Abstract
In activated macrophages, the anti-inflammatory cytokine IL-10 inhibits expression of molecules that propagate inflammation in a manner that depends on transcription factor STAT3. Expression of IL-10 is regulated post-transcriptionally by the RNA-binding protein tristetraprolin (TTP), which destabilizes IL-10 mRNA in activated macrophages. Using LPS-activated bone marrow-derived murine macrophages, we demonstrate that TTP is a negative regulator of the IL-10/STAT3 anti-inflammatory response. LPS-stimulated TTP-deficient macrophages overproduced IL-10, contained increased amounts of activated STAT3, and showed reduced expression of inflammatory cytokines, including cytokines encoded by TTP-target mRNAs. Thus, in LPS-stimulated TTP-deficient macrophages, increased IL-10/STAT3 anti-inflammatory control was dominant over the mRNA-stabilization of specific TTP targets. The TTP gene promoter contains a conserved STAT3 binding site and IL-10 induces STAT3 recruitment to this site. Correspondingly, STAT3 was required for efficient IL-10-induced TTP expression. Hence, by inducing TTP expression, STAT3 activates a negative regulatory loop that controls the IL-10/STAT3 anti-inflammatory response.
Introduction
IL-10 plays a key role in limiting inflammation and maintaining immune homeostasis. The anti-inflammatory function of IL-10 is demonstrated by the phenotype of IL-10-deficient mice which develop severe inflammatory bowel disease due to spontaneous chronic inflammation (1) and by patients with early-onset colitis who contain homozygous mutations in IL-10 receptor subunits (2). During acute inflammation, IL-10 is primarily produced by macrophages and dendritic cells (3) and functions to limit expression of pro-inflammatory cytokines and chemokines (4). Although IL-10 is necessary for attenuating inflammatory and autoimmune pathologies (1, 2, 5), negative control of IL-10 production is also critical, as excessive IL-10 could prevent beneficial inflammation and exert immunosuppressive effects (6–8).
IL-10 is negatively regulated at the post-transcriptional level through cis-acting adenine and uridine-rich elements (AREs)3 in the 3′-untranslated region of its mRNA which act together with the trans-acting ARE-binding protein, tristetraprolin (TTP, also known as TIS11, ZFP36, and Nup475) to destabilize IL-10 mRNA (9, 10). TTP is encoded by the gene Zfp-36 (referred to herein as Ttp) and is expressed at very low levels in unstimulated cells but is rapidly induced by LPS and TNF in macrophages (11). Ttp promoter analysis suggested that the response to mitogens is mediated by a series of cis-acting elements acting in concert to confer fully inducible transcription (12). Among these elements, a previously unknown cis-acting 10 base-pair sequence, termed TTP promoter element 1 (TPE1), was identified as a key site for serum-induced TTP expression (12).
Ttp knock-out (Ttp−/−) mice display a severe inflammatory phenotype that is largely attributed to increased production of TNF, a pro-inflammatory cytokine whose mRNA is subject to TTP-dependent destabilization (11, 13). While TTP destabilization of pro-inflammatory cytokine mRNAs negatively controls inflammation, the ability of TTP to destabilize IL-10 mRNA (9, 10) suggests that TTP may also be a negative regulator of anti-inflammatory responses, but it is not clear how TTP influences IL-10 signaling. TTP did not appear to affect the activation of STAT3 (14), a transcription factor that is critical for the anti-inflammatory effects of IL-10 (15, 16). Here we show that in LPS-stimulated bone marrow-derived murine macrophages (BMDMs), TTP modulates IL-10-STAT3 signaling by indirectly regulating STAT3 activation through control of endogenous IL-10 production. Furthermore, IL-10-activated STAT3 interacts with the Ttp promoter, which contains a consensus STAT3 binding site that overlaps with the previously reported TPE 1 sequence. These findings identify a negative feedback loop that limits IL-10 production and STAT3 activation in LPS-stimulated BMDMs.
