Abstract
The ideal anti-obesity drug would produce sustained weight loss with minimal side effects. The mechanisms that regulate energy balance have substantial built-in redundancy, overlap considerably with other physiological functions, and are influenced by social, hedonic and psychological factors that limit the effectiveness of pharmacological interventions. It is therefore unsurprising that anti-obesity drug discovery programmes have been littered with false starts, failures in clinical development, and withdrawals due to adverse effects that were not fully appreciated at the time of launch. Drugs that target pathways in metabolic tissues, such as adipocytes, liver and skeletal muscle, have shown potential in preclinical studies but none has yet reached clinical development. Recent improvements in the understanding of peptidergic signalling of hunger and satiety from the gastrointestinal tract mediated by ghrelin, cholecystokinin (CCK), peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), and of homeostatic mechanisms related to leptin and its upstream pathways in the hypothalamus, have opened up new possibilities. Although some have now reached clinical development, it is uncertain whether they will meet the strict regulatory hurdles required for licensing of an anti-obesity drug. However, GLP-1 receptor agonists have already succeeded in diabetes treatment and, owing to their attractive body-weight-lowering effects in humans, will perhaps also pave the way for other anti-obesity agents. To succeed in developing drugs that control body weight to the extent seen following surgical intervention, it seems obvious that a new paradigm is needed. In other therapeutic arenas, such as diabetes and hypertension, lower doses of multiple agents targeting different pathways often yield better results than strategies that modify one pathway alone. Some combination approaches using peptides and small molecules have now reached clinical trials, although recent regulatory experience suggests that large challenges lie ahead. In future, this polytherapeutic strategy could possibly rival surgery in terms of efficacy, safety and sustainability of weight loss.
Introduction
Obesity, most often defined as a body mass index (BMI) of ≥30 kg/m2 and caused by an imbalance between energy intake and expenditure, is widely recognised as the largest and fastest growing public health problem in the developed and developing world (https://apps.who.int/infobase/Publicfiles/SuRF2.pdf). Prevalence of the disorder in adults has more than tripled in the past decade, and obesity currently affects approximately 30–35% of the general population in the USA and 25% in the UK (National Audit Office, 2001) (http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles/obesity/statistics-on-obesity-physical-activity-and-diet-england-2010). It has been estimated that, in 2005, some 400 million adults worldwide were obese, with a total of 1.6 billion being overweight (http://www.who.int/mediacentre/factsheets/fs311/en/index.html). Of particular concern is the associated epidemic of obesity in children and adolescents; the current prevalence of 7–10% in these populations is predicted to at least double by 2025 (McPherson et al., 2007), and there is strong evidence of persistence into adulthood (Freedman et al., 2001; Daniels, 2006b).
Obesity is associated with substantial increases in morbidity, premature mortality, impaired quality of life and large healthcare costs (Kopelman, 2000; Fontaine et al., 2003; Haslam and James, 2005). The major comorbidities include type 2 diabetes, metabolic syndrome, hypertension, dyslipidaemia, myocardial infarction, stroke, certain cancers, sleep apnea and osteoarthritis (Flegal et al., 2007). Indeed, obesity is blamed as a major contributing factor in over 300,000 deaths annually in the United States, with the illness-related economic costs exceeding US$100 billion per annum (Daniels, 2006a). Although prevention through education and changes to the obesogenic environment are long-term goals, treatment is required for those who are already obese. Surprisingly, however, treatment options remain quite limited. Lifestyle changes in the form of dieting and/or exercise per se do not generally produce marked or sustainable weight loss (Dansinger et al., 2005; LeBlanc et al., 2011), whereas effective psychological therapies, such as cognitive behavioural therapy, cannot easily be delivered on a mass scale (e.g. Wing et al., 2006) and long-term results are disappointing. Bariatric surgery, such as Roux-en-Y bypass or gastric banding, is much more effective in terms of weight loss, comorbidity reduction and enhanced survival (Kral and Naslund, 2007; Sjostrom et al., 2007). However, owing to concerns about perioperative mortality, surgical complications and the frequent need for reoperation, these procedures tend to be reserved for the morbidly obese (Melnikova and Wages, 2006; Field et al., 2009).
An alternative strategy to surgery is to develop therapeutic agents that can reduce body weight by decreasing the consumption or absorption of food, and/or by increasing energy expenditure (Cooke and Bloom, 2006; Sargent and Moore, 2009). Unfortunately, although avidly pursued for more than half a century, this strategy has thus far only shown limited success. Many new agents that were heralded as the answer to the obesity problem were hastily withdrawn owing to an unacceptable side-effects burden. Indeed, a recent review rather pessimistically concluded that “the history of anti-obesity drug development is far from glorious, with transient magic bullets and only a handful of agents currently licensed for clinical use” (Rodgers et al., 2010). Therefore, new treatments for obesity that are both better tolerated and more efficacious are urgently needed (Halford et al., 2010; Kennett and Clifton, 2010; Rodgers et al., 2010; Vickers et al., 2011). In this context, major recent advances in our understanding of the basic neurobiology of appetite and energy homeostasis have identified numerous targets for potential anti-obesity drug development (Wilding, 2007; Heal et al., 2009; Halford et al., 2010). The aim of this Commentary is to place these developments in context by reviewing the pharmacotherapy of obesity in terms of its past, present and future.
