Abstract
Accumulation of all-trans-retinal (all-trans-RAL), reactive vitamin A aldehyde, is one of the key factors in initiating retinal photodamage. This photodamage is characterized by progressive retinal cell death evoked by light exposure in both an acute and chronic fashion. Photo-activated rhodopsin releases all-trans-RAL which is subsequently transported by ATP–binding cassette transporter 4 and reduced to all-trans-retinol by all-trans-retinol dehydrogenases located in photoreceptor cells. Any interruptions in the clearing of all-trans-RAL in the photoreceptors can cause an accumulation of this reactive aldehyde and its toxic condensation products. This accumulation may result in the manifestation of retinal dystrophy including human retinal degenerative diseases such as Stargardt’s disease and age-related macular degeneration. Here, we discuss the mechanisms of all-trans-RAL clearance in photoreceptor cells by sequential enzymatic reactions, the visual (retinoid) cycle, and potential molecular pathways of retinal photodamage. We also review recent imaging technologies to monitor retinal health status as well as novel therapeutic strategies preventing all-trans-RAL-associated retinal photodamage.
Introduction: All-trans-RAL in vision
Visual perception is established by sequential signal transduction via various neural cells from the outer retina to the visual cortex of the brain (http://webvision.med.utah.edu/). The ability to adapt to variations in environmental light conditions are controlled by two-classes of photoreceptor cells; the rod and cone of the retina (1). Rods and cones show distinct response kinetics and sensitivity covering a wide range of intensities and selected wavelengths of light ranging from ~360 to 620 nm. Visual pigments in the outer segments of rods and cones absorb light, which triggers the phototransduction cascade (2,3). To sustain visual perception, rapid restoration of the pre-illuminated physiological state is required. Dark-adapted photoreceptors carry 11-cis-retinal (11-cis-RAL), a light-sensitive visual chromophore derived from vitamin A. Production of 11-cis-RAL is conducted by several enzymatic reactions, called the retinoid visual (retinoid) cycle, occurring between photoreceptor cells and adjacent retinal pigmented epithelial cells (RPE) (4). All-trans-RAL is a major intermediate of the visual cycle. Continuous regeneration of the 11-cis chromophore from all-trans-RAL is essential for the renewal of light-sensitive visual pigments and determines photoreceptor survival in the vertebrate retina (4,5). Whereas deficient 11-cis-RAL production leads to congenital blindness in humans, accumulation of the photoisomerized chromophore, all-trans-RAL, also can be detrimental (6,7). Many biological problems occur when all-trans-RAL is not efficiently cleared from the internal membranes of retinal outer segment discs (8). Recently, our group provided evidence that transient accumulation of all-trans-RAL by delayed clearance from the retina is one of the key mechanisms in light-induced retinal degeneration (8–10). In this review, we discuss the pathological impact of delayed all-trans-RAL clearance in the retina using transgenic mice, focusing on two processes, translocation and reduction, that govern all-trans-RAL clearance in the retina. Additionally, we describe recent innovations in in vivo imaging of the retina as well as discuss novel pharmacological interventions against retinal photodamage mediated by all-trans-RAL.
Key emzymes for clearance of all-trans-RAL
The reaction from all-trans-RAL to all-trans-retinol in the photoreceptor is the first step of the visual cycle. Clearance of all-trans-RAL is achieved by two steps; 1) translocation of all-trans-RAL from the intradiscal space to cytoplasmic space across photoreceptor disc membranes by ABCA4 (11), and 2) reduction of all-trans-RAL to all-trans-retinol by all-trans-retinol dehydrogenase (RDH), mainly by RDH8 expressed in photoreceptor outer segment (POS) (12) (Fig. 1).
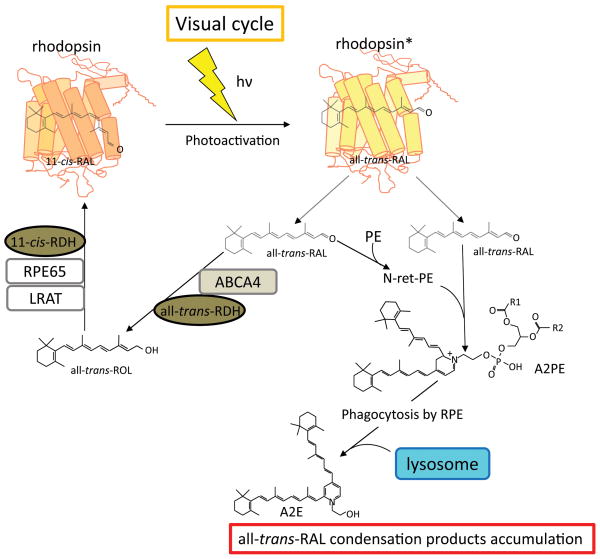
Figure 1. Process of all-trans-RAL clearance and accumulation of condensation byproducts.
All-trans-RAL is a one of the major vitamin A metabolites in the retina. In physiological conditions, all-trans-RAL is regenerated to the visual chromophore, 11-cis-retinal. The absorption of a photon (hν) by a visual pigment (rhodopsin) causes isomerization of 11-cis-RAL to all-trans-RAL, resulting in rhodopsin activation (rhodopsin*). The majority of all-trans-RAL is released from photoactivated rhodopsin into the cytosolic space of photoreceptor outer segments, and a fraction of all-trans-RAL is released to the intradiscal space. Clearance of all-trans-RAL is achieved via two processes. First all-trans-RAL is transported out from the intradiscal space into the cytosol by a photoreceptor specific ATP-binding transporter 4 (ABCA4) and reduced to all-trans-ROL by all-trans-RAL dehydrogenases (all-trans-RDHs; RDH8 and RDH12). Secondly all-trans-ROL diffuses into the RPE where it is esterified, isomerized and converted to 11-cis-RAL by sequential enzymatic reactions involving lecithin: retinol acyltransferase (LRAT), retinal pigment epithelium-specific 65 kDa protein (RPE65) and 11-cis-RDHs including RDH5, and then diffuses back into the photoreceptor where it regenerates rhodopsin. This 11-cis-RAL recycling system is termed the visual (retinoid) cycle. When clearing of all-trans-RAL is delayed, excess of all-trans-RAL accumulates in the form of its condensation products with PE in photoreceptor outer segments. N-retinylidene-phosphatidylethanolamine (N-ret-PE) and free all-trans-RAL are conjugated to form a phosphatidylpyridinium-bisretinoid (A2PE), a precursor of A2E, which escapes from ABCA4 transporting and accumulates in the intradiscal space. Accumulated A2PE is phagocytized by the RPE along with photoreceptor outer segments, and is converted to A2E by lysosomal digestion in the RPE.
