Abstract
GB virus type C (GBV-C) viremia is associated with reduced CD4+ T cell expansion following Interleukin 2 (IL-2) therapy and with a reduction in T cell activation in HIV-infected individuals. Mechanism(s) by which GBV-C might alter T-cell activation or IL-2 signaling have not been studied. Here, we assess IL-2 release, IL-2 receptor (IL-2R) expression, IL-2 signaling, and cell proliferation in Tet-off Jurkat cells expressing the GBV-C envelope glycoprotein (E2) following activation through the T cell receptor (TCR). TCR activation was induced by incubation in anti-CD3/CD28 antibodies. IL-2 release was measured by ELISA, STAT5 phosphorylation was assessed by immunoblot, and IL-2Rα (CD25) expression and cell proliferation were determined by flow cytometry. IL-2 and IL-2Rα steady-state mRNA levels were measured by real-time PCR. GBV-C E2 expression significantly inhibited IL-2 release, CD25 expression, STAT5 phosphorylation and cellular proliferation in Jurkat cells following activation through the TCR compared to control cell lines. Reducing E2 expression by doxycycline reversed the inhibitory effects observed in the E2-expressing cells. The N-terminal 219 a.a of E2 was sufficient to inhibit IL-2 signaling. Addition of purified recombinant GBV-C E2 protein to primary human CD4+ and CD8+ T cells inhibited TCR activation-induced IL-2 release and upregulation of IL-2Rα expression. These data provide evidence that the GBV-C E2 protein may contribute to the block in CD4+ T cell expansion following IL-2 therapy in HIV-infected individuals. Furthermore, the effects of GBV-C on IL-2 and IL-2 signaling pathways may contribute to the reduction in chronic immune activation observed in GBV-C/HIV co-infected individuals.
Keywords: HIV, GBV-C, T cell activation, IL-2, CD25, Proliferation
INTRODUCTION
GB virus C (GBV-C) is a human virus classified within the Flaviviridae which is not clearly associated with any disease [reviewed in (1–3)]. GBV-C infection frequently leads to persistent viremia and is highly prevalent, with approximately 1% to 4% of U.S. blood donors infected at the time of donation (2, 4). Due to shared routes of transmission, the virus is highly prevalent among HIV-infected individuals (up to 42%) (1, 2, 5). Several studies, though not all, observe an association between persistent GBV-C infection and prolonged survival in HIV-infected individuals (6–15). GBV-C is a lymphotropic virus, and infection modulates several host factors involved in HIV infection including expression of cytokines, chemokines and cellular receptors [reviewed in (16)]. These alterations in host lymphocyte factors may limit HIV infection and contribute to a protective effect of GBV-C coinfection observed in HIV-positive individuals.
Chronic HIV infection is characterized by persistent immune activation which contributes to T cell depletion, altered cytokine expression and loss of T cell function [reviewed in (17–19)]. Interleukin 2 (IL-2) is a critical cytokine required for T cell activation, proliferation, and function [reviewed in (20, 21)]. However, IL-2 also induces secretion of proinflammatory cytokines like IL-6, IL-1β and tumor necrosis factor alpha (TNF-α) (22–24), and is associated with increased levels of inflammatory markers like C-reactive protein (CRP) and D-dimer in the plasma of HIV-infected subjects, independent of HIV viral load (25). In addition, in vitro activation of peripheral blood mononuclear cells (PBMCs) with IL-2 increases HIV production (26, 27). Thus, IL-2 promotes HIV replication and contributes to HIV associated immune activation. Immune activation appears to be a better predictor of HIV disease progression than plasma HIV viral load (VL) (28, 29).
In studies of HIV-infected people, GBV-C viremia is associated with lower cell surface expression of T cell activation markers as compared to GBV-C non-viremic controls, independent of HIV VL (30–32). GBV-C viremia is also associated with a significant reduced CD4+ T cell expansion in HIV-infected subjects receiving intravenous IL-2 therapy compared to GBV-C non-viremic controls (33). Together, these findings suggest that GBV-C infection may alter T cell activation and IL-2 signaling pathways. In addition, GBV-C replication in peripheral blood mononuclear cells (PBMCs) is significantly reduced following in vitro activation with IL-2 and phytohemagglutinin (PHA) (34, 35), suggesting a potential bidirectional interaction between GBV-C and IL-2. Since IL-2 plays an important role in HIV infection and disease progression, the effects of GBV-C on IL-2 signaling pathways may contribute to the protective effect of GBV-C coinfection in HIV infected individuals. Previous studies demonstrated that GBV-C envelope glycoprotein (E2) inhibits HIV replication when added to cells (2, 36, 37), or when expressed in a CD4+ Jurkat T cell line (38). In this study we examined the role of the GBV-C E2 protein in the modulation of IL-2 production and IL-2 signaling pathways.
MATERIALS AND METHODS
Expression of GBV-C E2 proteins
The GBV-C E2 protein coding sequence without the C terminal transmembrane region (nt 1167–2161 based on GenBank AF 121950), E2 deletion mutants (N terminal 219 aa [nt 1167–1824], and C terminal 112 aa [nt 1824–2161]), and control sequences were ligated into a modified pTRE2-Hyg plasmid (Clontech Laboratories, Mountain View, CA) as previously described (38). This plasmid generates a bicistronic message encoding the GBV-C E2 sequence followed by the encephalomyocarditis virus (EMC) internal ribosomal entry site (IRES) that directs translation of GFP (39). Transcription of the bicistronic message is regulated by doxycycline as described (38).
Jurkat (tet-off) cell lines (Clontech, Inc) were transfected (Nucleofector II, Lonza Inc.) with plasmids encoding GBV-C E2 proteins. Control cell lines were generated by transfecting Jurkat (tet-off) cells with an E2 plasmid containing a frameshift mutation to abrogate protein expression (FS) or the empty vector expressing green fluorescent protein (GFP) (vector control; VC). Stable cell lines were generated after selection in hygromycin and neomycin (200 µg/ml) and GFP positive cells were bulk sorted using a BD FACSDiva (University of Iowa Flow Cytometry Facility). Expression of GBV-C E2 protein or E2 mutants were analyzed by immunoblot and GFP expression by flow cytometry (BD FACScan). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum (heat-inactivated), 2mM L-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin with hygromycin and neomycin (200 µg/ml). Insert and control sequences were confirmed by sequencing plasmid DNA (University of Iowa DNA Core Facility).