Materials and Methods
Reagents
Rabbit TTP antibody has been described (17). Tyr705-phosphorylated STAT3 (p-STAT3) and phosphorylated p38 (p-p38) antibodies were from Cell Signaling. STAT3, p38, and RNA polymerase II (Pol II) antibodies were from Santa Cruz Biotechnology. Actin antibody and LPS were from Sigma-Adrich. Etanercept (Enbrel™) was purchased from a pharmaceutical supplier. IL-10 antibody, IgG control antibody, and recombinant I L-10 were from eBioscience. Macrophage-colony stimulating factor (M-CSF) was from PeproTech.
Mice and bone marrow-derived macrophages
Ttp+/− (BL6) mice (13) were intercrossed to generate Ttp+/+, Ttp+/−, and Ttp−/− mice. To delete STAT3, Stat3F/F (BL6) mice (18) (Jackson Laboratory) were crossed with Mx1-Cre+/− (BL6) mice (19) (Jackson Laboratory) and the generated Stat3F/F/Mx1-Cre and Stat3F/F mice were injected 3 times with 250 μg of poly(IC) (Sigma) every other day. Three weeks after the final injection, bone marrow was collected and used to generate BMDMs in L-cell conditioned medium (20).
RNA and protein analysis
Total cellular RNA was extracted from BMDMs using TRIzol (Invitrogen) and reverse-transcribed with a SuperScript II First-Strand Synthesis kit (Invitrogen). qRT-PCR was performed with a SYBR green Kit (Applied Biosystems) and cyclophilin A mRNA was used for normalization. Primer sequences are available upon request. Proteins detected by immunoblotting were quantified with AlphaEaseFC software.
ELISA
BMDM supernatants were added to TNF ELISA (R&D Systems) and IL-6 ELISA (eBioscience) kits at dilutions of 1:2 and 1:50, respectively. Non-diluted supernatants were used with IL-10, IL-12, and IL-23 ELISA kits (eBioscience). Assays were performed as recommended by the manufacturer.
Chromatin immunoprecipitation (ChIP)
ChIP assay kit (Millipore) was used with slight modifications of the manufacturer’s protocol. Immunecomplexes were collected with protein G Dynabeads (Invitrogen). Purified DNA was analyzed by qRT PCR. Primer sequences are available upon request.
Online supplemental material
Supplementary Figure S1 shows that autocrine IL-10 is required for TTP-mediated control of IL-6, IL-12, and IL-23 expression. Supplementary Figure S2 shows that exogenous IL-10 represses cytokine gene transcription and activates STAT3 independently of TTP.
Results
Endogenous IL-10 is critical for TTP-mediated regulation of IL-6, IL-12, and IL-23
LPS-stimulated TTP-deficient BMDMs contain elevated amounts of both TNF and IL-10 mRNAs and secrete more TNF and IL-10 than wild-type control BMDMs ((9, 14, 21), Supplemental Fig. 1A, and our unpublished data). The increased TNF and IL-10 expression is due to the absence of TTP-mediated destabilization of TNF and IL-10 mRNAs. By negatively regulating IL-10 production, TTP may limit anti-inflammatory responses in LPS-stimulated macrophages. To investigate this potential role of TTP, the expression of additional LPS-inducible cytokines was examined in LPS-stimulated BMDMs generated from wild-type (Ttp+/+) and TTP-deficient (Ttp−/−) mice and was reduced in Ttp−/− BMDMs (Supplemental Fig. 1 A and B).
The increased IL-10 production by LPS-stimulated Ttp−/−BMDMs ((14) and data not shown), and the reduced expression of IL-6, IL-12, and IL-23 in these cells (Supplemental Fig. 1 A and B), suggested that IL-10 may be responsible for the latter effects. Indeed, IL-10 is known to inhibit IL-6, IL-12, and IL-23 expression in macrophages (4, 22). IL-10 neutralization, but not TNF neutralization, increased the amounts of IL-6, IL-12, and IL-23 mRNA and secreted proteins in Ttp−/− BMDMs to levels that were similar to those in activated Ttp+/+ BMDMs (Supplemental Fig. 1 A and B). TNF and IL-10 mRNA levels were also increased by IL-10 neutralization, although these mRNA levels remained elevated in LPS-stimulated Ttp−/−BMDMs (Supplemental Fig. 1A), indicating that endogenous IL-10 was specifically critical for TTP-mediated control of IL-6, IL-12, and IL-23 mRNAs in LPS-stimulated BMDMs.