ATP-binding cassette transporter 4 (ABCA4)
ABCA4 is a member of the ATP-binding cassette transporter family (ABC-transporters) which comprise one of the largest classes of proteins (13,14). ABC transporters utilize the energy of ATP hydrolysis to unidirectionally translocate a wide variety of substrates, ranging from ions to lipids and peptides, across cellular membranes (15). All ABC transporters share the same basic architecture (13,14). A minimum of four domains is required for the activity: two transmembrane domains and two nucleotide-binding domains which are known as ATP-binding cassettes. The function of transmembrane domains is to bind substrate and form a translocation path, whereas nucleotide-binding domains provide energy for transport by ATP hydrolysis. To date, 49 ABC transporters have been identified in the human genome (http://www.genenames.org/genefamily/abc.html). These are organized into seven subfamilies (ABC-A to ABC-G) (16). ABCA4 is one of 12 proteins of the ABC-A subfamily. A distinctive feature of family A members is the presence of large extracellular domains in the N-terminal half of the sequence.
The ABCA4 protein, also known as ABCR, is a ~250-kDa single chain protein localized to the incisures and margins of the outer segment disks of rod and cone photoreceptors (11). Preferred substrates for ABCA4 are all-trans-RAL and N-retinylidene-phosphatidylethanolamine (N-ret-PE). ABCA4 flips these substrates from the inside to the outside of disc membranes by utilizing energy from ATP hydrolysis. ABCA4 was initially observed in an electron microscopy study by Papermaster, et al. of immunohistochemically labeled frog photoreceptors (17). Later, a homologous protein was cloned and classified as a member of the ATP transporter superfamily, and shown to have the same localization in bovine outer segments (18). Human ABCA4 gene was initially discovered in Stargardt’s disease (STGD) patients, located on chromosome 1 at 1p22 (19). However, the presence of ABCA4 in cone cells was later demonstrated by immunohistochemistry and western blot (20).
Mutations in the ABCA4 gene have been linked to various retinal dystrophies including autosomal recessive, cone-rod dystrophy, retinitis pigmentosa and age-related macular degeneration (AMD) (21–25). STGD1 (Mendelian Inheritance in Man 248200) is a predominantly juvenile-onset macular dystrophy which is characterized by rapid irreversible loss of central vision with bilateral atrophy of photoreceptors and RPE cells of the central retina with an estimated prevalence of 1 in 10,000 (26–28). More than 600 disease-associated ABCA4 variants have been identified (29). Although the biological role of ABCA4 and its relevance to retinal degenerative diseases has been discovered, the structural and functional properties of ABCA4 remain largely undefined. Recently, Tsybovsky, et al. identified phosphorylation sites in cytoplasmic domains and investigated their putative functional implications using ABCA4 mutants. This study indicates that phosphorylation of these sites may represent a mechanism that modulates the function of ABCA4 although they are not essential for biological activity (30).
Retinoid dehydrogenases (RDHs)
RDHs belong to the short-chain dehydrogenase/reductase (SDR) family, which catalyzes NAD(H)-/NADP(H)-dependent oxidation/reduction reactions. The SDR family consists of functionally heterogeneous proteins involved in the metabolism of retinoids, steroids, prostaglandins, aliphatic alcohols and variety of xenobiotics (31). NADPH-dependent reduction of all-trans-RAL in photoreceptor outer segment (POS) is the first step in the regeneration of bleached visual pigments. Among RDHs which are reported to carry all-trans-RDH activity in vivo, RDH8 is the major all-trans-RDHs in rod and cone cells (12). RDH8 (also known as photoreceptor RDH, prRDH) was identified in 2000 by Rattner and colleagues (32). The human RDH8 gene is located in chromosome 19 at 19p13.2, and RDH8 protein expression is found in the retina. Immunohistochemistry with anti-RDH8 antibody reveals subcellular localization of RDH8 in the POS. RDH8 demonstrates a substrate and a cofactor preference for all-trans-RAL (32) and NADPH (33). The main phenotype of Rdh8−/− mice is delayed clearance of all-trans-RAL after bright light illumination (34), which is not accompanied by abnormal Meta-II decay of rhodopsin (34).
Rdh8−/− mice display: 1) accumulation of all-trans-RAL after intense illumination, 2) delayed dark adaptation, and 3) slightly increased accumulation of di-retinoid-pyridinium-ethanolamine (A2E), a product of all-trans-RAL conjugation with phosphatidylethanolamine, but no significant retinal degeneration was observed under room lighting conditions (34). A later study revealed that RDH8 is responsible for ~70% of the all-trans-RDH activity in the mouse retina, and RDH12 (35,36), which resides in the photoreceptor inner segments, carries on ~30% of this activity. Although retinas from Rdh8−/−Rdh12−/− mice had lost ~98% of their all-trans-RDH activity, these mice surprisingly still converted all-trans-RAL to all-trans-retinol in vivo. Other enzymes belong to alcohol dehydrogenase family members in the retina may contribute to the reduction of all-trans-RAL in the eye of knockout models (37,38). Indeed Rdh8−/−Rdh12−/− mice showed only mild retinal changes at 6 months of age when kept in a regular laboratory light/dark cyclic environment. Thus, less than 2% of total all-trans-RDH activity in photoreceptors is sufficient to maintain retinoid homeostasis in mice under such conditions (Fig. 2).
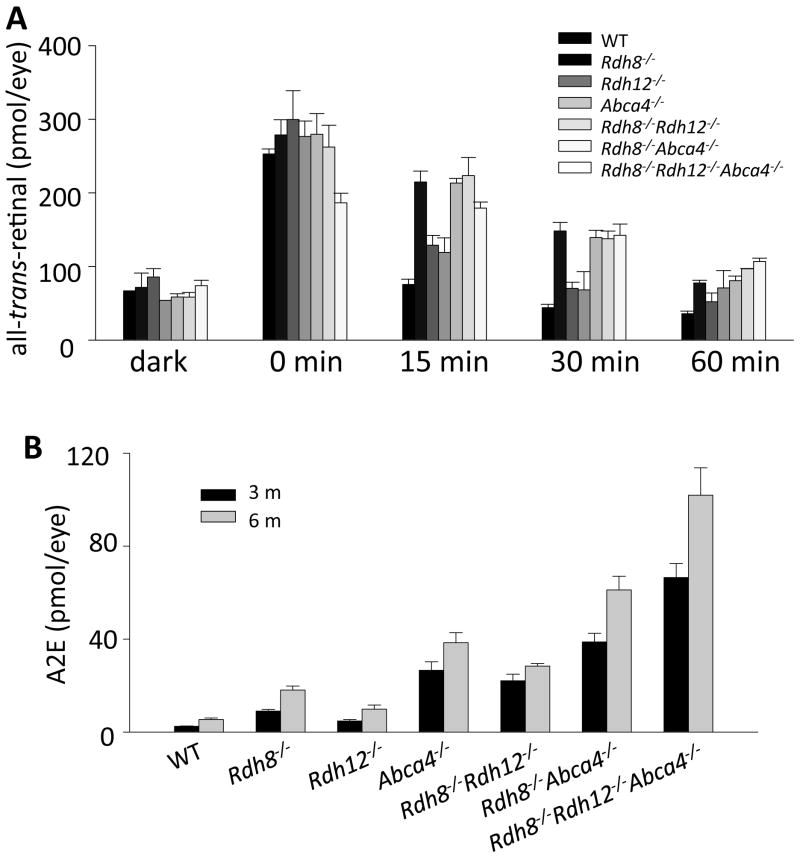
Figure 2. Clearance of all-trans-RAL and accumulation of condensation byproduct (A2E) in RDHs and ABCA4 deficient mice.