Recombinant GBV-C E2 protein fused to Fc at the C terminus was constructed by inserting the human IgG Fc coding sequence at nt 2161 of GBV-C E2 in the pSec vector (Invitrogen). CHO cells were transfected and selected on zeocin (400 µg/ml) and GFP expression. Following stable transfection, cells were adapted to serum free media as described (40). Fusion protein expressed in CHO cells was purified by protein G affinity chromatography and analyzed by SDS-PAGE and immunoblot analysis as described (40, 41). Purified human IgG protein was used as a negative control for the E2 – human Fc fusion protein.
Cell Stimulation
Jurkat cells (1×106 cells/ml) were stimulated with plate-bound anti-CD3 (5 µg/ml, OKT3 clone, eBioscience) and soluble CD28 (5µg/ml, clone CD28.2, BD Biosciences). Following obtaining written informed consent, peripheral blood mononuclear cells (PBMCs) were isolated from blood obtained from four healthy subjects using Ficoll-Hypaque density gradient centrifugation. This protocol was approved by the University of Iowa Institutional Review Board. PBMCs were washed with PBS and incubated with purified GBV-C E2 protein (20µg/ml) or purified human IgG (20µg/ml, Sigma) for 48 hours and stimulated with anti-CD3 (500ng/ml) and soluble CD28 (500ng/ml). Following 24 hours of stimulation, cells were analyzed for measurement of cytokine and cellular receptor expression. To measure STAT5 phosphorylation Jurkat cells were prepared as described (42). Briefly, cells were stimulated with 1µg/ml of anti-CD3 and soluble CD28 for 48 hours followed by 24 hours of serum starvation with or without doxycycline (1µg/ml). Cells were washed and incubated with IL-2 (250U/ml; Zeptometrix) for 15 minutes. Cell lysates were separated by polyacrylamide gel electrophoresis using 10% gels and membrane was incubated with anti-phospho STAT5 (pY694; BD Biosciences) or anti-STAT5 (BD Biosciences). Phosphorylation of STAT5 and total STAT5 expression was detected with Amersham ECL (GE Healthcare) using a Kodak Imager.
Cytokine quantitation
IL-2 cytokine released into cell culture supernatant was quantified using human IL-2 quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions.
mRNA expression
Total cellular RNA was extracted using RNeasy Mini Kit (Qiagen) following DNase treatment (RNase-Free DNase Set, Qiagen). Complementary DNA (cDNA) was generated using RT2 First Strand cDNA Kit (SABiosciences) and relative expression of IL-2 and CD25 mRNA was determined using RT2 qPCR primer assay for human IL2 and human CD25 (SABiosciences) and normalized to 18SrRNA using ABI 7500 Real Time PCR system.
Flow cytometry
Jurkat cells or PBMCs were incubated with the following antibodies from Becton Dickinson (BD) per manufacturer’s recommendation; CD3 (V450), CD4 (PE), CD8 (Alexa700) and CD25 (APC). Incubation was performed on ice for 1 hour and cells were subsequently washed 3 times with PBS. Data was acquired on BD LSR II flow cytometer using single stained CompBeads (BD Biosciences) for compensation. At least 10,000 total events were collected and FlowJo program (Tree Star Inc.) was used for data analysis. Jurkat cells expressing GBV-C E2 protein or vector control (FS) were stained with cell proliferation dye (eFlour450, eBioscience) and stimulated with plate-bound anti-CD3 (1µg/ml) and soluble CD28 (1µg/ml) and cell proliferation was measured by gating eFlour450 positive cells in flow cytometry.
Statistics
Statistics were performed using GraphPad software V4.0 (GraphPad Software Inc.). Comparisons between two groups were carried out using two-sided Student’s t tests. P values less than 0.05 were considered statistically significant.
RESULTS
GBV-C E2 protein expression inhibits IL-2 production
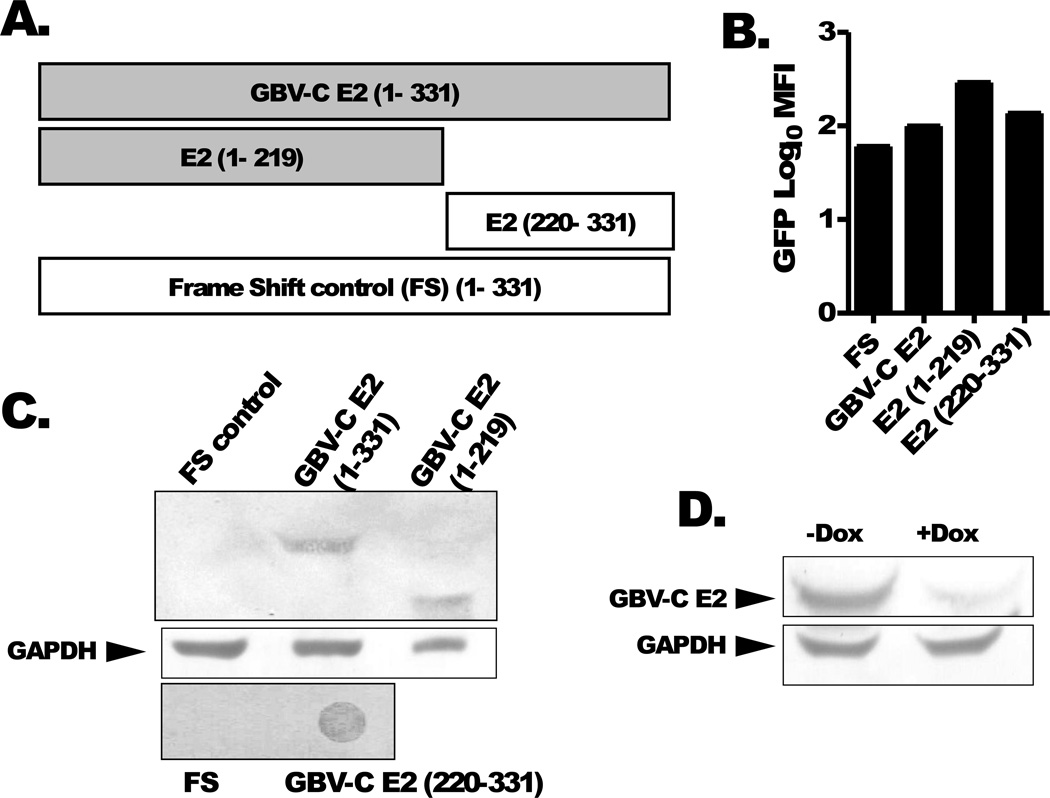
Three previously described CD4+ Jurkat (tet-off) T cell lines expressing GBV-C E2 protein (331 aa), the N terminal 219 aa, and the coding region for the E2 protein with a frame-shift to abolish protein translation (FS) were studied (38). In addition, a stable cell line expressing the E2 protein region from aa 220 to 331 was generated (Fig. 1A). All cell lines expressed GFP as determined by flow cytometry (Fig. 1B) and cellular lysates reacted with anti-his Ab (Qiagen) directed against C-terminal histidine (6xHis) tag in immunoblot analysis (Fig. 1C). Expression of GBV-C E2 RNA in Jurkat cells expressing the E2 frameshift construct was confirmed by RT-PCR and DNA sequencing as described (39). E2 expressing Jurkat cells maintained in doxycycline (dox, 1µg/ml) had reduced expression of E2 protein (Fig 1D).
