is a key negative regulator of GM-CSF expression by T cells, which likely regulates inflammation in health and disease.
Materials and Methods
Mice
C57BL/6 mice were from Charles River Laboratories. 2D2 mice were a kind gift from Prof. S. Anderton (Uni. of Edinburgh). stat1−/− mice were purchased from Taconic. il10−/− mice were a kind gift from MGC Foundation and were provided by Prof. Anne O’Garra (MRC NIMR, London). il27p28−/− mice were from by Lexicon Pharmaceuticals, Inc (The Woodlands, TX) (21). SOCS3 floxed (socs3)fl/fl mice (The Jackson Laboratory) were crossed with CD4-cre mice (European Mouse Mutant Archive) to generate mice that lacked SOCS3 in CD4+ cells only (CD4-cre/Socs3fl/fl). All animal maintenance and experiments were in compliance with the UK Home Office or in accordance with the federal and institutional guidelines of the University of Pennsylvania, School of Veterinary Medicine, and approved by the Queen’s University Ethical Review Committee or the University of Pennsylvania Institutional Animal Care and Use Committee.
Cell culture and T cell purification
Murine splenocytes were cultured in complete RPMI 1640 or X-VIVO-15 (Lonza). Cells were stimulated with soluble anti-CD3 and anti-CD28 (1 µg/ml each) antibodies at 2×106 cells/ml. For purified T cell cultures, cells were purified by magnetic microbead separation (Stemcell) and cultured as above at 106 cells/ml. For Th17 cell culture, cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) or X-VIVO-15 and activated as above. Where indicated, Jak Inhibitor 1 (0.001nM – 1nM; Calbiochem) was added to cultures.
Human PBMCs from healthy volunteers were purified by Ficoll density gradient centrifugation. PBMCs or purified CD4+ cells (Miltenyi) were cultured in RPMI 1640 or IMDM and activated as above at 2×106 cells/ml. All activation antibodies were from eBioscience.
T cell polarisation and cytokines
Non-Polarised (NP) conditions; no exogenous cytokines. Th1; IL-12 (10 ng/ml, eBioscience). Th17; TGF-β (2 ng/ml, R&D Systems) or IL-23 (50 ng/ml, eBioscience), IL-6 (200 U/ml, R&D Systems), IL-1β (10–20 ng/ml, Peprotech) and anti-IFN-γ antibody (10 µg/ml clone XMG1.2, BioXcell). For Human Th17 cells: IL-23 (10 ng/ml), anti-IFN-γ antibody (10 µg/ml) and anti-IL-4 antibody (5 µg/ml). GM-CSFhigh; anti-IFN-γ antibody and anti-IL-12 antibody (10 µg/ml clone C17.8, BioXcell). For re-activated murine Th17 cells; IL-23 (10 ng/ml, eBioscience) was added to cultures. Where indicated, IL-27 (10–20 ng/ml, eBioscience) was used. Where indicated, daily IL-2 (100 U/ml, R&D Systems) or anti-IFN-γ antibody was added.
Toxoplasma gondii infection
The ME49 strain of T. gondii was maintained and prepared as described (22). For infections, WT and il27p28−/− mice were administered 20 tissue cysts i.p. and treated with sulfadiazine (200 mg/L; Sigma) in drinking water from day 4 for 2 weeks. Mice were sacrificed 3–4 weeks post infection and GM-CSF expression by CD4+ T cells from spleen and CNS were assessed by flow cytometry.
Flow Cytometry
Cells were stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) and brefeldin A (1 µg/106 cells) for up to 5 hours before staining. Cells were surface stained, fixed, permeabilised and stained intracellularly with fluorescently conjugated antibodies (all eBioscience).
Real Time PCR
Extracted mRNA was converted to cDNA (Applied Biosystems). PCR amplification (QuantiTect SYBR Green; Qiagen) was carried out using primers for the csf-2 gene (7). Values were normalised to β-actin and compared to controls (unstimulated splenocytes).
Cytokine Quantification
GM-CSF protein was quantified by ELISA (R&D Systems).