(A) Clearance of all-trans-RAL was compared among 6-week old mice. After flash light exposure, eye retinoids were extracted and quantified by normal phase HPLC to evaluate effects of Rdh8, Rdh12, Abca4 genes and double or triple combinations of these genes on the clearance of all-trans-RAL from the retina. There was no difference in all-trans-RAL levels under fully dark-adapted condition between all these strains. Rdh8−/− mice displayed the most significant delay of all-trans-RAL clearance compared to other mice with only a single gene deletion. The double and triple genes deletions elongated all-trans-RAL clearance. (B) Amounts of A2E were quantified by reverse phase HPLC. Age-dependent accumulation of A2E was observed in mutant mice, and the accumulation levels were correlated with the delay of all-trans-RAL clearance. Bars indicate SD.
Role of all-trans-RAL in mediating photodamage to the retina
It has been demonstrated that the photoactivation of visual pigments is the essential trigger of light-induced retinal degeneration, which is supported by considerable evidence including: 1) photodamage is not inducible in visual chromophore deficient retina, such as retinoid isomerase, retinal pigment epithelium-specific 65 kDa protein (RPE65) or lecithin: retinol acyltransferase (LRAT) deficient mice (9,39), 2) there is clear correlation between rates of visual pigments regeneration and light-induced damage thresholds (40), and 3) slow regeneration rate of visual pigments can prevent light damage (39,41,42). However, it was not clear which processes in the visual cycle were critical in causing retinal photodamage. A potential role of all-trans-RAL in mediating retinal photo-damage has been suspected for over two decades (43). Still, there was lack of experimental evidence to indicate that free all-trans-RAL exists in the retina at levels adequate to cause photosensitized damage (44). Recently we reported that mice with genetic ablation of RDH8 and ABCA4, two important enzymes responsible for all-trans-RAL clearance from photoreceptors, develop light-dependent cone and rod dystrophy with characteristics similar to human macular degeneration (Fig. 3). These include lipofuscin/A2E accumulation, formation of drusen and basal laminar deposition, photoreceptor/RPE atrophy, complement deposition at Bruch’s membrane, and choroidal neovascularization (8). In contrast, mice that lack retinoids in the eye due to deletion of LRAT, an enzyme essential for retinoid storage in the RPE (45), namely Lrat−/−Rdh8−/−Abca4−/− mice failed to exhibit this retinal degeneration (9). Furthermore, Lrat−/−Rdh8−/− Abca4−/− mice supplemented with retinoids demonstrated light-induced retinal degeneration without A2E accumulation. These data suggest that all-trans-RAL but not A2E is a primary cause of retinal degeneration in Rdh8−/−Abca4−/−mice. Other data also suggest that all-trans-RAL causes greater mitochondrial oxidative stress-associated apoptosis than A2E (9). First, all-trans-RAL induced higher cytotoxicity than A2E in cultured RPE cells. Second, a caspase inhibitor (Z-VAD-fml) and a Bax inhibitor (Bax is a pro-apoptotic member of Bcl-2 family proteins mitochondrial-dependent apoptosis (46)) prevented cell death caused by all-trans-RAL in vitro. Third, oxidative phosphorylation in mitochondria was suppressed by all-trans-RAL but not A2E. Fourth, fruit flies (Drosophila melanogaster) with a pigment-cell-enriched dehydrogenase deficiency (homolog of mammalian RDH) underwent light-induced retinal degeneration (47). Together, these findings indicate that all-trans-RAL mediates phototoxiciy of the retina, and free all-trans-RAL is more cytotoxic than A2E, so that A2E production may actually lower all-trans-RAL toxicity.
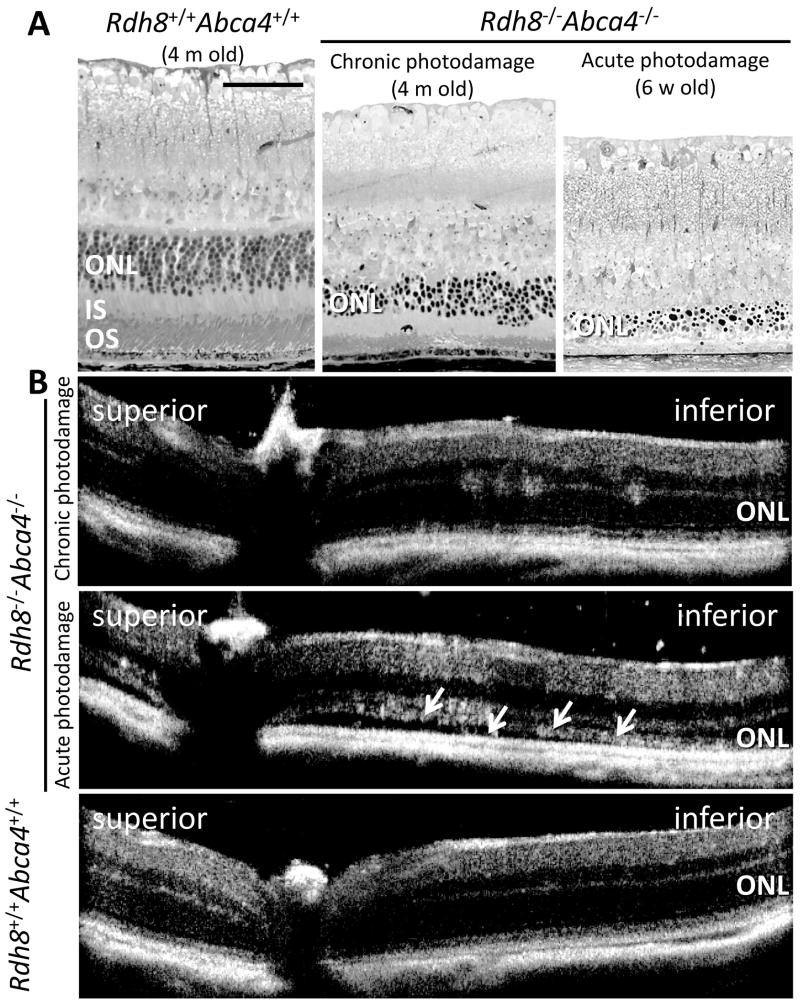
Figure 3. Chronic and acute retinal photodamage in Rdh8−/−Abca4−/−mice.