Figure 1. GBV-C E2 proteins and controls.
Schematic of GBV-C envelope protein E2 (nt 1167–2161; 331aa), N-terminal deletion mutant (aa 1–219; nt 1167–1824) and C terminal deletion mutant (aa 220–331; nt 1824–2161) and the 1–331 sequence with a frame shift inserted to abolish E2 expression (FS control). All of the recombinant proteins contained a C-terminal poly-histidine tag (A). Cell lines expressing the shaded constructs inhibited IL-2 release following T cell receptor activation, while non-shaded constructs did not. GFP expression was measured in the various cell lines (B). Immunoblot analysis using anti-His antibodies demonstrated recombinant GBV-C E2 protein expression in cell lines and deletion mutants (E2 1–219; E2 220–331) (panel C). The frameshift (FS) negative control is shown. GBV-C E2 protein expression was reduced more than 80% by maintaining Jurkat cells in doxycycline for 5 days (dox, 1 µg/mL) (D).
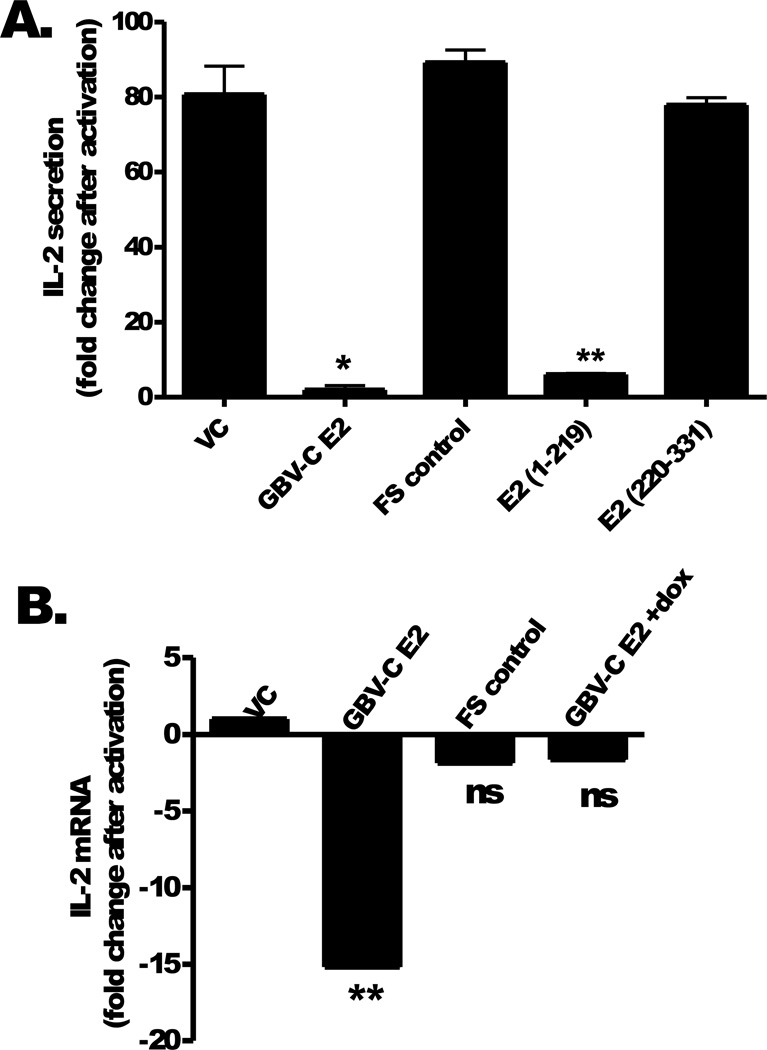
Following T cell receptor (TCR) activation with anti-CD3/CD28, IL-2 release into culture supernatants was significantly lower in Jurkat cells expressing GBV-C E2 protein compared to either the vector control (VC) or the frame-shift (FS) control (Fig 2A). The FS control confirms that IL-2 inhibition is due to GBV-C E2 protein expression and not through effects of GBV-C E2 RNA (Fig 2A). Examination of deletion mutants demonstrated that the N-terminal 219 a.a of E2 were required to inhibit IL-2 release following TCR stimulation, whereas the C-terminal region of E2 (aa 220 to 331) did not (Fig. 2A). Jurkat cells expressing GBV-C E2 protein had significantly lower steady state levels of IL-2 mRNA following activation compared to FS and VC control cells (Fig 2B). Reduction of GBV-C E2 protein expression by maintaining cells in doxycycline (dox, 1µg/mL) reversed the block in steady-state IL-2 mRNA levels following TCR activation (Fig 2B). Of note, no differences in 18S mRNA levels were observed between any of the Jurkat cell lines studied.