Statistics
Data were tested for statistical significance using unpaired, two-tailed, Student’s t tests for parametric data and Mann-Whitney tests for non-parametric data.
Results and Discussion
IL-27 suppresses GM-CSF expression in activated murine T cells
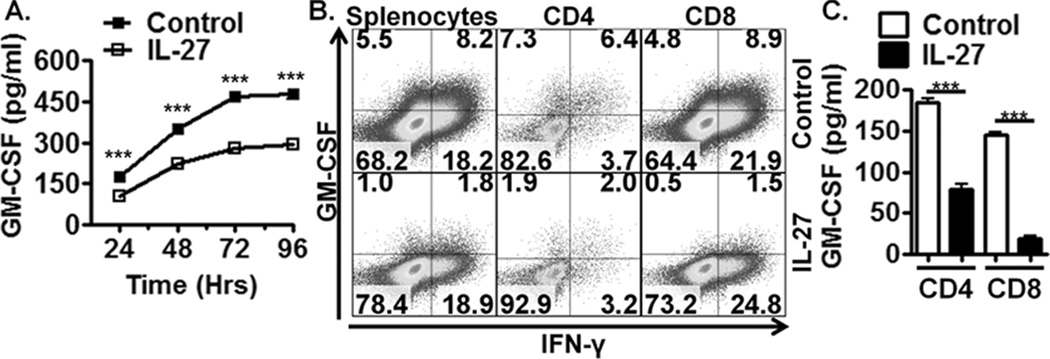
To test the hypothesis that IL-27 negatively regulates GM-CSF expression by activated T cells, splenocytes were activated in the presence or absence (+/−) of exogenous IL-27. Over 96 hrs, IL-27 suppressed GM-CSF protein expression (Fig. 1A) in both CD4+ and CD8+ T cells (Fig. 1B). To determine if IL-27 acted directly on T cells to suppress GM-CSF, purified CD4+ or CD8+ T cells were activated +/− IL-27. IL-27 consistently suppressed GM-CSF expression in purified cultures (Fig. 1C). These studies demonstrate that IL-27 can directly suppress GM-CSF expression by T cells.
Figure 1. IL-27 suppresses GM-CSF expression in activated murine T cells.
(A) ELISA of activated wild-type (WT) splenocyte supernatants stimulated with IL-27 for up to 96 hrs (n=4). (B) Flow cytometric analysis of activated WT splenocytes +/− IL-27 for 72 hrs gated on total live cells, CD4+ cells or CD8+ cells. (C) ELISA of activated purified CD4+ and CD8+ T cells cultured for 72 hrs (n=4). Data shown are from individual experiments representative of 2–3 replicate experiments.
IL-27 suppresses GM-CSF expression in Th1 but not Th17 polarising conditions
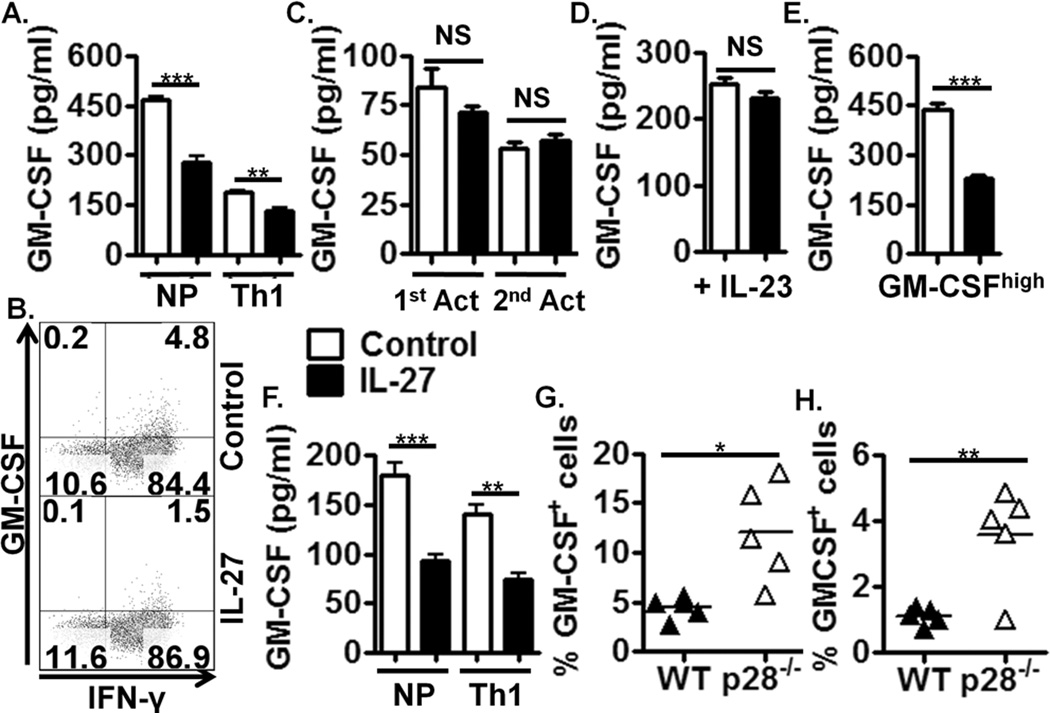
Given the importance of GM-CSF in the pathogenicity of Th1 cells in EAE (8), we examined if IL-27 suppressed GM-CSF expression by Th1 cells. GM-CSF production was diminished under Th1 polarising conditions (+ IL-12), consistent with recent findings (7). However, addition of IL-27 further suppressed GM-CSF expression (Fig. 2A, B, Supplemental Figure 1A), demonstrating synergistic actions of IL-27 and IL-12 in regulating GM-CSF production.
Figure 2. IL-27 suppresses GM-CSF expression in Th1 but not Th17 polarising conditions.
