Abstract
The opportunistic fungus Candida albicans is one of the leading causes of infections in immunocompromised patients, and innate immunity provides a principal mechanism for protection from the pathogen. In the present work, the role of integrin αXβ2 in the pathogenesis of fungal infection was assessed. Both purified αXβ2 and αXβ2-expressing human epithelial kidney 293 cells recognized and bound to the fungal hyphae of SC5314 strain of C. albicans but not to the yeast form or to hyphae of a strain deficient in the fungal mannoprotein, Pra1. The binding of the integrin to the fungus was inhibited by β-glucans but not by mannans, implicating a lectin-like activity in recognition but distinct in specificity from that of αMβ2. Mice deficient in αXβ2 were more prone to systemic infection with the LD50 fungal inoculum decreasing 3-fold in αXβ2-deficient mice compared with wild-type mice. After challenging i.v. with 1.5 × 104 cell/g, 60% of control C57BL/6 mice died within 14 d compared with 100% mortality of αXβ2-deficient mice within 9 d. Organs taken from αXβ2-deficient mice 16 h postinfection revealed a 10-fold increase in fungal invasion into the brain and a 2-fold increase into the liver. These data indicate that αXβ2 is important for protection against systemic C. albicans infections and macrophage subsets in the liver, Kupffer cells, and in the brain, microglial cells use αXβ2 to control fungal invasion.
Introduction
Candida albicans is a common opportunistic fungal pathogen. It is a dimorphic fungus existing as rounded yeast cells or as filamentous forms (1, 2). Although the yeast form can colonize mucosal membranes, it is thought that the filamentous form provides some protection to the microorganism against host defense systems, and the ability of C. albicans to rapidly and reversibly switch between yeast and filamentous morphologies is crucial to its pathogenicity (3–6). In recent years, Candida infections ranked as the fourth most common cause of nosocomial infections with immunocompromised patients being particularly susceptible (7, 8). Bloodstream fungal infections have an extremely high (30–70%, by different estimations) morbidity and mortality (8–11).
The innate immune system provides the principal protection against Candida infections. Polymorphonuclear leukocytes have been shown to be the primary components of the cellular immune defenses against Candida (12–14), and a protective role for macrophages in disseminated candidemia has also been suggested (13, 15, 16). The most prominent receptors on leukocytes used in fungal or microbial recognition are integrins of the β2 subfamily (17, 18). This subfamily of leukocyte receptors is composed of four members that share a common β2 subunit that associates noncovalently with one of four distinct but structurally homologous α subunits to form αMβ2 (Mac-1, CD11b/CD18, and CR3), αLβ2 (LFA-1 and CD11a/CD18), αXβ2 (p150,95 CD11c/CD18 and CR4), and αDβ2 (CD11d/CD18) (19–23). These cell surface receptors are expressed on monocytes, granulocytes, macrophages, and NK cells and have been implicated in diverse protective responses mediated by these cells, including phagocytosis, cell-mediated killing, chemotaxis, and cellular activation. Specifically, the β2 integrins mediate migration of leukocytes to sites of infection and adhesion to microorganisms with subsequent phagocytosis or killing of many pathogens (12, 17, 24). Patients with leukocyte adhesion deficiency-1 (LAD-1), a rare hereditary disease that is characterized by low expression (mild LAD-1) or complete absence (severe) of all four of the β2 integrins because of mutations in the ITGB2 (β2) gene (25, 26), are highly susceptible to a wide range of bacterial and fungal infections (27, 28) [and the increased sensitivity of such patients to C. albicans infections has been discussed (29)]. Although other leukocyte pattern recognition receptors, which recognize fungal β-glucans (Dectin-1 and TLR2 (30, 31) and mannan-specific TLR4 (32)), also participate in fungal recognition and apparently are essential in leukocyte activation and notably in activation of β2 integrins (33, 34), they do not directly facilitate leukocyte migration, adhesion, or phagocytosis.
Of the β2 integrins, αMβ2 has been specifically implicated in the recognition of C. albicans. Polymorphonuclear leukocytes and NK cells use αMβ2 to adhere only to the filamentous form but not to the yeast form of C. albicans (35, 36). C. albicans pH-regulated Ag 1 (Pra1) (37), also known as fibrinogen binding protein 1 (38) or C. albicans 58-kDa mannoprotein (39), was identified as the major ligand of αMβ2 among C. albicans proteins (40). Pra1p is a mannoprotein (1, 41) and is expressed on the surface of the hyphae but not on the yeast form of C. albicans (3, 41). Expression of Pra1p is strongly pH dependent and is also regulated by nutrition and certain other fungal genes (37, 41, 42). Disruption of the PRA1 gene protects the fungus against leukocyte killing in vitro and in vivo, impedes the innate immune response to infection, and increases overall fungal virulence and organ invasion in vivo (29, 43).
Although mutations in αM subunits have been previously described (44), it appears that the clinical manifestations of selective loss of αMβ2 are less severe than when all four β2-integrins are absent, which suggests that αMβ2 may share at least part of its surveillance functions with other β2 integrins, most likely with the integrin αXβ2 (19, 45). Integrin αXβ2 is present on the surface of all leukocyte subsets that express αMβ2, with the exception of dendritic cells, which have CD11c (αX) as a major surface marker. These integrins are ∼70% identical and also share a number of ligands, most notably fibrinogen (46), ICAM-1 (47), and iC3b, a component of the complement system (48). However, the functions of αXβ2 are less well studied, and its functions in innate immunity are still unclear. It was shown that αXβ2 is involved in macrophage-mediated phagocytosis of Mycobacterium tuberculosis (49) and Mycobacterium leprae (50) and may play a role in the development of gastric ulcers in chronic Helicobacter pylori infection (51).
The present study was undertaken to determine the role and significance of the αXβ2 in C. albicans pathogenicity and the effects of its elimination on host defense in vivo, using αXβ2-deficient mice in a model of systemic murine candidiasis.
Materials and Methods
C. albicans strains
C. albicans strain SC5314 was used in most in vitro and in vivo experiments. In some experiments, the Pra1-depleted strain CAMB5-18 (pra1::hisG/pra1::hisG iro1-ura3Δ/IRO1-URA3) was also used. This strain was derived from the strain CAMB435 by reversion of the iro1-ura3 deletion as previously described (52) and was characterized by us previously (29). All strains were routinely maintained on Difco Sabouraud Dextrose Agar (SDA) plates (BD Biosciences, Sparks, MD).
Animals
αX-Knockout (KO) mice (ΔαXβ2) were provided by Dr. C. M. Ballantyne (Baylor College of Medicine, Houston, TX). This mouse line was generated in parallel with other β2-KO lines (53). All these β2-KO murine lines have been used in a number of studies in comparison with the ΔαM mice (54–56), and there are no separate publications only characterizing the ΔαX mice. In our laboratory, these mice were backcrossed for more than 12 generations into a C57BL/J6 background. Before experiments, the genotypes of all mice were confirmed by PCR of blood DNA samples. Age-matched C57BL/J6 mice, obtained from The Jackson Laboratory (Bar Harbor, ME), were used as controls (wild-type [WT] mice). All protocols involving mice were approved by the Institutional Animal Care and Use Committee in accordance with the Public Health Service policy, the Health Research Extension Act (PL99-158), and the Cleveland Clinic policy. All experiments on mice involving C. albicans infections were carried out in a BSL2 facility. The mice were maintained during experiments on a 12-h alternating light/dark cycle and supplied with food (diet number 2918; Harlan Teklad, Madison, WI) and sterilized water ad libitum.
Cells
Human epithelial kidney 293 (HEK293) cells expressing αXβ2 (HEK293/αXβ2 cells) were prepared as described previously (57–59). Briefly, human full-length αX and β2 cDNAs were blunt-end cloned into TOPO-pCDNA 3.1(+) expression vector (Life Technologies, Carlsbad, CA). Plasmids were sequenced to ensure appropriate insert orientation and cotransfected into HEK293 cells using the LipofectAMINE Plus reagent (Life Technologies). To prepare control mock-transfected cells, the vector alone was used. Transfected cells were selected using neomycin sulfate (Life Technologies), and cells expressing the integrin were detected and sorted by flow cytometry (FACS) using a FACStar cell sorter (BD Biosciences) and anti-human CD18 mAb (clone IB4). The sorted cells were subcloned, and αXβ2 expression was characterized by FACS. The cell lines obtained were routinely grown in a monolayer in DMEM/F12 medium, supplemented with 10% FBS (all from BioWhittaker). For experiments, the cells were harvested using enzyme-free Cell Dissociation buffer (Life Technologies), washed with HBSS, and resuspended in HBSS containing 100 mM HEPES (pH 7.4), 5 mM CaCl2, and 5 mM MgCl2 (HBSS/HEPES). Monocytes were isolated from human donor blood using the Pan Monocyte Isolation Kit (Miltenyi Biotec) following the manufacturer’s instructions.
All studies involving human blood cells were performed in accord with protocols and policies approved by the Institutional Review Board at Cleveland Clinic and with the Helsinki Declaration of 1975 as revised in 2000.
Abs and inhibitors
mAbs used in this study were as follows: 44a (anti-human αM I-domain, IgG1), OKM1 (anti-human αM lectin domain, IgG2b), IB4 (anti-human β2, IgG2a). The hybridoma cell lines producing these mAbs were obtained from the American Type Culture Collection and adapted to growing in serum-free media in CELLine Bioreactor Flasks (Integra Biosciences, Hudson, NH) in the Cleveland Clinic Hybridoma Core. The mAbs were purified from conditioned media using recombinant protein G columns (Life Technologies).
The mAb clone 3.9 (anti-human αX, IgG1) and clone N418 (anti-mouse αX) were purchased from BioLegend (San Diego, CA), and mAb clone YW62.3 (anti-mouse CD45) was obtained from Serotec (Raleigh, NC).
Baker yeast β-glucan, mannan, and echistatin (60, 61) were purchased from Sigma-Aldrich (St. Louis, MO). The recombinant hookworm neutrophil inhibitory factor (NIF) was prepared as described previously (58).