Figure 2. GBV-C E2 protein inhibits T cell receptor (TCR) induced IL-2.
Following TCR activation with anti-CD3/CD28, IL-2 secretion was measured in Jurkat cells expressing GBV-C E2 protein (1–331), the C-terminal (1–219) and N-terminal (220–331) E2 deletions, the E2 coding region with a frame-shift control cell line (FS), or the vector control (VC). IL-2 release was significantly reduced in GBV-C E2 and E2 (1–219) expressing Jurkat cells (A). Jurkat cell IL-2 mRNA levels were measured in the various cell lines following TCR activation (B). Fold increase in IL-2 production was calculated by measuring IL-2 in the culture supernatant before and after activation. IL-2 mRNA levels were measured after activation and normalized to 18S rRNA levels. *P <0.05; **P <0.01; ns = not significant, p>0.05. Each experiment was performed in triplicate using three independent cultures and repeated at least three times on different days with consistent results.
GBV-C E2 protein effects on IL-2Rα and STAT5 phosphorylation
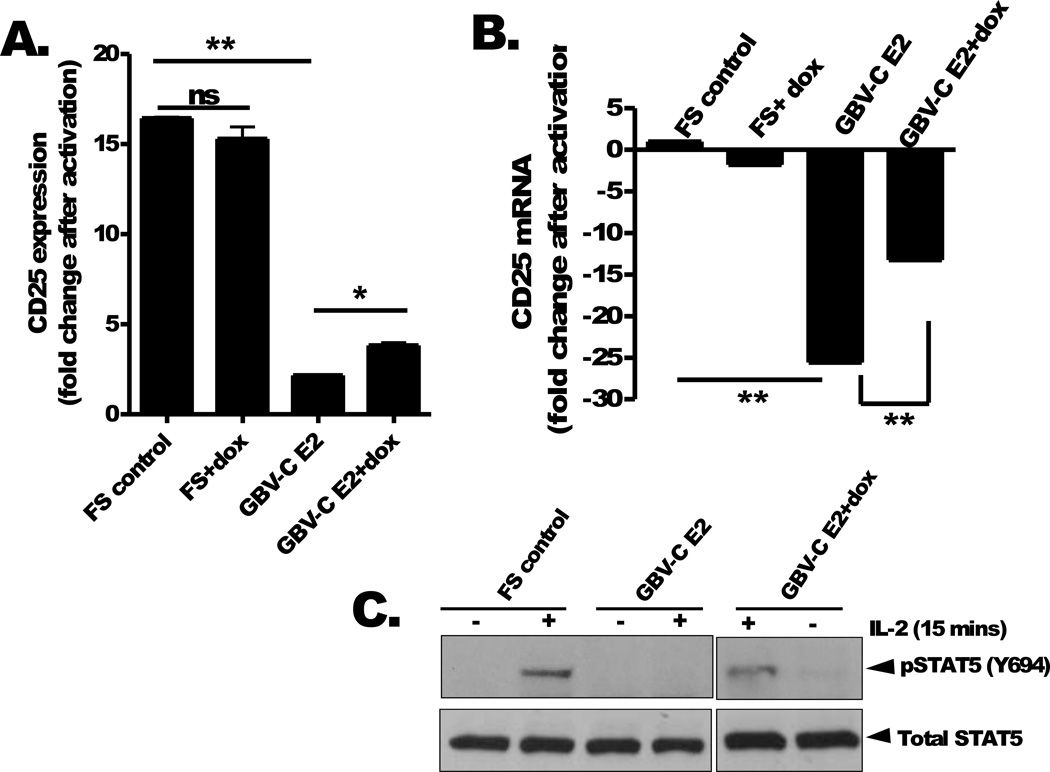
Surface expression of the alpha chain of IL-2 receptor (IL-2Rα; CD25) is increased by activation of T cells [reviewed in (43, 44)]. Following upregulation, CD25 interacts with IL-2Rβ (CD122) and IL-2γ (CD132) receptors to form the high affinity IL-2 receptor (IL-2R) that binds IL-2 and initiates IL-2 signaling [reviewed in (43, 44)]. In studies of HIV-infected people, GBV-C viremia is associated with lower CD25 expression on T cells and a reduced response to IL-2 therapy (30, 33). Consequently, we examined the effect of GBV-C E2 protein expression on Jurkat cell CD25 expression. CD25 expression was significantly reduced in cells expressing GBV-C E2 compared to the FS control following TCR activation (average fold increase in CD25 expression, 16.4 versus 2.16; p<0.001), and this was also partially reversed by growing the E2 expressing cells in doxycycline (3.7 fold; p<0.01) (Fig 3A). CD25 mRNA steady state levels increase following TCR activation, and this upregulation was also blocked in cells expressing the GBV-C E2 protein (Fig. 3B). This interference in the upregulation of CD25 steady state mRNA levels was also partially reversed by maintaining the cells in doxycycline to reduce GBV-C E2 expression (Fig. 3B). Maintaining Jurkat cells in doxycycline did not affect cell viability as previously described (Fig. 3A, 3B)(38).
Figure 3. GBV-C E2 protein reduces IL-2Rα expression and STAT5 phosphorylation.