(A) ELISA of activated Th1 polarised, WT splenocytes +/− IL-27 for 72 hrs (n=4). (B) Flow cytometric analysis of Th1 polarised, WT splenocytes +/− IL-27 for 72 hrs, gated on CD4+ cells. (C) ELISA of Th17 polarised WT splenocytes activated for 96 hrs (1st Act) and then reactivated (2nd Act) for 48hrs (n=4). During the first activation, cells were treated with IL-27 at 24hrs. (D) ELISA of Th17 cells polarised with IL-23 for 72 hrs. Cells were treated with IL-27 at 24hrs, (n=4). (E) ELISA of activated GM-CSFhigh polarised WT splenocytes treated with IL-27 for 72 hrs, (n=4). (F) ELISA of non-polarised and IL-12-polarised, MOG35–55-activated splenocytes from 2D2 mice +/− IL-27 for 72hrs, (n=4). (G)(H) Flow cytometric analysis of splenic effector T cells (G) and CNS infiltrating effector T cells (H) during acute and chronic T. gondii infection respectively, in WT and il27p28−/− mice. Data shown are from individual experiments representative of 2–4 replicate experiments or n=5 in in vivo studies.
As GM-CSF also contributes to the pathogenicity of Th17 cells (8), we examined the influence of IL-27 on Th17 cell-derived GM-CSF. As IL-27 directly suppresses Th17 differentiation (12), we activated splenocytes in Th17 polarising stimuli for 24 hrs before adding IL-27. Interestingly, we did not observe GM-CSF suppression by IL-27 in this system (Fig. 2C, Supplemental Figure 1B). To examine more thoroughly if IL-27 could modulate Th17 cell-derived GM-CSF, we reactivated differentiated Th17 cells under the influence of IL-23 +/− IL-27 (Fig. 2C). Again, we did not observe suppression of GM-CSF by IL-27. This is perhaps unsurprising considering published data demonstrating that differentiated Th17 cells lack responsiveness to IL-27 in vitro (12, 13). However, flow cytometric analysis of GM-CSF expression in these cultures only yielded positive GM-CSF staining in 1 of 6 repeated experiments (Supplemental Figure 2A), which parallels lower GM-CSF detection in these culture supernatants (Fig. 2C, Supplemental Figure 1B). Taken together, these data show that IL-27 suppresses GM-CSF production by Th1 but not Th17 cells in vitro.