TTP modulates STAT3 activation and cytokine transcription
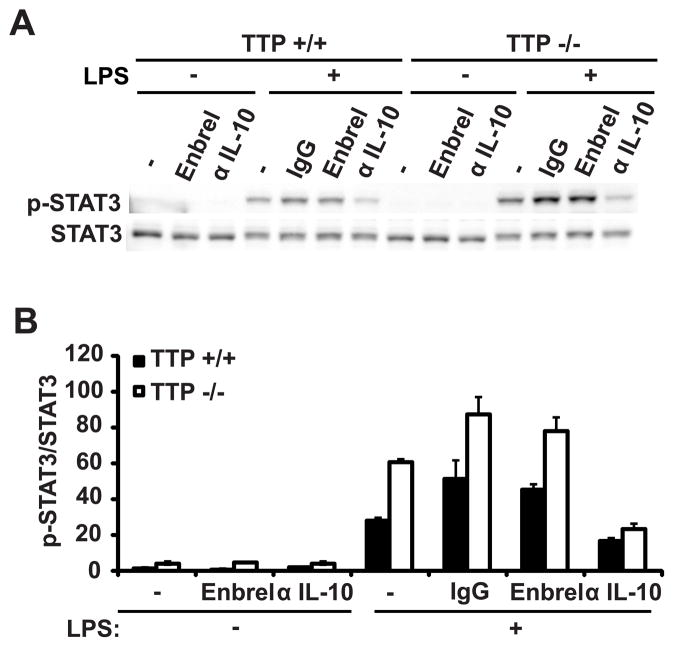
Since IL-10-induced activation of STAT3 is critical for the IL-10 anti-inflammatory response (15, 16), we examined the effect of TTP on STAT3 activation by immunoblotting with phosphorylated STAT3 (p-STAT3) antibody. In both Ttp+/+ and Ttp−/− BMDMs, STAT3 activation was induced by LPS, but STAT3 phosphorylation was increased in LPS-stimulated Ttp−/− BMDMs (Fig. 1 A and B). IL-10 neutralization attenuated the increased accumulation of STAT3 phosphorylation in Ttp−/− BMDMs (Fig. 1 A and B). IL-10 neutralization also reduced LPS-induced STAT3 phosphorylation in Ttp+/+ cells, whereas TNF neutralization had no effect on STAT3 phosphorylation in Ttp+/+ and Ttp−/−cells. Therefore, autocrine production of IL-10 is a major contributor to TTP-mediated control of STAT3 activation in macrophages.
FIGURE 1.
Autocrine IL-10 is required for TTP-mediated control of STAT3 activation. A, Ttp+/+ and Ttp−/− BMDMs were incubated with medium alone (−) or with (+) LPS (100 ng/ml) in the absence or presence of IgG control antibody (10 ug/ml), Enbrel (10 ug/ml), or anti-IL-10 antibody (1.0 ug/ml), as indicated for 4 hours. Lysates were prepared and examined for STAT3 phosphorylation by immunoblotting. B, Relative amounts of phosphorylated STAT3 were determined relative to total STAT3. Each measurement represents the mean and SD (n = 3).