Cell surface expression of the IL-2α receptor (CD25) on Jurkat cells expressing GBV-C E2 (1–331) or the frameshift control (FS) following T cell receptor activation (anti-CD3/CD28) was measured by flow cytometry. The fold increase in CD25 expression was calculated by measuring mean fluorescence intensity before and after stimulation. CD25 mRNA levels in Jurkat cell lines following TCR activation (B). GBV-C E2 and FS cells were maintained with or without doxycycline for 5 days (dox; 1 µg/mL) (B). Total and phosphorylated STAT5 expression following incubation with IL-2 (see methods for details) in FS and E2 expressing Jurkat cells maintained with and without dox for 5 days (C). *P <0.01; **P <0.001
Since CD25 expression is essential for IL-2 signaling, we further investigated the effect of GBV-C E2 protein expression on downstream IL-2 signaling pathways. Phosphorylation of STAT5 is rapidly detected after IL-2 interacts with the IL-2R, and is critical for IL-2 signaling (45). Following stimulation with IL-2, STAT5 phosphorylation was inhibited in GBV-C E2 expressing cells but not in FS control cells or GBV-C E2 expressing cells grown in doxycycline (Fig 3C). In the absence of IL-2, addition of doxycycline to E2 expressing Jurkat cells did not induce STAT5 phosphorylation indicating doxycycline alone does not induce activation or IL-2 signaling. Total STAT5 protein expression was not altered by GBV-C E2 protein expression (Fig 3C). Together, these data indicate that GBV-C E2 protein expression inhibits CD25 expression and IL-2 induced phosphorylation of STAT5.
GBV-C E2 protein reduces activation induced proliferation
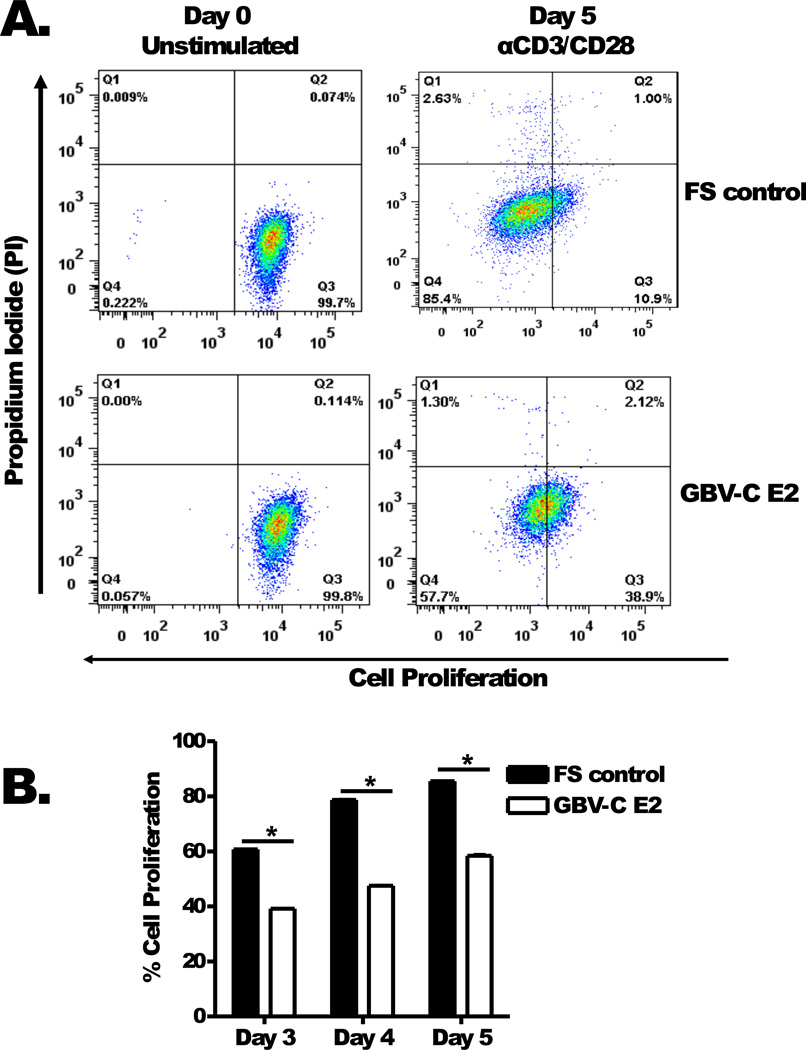
To determine whether the effect of GBV-C E2 expression on IL-2 release, CD25 expression and STAT5 phosphorylation affected T cell proliferation, Jurkat cells expressing GBV-C E2 or FS were assessed by flow cytometry. Jurkat cells expressing GBV-C E2 or the FS control were labelled with the proliferation dye eFluor450 (Day 0, Fig. 4A). Following TCR stimulation with anti-CD3/CD28, the Jurkat cells expressing GBV-C E2 protein demonstrated less proliferation than the FS control (Day 5, Fig 4A). This difference in proliferation between E2-expressing Jurkat cells and the FS control Jurkat cells was not seen when the cells were cultured without TCR activation (data not shown). The proliferation of these cell lines over five days following anti-CD3/CD28 stimulation is shown in figure 4B.
Figure 4. GBV-C E2 protein expression inhibits cell proliferation.
Proliferation of Jurkat cells expressing GBV-C E2 (1–331) or the frameshift control (FS) was measured at baseline (day 0) and following five days activation with anti-CD3/CD28 by flow cytometry (eFlour450). Propidium iodide (PI) staining was used to exclude dead cells (A). Cell proliferation was quantified on days 3 to 5 (B). *P <0.05. The data represent the average of three independent cultures.