As Th17 polarisation was initiated before addition of IL-27, Th17 polarising stimuli may have rendered these cells unresponsive to IL-27. It is also possible that Th17-polarising stimuli suppressed GM-CSF to such an extent that addition of IL-27 had no additive suppressive effect. This may potentially be due to the presence of TGF-β, which is known to inhibit GM-CSF (8).
To address this, we polarised Th17 cells in the absence of exogenous TGF-β (as described in (23)). While IL-23-polarised Th17 cells produced GM-CSF at levels equivalent to non-polarised T cells (Supplemental Figure 1B), IL-27 still did not suppress GM-CSF in these Th17 cultures (Fig. 2D, Supplemental Figure 1B).
Recently Codarri et al. showed that neutralisation of IFN-γ and IL-12 promotes the development of highly encephalitogenic GM-CSFhigh cells (7). To test if IL-27 also inhibits GM-CSF expression in this GM-CSFhigh population, splenocytes were activated in the presence of neutralizing anti-IFN-γ and anti-IL-12 +/− IL-27. Compared to non-polarising conditions, the proportion of CD4+ T cells expressing GM-CSF was enhanced and this was reduced by IL-27, as was GM-CSF secretion (Fig. 2E, Supplemental Figure 2B). These data suggest that IL-27 could limit the encephalitogenicity of GM-CSFhigh T cells.
To reflect in vivo T cell activation more accurately, splenocytes from 2D2 transgenic TCR mice were activated in vitro with MOG35–55 antigen under non-polarising and Th1 conditions +/− IL-27. In this model IL-27 suppressed GM-CSF production (Fig. 2F) demonstrating that GM-CSF expression by T cells in response to antigen can be suppressed by IL-27.
To determine if IL-27 regulated T cell-derived GM-CSF during CNS inflammation in vivo, WT and il27p28−/− mice were infected with T. gondii. Significantly higher proportions of GM-CSF-producing effector T cells were observed in spleens of il27p28−/− animals compared to WT during acute infection (Fig. 2G). IL-27p28 deficiency also resulted in higher proportions of GM-CSF-producing effector T cells in the CNS during chronic infection (Fig. 2H). These data demonstrate that IL-27 regulates GM-CSF expression by T cells in vivo, which may contribute to enhanced inflammation observed in IL-27R-deficient mice infected with T. gondii (20).
Suppression of GM-CSF expression by IL-27 is independent of IL-10, IL-2, IFN-γ and SOCS3 signalling but dependent on Jak2/Tyk2 activity and STAT1 signalling
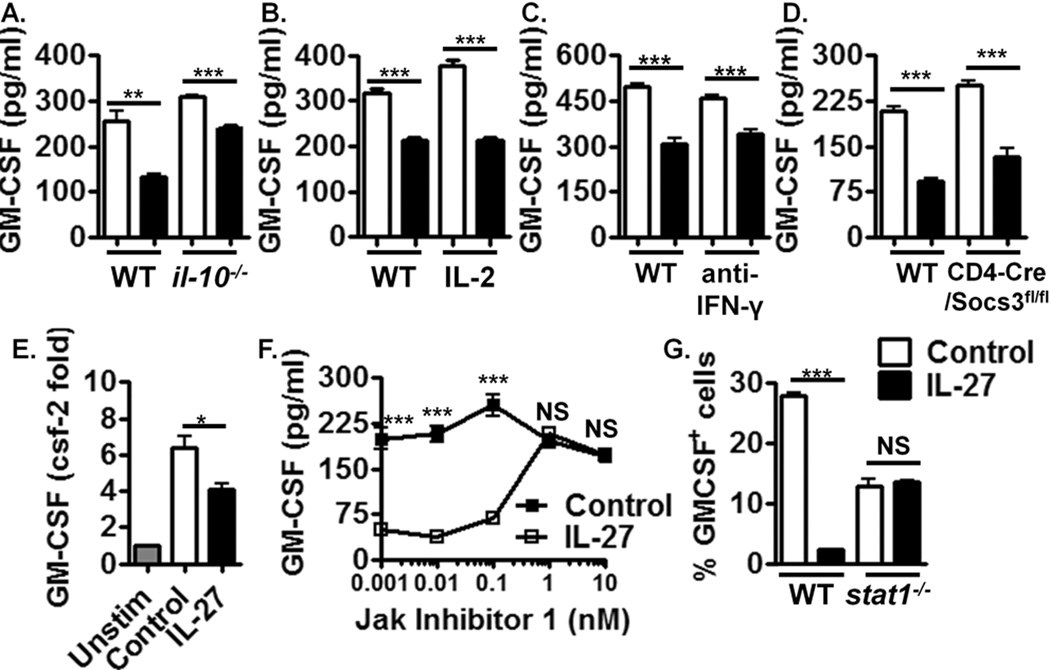
We next addressed the mechanism of GM-CSF suppression by IL-27. IL-10 is a potent inhibitor of GM-CSF (24) and as IL-27 has been demonstrated to induce IL-10 in T cells (15–17), we examined whether suppression of GM-CSF by IL-27 was dependent on IL-10. IL-27 suppressed GM-CSF expression in il-10−/− splenocytes (Fig. 3A, Supplemental Figure 2C) demonstrating that IL-10 is not required for GM-CSF suppression by IL-27.