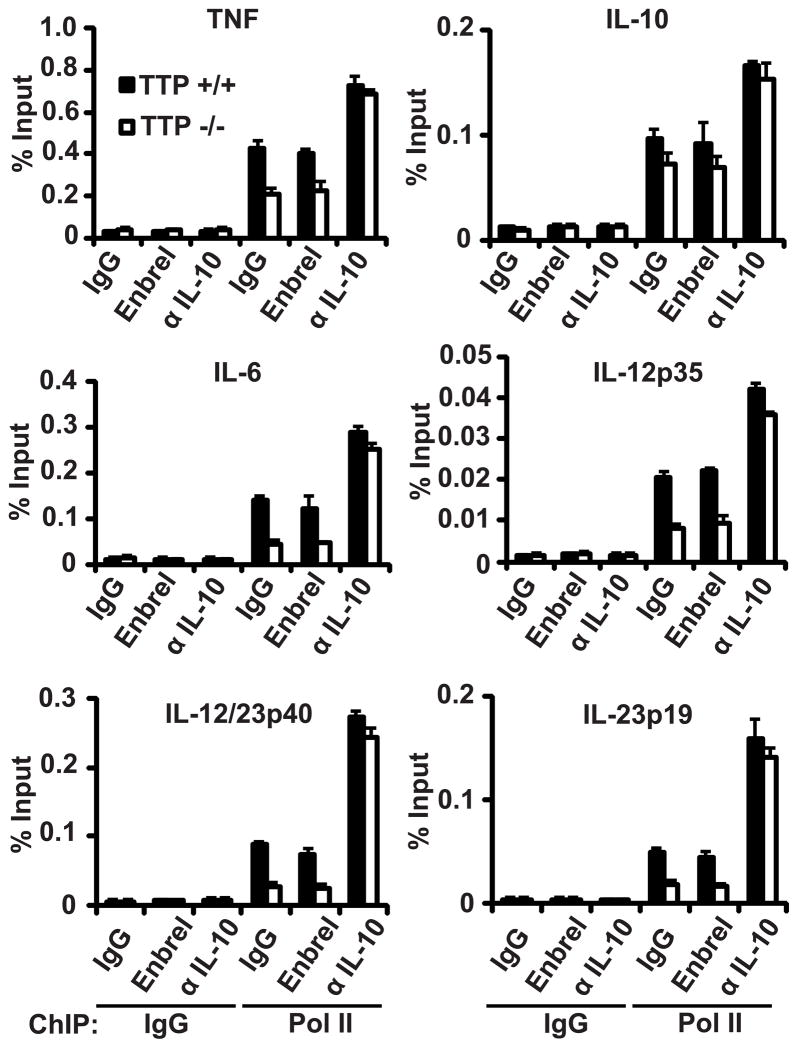
Next, we performed chromatin immunoprecipitation (ChIP) to test the effects of endogenous IL-10 and TTP on RNA polymerase II (Pol II) loading at the TNF, IL-6, IL-10, IL-12, and IL-23 gene promoters in LPS-stimulated BMDMs to determine if some of the effects on cytokine expression are transcriptionally mediated, since IL-10 may also regulate cytokine expression by destabilizing cytokine mRNA (23–25). Consistent with previous reports that IL-10 inhibition of cytokine gene expression is transcriptional (26, 27), Pol II recruitment to the examined cytokine gene promoters was reduced in LPS-stimulated Ttp−/−BMDMs (Fig. 2). This effect was autocrine IL-10-dependent, as IL-10 neutralization, but not TNF neutralization, potentiated LPS-induced Pol II recruitment to cytokine gene promoters to similar levels in Ttp+/+ and Ttp−/−BMDMs (Fig. 2). To test for IL-10 autocrine-independent effects of TTP on cytokine gene transcription, LPS-stimulated Ttp+/+ and Ttp−/− BMDMs were treated with recombinant mouse IL-10 and ChIP analysis was performed. Exogenous IL-10 reduced LPS-induced Pol II recruitment to similar levels in Ttp+/+ and Ttp−/− BMDMs (Supplemental Fig. 2A), indicating that the transcriptional effects of IL-10 are not entirely TTP-dependent. Consistent with these results, exogenous IL-10 induced similar amounts of STAT3 phosphorylation in Ttp+/+ and Ttp−/− BMDMs (Supplemental Fig. 2 B and C).
FIGURE 2.
Autocrine IL-10 production mediates TTP effects on IL-6, IL-12, and IL-23 expression at the transcriptional level. BMDMs were stimulated for 2 hours with LPS (100 ng/ml) and IgG (10 ug/ml), Enbrel (10 μg/ml), or anti-IL-10 antibody (1 μg/ml). ChIP was performed with IgG control or anti-Pol II antibody using fixed and sheared chromatin from Ttp+/+and Ttp−/−BMDMs. Presence of cytokine gene promoter sequences was examined by qRT-PCR. Each measurement represents the mean and SD (n = 4).