Tissue section preparation and assaying
Brains and livers from experimental mice were snap-frozen in OCT. Brain sections (6 μm thick) were processed for immunohistochemical staining with the following Abs: hamster anti-mouse CD11c (BioLegend), followed by biotinylated anti-hamster Ab and streptavidin Alexa Fluor 568. CD45 was stained using rat anti-mouse CD45 mAb (BD Biosciences) and rabbit–anti-rat Ab conjugated to Alexa Fluor 488. The slides were mounted using Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). To visualize the extent of the fungal cell invasion and proliferation, the brain and liver sections were stained with periodic acid–Schiff stain. The images were observed using a Leica DMR microscope equipped with ×10/0.4 and ×20/0.5 NA objective lenses (Leica Microsystems, Wetzlar, Germany) and photographed with a Qimaging Retiga ExiFas camera (Qimaging, Burnaby, British Columbia, Canada) using ImagePro 5.1 software (Media Cybernetics, Silver Spring, MD).The images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
C. albicans staining with soluble αXβ2
Recombinant αXβ2 was isolated from HEK293/αXβ2 cells with the method previously used by us to purify αMβ2 (40, 59). Briefly, 10 g cells was harvested, washed, and lysed with 1% Triton X-100 in TBS containing protease inhibitor mixture for mammalian cells (Sigma-Aldrich). The cell lysate was clarified by centrifugation, diluted with TBS containing CaCl2 and MgCl2 and loaded onto a column of immobilized IB4 (anti-β2). To prepare the immunoadsorbent, purified IB4 mAbs were coupled to cyanogen bromide-activated Sepharose 4B (GE Biosciences, Piscataway, NJ) following the manufacturer’s protocol to final concentrations of 2.2–2.8 mg immobilized proteins per 1 ml swollen gel. After washing with TBS containing 10 mM octyl-β-d-glucopyranoside (OG; Calbiochem, San Diego, CA) and CaCl2/MgCl2, bound protein was eluted with three column volumes of 20 mM sodium acetate buffer (pH 4.2), containing OG and Ca/Mg2+. Immediately after elution, 100 μl 1 M HEPES-NaOH (pH 8) was added to each 1 ml of the column eluate to neutralize the acidic pH. Protein fractions were pooled and dialyzed against HEPES-NaCl, OG, and Ca/Mg2+. The proteins were biotinylated using Sulfo-NHS-LC-Biotin (Pierce), according to the manufacturer’s protocol. To visualize αXβ2–C. albicans interaction, C. albicans strain SC5314 was allowed to germinate in RPMI 1640 medium for 2 h and then purified. Biotinylated αXβ2 was added to obtain a 1 μg/ml concentration, and the samples were incubated for 1 h at 37°C. After incubation, the fungi were washed with Dulbecco's PBS (D-PBS) and incubated with FITC–streptavidin conjugate for 30 min at room temperature. Subsequently, the fungi were again washed with D-PBS. Blankophor, the β-glucan, and chitin-specific dye were added, and the mixture was incubated for an additional 30 min (62). Finally, the fungi were washed with D-PBS and analyzed by fluorescence microscopy (Leica Microsystems) at a magnification of ×800.
Cell adhesion assays
Cell adhesion assays were performed as described previously (40, 63). Briefly, to determine cell adhesion to fungal hyphae, 48-well Costar tissue culture plates (Corning, Corning, NY) were precoated with polyvinylpyrrolidone (PVP; Sigma-Aldrich) and washed with HBSS, and aliquots of 5 × 105 C. albicans yeast were added and incubated overnight at 37°C to germinate. For adhesion to C. albicans yeast, the fungi were incubated in YNB broth to prevent germination. After incubation, the supernatant was removed and adherent fungi were carefully washed with HBSS. A total of 105 PMA-activated peripheral blood human monocytes or HEK293/αXβ2 cells were added in HBSS/HEPES and assay plates were incubated at 37°C for 1 h. Control wells were coated with PVP only. Each experimental point was in triplicate. Subsequently, plates were washed, and the number of adherent cells in each well was quantified using the CyQUANT Cell Proliferation Assay kit (Life Technologies) as described previously (40, 63). For inhibition assays, before addition to the plate wells, the HEK293/αXβ2 cells or isolated monocytes were preincubated with 10–20 μg/ml selected Abs, 1 mM β-glucan, 1 mM mannan, or 5 μM echistatin for 10 min at room temperature. Data from cell adhesion and migration (see below) are presented as percentage (mean ± SE) of total cells (to which was assigned the value of 100%) and represent the results of three independent experiments.
Cell migration assays
Human peripheral blood monocytes and HEK293/αXβ2 cell migration assays were performed in serum-free RPMI 1640 (monocytes) or DMEM/F-12 (HEK293/αXβ2) medium (Life Technologies) using modified Boyden chambers (Costar Transwell inserts in a 24-well plate format; Corning) with tissue culture-treated polycarbonate filters of 5-μm (for monocytes) or 8-μm pores (for HEK293/αXβ2 cells) as described previously (40, 63–65). The lower chambers contained 600 μl media with 106 C. albicans yeast, which were germinated overnight prior to beginning the analyses. The upper chambers contained final volumes of 200 μl HEK293/αXβ2 cell suspensions. The assays were initiated by addition of 50 μl cell suspension (105 cells/well) to 150 μl media in the upper chambers, and the plates were placed in a humidified incubator at 37°C and 5% CO2 for 8 h. For inhibition experiments, selected Abs, NIF, and glycans were added simultaneously with the cells to the upper chamber. After migration, nonmigrated cells were removed from upper chamber using cotton swabs. The migrated cells, present on the undersurface of the membrane as well as in the lower chamber, were quantified using the CyQUANT Cell Proliferation kit as described above and previously (63, 64).
Killing (phagocytosis) assay
A total of 105 C. albicans SC5314 strain cells in 0.25 ml high glucose RPMI 1640 medium containing 0.1 M HEPES (pH 7.8) were allowed to germinate in plastic tubes at 37°C for 1 h with slow agitation. The fungal cells were collected by centrifugation, washed twice with D-PBS, suspended in 0.25 ml HBSS/HEPES (pH 7.4), and mixed with 3 × 105 (1:3 ratio), 7 × 105 (1:7 ratio), or 1.1 × 106 (1:11 ratio) HEK293/αXβ2 or mock-transfected HEK293 cells. In control experiments, the HEK293/αXβ2 cells were preincubated with 20 μg/ml anti-αX mAbs 3.9 for 10 min at room temperature. The cell/fungal mixtures were incubated at 37°C with slow shaking for 2 h. To determine the extent of killing/phagocytosis, aliquots of the cell/fungal suspension were taken every 20 min, diluted with HBSS/HEPES, and subsequently plated in serial dilutions on SDA plates. The CFU were counted manually on day 2 using a Bel-Art Products Colony Counter. Cells were not lysed before plating, and all fungal cells that remained ingested were recorded as “killed.” Results were independently verified by a modification of the method of Lehrer et al. (40, 66). Briefly, at the experiment end point, an equal volume of 1% Tween 80 was added to the fungal/leukocyte mixture to lyse leukocytes, and C. albicans cell pellets obtained by centrifugation were resuspended in 0.25 ml 2.5 mM methylene blue (Sigma-Aldrich) in HBSS/HEPES. The number of viable (nonstained) cells was counted in a hemacytometer. Control samples contained C. albicans incubated without HEK293 cells. Results obtained by both methods of quantitation of fungal viability showed close correlation with variances in the 5–10% range.
Murine model of systemic disseminated candidiasis
The model of systematic candidiasis, described and applied to C57BL/6 by MacCallum and Odds (67), was used. Each experimental group contained 10 mice of 10–12 wk of age, and weights ranged from 19 to 21 g. C57BL/6 (control WT mice) and αXβ2-depleted mice (ΔαX mice) were injected with 105 or 3 × 105 C. albicans SC5314 strain in 0.1 ml sterile saline via the tail vein. Mice were returned to cages and monitored. To determine the degree of distress, we developed a scoring system, similar to one described for the determination of humane end points in a murine model of leukemia (68). The following symptoms were evaluated and scored as follows: coma, 15 points; weight loss of: 20%, 15 points, 15%, 11 points, and 10%, 8 points; abdominal swelling, 6 points; significant decrease in mobility, 4 points; hunched posture, 3 points; porphyrin “red tears,” 3 points; head pressing, 2 points; and spiky coat, 2 points. Each group was monitored daily over the 14-d period at the same time each day. The score was noncumulative and was recalculated for each mouse every 24 ± 1.5 h. The primary end point was the number of mice surviving on day 14 within each experimental group. When mice scored 15 or more points before 14 d, the animals were euthanized. At day 14, all surviving mice were euthanized and subjected to pathological examination. To determine the extent of C. albicans invasion and fungal burden in individual organs, liver, spleen, heart, lungs, and kidney were harvested, weighed, and homogenized in 5 ml PBS, and serial dilutions of the homogenates were plated onto SDA plates for CFU quantitation. In separate experiments, mice from each group of five mice were euthanized after 16 or 40 h of infection, and their organs were removed and examined to determine the degree of the fungal invasion and organ fungal burdens (67).
Statistical analyses
Statistical significance was determined using paired log-rank and Cox regression for mouse survival data or Student t test in all other cases. For all statistical calculations the statistical package in SigmaPlot, version 12.0 (Jandel Scientific Software, Chicago, IL) was used. Differences between groups were considered significant with p < 0.05. Data are expressed as means ± SD unless otherwise noted.
Results
Integrin αXβ2 is required to control C. albicans infection
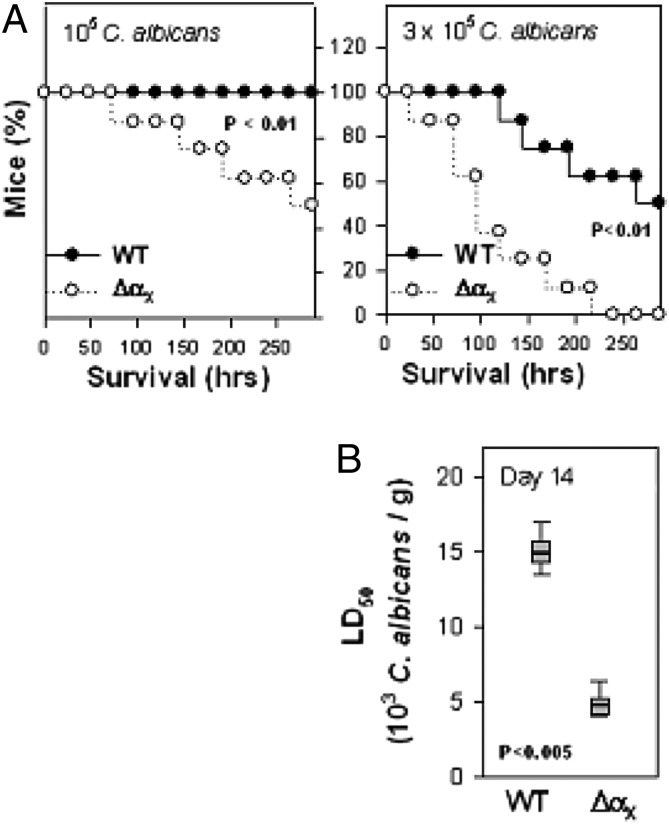
As a first step to assess the biological significance of αXβ2 in the context of the total host–pathogen relationship, transgenic mice that lack αX (ΔαX mice) were used in a murine model of disseminated candidiasis. In this assay, mice of both WT (C57BL/6 mice) and ΔαX lines were challenged with 105 or 3 × 105 C. albicans inocula via tail vein injection. All WT mice inoculated with 105 (0.5 × 10−4/g) C. albicans survived 14 d (336 h). αXβ2 elimination dramatically decreased mice survival; during these same 336 h, 50% of the ΔαX-mice reached the predetermined end point (see Materials and Methods for a complete list of “mortality” criteria) with a median survival time 264 h (Fig. 1A, left panel). A similar level of mortality occurred in WT mice only after introduction of a 3-fold higher inoculum (Fig. 1B). After challenge with the 3 × 105 C. albicans cells (∼1.5 × 10−4/g) inoculum, ∼50% of WT mice reached the end point within 12 d with a median survival time at 252 ± 12 h. In contrast, all ΔαX mice reached the end point with this inoculum within the first 9 d with a median survival time of 112 ± 8 h (p < 0.01; log-rank test) (Fig. 1A, right panel).