Figure 3. Suppression of GM-CSF expression by IL-27 is independent of IL-10, IL-2, IFN-γ and SOCS3 signalling but dependent on Jak2/Tyk2 activity and STAT1 signalling.
(A) ELISA of activated NP and Th1 polarised il-10−/− splenocytes treated with IL-27 for 72 hrs, (n=4). (B) ELISA of activated WT splenocytes supplemented with daily IL-2 +/− IL-27 for 72 hrs, (n=4). (C) ELISA of activated WT splenocytes cultured with neutralising anti-IFN-γ antibody +/− IL-27 for 72 hrs, (n=4). (D) ELISA of activated CD4-cre/Socs3fl/fl splenocytes treated with IL-27 for 72 hrs, (n=4). (E) Real Time PCR of GM-CSF mRNA expression +/− IL-27 at 3 hrs post T cell activation. Unstim = unstimulated cells, (n=4). (F) ELISA of activated WT splenocytes cultured +/− IL-27 with increasing concentrations of Jak Inhibitor 1 for 72hrs, (n=4). (G) Flow cytometric analysis of activated WT and stat1−/− splenocytes +/− IL-27 for 72hrs, (n=4). Data shown are from individual experiments representative of 2–4 replicate experiments.
IL-27 inhibits IL-2 expression by T cells (14). As IL-2 is an important T cell growth factor, IL-27 could suppresses T cell-derived GM-CSF via the deprivation of T cells of IL-2. However, daily addition of exogenous IL-2 did not abrogate GM-CSF suppression by IL-27 (Fig. 3B, Supplemental Figure 2D), demonstrating that IL-2 inhibition does not mediate this phenomenon.
As IFN-γ can inhibit GM-CSF expression (7) and is induced by IL-27 (22), we examined if IFN-γ mediated suppression of GM-CSF by IL-27. Addition of anti-IFN-γ did not prevent suppression of GM-CSF by IL-27 (Fig 3C, Supplemental Figure 2E), suggesting that IFN-γ does not mediate such suppression.
IL-27 induces SOCS3 (14), which regulates a range of cytokine signalling pathways (25) and thus could inhibit an inducer of GM-CSF. We examined the suppressive effect of IL-27 on GM-CSF expression in splenocytes from mice with SOCS3 deficiency restricted to CD4+ cells (CD4-cre/Socs3fl/fl). IL-27 suppressed GM-CSF expression in these cells (Fig. 3D, Supplemental Figure 2F), demonstrating that this mechanism is SOCS3-independent.
As suppression of GM-CSF was independent of several known regulatory mechanisms of IL-27 (Fig. 3A, B, D), we hypothesised that IL-27 directly suppresses early GM-CSF gene transcription. We observed suppression of csf-2 expression by IL-27 as early as 3 hours following T cell activation by real time PCR (Fig. 3E). Such rapid suppression suggests that IL-27 directly inhibits GM-CSF gene transcription, potentially via antagonising downstream TCR signalling targets that induce GM-CSF.