Rdh8−/−Abca4−/−mice exhibit severe retinal photodamage due to excess accumulation of all-trans-RAL. Epon-embedded retina cross-section images (A) and in vivo high-definition spectral-domain optical coherent tomography images (B) were obtained from Rdh8−/−Abca4−/−mice with chronic and acute photodamage. The disruption of the outer nuclear layer (ONL) with a decreasing number of photoreceptor cells was manifested compared to age-matched Rdh8+/+Abca4+/+ mouse retina (A left and middle panels) under regular cyclic light at 4 months of age. In vivo retinal image was obtained from these mice by spectral domain optical coherent tomography (SD-OCT). In Rdh8−/−Abca4−/−mice, disruption of ONL was demonstrated in the inferior retina (B upper panel) although Rdh8+/+Abca4+/+ retina maintained normal structure (B lower panel). Acute retinal photodamage was induced in Rdh8−/−Abca4−/−mice (6 weeks old) by intense light exposure (10,000 lux for 30 min) and retinal cross section images were obtained at 14 days after light exposure. Most of photoreceptors were disappeared and only debris of dead photoreceptor cells were accumulated in the subretinal space (A right panel). SD-OCT image showed only residual ONL layer and debris accumulation as well (white arrows in B middle panel). INL, inner nuclear layer; IS, inner segment; ONL, outer nuclear layer; OS, outer segment. Scale bar in A indicates 40 μm.
Delay in all-trans-RAL clearance and A2E accumulation
Delayed clearance of all-trans-RAL from the retina after light exposure results in production of A2E, which consists of two molecules of all-trans-RAL and one molecule of phosphatidylethanolamine (PE) (Fig 1). As previously mentioned in this review the clearance of all-trans-RAL is mediated by key enzymes which are specific to photoreceptors. To investigate the contribution of these responsible enzymes, RDH8, RDH12 and ABCA4, to all-trans-RAL clearance from retina, kinetics of all-trans-RAL after short term light exposure and A2E, end product of accumulated all-trans-RAL in chronic fashion, were compared in: Rdh8−/−, Rdh12−/−, Abca4−/−, Rdh8−/−Abca4−/−, Rdh12−/−Abca4−/− and Rdh8−/−Rdh12−/−Abca4−/− mice. RDH8 is the all-trans-RDH in the photoreceptor outer segments (5). RDH12 is also all-trans-RDH, but is located in the inner segments of photoreceptors (5). Interestingly, Rdh8−/−Rdh12−/−Abca4−/−mice at 6 weeks of age displayed retinal degeneration, whereas other mutant mice did not show apparent degeneration. Among tested animals, slower clearance of all-trans-RAL was detected in mice with RDH8 deficiency, suggesting that RDH8 is the most critical enzyme for clearing this molecule (Fig. 2A). Quantification of A2E was performed on 3- and 6-month-old mutant mice. Age-related accumulation of A2E was observed in all employed mice. Although clearance of all-trans-RAL after light was not significantly affected in mice with ABCA4 deficiency, an increase in A2E accumulation was associated with the loss of ABCA4 (Fig. 2B). Overall, slower clearance of all-tran-RAL is associated with a greater production of A2E in mice. In humans, lipofuscin accumulate with age in the RPE, especially in the macular region (48) and can account for up to 19% of the cytoplasmic space in elderly human RPE (49–51). Lipofuscin has been considered one of the major risk factors of several retinal diseases, including Best’s macular dystrophy, STGD and AMD (50,52–62).
Lipofuscin is a complex mixture of lipid-protein aggregates, and retinoid derivatives including A2E (63). The granules of lipofuscin are considered to form from the indigestible materials of phagocytized POS (64,65). Spatial localization of A2E in the RPE may vary based on the amount of accumulation (66) whereas lipofuscin granule accumulation is localized in the lysosomal storage bodies of the RPE (67,68). Since A2E was identified as the major orange-emitting fluorophore in the human RPE (69,70), the biosynthetic mechanism and pathological effects of A2E has been extensively studied. A2E is formed by condensation of PE with two molecules of all-trans-RAL followed by oxidation and hydrolysis of the phosphate ester (71). All-trans-RAL and N-ret-PE, a Schiff base adduct of all-trans-RAL and PE, are ABCA4 substrates, but phosphatidylpyridinium-bisretinoid (A2PE), a precursor of A2E, cannot be transported by ABCA4 transporter. A2PE therefore can accumulate in disc membranes. Eventually A2E accumulation is detected in the RPE by the resulting RPE’s phagocyctosis of the disc membranes (Fig. 1). Various mechanisms have been proposed to explain the toxicity of A2E, including: A2E’s properties as a cationic detergent (69), its physiologic interference with RPE function (72,73), and radical reactions induced by light-dependent A2E oxidation (74). Immunogenic properties of A2E have also been reported (75). These observations suggest a relationship between all-trans-RAL and A2E in the pathology of human retinal diseases including STGD and AMD.
Molecular pathways involved in all-trans-RAL-dependent retinal photodamage
Enzymatic reduction of all-trans-RAL to all-trans-retinol is a relatively slow process (43). Thus, rhodopsin regeneration is a prerequisite for the build-up of free all-trans-RAL during light perception. Free all-trans-RAL is not only toxic as a reactive aldehyde, but it also is a potent photosensitizer when photoactivated by UVA and blue light (43). Importantly, it has been shown that photoexcited all-trans-RAL inactivates ABCA4, which is involved in removal of all-trans-RAL from the discs (76). Inactivation of ABCA4 may lead to a further increase in the accumulation of all-trans-RAL. In cultured human RPE derived cells (ARPE-19), 10 μM of all-trans-RAL showed cytotoxicity and increased intracellular Ca2+, one of the early events of cell death. These affects were observed in less than 1 min after co-incubation with all-trans-RAL (9). Rod outer segments contain 5 mM of rhodopsin (77), which when bleached, yield equivalent concentrations of all-trans-RAL. Even bleaching of less than 0.5% of total amounts of rhodopsin will generate toxic levels of all-trans-RAL if this retinoid is not properly and quickly cleared from the retina. If a sufficient supply of 11-cis-RAL is provided, but either ABCA4 or all-trans-RDH is inactive, the concentration of accumulated all-trans-RAL in the retina can easily reach levels sufficient to cause cell toxicity and apoptosis.