STAT3 mediates IL-10-induced TTP expression
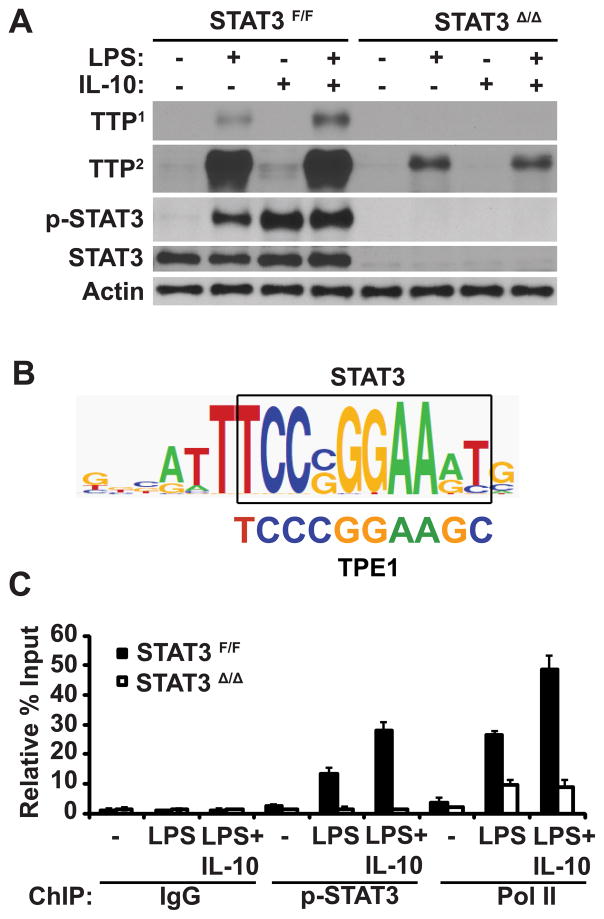
To determine the role of STAT3 in LPS-induced TTP expression, STAT3-deleted (Stat3Δ/Δ) and control (Stat3F/F) BMDMs were generated. In Stat3F/F BMDMs, STAT3 phosphorylation was induced by either LPS or IL-10 stimulation, whereas in Stat3Δ/Δ BMDMs STAT3 phosphorylation was not detected (Fig. 3A). Importantly, TTP protein and mRNA amounts were substantially reduced in LPS-stimulated Stat3Δ/Δ BMDMs relative to Stat3F/F cells (Fig. 3A and data not shown). IL-10 supplementation augmented STAT3 activation and TTP protein expression in Stat3F/F BMDMs, but not in Stat3Δ/Δ BMDMs (Fig. 3 A), indicating that STAT3 is required for IL-10-induced TTP expression in LPS-stimulated BMDMs. In Stat3F/F BMDMs, IL-10 also induced TTP protein expression, albeit to a lower extent than LPS, and that was also STAT3-dependent, as IL-10-induced TTP expression was not detected in Stat3Δ/Δ BMDMs (Fig. 3A).
FIGURE 3.
STAT3 mediates IL-10-induced TTP expression. A, TTP expression in Stat3F/F and Stat3Δ/Δ BMDMs. BMDMs were incubated with medium alone or with LPS (10 ng/ml), IL-10 (10 ng/ml), or both LPS and IL-10 (LPS+IL-10), as indicated. After 4 hours, lysates were prepared and analyzed by immunoblotting for TTP expression, STAT3 phosphorylation, and actin content. TTP1 and TTP2 indicate 30 sec and 4 min film exposures, respectively. B, STAT3 consensus binding site sequence and TPE1 sequence alignment. The STAT3 consensus binding site sequence was obtained using TESS software (http://www.cbil.upenn.edu/tess/). C, IL-10 promotes recruitment of activated STAT3 and Pol II to the Ttp promoter in Stat3F/F, but not in Stat3Δ/Δ, BMDMs. BMDMs were incubated with medium alone (−) or with LPS or LPS+IL-10 for 15 min and ChIP was performed with IgG control, anti-p-STAT3, and anti-Pol II antibodies as described in Fig. 2. Each measurement represents the mean and SD (n = 3).