To determine the signalling pathway via which IL-27 mediates suppression of GM-CSF, we examined Jak/STAT signalling using a Janus kinase (Jak) inhibitor, Jak Inhibitor 1 (JI-1) (Fig. 3F). At concentrations of JI-1 that selectively inhibited Jak2 and Tyk2 (1 nM), suppression of GM-CSF was lost (Fig. 3F, Supplemental Figure 2G), demonstrating that suppression of GM-CSF by IL-27 is mediated by Jak2/Tyk2 activity.
STAT1 can be directly phosphorylated by Jak2, and mediates several anti-inflammatory properties of IL-27 (16, 17, 19). We therefore examined if suppression of GM-CSF by IL-27 was dependent on STAT1. In cultures of activated stat1−/− splenocytes, IL-27 did not suppress GM-CSF expression (Fig. 3G), demonstrating that STAT1 signalling is required for IL-27-mediated suppression of GM-CSF. Interestingly, suppression of GM-CSF by IL-12 was also lost in stat1−/− mice (data not shown), suggesting that both cytokines may suppress GM-CSF via similar mechanisms.
IL-27 suppresses GM-CSF expression in human T cells
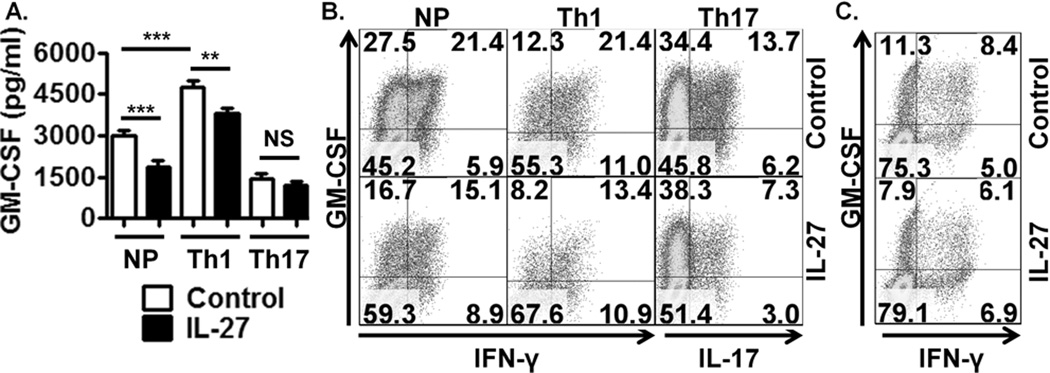
To translate findings from murine to human systems, we examined if IL-27 suppressed GM-CSF expression by human T cells. PBMCs from healthy donors were activated under NP, Th1 and Th17 conditions +/− IL-27. Robust GM-CSF expression was observed in this system (Fig. 4A, B). Even in such potent GM-CSF-producing cultures, IL-27 suppressed GM-CSF in NP and Th1 polarised conditions (Fig. 4A, B), consistent with murine observations (Fig. 1 and 2). Interestingly, IL-12 did not suppress GM-CSF in human T cells, the opposite of that observed in our murine model (Fig. 2 and 4A, Supplemental Figure 1A, C), suggesting differential kinetics of GM-CSF regulation by IL-12 in murine and human systems. However, IL-27 consistently suppressed both proportions of GM-CSF+ cells and secreted GM-CSF in supernatants of both murine and human cultures (Fig. 1 and 4, Supplemental Figure 1C), demonstrating that IL-27 is a conserved negative regulator of GM-CSF.
Figure 4. IL-27 suppresses GM-CSF expression in human T cells.
(A) ELISA of activated NP, Th1 and Th17 polarised human PBMC cultures treated with IL-27 for 120 hrs. Results are pooled from 5 donors. (B) Flow cytometric analysis of activated NP, Th1 and Th17 polarised PBMCs, gated on CD4+ cells, +/− IL-27 for 120 hrs, representative of 4 donors. (C) Flow cytometric analysis of activated, purified CD4+ T cells +/− IL-27 for 24 hrs, representative of 5 donors.