All-trans-RAL can mediate the generation of superoxide radical anion, singlet oxygen, and peroxides when irradiated with UVA or blue light (43). Recent cell culture studies demonstrated that aldehydes including all-trans-RAL can produce reactive oxygen species (ROS) in NADPH oxidase-dependent manner (10,78). Unless effective antioxidants and repair enzymes offer protection, ROS produced by all-trans-RAL can cause oxidative damage to lipids and proteins that compromise their structures and functions. The RPE phagocytoses 10% of the outer segment discs daily which then undergo lysosomal degradation (69). However, oxidatively damaged compounds are no longer susceptible to degradation by lysosomal enzymes, and/or can in turn inactivate these enzymes. Because lysosomal degradation of photoreceptor outer segments is incomplete, ipofuscin/debris accumulates in the RPE. Indeed, the primary components of lipofuscin are all-trans-RAL conjugates such as A2E and all-trans-RAL-dimer (79). Photoactivation of lipofuscin by blue light also generates ROS that induce further oxidation of intragranular components (79), some of which could leak out of the granule and cause damage leading to RPE dysfunction or even death (74). Some oxidative products affect gene expression in the RPE, resulting in release of pro-inflammatory and pro-angiogenic cytokines (43). Currently drusen are proposed to represent breakdown products of the RPE (80). Thus exocytosed lipofuscin and side-products, formed by enzymes activated by all-trans-RAL and its conjugates, may contribute to the formation of age-related drusen located between the RPE and Bruch’s membrane. Some components of those deposits exhibit photosensitizing properties and others include oxidatively modified products with pro-angiogenic and pro-inflammatory properties (80). Therefore, all-trans-RAL-associated oxidative stress contributes to age-related retinal changes. Further studies regarding all-trans-RAL inducible oxidative stress and mechanisms involved in activating inflammatory responses are essential to devise successful therapeutics for age-related blinding diseases.
In vivo imaging of retinal photodamage
Recent advances in in vivo imaging technology such as a scanning laser ophthalmoscopy (SLO) and two-photon microscopic imaging (TPM) have enabled us to obtain high–resolution images from retinas and have been applied to a variety of experiments (81–87). Fundus autofluorescence (AF) can be monitored by SLO (typically using 488 nm excitation; emission filter, 500–700 nm) and has been utilized as one of the biomarkers for several types of retinal degenerative diseases (82,88). A2E and other bisretinoids give rise to elevated AF due to intramolecular conjugated double bonds within retinoid-derived fluorophores. Additional evidence of fundus AF and bisretinoids is extensively covered elsewhere (44).
In vivo SLO and TPM imaging ofRdh8−/−Abca4−/−mice, which display age-related A2E accumulation, showed a good correlation between intensity of fundus AF and amounts of accumulated A2E (85,87). A2E accumulation is accompanied by age-related retinal degeneration under room light condition (Figure 2B), and A2E production in Rdh8−/−Abca4−/− mice is more closely associated with age-related degeneration than light-induced acute degeneration. Although accumulation of A2E is an important hallmark for age-related retinal degeneration in Rdh8−/−Abca4−/− mice and intensity of AF is well correlated with A2E amounts in the RPE (Fig. 4), progression of age-related retinal changes in Rdh8−/−Abca4−/− mice is not directly corresponding with fundus AF intensity. The fundus AF increased uniformly across the entire retina (Fig. 4A), but degenerative retinal changes were dominantly observed in the inferior retina (Fig. 3) (8). In addition to this intriguing phenomenon, the spatial distribution of A2E and its oxides was determined by using the high molecular specificity of matrix-assisted laser desorption-ionization imaging mass spectrometry. This technique showed a broad accumulation of these retinoid byproducts distributed across the entire mouse fundus of Abca4−/−mice (66). Noteworthy are the several clinical studies having investigated the relationship between abnormal intensity of AF and the progression of retinal degeneration in AMD and Stargardt’s disease (STDG) patients, but this relationship is still controversial (61,89–91).
Figure 4. In vivo imaging of age-dependent accumulation of all-trans-RAL condensation products in mouse eye.
In vivo fundus images by scanning laser ophthalmoscopy (SLO) in autofluorescent mode (AF mode) (A) and ex vivo images of the RPE by two-photon microscopy (TPM) (B) were obtained from Rdh8−/−Abca4−/−mice. (A) Age-dependent increase of AF levels was observed across the entire fundus in Rdh8−/−Abca4−/−mouse eye but only low level AF was observed in age-matched Rdh8+/+Abca4+/+mouse. Infiltration of inflammatory cells which engulfed photoreceptor debris was observed as white dots (yellow arrows) sporadically in the fundus ofRdh8−/−Abca4−/−mice. These SLO images were obtained at AF mode with 3 second exposure to 488 nm excitation. (B) Higher intensity of autofluorescence which indicates higher level accumulation of all-trans-RAL condensation products was specifically detected by TPM using 850 nm excitation in the cytoplasmic space of the RPE in Rdh8−/−Abca4−/−mouse eye at 6 months of age (B left panel) when compared to those of age-matched Rdh8+/+Abca4+/+ mice (B right panel). Scale bar in B indicates 30 μm.
AF measurements from other retinoid derivatives, such as retinyl esters (mostly all-trans-retinyl palmitate) were tested using the autofluorescent mode in SLO, using a 488 nm excitation, in Rpe65−/−mice, which are characterized as having an over accumulation of retinyl esters in the retinosomes of the RPE (92). Theoretically, this imaging condition is not able to detect AF of retinyl esters, but AF intensity measured by this mode correlates well with A2E amounts in the RPE (87). Further clinical and animal model studies are required to draw conclusions as to whether or not AF can serve as a reliable marker for disease progression inpatients with AMD, STGD and other retinopathies with related pathologies.