FIGURE 1.
The effect of αXβ2 elimination on C. albicans virulence in a murine model of disseminated candidiasis. (A) The Kaplan–Meier (cumulative) graphic of murine survival. A total of 105 (left panel) or 3 × 105 (right panel) of strain SC5314 C. albicans were introduced in 100 μl D-PBS via tail vein injection into WT (●) or ΔαX (○) mice (n = 10 in each group). After administration, the mice were inspected on a 12 ± 2 h basis and euthanized when they became moribund (e.g., at 20% weight loss). (B) LD50 dose of C. albicans. The median survivors of both groups are calculated as medians (25th and 75th). The p values were calculated by log-rank test.
Elimination of αMβ2 decreases resistance of brain and liver to C. albicans invasion
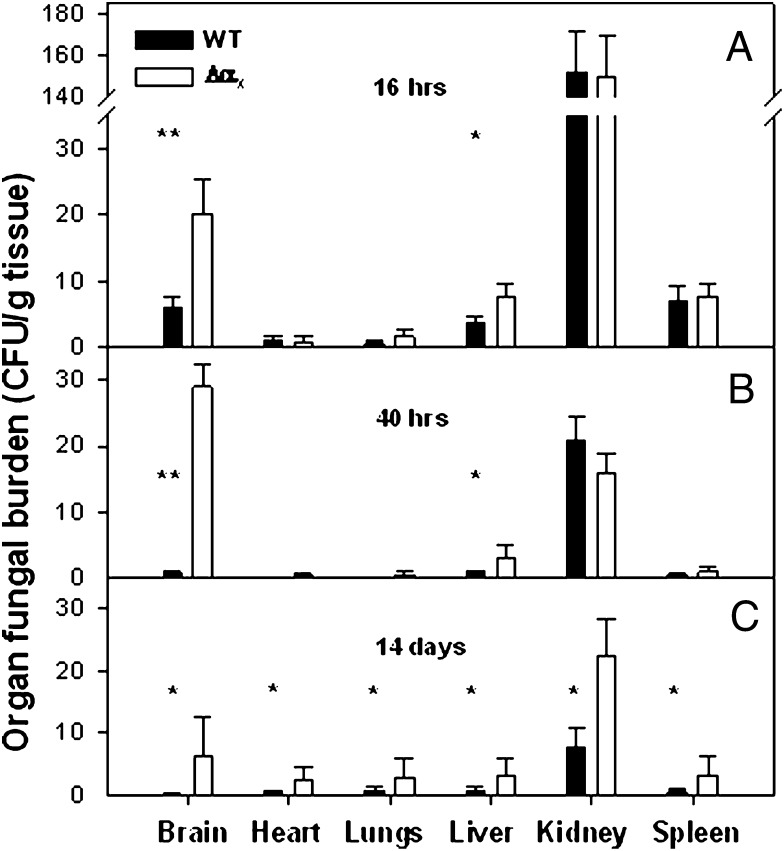
The substantial increase in susceptibility of the ΔαX mice to the Candida infection indicates that αXβ2 plays a significant role in antifungal protection and innate immunity. This interpretation was further corroborated by pathological examination of infected mice. To determine the impact of αXβ2 deletion on the rate of fungal colonization, WT and the ΔαX mice were challenged i.v. with 105 C. albicans, and selected organs (brain, kidney, lung, heart, spleen, and liver) were recovered 16 and 40 h postinfection and at day 14 from mice that survived. Tissue targeting and invasion, fungal dissemination, and organ fungal burden were assessed in recovered organs. Consistent with previous studies (69), high fungal burden was present in the kidneys at all times postinfection and was similar at 16 and 40 h (p = 0.96; Student t test) in both mouse strains (Fig. 2A, 2B). There was no significant difference (p > 0.05) in fungal burdens in the spleen, heart, and lungs at both early time points; but at day 14, differences in fungal burden in the surviving WT and ΔαX mice become evident (p < 0.05). At the day 14 survival point, fungal burden in the kidney was significantly elevated (2.8-fold difference; p < 0.01) in ΔαX mice (2 × 104 ± 3.2 × 103 CFU/g) compared with WT mice (7.7 × 103 ± 1.2 × 103) (Fig. 2C). In contrast, fungal burdens in brains and livers recovered from ΔαX mice were substantially elevated compared with the corresponding WT organs: at 16 h, ΔαX brain had 2 × 104 ± 2.4 × 103 CFU/g tissue, whereas WT brains had 6.1 × 103 ± 7 × 102 CFU/g (10-fold raise; p < 0.01); ΔαX liver had 7.6 × 103 ± 1.3 × 103 CFU/g, 2-fold rise (p < 0.05), whereas WT liver had a fungal burden of 3.5 × 103 ± 360 CFU/g (Fig. 2A). These differences increased over time: at 40 h, 2.9 × 104 ± 2.3 × 103 CFU/g ΔαX brain compared with 700 ± 120 CFU/g WT brain (40-fold rise; p < 0.005) and 3 × 103 ± 220 CFU/g ΔαX liver compared with 480 ± 110 CFU/g WT liver (6-fold rise; p < 0.01) (Fig. 2B). These differences were sustained at day 14 in all surviving mice: 6400 ± 1820 versus 200 ± 160 CFU/g (32-fold; p < 0.01) and 3100 ± 1200 versus 700 ± 400 CFU/g (4-fold; p < 0.01) for brain and liver of ΔαX and WT mice, respectively (Fig. 2C).
FIGURE 2.
The effect of αXβ2 elimination on C. albicans dissemination. Challenged i.v. with SC5314 105 C. albicans, WT (▪), or ΔαX (□) mice were euthanized after 16 h (A) or 40 h (B) of infection; their organs (brain, heart, lungs, liver, left kidney, and spleen) were removed, homogenized, and plated in serial dilution onto agar plates. (C) The organ fungal burdens of mice who survived to end point are presented. The results are presented as mean ± SD of two independent experiments (n = 3). *p < 0.05, **p < 0.005 (t test).
Residential macrophages require αXβ2 to control C. albicans invasion in vivo
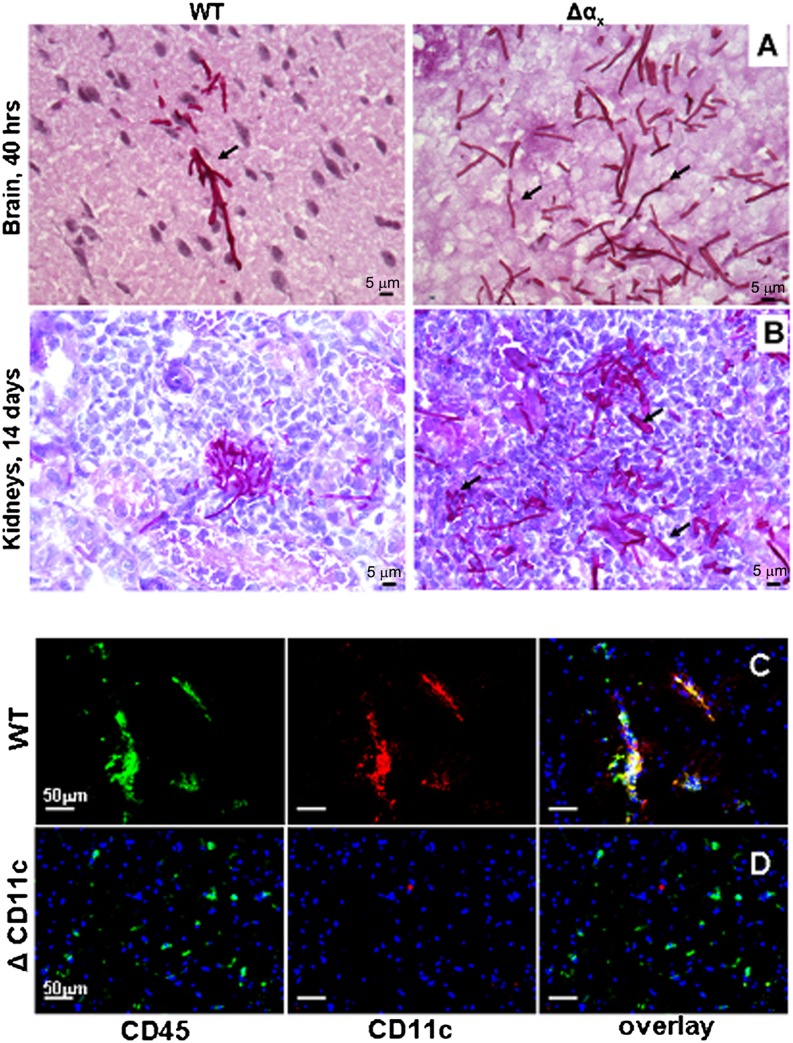
The results of fungal burden studies were further confirmed in histological sections of tissues from the infected organs. In the sections of WT brains obtained at 40 h postinfection, staining with periodic acid–Schiff reagent revealed only several scattered fungal hyphae (Fig. 3A, left panel). In contrast, in the brains of ΔαX mice 40 h postinfection, C. albicans formed a visible network of numerous fungal hyphae (Fig. 3A, right panel). After 14 d of infection, in the kidney sections of WT mice, C. albicans formed single scattered colonies (Fig. 3B, left panel), whereas in kidney sections of ΔαX mice, the fungal colonies were numerous and showed evidence of extensive organ colonization (arrows in Fig. 3B, right panel).
FIGURE 3.