IL-27 did not suppress GM-CSF production in human Th17 cultures as measured by ELISA (Fig. 4A). Despite confirmation of IL-27 bioactivity through inhibition of Th17 polarisation (Fig. 4B), the percentage of Th17 cells (based on total IL-17+ cells) producing GM-CSF was unaffected (68% in control cells compared to 71% in IL-27 treated cells (Supplemental Figure 1F)). This further demonstrates IL-27 does not suppress GM-CSF production in committed Th17 cells. IL-27 also directly suppressed GM-CSF by purified NP and Th1 polarised CD4+ T cells (Fig. 4C, Supplemental Figure 1D, G), but not in Th17 polarised purified CD4+ T cell cultures (based on the percentage of total IL-17+ cells that co-expressed GM-CSF (Supplemental Figure 1E, H)). These data show that IL-27 directly regulates GM-CSF expression by human Th1, but not Th17, cells.
In this study we report that IL-27 suppresses GM-CSF in activated murine and human T cells, however importantly, GM-CSF was never completely abolished. This suggests that IL-27 selectively inhibits only some of the signals that induce GM-CSF expression. Understanding these mechanisms would be important in the development of novel GM-CSF-directed therapies, such that selective antagonism of the GM-CSF system could be appropriated to maximise preservation of normal immune function, but limit GM-CSF-associated immunopathogenesis.
Given the importance of GM-CSF in APC function during inflammation, inhibition by IL-27 may suppress inflammation by muting APC activation, further limiting T cell activation. Indeed, T cell-derived GM-CSF contributes to EAE pathogenesis by potentiating APC functions in the CNS, particularly in microglia, which augments recruitment and activation of T cells and other myeloid cells (5–7). This serves to propagate the local inflammatory response which drives tissue destruction. Thus, strategies that inhibit the pathogenic signature of effector T cells, such as high GM-CSF expression (7, 8) inhibit establishment and/or propagation of local inflammatory responses. In this regard, GM-CSF suppression by IL-27 may be an endogenous anti-inflammatory mechanism and may hold potential for therapeutic exploitation in inflammatory disease.
Supplementary Material
Acknowledgments
This work was funded by the Department of Employment and Learning (DEL). PF is supported by Science Foundation Ireland and National Children’s Research Centre. CH is supported by the NIH.
References
- 1.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol. 2005;25:405–428. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 2.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 3.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 4.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 5.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marusic S, Miyashiro JS, Douhan J, 3rd, Konz RF, Xuan D, Pelker JW, Ling V, Leonard JP, Jacobs KA. Local delivery of granulocyte macrophage colonystimulating factor by retrovirally transduced antigen-specific T cells leads to severe, chronic experimental autoimmune encephalomyelitis in mice. Neurosci Lett. 2002;332:185–189. doi: 10.1016/s0304-3940(02)00947-3. [DOI] [PubMed] [Google Scholar]
- 7.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 8.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman DL, Chodakewitz JA, Bartiss AH, Mellors JW. Granulocytemacrophage colony-stimulating factor enhances selective effector functions of tissuederived macrophages. Blood. 1988;72:573–578. [PubMed] [Google Scholar]
- 10.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 11.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 13.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst, and C. A. Hunter M. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 21.O'Hara Hall A, Beiting DP, Tato C, John B, Oldenhove G, Pritchard GH, Lombana CG, Silver JS, Bouladoux N, Grainger J, Tait Wojno ED, Stumhofer JS, Harris TH, Wagage S, Roos DS, Scott P, Turka LA, Reiner SL, Cua D, Belkaid Y, Merle Elloso M, Hunter CA. Distinct roles for IL-27 and IFN-γ in the development of T-bet+ Treg required to limit infection-induced pathology. Immunity. 2012 doi: 10.1016/j.immuni.2012.06.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 23.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O'Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehler L, Kollars M, Bohle B, Berer A, Reiter E, Lechner K, Geissler K. Interleukin-10 inhibits burst-forming unit-erythroid growth by suppression of endogenous granulocyte-macrophage colony-stimulating factor production from T cells. Exp Hematol. 1999;27:217–223. doi: 10.1016/s0301-472x(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.