Therapeutic approaches to prevent all-trans-RAL-associated retinal degeneration
Since pathogenic roles of all-trans-RAL in retinal degeneration are implicated in mice models, which recapitulate the features of human retinal diseases, all-trans-RAL can be a promising molecular target to prevent progression of several types of retinal degenerations. To date, there is no efficacious treatment for patients with dry-type AMD, STGD and other degenerative retinal diseases, to prevent, halt, or slow the disease process; unlike in the wet type of AMD where recent breakthrough using anti-vascular endothelial growth factor therapy have yielded positive results (93,94). Two different therapeutic interventions have been proposed to reduce the toxicity of all-trans-RAL: 1) visual cycle inhibitors to produce less all-trans-RAL after light exposure and 2) scavengers of all-trans-RAL to trap toxic free all-trans-RAL by forming Schiff base interactions (95). Sieving, et al. showed protective effects of 13-cis-retinoic acid, which has RDH5 inhibitory effects in a mouse model of light-induced retinal degeneration (42). Additionally Radu, et al. found reduced amounts of accumulated A2E in Abca4−/− mice in the presence of the visual cycle inhibitor, 13-cis-retinoic acid (96) and fenretinide (4-HPR) with abilities to reduce in serum retinoid binding proteins thus resulting in lower concentration of ocular retinoids (97). It is also known that retinoid isomerase (RPE65) activity is inhibited by 13-cis-retinoic acid and 4-HPR as well as all-trans retinoic acid (98). These studies provide the evidence that inhibition of the visual cycle is beneficial in preventing light-induced retinal degeneration and the accumulation of toxic all-trans-RAL condensation products. In 2005, retinylamine (Ret-NH2) was found to inhibit RPE65 activity, and thus can function as a visual cycle inhibitor (99). Administration of Ret-NH2 not only prevented light-induced retinal degeneration in BALB/c mice (41), but also ameliorated age-related retinal degeneration with less accumulation of A2E in Rdh8−/−Abca4−/− mice (8). Visual cycle inhibitors are effective in preventing all-trans-RAL-associated retinal degeneration; however, these drugs may induce retinal degeneration by depleting the supply of the visual chromophore. To overcome this problem, we tested the idea that direct trapping of all-trans-RAL by amine drugs in form of Schiff base can lower intraocular the all-trans-RAL concentration and ameliorate progression of retinal degeneration. Recently multiple FDA-approval drugs with primary amino group were administrated to Rdh8−/−Abca4−/− mice. Some of these drugs did not inhibit chromophore regeneration and formed Schiff base adducts with all-trans-RAL, thereby lowering the peak concentration of free all-trans-RAL. Importantly, these drugs protected the retina from light-induced and age-related retinal degeneration in Rdh8−/−Abca4−/− mice (95). Of note, Ret-NH2 exhibits dual properties working as both a visual cycle inhibitor and all-trans-RAL scavenger (Fig. 5). Alternatively, overexpression of RDH8 or ABCA4 using established gene delivery methods like adeno-associated virus can be another approach to prevent accumulation of all-trans-RAL after light exposure. In fact, clinical studies for ocular gene therapy have been conducted in patients with RPE65 mutations, and encouraging results have been reported (100,101). Further elucidation of the mechanisms of all-trans-RAL toxicity can improve on these pharmacological treatments, and the outcomes generated in these studies can be applied to the clinical setting where the detection of early pathological changes associated with all-trans-RAL is able to be monitored. This connection from bench to bedside may promote the development of prophylactic treatments and aid in preventing the progression of retinal dysfunction before visual acuity is adversely affected.
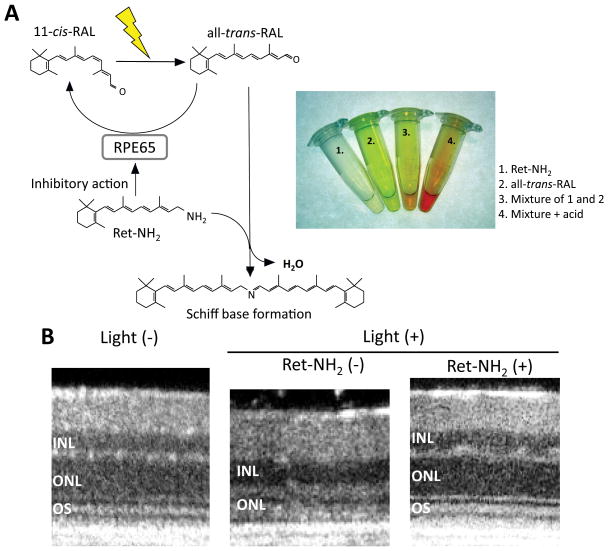
Figure 5. Pharmacological innovation to protect photodamage mediated by all-trans-RAL.
Accumulation of all-trans-RAL is prevented by two pharmacological actions of retinylamine (Ret-NH2). First, free all-trans-RAL is neutralized by Schiff base formation between all-trans-RAL and Ret-NH2 (A). This chemical reaction can be monitored in a color change in the reaction-mixture. Second, free all-trans-RAL generation can be decreased by the inhibitory action of Ret-NH2 in the visual cycle. Specific-binding of Ret-NH2 to RPE65 can prevent an isomerization reaction and slow down regeneration of 11-cis-RAL, which can consequently decrease free all-trans-RAL during light exposure. These two pharmacological actions can protect Rdh8−/−Abca4−/−retina from photodamage (B). Representative in vivo retinal images by high-definition SD-OCT clearly reveal that Rdh8−/−Abca4−/− mice treated with Ret-NH2 can maintain normal morphology of the retina whereas outer nuclear layer (ONL) are severely degenerated in vehicle treated mice. INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segment.
Conclusion
Photodamage can be mediated by all-trans-RAL and its condensation products, therefore, efficient transport and reduction of all-trans-RAL by ABCA4 and all-tans-RDHs in the photoreceptor is important for maintaining the health of the retina. Recent understanding the role all-trans-RAL plays in phototoxicity in addition to the advancement of in vivo imaging may contribute to the future development of new methods to fight retinal degenerative diseases.
Acknowledgments
We specially thank Dr. Krzysztof Palczewski (Case Western Reserve Univ.) for his generous support. We also thank Drs. S. Howell, H. Fujioka. M. Hitomi, S. Roos and L. Perusek (Case Western Reserve Univ.), P. Palczewska and Z. Dong (Polgenix, Inc.) for technical support. This work was supported in part by funding from the National Institutes of Health (EY019031, EY019880, EY009339, EY 021126, P30 EY11373); the Research to Prevent Blindness Foundation; Foundation Fighting Blindness; Fight for Sight and the Ohio Lions Eye Research Foundation.
Abbreviations used
- ABCA4
ATP–binding cassette transporter 4
- ABCR
ATP- Binding Cassette Transporter, Retina-Specific
- all-trans-RAL
all-trans-retinal
- AMD
age–related macular degeneration
- A2E
di–retinoid–pyridinium–ethanolamine
- A2PE
phosphatidylpyridinium-bisretinoid
- 11-cis-RAL
11-cis-retinal
- LRAT
lecithin: retinol acyltransferase
- PE
phosphatidylethanolamine
- POS
photoreceptor outer segment
- Ret-NH2
retinylamine
- RDH
retinol dehydrogenase
- ROS
reactive oxygen species
- RP
retinitis pigmentosa
- RPE
retinal pigmented epithelium
- RPE65
retinal pigment epithelium–specific protein 65 kDa or retinoid isomerase
- SDR
short-chain dehydrogenase/reductase
- SLO
scanning laser ophthalmoscopy
- SD–OCT
spectral domain–optical coherent tomography
- STGD
Stargardt’s disease
Biographies
Tadao Maeda obtained his MD, PhD degree in biochemistry/ophthalmology from the Sapporo Medical University in 2001. At the University of Washington, Tadao finished his postdoctoral work which focused on retinal degeneration and its relationship to the visual cycle. Following postdoctoral work he joined the Department of Pharmacology/Vision Science Research Center at Case Western Reserve University, where he currently works as a Senior Instructor and co-director of animal imaging core. His main interest is the characterization of various retinal dystrophy mouse models, which may contribute to the development of pharmacological and gene therapies in the future.