GBV-C E2 protein reduces IL-2 and CD25 expression in primary human T cells
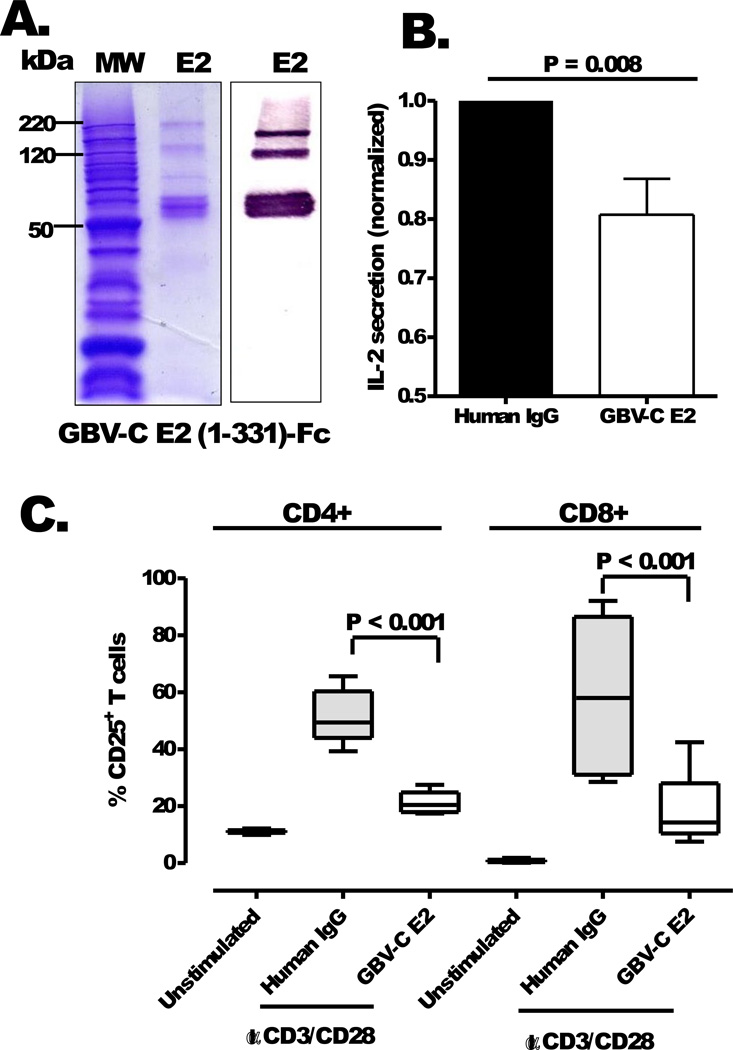
To determine if the addition of E2 to cells altered activation following TCR engagement, IL-2 release was measured in peripheral blood mononuclear cells (PBMCs) incubated with highly purified recombinant GBV-C E2-Fc fusion protein (Fig. 5A) or human IgG control protein. IL-2 production was significantly lower in PBMCs from healthy donor PBMCs (n=4) incubated with GBV-C E2 protein (20 µg/mL) compared to cells incubated with human IgG (20 µg/mL; P = 0.008; Fig. 5B). Similarly, and consistent with the findings observed in the Jurkat cells expressing E2 protein, the addition of E2-Fc to primary CD4+ and CD8+ T cells obtained from the four healthy donors significantly blocked CD25 upregulation following anti-CD3/CD28 stimulation compared to cells incubated with IgG (20 µg/mL; P <0.001; Fig. 5C).
Figure 5. GBV-C E2 protein inhibits IL-2 secretion and IL-2Rα (CD25) expression in primary human T cells.
SDS-PAGE and immunoblot analysis of purified recombinant GBV-C E2 protein fused to human IgG Fc (E2-Fc; panel A). Bands in the gel represents monomeric (60 kDa), dimeric (120 kDa) and trimeric (180 kDa) forms of recombinant E2-Fc protein. IL-2 release (B) from primary human peripheral blood mononuclear cells and CD25 expression (C) on CD4+ and CD8+ T cells following activation with anti-CD3/CD28 antibodies in media containing recombinant E2 (20 µg/mL) or human IgG control (20 µg/mL). Data represent average results obtained using PBMCs from four healthy subjects. IL-2 secretion by PBMCs incubated with GBV-C E2-Fc recombinant protein was normalized to IL-2 produced by control PBMCs incubated with the same concentration of human IgG.
DISCUSSION
Persistent immune activation and immune dysfunction are characteristic features of chronic HIV infection that contribute to HIV-associated immunodeficiency [reviewed in (17, 19)]. Previous studies suggested a potential interaction between GBV-C and IL-2 in vitro and in vivo (33, 35, 46). IL-2 is a pleiotropic cytokine essential for normal T cell function; however, IL-2 also promotes HIV replication and activation induced cell death (AICD) of T cells (26, 47, 48). A recent study found an association between GBV-C viremia and reduced T cell AICD, further suggesting that GBV-C viremia may reduce activation and alter IL-2 response (35). Here, we show that GBV-C E2 expression significantly inhibited IL-2 production and significantly blocked the upregulation of steady-state IL-2 mRNA levels following TCR engagement (Fig. 2). The region within the E2 protein required to alter IL-2 expression resided in the N terminal 219 a.a, as the expression of aa 220 to 331 of the GBV-C E2 protein did not affect IL-2 (Fig 2C). Studies are underway to further characterize the precise domain within GBV-C E2 required to alter IL-2 expression.
Clinical studies have suggested interactions between GBV-C and T cell activation and proliferation. Specifically, HIV-positive subjects with GBV-C coinfection had significantly reduced CD4+ T cell expansion following intravenous IL-2 therapy compared to subjects without GBV-C infection (33). Subjects without GBV-C infection had a significant increase in CD4+ T cell count after IL-2 therapy whereas those with GBV-C did not have a significant increase in CD4+ T cell counts following IL-2 therapy (33). In addition, PBMC proliferation ex vitro was reduced in GBV-C viremic subjects following activation with IL-2 compared to PBMCs from subjects without GBV-C infection (35). Furthermore, GBV-C and HIV coinfected subjects had reduced CD25 expression on CD4+ and CD8+ T cells compared to HIV mono-infected subjects (30). In this study, either the expression of the GBV-C E2 protein in Jurkat cells or the addition of recombinant E2 protein to primary CD4+ and CD8+ T cells reduced the expression of CD25 following TCR engagement compared to control cells (Fig. 3A and Fig. 5C). E2 protein expression was required for these effects, as cells expressing the GBV-C RNA region with a frame-shift to prevent E2 expression and reducing E2 expression by maintaining cells in doxycycline reversed the inhibition of CD25 expression (Fig. 3A).
Because GBV-C E2 expression is likely to be higher in the stably transfected Jurkat cells than that produced during natural infection, the effect of E2 on IL-2 release and IL-2 signaling may be less potent in vivo. However, as noted, GBV-C coinfection with HIV is associated with a significant block in CD4 cell proliferation following administration of recombinant IL-2 (33). The in vitro data demonstrating that E2 expression inhibits IL-2 release, CD25 (IL-2Rα) expression, IL-2 signaling (measured by STAT5 phosphorylation) and cellular proliferation following TCR activation provides evidence supporting a causal role of GBV-C for the apparent interaction observed in the clinical study (33). Reducing GBV-C E2 expression by maintaining the cells in doxycycline reduced the extent of IL-2 signaling reduction. The fact that GBV-C E2 expressing cells still inhibited IL-2 after incubation in doxycycline suggests that low levels of IL-2 are sufficient to have a measurable effect on TCR-mediated signaling.
