Abstract
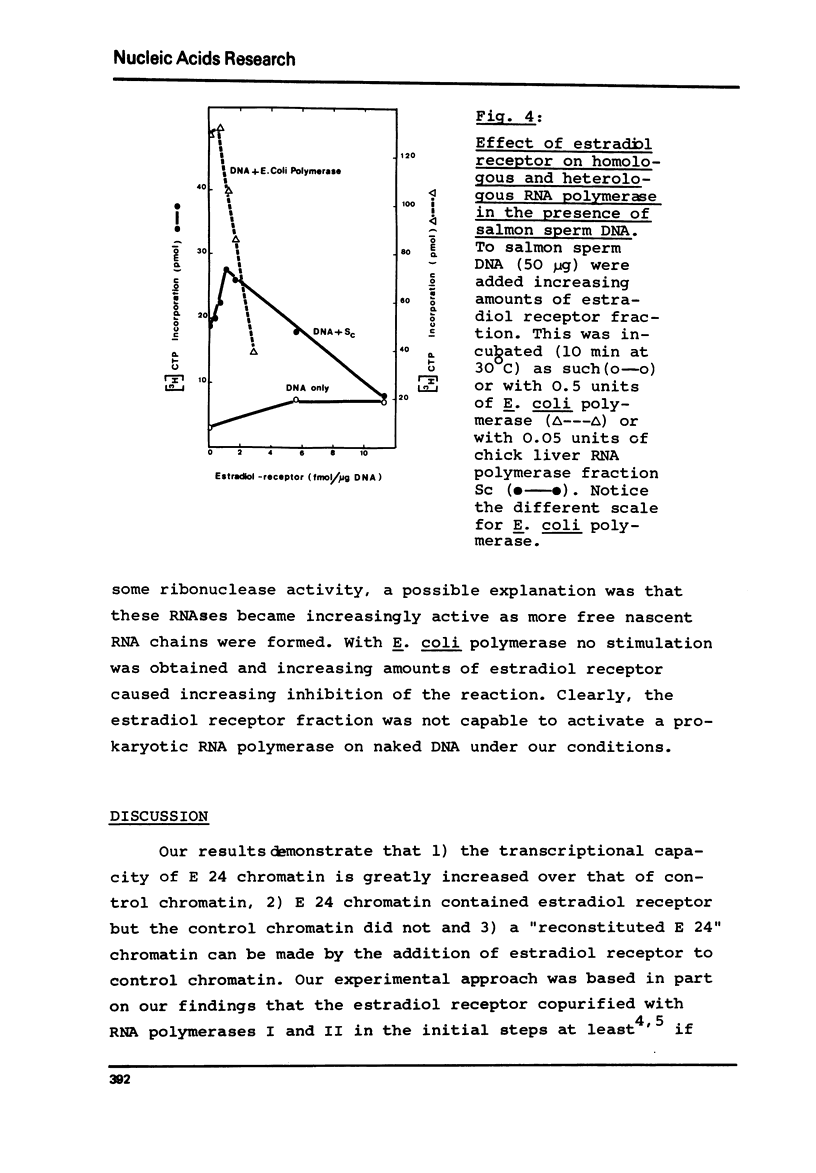
The endogenous transcriptional capacity (RNA polymerase I and II activity) of liver chromatin from chicks treated with 17 beta-estradiol for 24 h (E 24) was double that of the controls. E 24 chromatin contained estradiol receptor activity while control chromatin did not. Its presence suggested an implication in the enhanced activities of RNA polymerases of E 24 chromatin. When semi-purified estradiol receptor was added to control chromatin, the endogenous transcriptional capacity of this chromatin was greatly increased. Studies with alpha-amanitin showed that both RNA polymerase I and II were stimulated by the estradiol receptor. This stimulation was observed as long as homology of the system was maintained. Solubilized homologous RNA polymerases were stimulated much less by the hormone complex in the presence of heterologous DNA than with homologous chromatin. Prokaryotic RNA polymerase could not be stimulated by chick liver estradiol receptor in the presence of heterologous DNA.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud M., Beziat Y., Borgna J. L., Guilleux J. C., Mousseron-Canet M. Le récepteur de l'oestradiol, l'AMP cyclique et la RNA polymérase nucléolaire dans l'uterus de génisse. Stimulation de la biosynthèse de RNA in vitro. Biochim Biophys Acta. 1971 Dec 16;254(2):241–254. [PubMed] [Google Scholar]
- Arnaud M., Beziat Y., Guilleux J. C., Hough A., Hough D., Mousseron-Canet M. Les récepteurs de l'oestradiol dans l'utérus de génisse. Stimulation de la biosynthèse de RNA in vitro. Biochim Biophys Acta. 1971 Feb 25;232(1):117–131. [PubMed] [Google Scholar]
- Buller R. E., Schwartz R. J., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. In vitro effect of receptor subunits on gene transcription. J Biol Chem. 1976 Sep 10;251(17):5178–5186. [PubMed] [Google Scholar]
- Dierks-Ventling C., Jost J. P. Effect of 17 beta-estradiol on the synthesis of non-histone nuclear proteins in chick liver. Eur J Biochem. 1974 Dec 16;50(1):33–40. doi: 10.1111/j.1432-1033.1974.tb03870.x. [DOI] [PubMed] [Google Scholar]
- Ehresmann B., Imbault P., Weil J. H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal Biochem. 1973 Aug;54(2):454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Joss U., Bassand C., Dierks-Ventling C. Rapid appearance of estrogen receptor in chick liver nuclei: partial inhibition by cycloheximide. FEBS Lett. 1976 Jul 15;66(2):293–298. doi: 10.1016/0014-5793(76)80525-x. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Keller R., Dierks-Ventling C. Deoxyribonucleic acid and ribonucleic acid synthesis during phosvitin induction by 17beta-estradiol in immature chicks. J Biol Chem. 1973 Aug 10;248(15):5262–5266. [PubMed] [Google Scholar]