Marcin Golczak obtained his Master’s degree in Biotechnology from the Wroclaw University of Technology, Poland in 1999. He continued research working on calcium binding proteins at Nencki Institute of Experimental Biology, Polish Academy of Science in Warsaw, Poland, where he received his PhD in 2003. During his subsequent postdoctoral research in Krzysztof Palczewski’s laboratory at the University of Washington and Case Western Reserve University he focused on vitamin A metabolism in particular, the enzymatic pathway called retinoid cycle that leads to regeneration of the visual chromophore. He is currently an Instructor in the Department of Pharmacology at Case Western Reserve University. His main interest is the development of small molecule-based therapeutic strategies against light-induced retinal degeneration.
Akiko Maeda received her MD, PhD from the Sapporo Medical University in Japan. Following her postdoctoral work in retinal biochemistry and pharmacology both at the University of Washington and Case Western Reserve University, she joined Department of Ophthalmology & Visual Sciences at Case Western Reserve University in 2009. Her main interest involves the characterization of retinal degeneration and inflammation in retinal diseases, which can be applied to future therapies aimed at alleviating disease progression
Footnotes
This invited paper is part of the Symposium in Print “Retinal Photodamage”
References
- 1.Baylor D. How photons start vision. Proc Natl Acad Sci USA. 1996;93:560–565. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palczewski K. Chemistry and Biology of Vision. J Biol Chem. 2011;287:1612–1619. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 5.Kiser PD, Golczak M, Maeda A, Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 2012;1821:137–151. doi: 10.1016/j.bbalip.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010;31:284–295. doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Okano K, Maeda T, Chauhan V, Golczak M, Maeda A, Palczewski K. Mechanism of All-trans-retinal Toxicity with Implications for Stargardt Disease and Age-related Macular Degeneration. J Biol Chem. 2012;287:5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91:788–792. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 14.Kos V, Ford RC. The ATP-binding cassette family: a structural perspective. Cell Mol Life Sci. 2009;66:3111–3126. doi: 10.1007/s00018-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 16.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papermaster DS, Schneider BG, Zorn MA, Kraehenbuhl JP. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Biol Chem. 1978;78:415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 19.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 20.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 21.Rivera A, White K, Stohr H, Steiner K, Hemmrich N, Grimm T, Jurklies B, Lorenz B, Scholl HP, Apfelstedt-Sylla E, Weber BH. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26:242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- 23.Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 25.Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 26.Paskowitz DM, LaVail MM, Duncan JL. Light and inherited retinal degeneration. Br J Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30:63–68. doi: 10.1080/13816810802695550. [DOI] [PubMed] [Google Scholar]
- 28.Weleber RG. Stargardt’s macular dystrophy. Arch Ophthalmol. 1994;112:752–754. doi: 10.1001/archopht.1994.01090180050033. [DOI] [PubMed] [Google Scholar]
- 29.Allikmets R. Stargardt disease: from gene discovery to therapy. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics and Therapeutics. Humana Press; Totowa: 2007. pp. 105–118. [Google Scholar]
- 30.Tsybovsky Y, Wang B, Quazi F, Molday RS, Palczewski K. Posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4. Biochemistry. 2011;50:6855–6866. doi: 10.1021/bi200774w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jornvall H. Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact. 2003;143–144:247–253. doi: 10.1016/s0009-2797(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 32.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 33.Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 34.Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrispell JD, Feathers KL, Kane MA, Kim CY, Brooks M, Khanna R, Kurth I, Hubner CA, Gal A, Mears AJ, Swaroop A, Napoli JL, Sparrow JR, Thompson DA. Rdh12 activity and effects on retinoid processing in the murine retina. J Biol Chem. 2009;284:21468–21477. doi: 10.1074/jbc.M109.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martras S, Alvarez R, Martinez SE, Torres D, Gallego O, Duester G, Farres J, de Lera AR, Pares X. The specificity of alcohol dehydrogenase with cis-retinoids. Activity with 11-cis-retinol and localization in retina. Eur J Biochem. 2004;271:1660–1670. doi: 10.1111/j.1432-1033.2004.04058.x. [DOI] [PubMed] [Google Scholar]
- 38.Pares X, Julia P, Farres J. Properties of rat retina alcohol dehydrogenase. Alcohol. 1985;2:43–46. doi: 10.1016/0741-8329(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 39.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 40.Redmond TM, Weber CH, Poliakov E, Yu S, Gentleman S. Effect of Leu/Met variation at residue 450 on isomerase activity and protein expression of RPE65 and its modulation by variation at other residues. Mol Vis. 2007;13:1813–1821. [PubMed] [Google Scholar]
- 41.Maeda A, Maeda T, Golczak M, Imanishi Y, Leahy P, Kubota R, Palczewski K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol. 2006;70:1220–1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieving PA, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson TJ, Bush RA, Thompson DA. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc Natl Acad Sci USA. 2001;98:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozanowska M, Sarna T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- 44.Sparrow JR, Wu Y, Nagasaki T, Yoon KD, Yamamoto K, Zhou J. Fundus autofluorescence and the bisretinoids of retina. Photochem Photobiol Sci. 2010;9:1480–1489. doi: 10.1039/c0pp00207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin–retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tawa P, Tam J, Cassady R, Nicholson DW, Xanthoudakis S. Quantitative analysis of fluorescent caspase substrate cleavage in intact cells and identification of novel inhibitors of apoptosis. Cell Death Differ. 2001;8:30–37. doi: 10.1038/sj.cdd.4400769. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Wang T, Jiao Y, von Lintig J, Montell C. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol. 2010;20:93–102. doi: 10.1016/j.cub.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–607. [PubMed] [Google Scholar]
- 49.Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27:145–152. [PubMed] [Google Scholar]
- 50.Feeney–Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200. [PubMed] [Google Scholar]
- 51.Davies S, Elliott MH, Floor E, Truscott TG, Zareba M, Sarna T, Shamsi FA, Boulton ME. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med. 2001;31:256–265. doi: 10.1016/s0891-5849(01)00582-2. [DOI] [PubMed] [Google Scholar]
- 52.Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30:1691–1699. [PubMed] [Google Scholar]
- 53.Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt’s disease–Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36:2327–2331. [PubMed] [Google Scholar]