The effect of αXβ2 elimination on C. albicans invasion of the brain. Histological sections of murine brains taken after 16 h of infection (A) or of murine kidney obtained after 14 d of infection (B) from WT (left two photos) or from ΔαX mice (right two photos) were stained with hematoxylin. Arrows point on C. albicans hyphae (A) and on the signs of further organ colonization (B). Scale bars (A, B), 5 μm. Sections of brains taken from WT (C) or ΔαX (D) mice 40 h postinfection were stained with Alexa Fluor 488-labeled anti-CD45 mAbs (hematopoietic cells marker, left panels, green fluorescence) and Alexa Fluor 558-labeled anti-CD11c mAbs (anti-β2, middle panels, red fluorescence). In the right panels, the overlay of the sample’s green and red fluorescence is presented. The cellular nuclei are stained with DAPI (blue fluorescence). Scale bars (C, D), 50 μm.
To ensure that αXβ2 deletion affects only leukocytes, the sections of brains from WT (Fig. 3C) and ΔαX (Fig. 3D) mice at 40 h of infection were immunostained with Abs against the common hematopoietic cell surface marker CD45 (anti-Ly5, labeled with Alexa Fluor 488, green fluorescence), anti-CD11c (anti-αX, labeled with Alexa Fluor 568, red fluorescence), and DAPI (to visualize nuclei, blue fluorescence). The fluorescence overlays demonstrate that only hematopoietic cells (leukocytes) are CD11c+ within this organ. The images also show that CD45+CD11c+ cells in the brain of the WT mice group form filamentous structures most likely along fungal hyphae. In contrast, CD45+CD11c− cells in the brains of the ΔαX mice do not organize but instead remained dispersed (Fig. 3C, 3D). These results indicate that subsets of brain residential leukocytes may use αXβ2 for localization to the fungus.
Activated monocytes use both αMβ2 and αXβ2 for fungal recognition
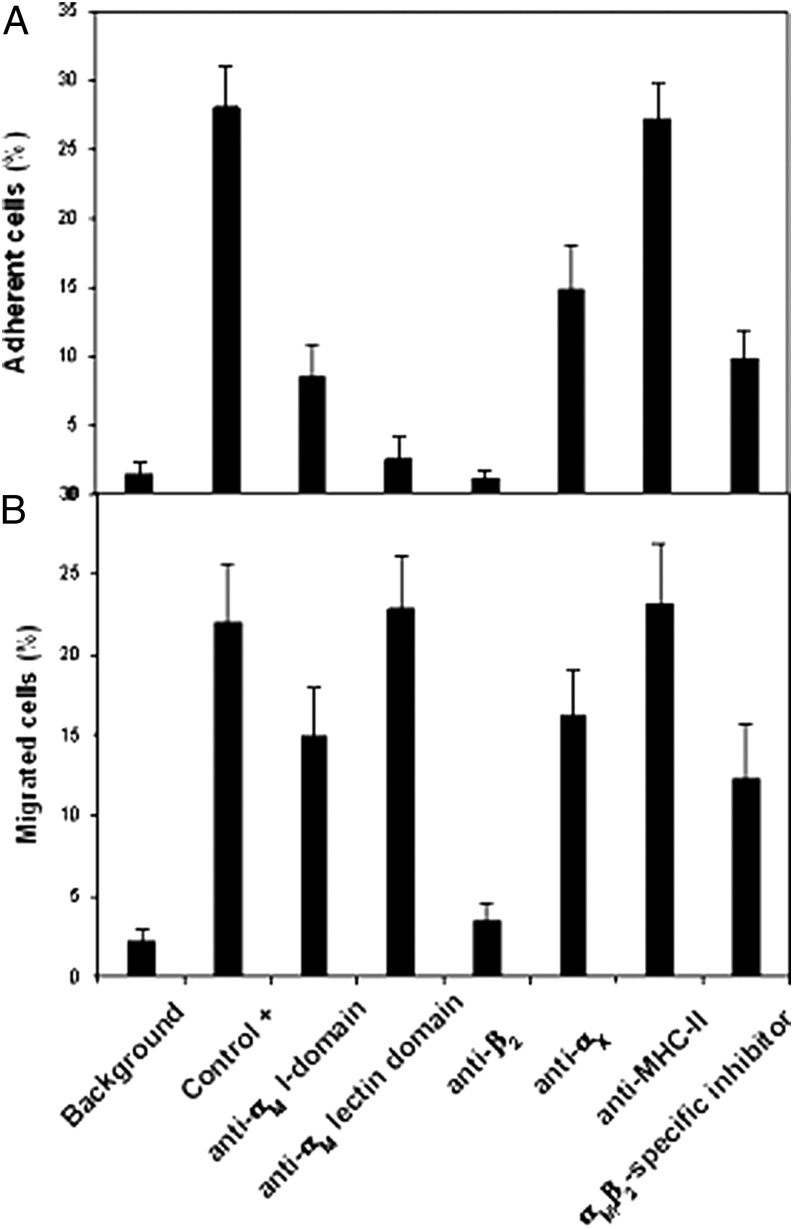
Previous studies demonstrated that NK lymphocytes (35, 61) and neutrophils (40) use integrin αMβ2 but not αXβ2 for C. albicans recognition. With our data on organ fungal burden suggesting that αXβ2 deficiency affected residential tissue macrophages, the microglia in a brain, and Kupffer cells a in liver, we chose monocytes as representative primary cell to assess αXβ2 involvement in leukocyte adhesion to C. albicans. Human peripheral blood monocytes were isolated, stimulated with PMA to activate their integrins, and added to germinated SC5314 fungus. In the absence of inhibitors, 28 ± 6% of the activated monocytes adhered to the fungal hyphae as compared with only 2% monocyte adhesion to the fungal yeast form (defined as nonspecific adhesion). Both anti-αM I-domain mAb 44a and the αMβ2-specific ligand NIF decreased monocyte adhesion to the fungal hyphae by ∼3-fold, to 8 ± 4%. Anti-αX mAb inhibited cell adhesion only ∼50%, to 15 ± 6% of the total cells. The anti-αM lectin domain mAb OKM1 and anti-β2 mAb IB4 completely inhibited monocyte adhesion, whereas the irrelevant control, mAb W6/32, had no effect (Fig. 4A). A similar specificity was demonstrated in migration assays (Fig. 4B). When monocytes were allowed to migrate (through 5-μm pores) overnight to germinated C. albicans, anti-β2 mAb completely inhibited the migration, anti-αM I-domain mAb, and anti-αX mAb inhibited 40–50%, and the control irrelevant anti-MHC class II (MHC-II) mAbs had no effect. However, a difference between migration and adhesion assays was noted. Anti-αM lectin-domain mAb OKM1 completely inhibited adhesion of monocytes to the fungus, whereas this mAb had no effect on the monocyte migration to the fungus. This difference may reflect different roles of the fungal carbohydrates (e.g., β-glucans and mannans) recognized by the lectin domain of αMβ2 on the migratory and adhesive responses of monocytes. These results demonstrate that monocytes can use αXβ2 as well as αMβ2 to engage C. albicans, and taken together, the two integrins are the major mediators of monocyte adhesion and migration to the fungus.
FIGURE 4.
Effect of selected Abs and inhibitors on adhesive (A) and migratory (B) responses to C. albicans of PMA-activated human monocytes. Isolated human peripheral blood monocytes were preincubated for 10 min on ice in the presence of 5 mM PMA with or without inhibitors. (A) Adhesion to C. albicans. The plates were incubated at 37°C for 25 min, and then, nonadherent cells were removed by washing, and the adherent cells were quantified using the CyQUANT Cell Proliferation Assay kit. (B) Migration to C. albicans. A total of 5 × 105 monocytes/well were allowed to migrate overnight through polycarbonate membrane with 5-μm porosity to 106 pregerminated C. albicans of WT SC5314 strain. The number of migrated cells was quantified in the lower chamber and in the “fungal side” of the membrane using CyQUANT Cell Proliferation kits. As inhibitors, the following mAbs were used in 10 μg/ml concentration: anti-αM I-domain (44a), anti-αM lectin domain (OKM1), anti-β2 (IB4), anti-αX (3.9), irrelevant anti–MHC-II (W6/32), and 5 μM recombinant NIF. Migration or adhesion in the absence of C. albicans or in the absence of inhibitors was used as negative (background) and positive controls, respectively. Results are presented as percentage (mean ± SE) of total cells (to which was assigned the value of 100%) and represent the results of three independent experiments, each in triplicate.
Purified recombinant αXβ2 recognizes the hyphae of WT C. albicans but not Pra1-deficient fungi
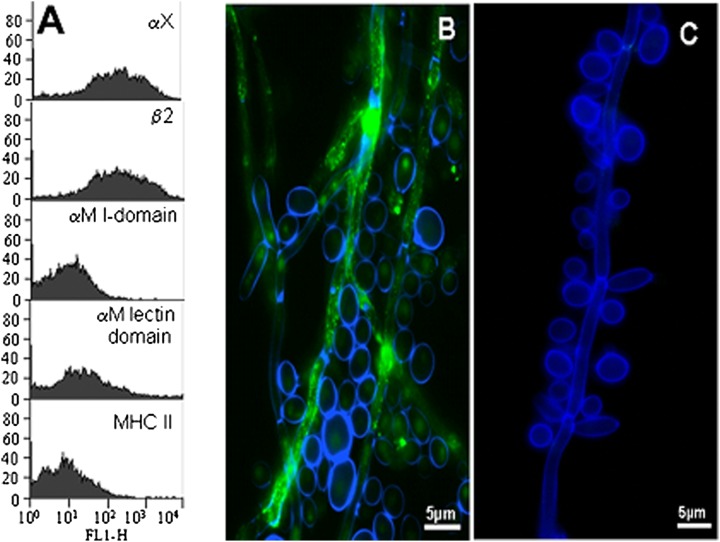
To access the specificity of αXβ2–C. albicans interaction, a HEK293 cell line stably expressing the integrin was developed. Full-length cDNAs of human αX and β2 were cotransfected into HEK293, and positive cells were sorted and subcloned. The established cell line (HEK293/αXβ2 cells) then was examined by FACS using a panel of Abs: anti-human αX (mAb 3.9), anti-human β2 (IB4), anti-human αM I-domain (44a), anti-human αM lectin domain (OKM1), and control irrelevant mAb W6/32 (anti-human MHC-II). As expected, αX and β2 were highly expressed on the HEK293/αXβ2 cell surface (Fig. 5A). Although αX is highly homologous to αM, the anti-αM I-domain mAb 44a, which block binding of most ligands to αMβ2, did not recognize the HEK293/αXβ2 cells. Surprisingly, the anti-αM lectin domain mAb OKM1, which inhibits the binding of polysaccharides such as β-glucans and mannans to αMβ2 (70), also showed weak reactivity with αXβ2 (Fig. 5A) but not with mock-transfected HEK293 cells (data not shown). Thus, the αX subunit may contain structures similar to the αM lectin domain and therefore may recognize fungal polysaccharides and serve as a pattern recognition receptor.