Analysis of the Ttp promoter for potential STAT3 binding sites identified the previously characterized TPE1 sequence (12) as highly homologous to a STAT3 consensus binding site (Fig. 3B). The documented role of the TPE1 sequence in serum-induced Ttp expression in fibroblasts suggested that STAT3 interaction with the Ttp promoter may contribute to LPS-induced Ttp transcription. To examine this possibility, we performed ChIP analyses to measure STAT3 and Pol II recruitment to the Ttp promoter. p-STAT3 was detected at the Ttp promoter in LPS-stimulated Stat3F/FBMDMs, but not in Stat3Δ/Δ BMDMs, and exogenous IL-10 further enhanced p-STAT3 recruitment in LPS-stimulated Stat3F/F, but not in Stat3Δ/Δ, BMDMs (Fig. 3C). Pol II was also recruited to the Ttp promoter in LPS-stimulated Stat3F/F BMDMs and IL-10 further enhanced its recruitment. In contrast to Stat3F/F BMDMs, LPS-induced Pol II recruitment to the Ttp promoter was reduced and no longer responsive to IL-10 in Stat3Δ/Δ BMDMs (Fig. 3C), indicating that STAT3 is required for IL-10-induced Pol II recruitment to the Ttp promoter in LPS-stimulated BMDMs.
Discussion
Our studies demonstrate that TTP regulation of autocrine IL-10 production is important for LPS-induced STAT3 activation, and one important target for STAT3 identified in our study is the Ttp gene. Taken together, our findings indicate the existence of a negative feedback loop in which IL-10-activated STAT3 induces TTP expression, which in turn, reduces IL-10 production and thereby negatively regulates IL-10 activation of STAT3 in LPS-stimulated macrophages. In addition, because IL-6 and IL-23p19 mRNAs are known TTP targets (28), our data show that the ability of TTP to destabilize particular mRNAs does not automatically confer their overexpression in LPS-stimulated Ttp−/− macrophages. Rather, expression of some TTP-target mRNAs is reduced in LPS-stimulated Ttp−/− BMDMs due to an increased IL-10/STAT3 anti-inflammatory response that acts in a dominant manner over the mRNA-stabilizing effect in Ttp−/−BMDMs. Basal levels for each of the TTP target mRNAs, however, are elevated in Ttp−/− BMDMs (data not shown), indicating that in the absence of LPS-stimulation and STAT3 activation, mRNA stabilization is the dominant regulator of these mRNA levels in Ttp−/−BMDMs. The mRNA selective effects may be influenced by cytokine expression kinetics, since TNF and IL-10 mRNA expression precedes that of IL-6, IL-12, and IL-23 mRNAs (our unpublished data) and TTP regulates cytokine mRNA stability in a temporal manner in LPS-stimulated macrophages (28). LPS-induced TTP, which precedes that of TNF (11), may target the early expression of TNF and IL-10 mRNAs, whereas later in the LPS response, reduced TTP activity may allow IL-6, IL-12, and IL-23 mRNAs to be dominantly regulated by TTP control of IL-10 mRNA.
It was unknown how TTP could regulate the IL-10-induced anti-inflammatory response in macrophages because STAT3 activation appeared unaffected by TTP expression in macrophages stimulated with LPS for 30 min (14). In agreement with these observations, increased STAT3 activation was detected in Ttp−/−BMDMs stimulated with LPS for 4 or more hours, but not in Ttp−/−BMDMs stimulated with LPS for 30 min (data not shown). Because STAT3 activation remains low in Ttp+/+ and Ttp−/−BMDMs after 30 min of LPS simulation (data not shown and (14)), TTP-mediated control of STAT3 may require more than 30 min of LPS-stimulation for efficient autocrine IL-10 activity.
In addition to STAT3, other STAT transcription factors can induce Ttp expression. IFN-γ and IL-4 activation of STAT1 and STAT6, respectively, are reported to induce Ttp expression (29, 30). Although these studies did not investigate the effects of IFN-γ and IL-4 autocrine signaling or TTP-mediated control of IFN-γ and IL-4 mRNAs, they demonstrate that STAT induction of Ttp is not limited to STAT3. Our studies raise the possibility that a common mechanism among cytokine-activated STATs may involve the induction of Ttp expression to feedback regulate STAT activation.
Supplementary Material
Acknowledgments
We are indebted to Elizabeth Kennington for kindly providing TTP antibody, to Hisanobu Ogata and Weizhou Zhang for their assistance in generating Stat3Δ/Δ mice, and to Christina Camacho for assistance with figures.