- 54.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 55.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 56.Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61:448–453. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 57.Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 58.Lopez PF, Maumenee IH, de la Cruz Z, Green WR. Autosomal-dominant fundus flavimaculatus. Clinicopathologic correlation. Ophthalmology. 1990;97:798–809. doi: 10.1016/s0161-6420(90)32508-3. [DOI] [PubMed] [Google Scholar]
- 59.Rabb MF, Tso MO, Fishman GA. Cone-rod dystrophy. A clinical and histopathologic report. Ophthalmology. 1986;93:1443–1451. doi: 10.1016/s0161-6420(86)33547-4. [DOI] [PubMed] [Google Scholar]
- 60.Weingeist TA, Kobrin JL, Watzke RC. Histopathology of Best’s macular dystrophy. Arch Ophthalmol. 1982;100:1108–1114. doi: 10.1001/archopht.1982.01030040086016. [DOI] [PubMed] [Google Scholar]
- 61.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 62.Smith RT, Gomes NL, Barile G, Busuioc M, Lee N, Laine A. Lipofuscin and autofluorescence metrics in progressive STGD. Invest Ophthalmol Vis Sci. 2009;50:3907–3914. doi: 10.1167/iovs.08-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murdaugh LS, Mandal S, Dill AE, Dillon J, Simon JD, Gaillard ER. Compositional studies of human RPE lipofuscin: mechanisms of molecular modifications. J Mass Spectrom. 2011;46:90–95. doi: 10.1002/jms.1865. [DOI] [PubMed] [Google Scholar]
- 64.Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci. 1989;30:82–89. [PubMed] [Google Scholar]
- 65.Feeney-Burns L, Eldred GE. The fate of the phagosome: conversion to ‘age pigment’ and impact in human retinal pigment epithelium. Trans Ophthalmol Soc UK. 1983;103:416–421. [PubMed] [Google Scholar]
- 66.Grey AC, Crouch RK, Koutalos Y, Schey KL, Ablonczy Z. Spatial localization of A2E in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:3926–3933. doi: 10.1167/iovs.10-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sparrow JR, Kim SR, Cuervo AM, Bandhyopadhyayand U. A2E, a pigment of RPE lipofuscin, is generated from the precursor, A2PE by a lysosomal enzyme activity. Adv Exp Med Biol. 2008;613:393–398. doi: 10.1007/978-0-387-74904-4_46. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275:29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 69.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 70.Eldred GE. Age pigment structure. Nature. 1993;364:396. doi: 10.1038/364396a0. [DOI] [PubMed] [Google Scholar]
- 71.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 72.Vives-Bauza C, Anand M, Shirazi AK, Magrane J, Gao J, Vollmer-Snarr HR, Manfredi G, Finnemann SC. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–24780. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yasukawa T, Wiedemann P, Hoffmann S, Kacza J, Eichler W, Wang YS, Nishiwaki A, Seeger J, Ogura Y. Glycoxidized particles mimic lipofuscin accumulation in aging eyes: a new age-related macular degeneration model in rabbits. Graefes Arch Clin Exp Ophthalmol. 2007;245:1475–1485. doi: 10.1007/s00417-007-0571-z. [DOI] [PubMed] [Google Scholar]
- 74.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 75.Radu RA, Hu J, Yuan Q, Welch DL, Makshanoff J, Lloyd M, McMullen S, Travis GH, Bok D. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem. 2011;286:18593–18601. doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 77.Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Biol Chem. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lochner JE, Badwey JA, Horn W, Karnovsky ML. all-trans-Retinal stimulates superoxide release and phospholipase C activity in neutrophils without significantly blocking protein kinase C. Proc Natl Acad Sci USA. 1986;83:7673–7677. doi: 10.1073/pnas.83.20.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Rozanowska MB, Bonilha VL, Rayborn ME, Salomon RG, Sparrow JR, Boulton ME, Hollyfield JG, Crabb JW. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7:1397–1405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44:1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 81.Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, Hohman TC, Holz FG. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1–6. doi: 10.1167/iovs.10-5619. [DOI] [PubMed] [Google Scholar]
- 82.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 83.Fleckenstein M, Charbel Issa P, Helb HM, Schmitz-Valckenberg S, Finger RP, Scholl HP, Loeffler KU, Holz FG. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:4137–4144. doi: 10.1167/iovs.08-1967. [DOI] [PubMed] [Google Scholar]
- 84.Rossi EA, Chung M, Dubra A, Hunter JJ, Merigan WH, Williams DR. Imaging retinal mosaics in the living eye. Eye (London, England) 2011;25:301–308. doi: 10.1038/eye.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han M, Bindewald-Wittich A, Holz FG, Giese G, Niemz MH, Snyder S, Sun H, Yu J, Agopov M, La Schiazza O, Bille JF. Two–photon excited autofluorescence imaging of human retinal pigment epithelial cells. J Biomed Opt. 2006;11:010501. doi: 10.1117/1.2171649. [DOI] [PubMed] [Google Scholar]
- 86.Palczewska G, Maeda T, Imanishi Y, Sun W, Chen Y, Williams DR, Piston DW, Maeda A, Palczewski K. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med. 2010;16:1444–1449. doi: 10.1038/nm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiose S, Chen Y, Okano K, Roy S, Kohno H, Tang J, Pearlman E, Maeda T, Palczewski K, Maeda A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011;286:15543–15555. doi: 10.1074/jbc.M111.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmitz-Valckenberg S, Fleckenstein M, Scholl HP, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009;54:96–117. doi: 10.1016/j.survophthal.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, Sehmi K, Fitzke FW, Holz FG, Tufail A. Evaluation of autofluorescence imaging with the scanning laser ophthalmoscope and the fundus camera in age-related geographic atrophy. Am J Ophthalmol. 2008;146:183–192. doi: 10.1016/j.ajo.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Gomes NL, Greenstein VC, Carlson JN, Tsang SH, Smith RT, Carr RE, Hood DC, Chang S. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50:3953–3959. doi: 10.1167/iovs.08-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burke TR, Rhee DW, Smith RT, Tsang SH, Allikmets R, Chang S, Lazow MA, Hood DC, Greenstein VC. Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1) Invest Ophthalmol Vis Sci. 2011;52:8006–8015. doi: 10.1167/iovs.11-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 93.Davis J, Olsen TW, Stewart M, Sternberg P., Jr How the comparison of age–related macular degeneration treatments trial results will impact clinical care. Am J Opthalmol. 2011;152:509–514. doi: 10.1016/j.ajo.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 95.Maeda A, Golczak M, Chen Y, Okano K, Kohno H, Shiose S, Ishikawa K, Harte W, Palczewska G, Maeda T, Palczewski K. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2011;8:170–178. doi: 10.1038/nchembio.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc Natl Acad Sci USA. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 98.Gollapalli DR, Rando RR. The specific binding of retinoic acid to RPE65 and approaches to the treatment of macular degeneration. Proc Natl Acad Sci USA. 2004;101:10030–10035. doi: 10.1073/pnas.0401936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci USA. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao H, Molday RS, Hu J. Gene therapy: light is finally in the tunnel. Protein Cell. 2011;2:973–989. doi: 10.1007/s13238-011-1126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 Gene Therapy Readministration in Three Adults with Congenital Blindness. Sci Transl Med. 2012;4:120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]