FIGURE 5.
(A) Characterization of HEK293/αXβ2 cells by reactivity mAbs; (B, C) binding of purified αXβ2 to germinated C. albicans. (A) Characterization of HEK293/αXβ2 cells by FACS. A panel of Abs was used to examine the expression of αXβ2 Ags: anti-human αX (mAb 3.9), anti-human β2 (IB4), anti-human αM I-domain (44a), anti-human αM lectin domain (OKM1), and negative control irrelevant mAb W6/32 (anti-human MHC-II). Germinated C. albicans of WT strain SC5314 (B) or Pra1-depleted strain CAMB5-18 (C) labeled with Blankophore dye (blue fluorescence) was stained with and FITC-labeled purified αXβ2 (green fluorescence) and photographed using fluorescence microscopy. Scale bars (B, C), 5 μm.
Both αMβ2 and αXβ2 integrins have many ligands in common. Because the C. albicans hyphae surface protein Pra1 was identified as a major C. albicans ligand for αMβ2 (40), we considered whether this protein might also serve as a ligand for αXβ2. We tested HEK293/αXβ2 cells as a source of αXβ2 protein and the ability of purified αXβ2 to recognize two C. albicans strains, SC5314 (WT) and Pra1-nul mutant (CAMB5-18). The yeast of both fungal strains were allowed to germinate overnight for maximal Pra1 expression in the WT strain and the fungal cell wall chitin and glucans were stained with blue fluorescent dye Blankophor (62). Labeled fungi then were incubated with FITC-labeled αXβ2 and examined by fluorescence microscopy. The microphotograph shown in Fig. 5B clearly demonstrates that soluble αXβ2 (green fluorescence) bound the hyphal form of WT fungus but not the rounded yeast form of C. albicans (Fig. 5B). No binding to the Pra1-deficient strain CAMB5-18 (Fig. 5C) was detected, indicating that αXβ2 binds to Pra1 or to a C. albicans hyphal constituent regulated by Pra1.
Upon expression of αXβ2 HEK293 cells acquire ability to recognize, bind, and phagocytose C. albicans
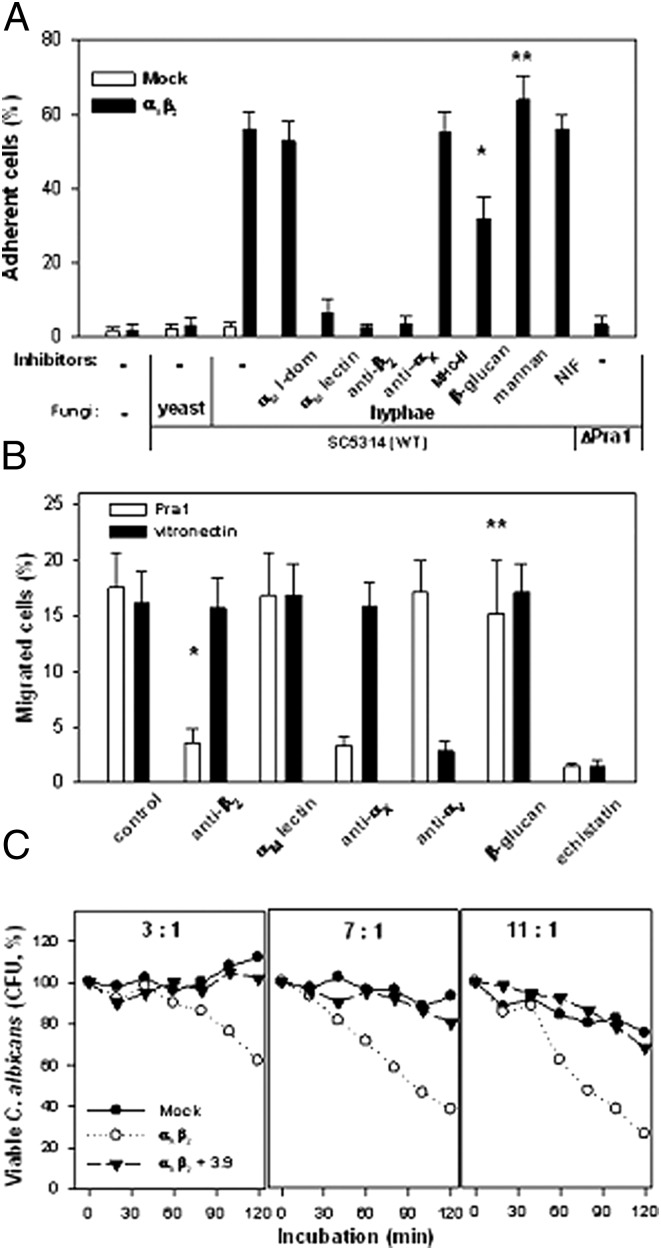
In the next set of experiments, the ability of αXβ2 to support HEK293 cell migration to C. albicans and adhesion to the fungi with subsequent phagocytosis was explored. HEK293 cells, expressing either αXβ2 or mock transfected, were added to plates with germinated C. albicans of SC5314 or CAMB5-18 strains. In some cases, plates with nongerminated fungi were also tested. After 30 min, unbound cells were washed away, and adherent cells were quantified using the CyQUANT fluorescent dye. In the absence of inhibitors, 58 ± 5% of αXβ2 cells adhered to the hyphal form of SC5314 strain, whereas they failed to adhere to the yeast form of this fungal strain. The control mock-transfected HEK293 cells did not adhere to any form of C. albicans SC5314 strain (Fig. 6A), suggesting that the adhesion is αXβ2 dependent. This conclusion was confirmed using a panel of αXβ2 and αMβ2 inhibitors. The adhesion of HEK293/αXβ2 was completely inhibited with anti-αX (3.9) and anti-β2 (IB4) mAbs, whereas the anti-αM lectin domain mAb OKM1 inhibited 80 ± 10% of the cell adhesion. In contrast, the anti-αM I-domain blocking mAbs 44a and NIF, a specific inhibitor of ligand binding to αMβ2 (58, 71) (NIF) as well as control W6/32 anti–MHC-II mAb, were ineffective. Soluble β-glucans at a concentration of 1 μg/ml inhibited adhesion of the HEK293/αXβ2 cells by 50 ± 5%. Surprisingly, baker yeast mannans, which blocks HEK293/αMβ2 adhesion to C. albicans (36), did not block adhesion of HEK293/αXβ2 cells (Fig. 6A). In control experiments, neither β-glucan nor mannan was able to inhibit αXβ2-supported adhesion to another αMβ2 ligand—the fibrinogen peptide P2C, a nonglycosylated ligand that is also recognized by αXβ2 (59, 72) (results not shown). Both cell lines were not able to adhere to germinated Pra1-deficient C. albicans strain CAMB5-18 (40) (Fig. 6A), again indicating that C. albicans recognition by αXβ2 is Pra1 dependent.
FIGURE 6.
Upon expression on surface of HEK293 cells αXβ2 supports adhesive, migratory and phagocytic activities of the cells toward C. albicans. (A) HEK293/αXβ2 cell adhesion to C. albicans. A total of 5 × 105 HEK293/αXβ2 (▪) or mock-transfected (□) cells were added to wells of tissue culture plates containing germinated (hyphae) or nongerminated (yeast) C. albicans of WT (SC5314) or ΔPra1 (CAMB5-18) strains. The plates were incubated at 37°C for 1 h, then nonadherent cells were removed by washing, and the adherent cells were quantified using the CyQUANT Cell Proliferation Assay kit. For inhibition assays, before addition to the plate wells, the cells were preincubated with 10 μg/ml of the Abs: anti-αM I-domain (44a), anti-αM lectin domain (OKM1), anti-β2 (IB4), anti-αX (3.9), and irrelevant anti–MHC-II (W6/32) or with 1 mM β-glucan, 1 mM mannan, or 5 μM recombinant NIF for 10 min at room temperature. (B) HEK293/αXβ2 cell migration to C. albicans. Cell migration was measured in Boyden chambers (Costar Transwell with 8-μm porosity in a 24-well format). A total of 105 HEK293/αXβ2 cells were added to the upper chamber, whereas lower chambers contained 106 germinated C. albicans cells of WT SC5314 strain (□) or 10 mM vitronectin (▪) in serum-free DMEM/F-12 medium. The inhibitors mAbs IB4, OKM1, 3.9 (see above), anti-αV (27217E6), and 1 mM β-glucan or echistatin were added with the cells to the upper chamber. Plates were incubated for 8 h in a humidified incubator at 37°C and 5% CO2. The migrated cells were counted using the CyQUANT Cell Proliferation kit. (C) Phagocytosis of C. albicans by HEK293/αXβ2 cells. A total of 105 germinated C. albicans SC5314 strain cells were incubated with 3 × 105 (1:3 ratio), 7 × 105 (1:7 ratio), or 1.1 × 106 (1:11 ratio) mock-transfected HEK293 cells (●) or HEK293/αXβ2 cells in the presence (▴) or absence (○) of anti-αX mAbs 3.9. Fungal survival was determined every 20 min by plating the sample aliquots onto SDA plates in serial dilutions. Results are presented as percentage (mean ± SE) of total cells (to which was assigned the value of 100%) and represent the results of three independent experiments, each in triplicate. *p < 0.05 (t test), **p > 0.05.
Next, we tested migration of αXβ2 cells to C. albicans conditioned medium, a source of soluble Pra1 (40), in the presence or absence of integrin inhibitors. After 8 h, in the absence of inhibitors, 17 ± 4% αXβ2 cells migrated to fungal supernatant. The anti-β2 mAb IB4, at 20 μg/ml, reduced cell migration to fungal supernatant to 4 ± 1%. The same effect was observed in the presence of anti-αX mAb 3.9, which also inhibited migration to C. albicans supernatant. As a control, we also measured migration of the cells to vitronectin, which is mediated primarily by endogenous αV integrins on these cells. Anti-αV mAb 272-17E6 inhibited migration of the cells to vitronectin to 4.4 ± 3.5% but had no affect on migration of the cells to the fungal supernatant. Echistatin, a snake venom disintegrin that inhibits ligand recognition by most integrins, including the β2 and αV integrins (61), completely reduced cell migration to both Candida supernatant and vitronectin (Fig. 6B). Taken together, these data indicate that migration of αXβ2 cells to C. albicans proteins is integrin dependent, and αXβ2 is implicated in this response of the HEK293/αXβ2 cells. Notably, β-glucan and anti-αM lectin domain mAb OKM1, which inhibited adhesion of αXβ2 cells to C. albicans, did not affect migration of these cells to the fungal extracellular proteins (Fig. 6B), suggesting that the lectin specificity of αXβ2 is involved in adhesion but not migration to C. albicans.