- Kalimi M., Tsai S. Y., Tsai M. J., Clark J. H., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Correlation between nuclear-bound estrogen receptor and chromatin initiation site for transcription. J Biol Chem. 1976 Jan 25;251(2):516–523. [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Gniazdowski M., Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. 1. Large-scale solubilization and separation of A and B calf-thymus RNA-polymerase activities. Eur J Biochem. 1972 Jul 13;28(2):269–276. doi: 10.1111/j.1432-1033.1972.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Huang R. C. Chromatin directed transcription of 5S and tRNA genes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1082–1086. doi: 10.1073/pnas.72.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Nuclear estrogen receptor of chick liver. Biochim Biophys Acta. 1972 Jan 28;261(1):236–244. doi: 10.1016/0304-4165(72)90334-0. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Totsuka A., Zahn R. Association of an estradiol receptor with the DNA-dependent RNA polymerase I from immature quail oviduct. Biochim Biophys Acta. 1974 Oct 11;366(2):224–233. doi: 10.1016/0005-2787(74)90336-0. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Isolation and partial characterization. J Biol Chem. 1974 Jan 10;249(1):241–248. [PubMed] [Google Scholar]
- Schwartz R. J., Kuhn R. W., Buller R. E., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. In vitro effects of purified hormone-receptor complexes on the initiation of RNA synthesis in chromatin. J Biol Chem. 1976 Sep 10;251(17):5166–5177. [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., Chytil F., O'Malley B. W. Progesterone-binding components of chick oviduct. V. Exchange of progesterone-binding capacity from target to nontarget tissue chromatins. J Biol Chem. 1972 Mar 10;247(5):1368–1374. [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., O'Malley B. W. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971 Jul 10;246(13):4188–4197. [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., Schwartz R., Kalimi M., Clark J. H., O'Malley B. W. Effects of estrogen on gene expression in chick oviduct: nuclear receptor levels and initiation of transcription. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4228–4232. doi: 10.1073/pnas.72.11.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]