GBV-C E2 protein also inhibited the upregulation of IL-2 and CD25 steady-state mRNA levels following TCR activation, contributing to the observed reduction in cellular proliferation (Fig. 4). Although reducing cellular proliferation will also lead to reduced IL-2 and IL2R expression, the effect of E2 protein specifically blocked TCR signaling, as both IL-2 production and CD25 expression were blocked within 24 hrs, thus prior to significant cellular proliferation. Finally, exposure of primary human CD4+ and CD8+ T cells to recombinant GBV-C E2 protein recapitulated the IL-2 and CD25 findings observed in Jurkat cells expressing E2 protein (Fig. 5B and 5C), suggesting that GBV-C particles containing E2 protein may influence TCR signaling in bystander cells.
GBV-C infection is characterized by high levels of replication in HIV-infected people, and on average, there are 5.45 × 107 genome equivalents of GBV-C found per mL serum (35). If there are 180 copies of GBV-C envelope glycoproteins per virion as there are for other flaviviruses [reviewed in (2)], approximately 1 × 1010 copies of E2 are present in each mL of plasma. Furthermore, virus is produced by B and T lymphocytes (34), thus E2 production is predominantly in lymphoid tissue. Thus, even modest effects of GBV-C E2 protein on IL-2 homeostasis and T cell activation may result in global alteration of T cell function. Since GBV-C is not associated with any known human disease, the association between GBV-C and reduced T cell activation and response to therapeutic IL-2 administration, although measurable, are not potent enough to lead to immunodeficiency. For immune-mediated disease however, these effects may be beneficial, and potentially this relates to the improved survival observed in many, though not all studies of GBV-C infection in HIV-infected cohorts (6–12).
In conclusion, these data provide in vitro evidence to support a causal role for the effect of GBV-C viremia on T cell activation and IL-2 mediated proliferation observed in epidemiological studies (30, 33). The effects on T cell activation and proliferation are mediated at least in part by the N-terminal 219 aa of the GBV-C envelope glycoprotein E2 in vitro. A previous study found that aa 276–292 of GBV-C E2 protein are sufficient to inhibit HIV replication at the entry step (38). Since GBV-C E2 protein containing this region did not interfere with activation or IL-2 signaling, it is clear that different E2 regions are involved in the interaction between HIV inhibition and the modulation of T cell activation and proliferation. Furthermore, the effects of GBV-C E2 protein on IL-2 signaling pathways may contribute to the reduction in HIV-associated immune activation observed in GBV-C/HIV coinfected individuals. Given the pleiotropic effects of IL-2 on immune system, studies on the effects of GBV-C infection on other immune cell functions are warranted.
ACKNOWLEDGEMENTS
We thank our volunteer blood donors for participating in these studies. We also thank Dr. Alan Landay (Rush University) for helpful discussions and advice.
This work was supported by Merit Review grants from the Department of Veterans Affairs (JTS, JX), and by a grant National Institute of Allergy and Infectious Diseases (RO1 AI-58740, JTS).
Footnotes
This work was presented in part at the International AIDS Society Meeting, July 2011 and Keystone Symposia on Viral Immunity and Host Gene Influence, March 2012
Conflicts of Interest:
Drs. Xiang, McLinden and Stapleton have patents related to the use of GBV-C as potential therapy for HIV.
REFERENCES
- 1.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol. 2011;92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat. 2009;16:757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23:137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton JT, Williams CF, Xiang J. GB virus type C: a beneficial infection? J Clin Microbiol. 2004;42:3915–3919. doi: 10.1128/JCM.42.9.3915-3919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rey D, Vidinic-Moularde J, Meyer P, Schmitt C, Fritsch S, Lang JM, Stoll-Keller F. High prevalence of GB virus C/hepatitis G virus RNA and antibodies in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2000;19:721–724. doi: 10.1007/s100960000352. [DOI] [PubMed] [Google Scholar]
- 6.Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton JT. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 7.Nunnari G, Nigro L, Palermo F, Attanasio M, Berger A, Doerr HW, Pomerantz RJ, Cacopardo B. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Ann Intern Med. 2003;139:26–30. doi: 10.7326/0003-4819-139-1-200307010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Xiang J, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 9.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, Schmidt RE, Manns MP. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 10.Yeo AE, Matsumoto A, Hisada M, Shih JW, Alter HJ, Goedert JJ. Effect of hepatitis G virus infection on progression of HIV infection in patients with hemophilia. Multicenter Hemophilia Cohort Study. Ann Intern Med. 2000;132:959–963. doi: 10.7326/0003-4819-132-12-200006200-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lefrere JJ, Ferec C, Roudot-Thoraval F, Loiseau P, Cantaloube JF, Biagini P, Mariotti M, LeGac G, Mercier B. GBV-C/hepatitis G virus (HGV) RNA load in immunodeficient individuals and in immunocompetent individuals. J Med Virol. 1999;59:32–37. doi: 10.1002/(sici)1096-9071(199909)59:1<32::aid-jmv6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda H, Fukuda Y, Hayakawa T, Takamatsu J, Saito H. Effect of GB virus C/hepatitis G virus coinfection on the course of HIV infection in hemophilia patients in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:209–213. doi: 10.1097/00042560-199803010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Heringlake S, Ockenga J, Tillmann HL, Trautwein C, Meissner D, Stoll M, Hunt J, Jou C, Solomon N, Schmidt RE, Manns MP. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? J Infect Dis. 1998;177:1723–1726. doi: 10.1086/517431. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkman P, Flamholc L, Naucler A, Molnegren V, Wallmark E, Widell A. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS. 2004;18:877–886. doi: 10.1097/00002030-200404090-00005. [DOI] [PubMed] [Google Scholar]