The ability of αXβ2 to promote antifungal activity of HEK293 cells was also tested. Because fibroblasts such as HEK293 possess weak endogenous antifungal activity and are able to internalize pathogens by passive endocytosis (73, 74), we wished to distinguish passive nonspecific endocytosis from specific phagocytosis, and various ratios of fungi/cells were tested in killing assays. Germinated C. albicans cells of the SC5314 strain were coincubated with αXβ2 or mock-transfected HEK293 at fungi/HEK293 cell ratios of 1:3, 1:7, or 1:11 for 2 h. Aliquots were taken every 20 min, and the amounts of viable fungi remaining were quantified as CFU by plating aliquots in a series of dilutions onto agar plates. Only 58 ± 6% of the initial fungi remained viable after a 2-h incubation with the αXβ2 cells at the 1:3 ratio. At a 1:7 ratio, the amount of surviving fungi decreased to 42 ± 5% and fell to 28 ± 6% at a 1:11 ratio. In contrast, with mock-transfected HEK293 cells, fungal survival was 76 ± 6 and 82 ± 5% at the 1:11 and 1:7 ratios, respectively, and all fungi survived at the ratio 1:3 (Fig. 6C). Preincubation of αXβ2 cells with anti-αX blocking mAb 3.9 completely inhibited the antifungal activity of the αXβ2 cells to levels observed with mock-transfected HEK293 cells (p > 0.5). These results indicate that αXβ2 is able to promote phagocytosis of C. albicans.
Discussion
In the present work, we demonstrate the importance of integrin αXβ2 for protection against C. albicans systemic infection by the innate immune system. Although the ability of αXβ2 to recognize C3bi and thereby to assist in the elimination of opsonized particles has been described previously (48), to our knowledge, our study is the first demonstration of involvement of this integrin in direct pathogen recognition and elimination by leukocytes as well as its critical importance in the control of fungal invasion to brain and liver by certain subsets of tissue residential macrophages (see below).
Our data on the murine organ fungal burdens indicate that the αXβ2 elimination affects mainly the liver and brain, dramatically increasing invasion and propagation of the fungus in these organs. This effect of αXβ2 became evident at the earliest stages of infection: as early as16 h after the challenge, a 2-fold difference (p < 0.05) emerged in fungal burdens in the livers of ΔαX and WT mice and a 10-fold difference (p < 0.01) in the brains. At 40 h postinfection, this difference in the susceptibility of the αXβ2-deficient mice and WT animals reached 6-fold (p < 0.01) in the liver and >40-fold (p < 0.005) in the brain.
The integrins of the β2 subfamily, known collectively as “leukocyte integrins,” are expressed predominantly on the surface of leukocytes (23, 75). In our experiments, immunostaining of infected brain and liver sections revealed that only the CD45+ hematopoietic cells in these tissues express αX [also see (76)], and thus, αXβ2 elimination is likely to affect leukocyte function only.
The kidney and the brain are the primary targets for C. albicans during systemic infection. The fungi invade these organs directly from the bloodstream, and invasion can start during the first minutes postinfection (67). The blood immune mechanisms (e.g., monocytes, neutrophils, NK lymphocytes, and cells of the blood–brain barrier) provide little protection from neuroinvasion during the initial stages of systemic infection. Blood cells can clarify the bloodstream of sublethal doses of C. albicans only after 20 h of infection, and, in the case of near-lethal doses, fungal CFUs can be detected in the blood even after 24–30 h postinfection (67). The i.v. route for the fungal injection bypasses possible contact of the fungi with tissue macrophages. To circumvent the blood–brain barrier, C. albicans uses a unique mechanism of invasion: upon binding to gp96 heat shock protein and/or to N-cadherin on the surface of normally nonphagocytic brain microvascular epithelial cells, fungi stimulate their own uptake (77, 78). Therefore, in our model, the difference in organ fungal burdens of WT and ΔαX in mice appears to be due to differences in activity of the organ-resident macrophage subsets, microglial cells in brain, and Kupffer cells in liver.
Existing literature present extensive evidence that microglia play the principal role in the protection against C. albicans intracerebral infections. Direct proof of their crucial role was provided by the demonstration that intracerebral transfer of microglial cells provides complete protection (100% survival) against subsequent intracerebral challenge with a lethal inoculum of the fungus. After i.v. challenge with near-lethal C. albicans inoculums, the concentration of fungal CFUs in the brain rapidly increases and reaches maximal level at ∼24 h infection. Then, the fungal burden in brain stabilizes and remains at this level until days 7–8 with a subsequent slow decline (67). This time course implies that 24 h is sufficient for microglial activation and conversion to “brain macrophages,” and the migration to the fungus to contain infection and corresponds well with our data, demonstrating that after 40 h most brain CD45+CD11c+ cells in WT have migrated and assembled around the hyphal-like structures of C. albicans. In αXβ2-deficient mice, the CD45+CD11c− cells in the brain remained diffusely distributed, suggesting that αXβ2 is required for these cells to migrate to and recognize C. albicans.
Kupffer cells are the specialized phagocytic cells found on the luminal surface of hepatic sinusoids (79). These cells are of monocytoid lineage (79, 80) and express both αXβ2 and αMβ2 integrins (81–83), and their importance for protection against C. albicans invasion has been demonstrated previously (84–87). The possible involvement of integrin αXβ2 in phagocytosis of C. albicans by microglial and Kupffer cells has been proposed (84, 88, 89). Taken together, these data suggest that αXβ2 but not αMβ2 is critical in antifungal activity of tissue-resident macrophage subsets. This conclusion is consistent with the previous report demonstrating that increased expression of αXβ2 results in enhanced phagocytosis of M. tuberculosis by human macrophages (49).
Integrins on the surface of nonstimulated leukocytes are expressed in inactive “closed” conformation and require activation to recognize their ligands with high affinity. During inflammation, various physiological agonists induce activation of specific integrins. Thus, αXβ2 may become activated, whereas αMβ2 remains in an inactive conformation or vice versa, and therefore, these two β2 integrins may differentially participate in leukocyte function despite both being expressed on the leukocyte surface (19). Activation of peripheral blood monocytes in vitro with PMA results in activation of all leukocyte integrins. For this reason, anti-αXβ2 mAbs block adhesion of PMA-activated monocytes only partially. The only anti-αM mAb that blocks adhesion to the fungus is directed to the αM lectin domain, and as we have shown, this mAb also cross-reacts with a previously unrecognized lectin domain within the αX subunit.
β-Glucans and mannans are important immunomodulators, and their binding by leukocytes is implemented by integrin αMβ2 (90, 91). Upon ligation with the integrin, β-glucans activate αMβ2 and stabilize it in an intermediate active conformation (92).Unlike αMβ2, where the carbohydrate binding and sugar selectivity of its αM-lectin domain are well characterized (e.g., (93–95)), there is no evidence in the literature for recognition of fungal glycans or bacterial LPS by αXβ2. Therefore, our observation that activity of αXβ2 is modulated by fungal β-glucans is a novel finding of our present work. On the basis of ∼70% homology between αM and αX and that the OKM1 mAb, which blocks glycan binding to αM (70), weakly cross-reacts with αX, we anticipate certain similarities in the sugar specificity of these integrin subunits. However, αMβ2 and αXβ2 demonstrated clear distinction in carbohydrate selectivity: although αMβ2 recognizes both β-glucans and mannans, the activity of αXβ2 appears to be modulated by β-glucans but not by mannans. In our experiments, mannans, unlike β-glucans, were not able to inhibit adhesion of HEK293/αXβ2 to C. albicans hyphae. The observed differences in sugar selectivity of the integrins may play an important role in the regulation of leukocyte activation and differentiation (95, 96).
In the present work, direct interaction between purified αXβ2 and Pra1 was not tested directly. Therefore, we cannot exclude the possibility that another C. albicans hyphal protein that is regulated by Pra1 may serve as a ligand for αXβ2. However, the existing literature provides no evidence for such a molecule. Thus, our findings that purified αXβ2 interacts with C. albicans hyphae but not with the yeast form and that the HEK293/αXβ2 cells recognize and adhere to hyphae of WT C. albicans strain SC5314, but not of Pra1-deficient strain CAMB5-18, provide strong evidence that Pra1 serves as C. albicans ligand for αXβ2.
The integrin αXβ2 is usually present on the surface of leukocyte subsets together with another member of the β2 integrin family, αMβ2, to which such primary antipathogen leukocyte activities, such as recognition of bacterial LPS and fungal mannoproteins, are traditionally ascribed (12, 17, 24). We speculate that αMβ2 and αXβ2 integrins may play complementary roles in executing cellular immunity or that different cellular agonists may favor activation and utilization of one particular integrin. Our data showing significantly reduced resistance of αXβ2-deficient mice to Candida invasion and the αXβ2 requirement for fungal recognition and killing by macrophages clearly demonstrate that αXβ2 plays an independent role in the defense against fungal infections and does not simply serve as an auxiliary receptor for pathogens, secondary to αMβ2.
Taken together, these data clearly demonstrate the importance of αXβ2 in protection against C. albicans systemic infection, and this protective effect is mediated by subsets of tissue residential macrophages.
Acknowledgments
We thank Dr. Christy A. Ballantyne of Baylor College of Medicine for the ΔαX mice, Dr. William A. Fonzi of Georgetown University for C. albicans ΔPra1 strain, Earl Poptic from Cleveland Clinic Hybridoma Core for help with large-scale Ab preparation, Carla Drumm (Cleveland Clinic) for help with mouse husbandry, and Rajani Tendulkar (Cleveland Clinic) for excellent administrative support of the project.
This work was supported by National Institute for Allergy and Infectious Diseases Grant AIO 80596.