Footnotes
AG was supported by a National Cancer Institute-sponsored Cancer Therapeutic Training Program and a NIH Research Supplement to Promote Diversity in Health-Related Research to CA118165. SG was supported by the Crohn’s and Colitis Foundation of America (CDA #2693), a Pathway to Independence award from NIH (K99-DK088589), and a UCSD DDRDC Pilot Grant (DK080506). Work was supported by NIH grants to M.K., who is an American Cancer Society Research Professor. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used in this paper: ARE, adenine and uridine-rich element; BMDM, Bone marrow-derived macrophage; ChIP, chromatin immunoprecipitation; Pol II, RNA polymerase II; qRT-PCR, quantitative RT-PCR, TTP, tristetraprolin; TPE1, TTP promoter element 1
The authors have no conflicting financial interests.
References
- 1.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 2.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Muller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol. 2006;36:3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 4.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Docke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salhi A, Rodrigues V, Jr, Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, Rafati S, Chevillard C, Prata A, Alcais A, Argiro L, Dessein A. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 7.Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S, Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;71:630–637. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Grondal G, Kristjansdottir H, Gunnlaugsdottir B, Arnason A, Lundberg I, Klareskog L, Steinsson K. Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum. 1999;42:1649–1654. doi: 10.1002/1529-0131(199908)42:8<1649::AID-ANR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Stoecklin G, Tenenbaum SA, Mayo T, Chittur SV, George AD, Baroni TE, Blackshear PJ, Anderson P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem. 2008:11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tudor C, Marchese FP, Hitti E, Aubareda A, Rawlinson L, Gaestel M, Blackshear PJ, Clark AR, Saklatvala J, Dean JL. The p38 MAPK pathway inhibits tristetraprolin-directed decay of interleukin-10 and pro-inflammatory mediator mRNAs in murine macrophages. FEBS Lett. 2009;583:1933–1938. doi: 10.1016/j.febslet.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 12.Lai WS, Thompson MJ, Taylor GA, Liu Y, Blackshear PJ. Promoter analysis of Zfp-36, the mitogen-inducible gene encoding the zinc finger protein tristetraprolin. J Biol Chem. 1995;270:25266–25272. doi: 10.1074/jbc.270.42.25266. [DOI] [PubMed] [Google Scholar]
- 13.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 14.Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, Vogl C, Strobl B, Muller M, Blackshear PJ, Poli V, Lang R, Murray PJ, Kovarik P. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183:1197–1206. doi: 10.4049/jimmunol.0803883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 16.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 17.Cao H, Tuttle JS, Blackshear PJ. Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein. J Biol Chem. 2004;279:21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 19.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 20.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 21.Carballo E, Gilkeson GS, Blackshear PJ. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (−/−) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. J Clin Invest. 1997;100:986–995. doi: 10.1172/JCI119649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol. 2005;17:649–659. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 23.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267:23301–23308. [PubMed] [Google Scholar]
- 24.Brown CY, Lagnado CA, Vadas MA, Goodall GJ. Differential regulation of the stability of cytokine mRNAs in lipopolysaccharide-activated blood monocytes in response to interleukin-10. J Biol Chem. 1996;271:20108–20112. doi: 10.1074/jbc.271.33.20108. [DOI] [PubMed] [Google Scholar]
- 25.Denys A, I, Udalova A, Smith C, Williams LM, Ciesielski CJ, Campbell J, Andrews C, Kwaitkowski D, Foxwell BM. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–4845. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 26.Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratochvill F, Machacek C, Vogl C, Ebner F, Sedlyarov V, Gruber AR, Hartweger H, Vielnascher R, Karaghiosoff M, Rulicke T, Muller M, Hofacker I, Lang R, Kovarik P. Tristetraprolin-driven regulatory circuit controls quality and timing of mRNA decay in inflammation. Mol Syst Biol. 2011;7:560. doi: 10.1038/msb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Muller M, Blackshear PJ, Kovarik P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood. 2006;107:4790–4797. doi: 10.1182/blood-2005-07-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Nakajima H, Ikeda K, Maezawa Y, Suto A, Takatori H, Saito Y, Iwamoto I. IL-4-Stat6 signaling induces tristetraprolin expression and inhibits TNF- alpha production in mast cells. J Exp Med. 2003;198:1717–1727. doi: 10.1084/jem.20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.