- 15.Van der Bij AK, Kloosterboer N, Prins M, Boeser-Nunnink B, Geskus RB, Lange JM, Coutinho RA, Schuitemaker H. GB virus C coinfection and HIV-1 disease progression: The Amsterdam Cohort Study. J Infect Dis. 2005;191:678–685. doi: 10.1086/427559. [DOI] [PubMed] [Google Scholar]
- 16.Bhattarai N, Stapleton JT. GB virus C: the good boy virus? Trends Microbiol. 2012 doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pett SL. Immunotherapies in HIV-1 infection. Curr Opin HIV AIDS. 2009;4:188–193. doi: 10.1097/COH.0b013e328329d090. [DOI] [PubMed] [Google Scholar]
- 18.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 20.Pett SL, Kelleher AD, Emery S. Role of interleukin-2 in patients with HIV infection. Drugs. 2010;70:1115–1130. doi: 10.2165/10898620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J Allergy Clin Immunol. 2002;109:758–770. doi: 10.1067/mai.2002.124259. [DOI] [PubMed] [Google Scholar]
- 22.Fortis C, Soldini L, Ghezzi S, Colombo S, Tambussi G, Vicenzi E, Gianotti N, Nozza S, Veglia F, Murone M, Lazzarin A, Poli G. Tumor necrosis factor alpha, interleukin 2, and soluble interleukin 2 receptor levels in human immunodeficiency virus type 1-infected individuals receiving intermittent cycles of interleukin 2. AIDS Res Hum Retroviruses. 2002;18:491–499. doi: 10.1089/088922202317406637. [DOI] [PubMed] [Google Scholar]
- 23.Sereti I, Herpin B, Metcalf JA, Stevens R, Baseler MW, Hallahan CW, Kovacs JA, Davey RT, Lane HC. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. AIDS. 2001;15:1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 24.Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 1993;53:2597–2602. [PubMed] [Google Scholar]
- 25.Porter BO, Shen J, Kovacs JA, Davey RT, Rehm C, Lozier J, Csako G, Nghiem K, Costello R, Lane HC, Sereti I. Interleukin-2 cycling causes transient increases in high-sensitivity C-reactive protein and D-dimer that are not associated with plasma HIV-RNA levels. AIDS. 2009;23:2015–2019. doi: 10.1097/QAD.0b013e32832d72c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinter AL, Poli G, Fox L, Hardy E, Fauci AS. HIV replication in IL-2-stimulated peripheral blood mononuclear cells is driven in an autocrine/paracrine manner by endogenous cytokines. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]
- 27.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 28.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 29.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 30.Maidana-Giret MT, Silva TM, Sauer MM, Tomiyama H, Levi JE, Bassichetto KC, Nishiya A, Diaz RS, Sabino EC, Palacios R, Kallas EG. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS. 2009;23:2277–2287. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze-Zander C, Neibecker M, Othman S, Tural C, Clotet B, Blackard JT, Kupfer B, Luechters G, Chung RT, Rockstroh JK, Spengler U. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir Ther. 2010;15:745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, Spengler U. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS. 2003;17:1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton JT, Chaloner K, Zhang J, Klinzman D, Souza IE, Xiang J, Landay A, Fahey J, Pollard R, Mitsuyasu R. GBV-C viremia is associated with reduced CD4 expansion in HIV-infected people receiving HAART and interleukin-2 therapy. AIDS. 2009;23:605–610. doi: 10.1097/QAD.0b013e32831f1b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 35.Rydze RT, Bhattarai N, Stapleton JT. GB virus C infection is associated with a reduced rate of reactivation of latent HIV and protection against activation-induced T cell death. Antivir Ther. 2012 doi: 10.3851/IMP2309. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koedel Y, Eissmann K, Wend H, Fleckenstein B, Reil H. Peptides derived from a distinct region of GB virus C glycoprotein E2 mediate strain-specific HIV-1 entry inhibition. J Virol. 2011;85:7037–7047. doi: 10.1128/JVI.02366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung S, Eichenmuller M, Donhauser N, Neipel F, Engel AM, Hess G, Fleckenstein B, Reil H. HIV entry inhibition by the envelope 2 glycoprotein of GB virus C. AIDS. 2007;21:645–647. doi: 10.1097/QAD.0b013e32803277c7. [DOI] [PubMed] [Google Scholar]
- 38.Xiang J, McLinden JH, Kaufman TK, Mohr EL, Bhattarai N, Chang Q, Stapleton JT. Characterization of a peptide domain within the GB Virus C envelope glycoprotein (E2) that inhibits HIV replication. Virology. 2012;430:53–62. doi: 10.1016/j.virol.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang J, McLinden JH, Chang Q, Kaufman TM, Stapleton JT. An 85-aa segment of the GB virus type C NS5A phosphoprotein inhibits HIV-1 replication in CD4+ Jurkat T cells. Proc Natl Acad Sci U S A. 2006;103:15570–15575. doi: 10.1073/pnas.0604728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLinden JH, Kaufman TM, Xiang J, Chang Q, Klinzman D, Engel AM, Hess G, Schmidt U, Houghton M, Stapleton JT. Characterization of an immunodominant antigenic site on GB virus C glycoprotein E2 that is involved in cell binding. J Virol. 2006;80:12131–12140. doi: 10.1128/JVI.01206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohr EL, Xiang J, McLinden JH, Kaufman TM, Chang Q, Montefiori DC, Klinzman D, Stapleton JT. GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates. J Immunol. 2010;185:4496–4505. doi: 10.4049/jimmunol.1001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camargo JF, Quinones MP, Mummidi S, Srinivas S, Gaitan AA, Begum K, Jimenez F, VanCompernolle S, Unutmaz D, Ahuja SS, Ahuja SK. CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation. J Immunol. 2009;182:171–182. doi: 10.4049/jimmunol.182.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev. 2011;241:63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 46.George SL, Xiang J, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology. 2003;316:191–201. doi: 10.1016/s0042-6822(03)00585-3. [DOI] [PubMed] [Google Scholar]
- 47.Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG. The role of the common cytokine receptor gamma-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol. 1999;163:3131–3137. [PubMed] [Google Scholar]
- 48.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]