- D-PBS
- Dulbecco's PBS
- HEK293
- human epithelial kidney 293
- HEK293/αXβ2 cell
- the HEK293 cell expressing αXβ2 on its surface
- KO
- knockout
- LAD-1
- leukocyte adhesion deficit, type 1
- MHC-II
- MHC class II
- NIF
- canine hookworm neutrophil inhibitory factor
- OG
- octyl-β-d-glucopyranoside
- Pra1
- pH-regulated Ag 1
- SDA
- Sabouraud Dextrose Agar
- WT
- wild-type
- ΔαX mice
- mice depleted in αXβ2.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chaffin W. L., López-Ribot J. L., Casanova M., Gozalbo D., Martínez J. P. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62: 130–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corner B. E., Magee P. T. 1997. Candida pathogenesis: unravelling the threads of infection. Curr. Biol. 7: R691–R694 [DOI] [PubMed] [Google Scholar]
- 3.Muhlschlegal F., Fonzi W. A., Hoyer L. W., Payne T., Poulet F. M., Clevenger J., Latgé J. P., Calera J., Beauvais A., Paris S., et al. 1998. Molecular mechanisms of virulence in fungus-host interactions for Aspergillus fumigatus and Candida albicans. Med. Mycol. 36(Suppl 1): 238–248 [PubMed] [Google Scholar]
- 4.Calderone R. A., Fonzi W. A. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9: 327–335 [DOI] [PubMed] [Google Scholar]
- 5.Lo H. J., Köhler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G. R. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949 [DOI] [PubMed] [Google Scholar]
- 6.Saville S. P., Lazzell A. L., Monteagudo C., Lopez-Ribot J. L. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snydman D. R. 2003. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 123(Suppl. 5)500S–503S [DOI] [PubMed] [Google Scholar]
- 8.Abelson J. A., Moore T., Bruckner D., Deville J., Nielsen K. 2005. Frequency of fungemia in hospitalized pediatric inpatients over 11 years at a tertiary care institution. Pediatrics 116: 61–67 [DOI] [PubMed] [Google Scholar]
- 9.Cole G. T., Halawa A. A., Anaissie E. J. 1996. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin. Infect. Dis. 22(Suppl. 2): 73–88 [DOI] [PubMed] [Google Scholar]
- 10.Wingard J. R. 1994. Infections due to resistant Candida species in patients with cancer who are receiving chemotherapy. Clin. Infect. Dis. 19(Suppl. 1): S49–S53 [DOI] [PubMed] [Google Scholar]
- 11.Richardson M. D. 2005. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56(Suppl. 1): i5–i11 [DOI] [PubMed] [Google Scholar]
- 12.Diamond R. D. 1993. Interactions of phagocytic cells with Candida and other opportunistic fungi. Arch. Med. Res. 24: 361–369 [PubMed] [Google Scholar]
- 13.Netea M. G., Gijzen K., Coolen N., Verschueren I., Figdor C. G., Van der Meer J. W., Torensma R., Kullberg B. J. 2004. Human dendritic cells are less potent at killing Candida albicans than both monocytes and macrophages. Microbes Infect. 6: 985–989 [DOI] [PubMed] [Google Scholar]
- 14.Mahanty S., Greenfield R. A., Joyce W. A., Kincade P. W. 1988. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect. Immun. 56: 3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baghian A., Lee K. W. 1988. Role of activated macrophages in resistance to systemic candidosis. J. Leukoc. Biol. 44: 166–171 [DOI] [PubMed] [Google Scholar]
- 16.Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. 1986. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51: 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayadas T. N., Cullere X. 2005. Neutrophil β2 integrins: moderators of life or death decisions. Trends Immunol. 26: 388–395 [DOI] [PubMed] [Google Scholar]
- 18.McFarland H. I., Nahill S. R., Maciaszek J. W., Welsh R. M. 1992. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J. Immunol. 149: 1326–1333 [PubMed] [Google Scholar]
- 19.Arnaout M. A., Lanier L. L., Faller D. V. 1988. Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J. Cell. Physiol. 137: 305–309 [DOI] [PubMed] [Google Scholar]
- 20.Larson R. S., Springer T. A. 1990. Structure and function of leukocyte integrins. Immunol. Rev. 114: 181–217 [DOI] [PubMed] [Google Scholar]
- 21.Arnaout M. A., Michishita M., Sharma C. P. 1992. On the regulation of β2 integrins. Adv. Exp. Med. Biol. 323: 171–179 [DOI] [PubMed] [Google Scholar]
- 22.Stewart M., Thiel M., Hogg N. 1995. Leukocyte integrins. Curr. Opin. Cell Biol. 7: 690–696 [DOI] [PubMed] [Google Scholar]
- 23.Harris E. S., McIntyre T. M., Prescott S. M., Zimmerman G. A. 2000. The leukocyte integrins. J. Biol. Chem. 275: 23409–23412 [DOI] [PubMed] [Google Scholar]
- 24.Bajtay Z., Speth C., Erdei A., Dierich M. P. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 173: 4775–4778 [DOI] [PubMed] [Google Scholar]
- 25.Kuijpers T. W., van de Vijver E., Weterman M. A., de Boer M., Tool A. T., van den Berg T. K., Moser M., Jakobs M. E., Seeger K., Sanal O., et al. 2009. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113: 4740–4746 [DOI] [PubMed] [Google Scholar]
- 26.Anderson D. C., Schmalstieg F. C., Shearer W., Becker-Freeman K., Kohl S., Smith C. W., Tosi M. F., Springer T. 1985. Leukocyte LFA-1, OKM1, p150,95 deficiency syndrome: functional and biosynthetic studies of three kindreds. Fed. Proc. 44: 2671–2677 [PubMed] [Google Scholar]
- 27.Andrews T., Sullivan K. E. 2003. Infections in patients with inherited defects in phagocytic function. Clin. Microbiol. Rev. 16: 597–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klempner M. S., Malech H. L. 2003. Phagocytes: normal and abnormal neutrophil host defenses. In Infectious Diseases, 3rd ed. Gorbach S. L., Bartlett J. G., Blacklow N. R., eds. Lippincott Williams & Wilkins, Philadelphia: p. 14‑39 [Google Scholar]
- 29.Soloviev D. A., Jawhara S., Fonzi W. A. 2011. Regulation of innate immune response to Candida albicans infections by αMβ2-Pra1p interaction. Infect. Immun. 79: 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown G. D., Taylor P. R., Reid D. M., Willment J. A., Williams D. L., Martinez-Pomares L., Wong S. Y., Gordon S. 2002. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196: 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netea M. G., Sutmuller R., Hermann C., Van der Graaf C. A., Van der Meer J. W., van Krieken J. H., Hartung T., Adema G., Kullberg B. J. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172: 3712–3718 [DOI] [PubMed] [Google Scholar]
- 32.Netea M. G., Van Der Graaf C. A., Vonk A. G., Verschueren I., Van Der Meer J. W., Kullberg B. J. 2002. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185: 1483–1489 [DOI] [PubMed] [Google Scholar]
- 33.Harokopakis E., Albzreh M. H., Martin M. H., Hajishengallis G. 2006. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 176: 7645–7656 [DOI] [PubMed] [Google Scholar]
- 34.Sorgi C. A., Secatto A., Fontanari C., Turato W. M., Belangér C., de Medeiros A. I., Kashima S., Marleau S., Covas D. T., Bozza P. T., Faccioli L. H. 2009. Histoplasma capsulatum cell wall β-glucan induces lipid body formation through CD18, TLR2, and dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection. J. Immunol. 182: 4025–4035 [DOI] [PubMed] [Google Scholar]
- 35.Forsyth C. B., Mathews H. L. 1996. Lymphocytes utilize CD11b/CD18 for adhesion to Candida albicans. Cell. Immunol. 170: 91–100 [DOI] [PubMed] [Google Scholar]
- 36.Forsyth C. B., Plow E. F., Zhang L. 1998. Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CD18 subunit. J. Immunol. 161: 6198–6205 [PubMed] [Google Scholar]
- 37.Sentandreu M., Elorza M. V., Sentandreu R., Fonzi W. A. 1998. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J. Bacteriol. 180: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Ribot J. L., Sepúlveda P., Cervera A. M., Roig P., Gozalbo D., Martínez J. P. 1997. Cloning of a cDNA fragment encoding part of the protein moiety of the 58-kDa fibrinogen-binding mannoprotein of Candida albicans. FEMS Microbiol. Lett. 157: 273–278 [DOI] [PubMed] [Google Scholar]
- 39.Casanova M., Lopez-Ribot J. L., Monteagudo C., Llombart-Bosch A., Sentandreu R., Martinez J. P. 1992. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect. Immun. 60: 4221–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soloviev D. A., Fonzi W. A., Sentandreu R., Pluskota E., Forsyth C. B., Yadav S. P., Plow E. F. 2007. Identification of pH-regulated antigen 1 released from Candida albicans as the major ligand for leukocyte integrin αMβ2. J. Immunol. 178: 2038–2046 [DOI] [PubMed] [Google Scholar]
- 41.De Bernardis F., Mühlschlegel F. A., Cassone A., Fonzi W. A. 1998. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect. Immun. 66: 3317–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramon A. M., Porta A., Fonzi W. A. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181: 7524–7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo S., Poltermann S., Kunert A., Rupp S., Zipfel P. F. 2009. Immune evasion of the human pathogenic yeast Candida albicans: Pra1 is a Factor H, FHL-1 and plasminogen binding surface protein. Mol. Immunol. 47: 541–550 [DOI] [PubMed] [Google Scholar]
- 44.Hogg N., Bates P. A. 2000. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biol. 19: 211–222 [DOI] [PubMed] [Google Scholar]
- 45.Lanier L. L., Arnaout M. A., Schwarting R., Warner N. L., Ross G. D. 1985. p150/95, third member of the LFA-1/CR3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur. J. Immunol. 15: 713–718 [DOI] [PubMed] [Google Scholar]
- 46.Nham S. U. 1999. Characteristics of fibrinogen binding to the domain of CD11c, an α subunit of p150,95. Biochem. Biophys. Res. Commun. 264: 630–634 [DOI] [PubMed] [Google Scholar]
- 47.Frick C., Odermatt A., Zen K., Mandell K. J., Edens H., Portmann R., Mazzucchelli L., Jaye D. L., Parkos C. A. 2005. Interaction of ICAM-1 with β2-integrin CD11c/CD18: characterization of a peptide ligand that mimics a putative binding site on domain D4 of ICAM-1. Eur. J. Immunol. 35: 3610–3621 [DOI] [PubMed] [Google Scholar]
- 48.Bilsland C. A., Diamond M. S., Springer T. A. 1994. The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous beta subunit and localization of a ligand recognition site to the I domain. J. Immunol. 152: 4582–4589 [PubMed] [Google Scholar]
- 49.Rosas-Taraco A. G., Salinas-Carmona M. C., Revol A., Rendon A., Caballero-Olin G., Arce-Mendoza A. Y. 2009. Expression of CDllc in blood monocytes as biomarker for favorable response to antituberculosis treatment. Arch. Med. Res. 40: 128–131 [DOI] [PubMed] [Google Scholar]
- 50.Schlesinger L. S., Horwitz M. A. 1991. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-γ activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147: 1983–1994 [PubMed] [Google Scholar]
- 51.Hellmig S., Mascheretti S., Renz J., Frenzel H., Jelschen F., Rehbein J. K., Fölsch U., Hampe J., Schreiber S. 2005. Haplotype analysis of the CD11 gene cluster in patients with chronic Helicobacter pylori infection and gastric ulcer disease. Tissue Antigens 65: 271–274 [DOI] [PubMed] [Google Scholar]
- 52.Porta A., Ramon A. M., Fonzi W. A. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181: 7516–7523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu H., Smith C. W., Perrard J., Bullard D., Tang L., Shappell S. B., Entman M. L., Beaudet A. L., Ballantyne C. M. 1997. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1‑deficient mice. J. Clin. Invest. 99: 1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenkranz A. R., Coxon A., Maurer M., Gurish M. F., Austen K. F., Friend D. S., Galli S. J., Mayadas T. N. 1998. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J. Immunol. 161: 6463–6467 [PubMed] [Google Scholar]
- 55.Ding Z. M., Babensee J. E., Simon S. I., Lu H., Perrard J. L., Bullard D. C., Dai X. Y., Bromley S. K., Dustin M. L., Entman M. L., et al. 1999. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163: 5029–5038 [PubMed] [Google Scholar]
- 56.Pluskota E., Woody N. M., Szpak D., Ballantyne C. M., Soloviev D. A., Simon D. I., Plow E. F. 2008. Expression, activation, and function of integrin αMβ2 (Mac-1) on neutrophil-derived microparticles. Blood 112: 2327–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Plow E. F. 1996. A discrete site modulates activation of I domains: application to integrin αMβ2. J. Biol. Chem. 271: 29953–29957 [DOI] [PubMed] [Google Scholar]
- 58.Zhang L., Plow E. F. 1996. Overlapping, but not identical sites, are involved in the recognition of C3bi, NIF, and adhesive ligands by the αMβ2 integrins. J. Biol. Chem. 271: 18211–18216 [DOI] [PubMed] [Google Scholar]
- 59.Solovjov D. A., Pluskota E., Plow E. F. 2005. Distinct roles for the α and β subunits in the functions of integrin αMβ2. J. Biol. Chem. 280: 1336–1345 [DOI] [PubMed] [Google Scholar]
- 60.Kumar C. C., Nie H., Rogers C. P., Malkowski M., Maxwell E., Catino J. J., Armstrong L. 1997. Biochemical characterization of the binding of echistatin to integrin αvβ3 receptor. J. Pharmacol. Exp. Ther. 283: 843–853 [PubMed] [Google Scholar]
- 61.Forsyth C. B., Mathews H. L. 2002. Lymphocyte adhesion to Candida albicans. Infect. Immun. 70: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rüchel R., Schaffrinski M. 1999. Versatile fluorescent staining of fungi in clinical specimens by using the optical brightener Blankophor. J. Clin. Microbiol. 37: 2694–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soloviev D. A., Pluskota E., Plow E. F. 2006. Cell adhesion and migration assays. In Methods in Molecular Medicine. Wang Q., ed. Vol. 129 Humana Press, Totowa, NJ, p. 267–278 [DOI] [PubMed] [Google Scholar]
- 64.Pluskota E., Soloviev D. A., Bdeir K., Cines D. B., Plow E. F. 2004. Integrin αMβ2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J. Biol. Chem. 279: 18063–18072 [DOI] [PubMed] [Google Scholar]
- 65.Forsyth C. B., Solovjov D. A., Ugarova T. P., Plow E. F. 2001. Integrin αMβ2-mediated cell migration to fibrinogen and its recognition peptides. J. Exp. Med. 193: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehrer R. I., Cline M. J. 1969. Interaction of Candida albicans with human leukocytes and serum. J. Bacteriol. 98: 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacCallum D. M., Odds F. C. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48: 151–161 [DOI] [PubMed] [Google Scholar]
- 68.Aldred A. J., Cha M. C., Meckling-Gill K. A. 2002. Determination of a humane endpoint in the L1210 model of murine leukemia. Contemp. Top. Lab. Anim. Sci. 41: 24–27 [PubMed] [Google Scholar]
- 69.Spellberg B., Ibrahim A. S., Edwards J. E., Jr., Filler S. G. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192: 336–343 [DOI] [PubMed] [Google Scholar]
- 70.Ross G. D., Cain J. A., Lachmann P. J. 1985. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J. Immunol. 134: 3307–3315 [PubMed] [Google Scholar]
- 71.Zhang L., Plow E. F. 1997. Identification and reconstruction of the binding site within αMβ2 for a specific and high affinity ligand, NIF. J. Biol. Chem. 272: 17558–17564 [DOI] [PubMed] [Google Scholar]
- 72.Ugarova T. P., Solovjov D. A., Zhang L., Loukinov D. I., Yee V. C., Medved L. V., Plow E. F. 1998. Identification of a novel recognition sequence for integrin αMβ2 within the γ-chain of fibrinogen. J. Biol. Chem. 273: 22519–22527 [DOI] [PubMed] [Google Scholar]
- 73.Ginsburg I., Sela M. N., Morag A., Ravid Z., Duchan Z., Ferne M., Rabinowitz-Bergner S., Thomas P. P., Davies P., Niccols J., et al. 1981. Role of leukocyte factors and cationic polyelectrolytes in phagocytosis of group A streptococci and Candida albicans by neutrophils, macrophages, fibroblasts and epithelial cells: modulation by anionic polyelectrolytes in relation to pathogenesis of chronic inflammation. Inflammation 5: 289–312 [DOI] [PubMed] [Google Scholar]
- 74.Bodo M., Becchetti E., Baroni T., Mocci S., Merletti L., Giammarioli M., Calvitti M., Sbaraglia G. 1995. Internalization of Candida albicans and cytoskeletal organization in macrophages and fibroblasts treated with concanavalin A. Cell. Mol. Biol. (Noisy-le-grand) 41: 297–305 [PubMed] [Google Scholar]
- 75.Margadant C., Monsuur H. N., Norman J. C., Sonnenberg A. 2011. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23: 607–614 [DOI] [PubMed] [Google Scholar]
- 76.Becher B., Antel J. P. 1996. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18: 1–10 [DOI] [PubMed] [Google Scholar]
- 77.Jong A. Y., Stins M. F., Huang S. H., Chen S. H., Kim K. S. 2001. Traversal of Candida albicans across human blood‑brain barrier in vitro. Infect. Immun. 69: 4536–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y., Mittal R., Solis N. V., Prasadarao N. V., Filler S. G. 2011. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 7: e1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naito M., Hasegawa G., Takahashi K. 1997. Development, differentiation, and maturation of Kupffer cells. Microsc. Res. Tech. 39: 350–364 [DOI] [PubMed] [Google Scholar]
- 80.Lawson L. J., Perry V. H., Gordon S. 1992. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48: 405–415 [DOI] [PubMed] [Google Scholar]
- 81.Akiyama H., McGeer P. L. 1990. Brain microglia constitutively express β2 integrins. J. Neuroimmunol. 30: 81–93 [DOI] [PubMed] [Google Scholar]
- 82.Helmy K. Y., Katschke K. J., Jr., Gorgani N. N., Kljavin N. M., Elliott J. M., Diehl L., Scales S. J., Ghilardi N., van Lookeren Campagne M. 2006. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124: 915–927 [DOI] [PubMed] [Google Scholar]
- 83.Hinglais N., Kazatchkine M. D., Mandet C., Appay M. D., Bariety J. 1989. Human liver Kupffer cells express CR1, CR3, and CR4 complement receptor antigens: an immunohistochemical study. Lab. Invest. 61: 509–514 [PubMed] [Google Scholar]
- 84.Lionakis M. S., Lim J. K., Lee C. C., Murphy P. M. 2011. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun. 3: 180–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redmond H. P., Shou J., Gallagher H. J., Kelly C. J., Daly J. M. 1993. Macrophage-dependent candidacidal mechanisms in the murine system: comparison of murine Kupffer cell and peritoneal macrophage candidacidal mechanisms. J. Immunol. 150: 3427–3433 [PubMed] [Google Scholar]
- 86.Blasi E., Mazzolla R., Barluzzi R., Bistoni F. 1991. Microglial cell-mediated anti-Candida activity: temperature, ions, protein kinase C as crucial elements. J. Neuroimmunol. 34: 53–60 [DOI] [PubMed] [Google Scholar]
- 87.Neglia R., Colombari B., Peppoloni S., Orsi C., Tavanti A., Senesi S., Blasi E. 2006. Adaptive response of microglial cells to in vitro infection by Candida albicans isolates with different genomic backgrounds. Microb. Pathog. 41: 251–256 [DOI] [PubMed] [Google Scholar]
- 88.Toth C. A., Thomas P. 1992. Liver endocytosis and Kupffer cells. Hepatology 16: 255–266 [DOI] [PubMed] [Google Scholar]
- 89.Yan J., Vetvicka V., Xia Y., Hanikýrová M., Mayadas T. N., Ross G. D. 2000. Critical role of Kupffer cell CR3 (CD11b/CD18) in the clearance of IgM-opsonized erythrocytes or soluble β-glucan. Immunopharmacology 46: 39–54 [DOI] [PubMed] [Google Scholar]
- 90.Vetvicka V., Dvorak B., Vetvickova J., Richter J., Krizan J., Sima P., Yvin J.-C. 2007. Orally administered marine (1→3)-β-d-glucan Phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol. 40: 291–298 [DOI] [PubMed] [Google Scholar]
- 91.Chan G. C., Chan W. K., Sze D. M. 2009. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien X. M., Heflin K. E., Lavigne L. M., Yu K., Kim M., Salomon A. R., Reichner J. S. 2012. Lectin site ligation of CR3 induces conformational changes and signaling. J. Biol. Chem. 287: 3337–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thornton B. P., Vĕtvicka V., Pitman M., Goldman R. C., Ross G. D. 1996. Analysis of the sugar specificity and molecular location of the β-glucan‑binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156: 1235–1246 [PubMed] [Google Scholar]
- 94.Xia Y., Vetvicka V., Yan J., Hanikýrová M., Mayadas T., Ross G. D. 1999. The β-glucan‑binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 162: 2281–2290 [PubMed] [Google Scholar]
- 95.Ross G. D. 2000. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/αMβ2-integrin glycoprotein. Crit. Rev. Immunol. 20: 197–222 [PubMed] [Google Scholar]
- 96. Agarwal, S., C. A. Specht, H. Huang, G. R. Ostroff, S. Ram, P. A. Rice, and A. Levitzki. 2011. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. MBio 2: e00178-11. [DOI] [PMC free article] [PubMed]