To set the stage, it is important to recognise that, following preclinical identification of potentially important new therapeutic agents, human drug trials progress through several phases of development. Phase I typically focuses on tolerability, safety and pharmacokinetics; Phase II on proof of concept (mechanism, efficacy and safety); Phase III on confirmation of efficacy and side-effect profile in large-scale multi-centre trials; and Phase IV on long-term monitoring and data collection following governmental approval. Application for approval from the United States Food and Drug Administration (FDA) or the European Medicines Agency (EMA) is made after Phase III.
The past
Centrally acting sympathomimetics, such as the amphetamine derivatives desoxyephedrine, phentermine and diethylpropion, were among the earliest pharmacological agents used for weight loss (Colman, 2005; Wilding, 2007). They were popular in the 1950s and 1960s, but growing concerns about cardiovascular risk and abuse potential led to a marked decline in their use by the early 1970s. Although still available in many countries, phentermine and diethylpropion were largely superseded in the 1970s and 1980s by the serotonin (5-HT)-releasing agents fenfluramine and dexfenfluramine. It was known from the outset that agents of this series had the potential to produce primary pulmonary hypertension, but the risk was deemed sufficiently low against the weight loss benefits. In the early 1990s, evidence of superior efficacy over either compound given alone led to the widespread use in the United States of combined treatment with phentermine and fenfluramine (Weintraub et al., 1992). However, within only a few years, reports of cardiac valvulopathy (Connolly et al., 1997), particularly when these agents were combined with phentermine, led the manufacturers to withdraw fenfluramine and dexfenfluramine from the market.
Until very recently, three agents were approved in Europe for the long-term clinical management of obesity and related metabolic syndrome: sibutramine (trade names Meridia® and Reductil®), rimonabant (Acomplia®) and orlistat (Xenical® and Alli®). Sibutramine, a dual monoamine (noradrenaline and serotonin)-reuptake inhibitor, was introduced to clinical practice in the late 1990s (McNeely and Goa, 1998; Luque and Rey, 2002) and is believed to achieve very modest weight loss by decreasing energy intake and increasing energy expenditure. However, cumulative clinical experience has identified some adverse effects, the most serious of which (cardiovascular risk) emerged as a result of a post-marketing clinical trial (James et al., 2010) and led in January 2010 to the suspension of marketing authorisations by the EMA (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/01/WC500069995.pdf). There is substantial evidence that phytocannabinoids (i.e. cannabis and its constituents) and endocannabinoids (e.g. anandamide) stimulate appetite in animals and humans, and that these effects are mediated via cannabinoid CB1 receptors in the brain and/or periphery (Kirkham, 2009). Rimonabant, a cannabinoid CB1 receptor antagonist/inverse agonist (see Box 1 for Glossary) developed in the mid-1990s, suppresses appetite and weight gain in experimental animals and, in four major clinical trials, has consistently been found to produce a placebo-subtracted weight loss of 4–5 kg (Despres et al., 2005; Van Gaal et al., 2005; Pi-Sunyer et al., 2006; Scheen et al., 2006). Although never approved in the United States, rimonabant (Acomplia®) was licensed in Europe as an anti-obesity agent by the EMA in June 2006. However, by October 2008, burgeoning reports of serious psychiatric problems (such as anxiety, depression and suicide) led to suspension of marketing authorisations by the EMA (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf). This decision in turn rapidly led to the termination of several CB1-receptor-antagonist-based anti-obesity drug development programmes [including those for rimonabant, taranabant, otenabant, surinabant and ibipinabant (Plieth, 2008)].
Box 1.
Glossary
- Agonist:
a substance that binds to a receptor molecule and produces a stimulus that results in a measurable response in the tissue.
- Antagonist:
a substance that binds to the same receptor as an agonist but fails to elicit a stimulus (so that no tissue response is produced). An antagonist can, however, block or reverse the effects of an agonist or inverse agonist.
- Inverse agonist:
in tissues in which receptors exhibit constitutive activity, an inverse agonist will bind to the same receptor as an agonist, but the resulting stimulus produces the opposite response in the tissue.
- Antagonist/inverse agonist:
a substance that binds to the same receptor as an agonist, but its effects vary as a function of the presence (antagonist action) or absence (inverse agonist action) of the agonist. A neutral antagonist has no inverse agonist activity.
The FDA approved orlistat for the treatment of obesity in 1998. Unlike the other weight loss agents mentioned above, which reduce appetite and/or enhance energy expenditure, orlistat inhibits pancreatic lipases, thereby reducing fat absorption from the gut by ∼30% (Borgstrom, 1988). Weight loss is relatively modest [circa 3 kg at 12 months (Li et al., 2005)], but of sufficient magnitude to have beneficial effects on cardiovascular risk, as reflected by a lowering of low-density-lipoprotein (LDL) cholesterol, blood pressure and glycaemia (Broom et al., 2002; Torgerson et al., 2004). Compared with other agents, adverse effects are limited, but include diarrhoea, flatulence, bloating, abdominal pain and dyspepsia (Bray and Greenway, 2007). At the time of writing, orlistat is the only weight loss agent approved for long-term clinical use in Europe.
The present
Despite the withdrawal of rimonabant and the demise of several CB1-receptor-antagonist development programmes, there are reasons to believe that we have not yet reached the end of the line for anti-obesity treatments targeting the CB1 receptor (for reviews, see Kunos et al., 2008; Bermudez-Silva et al., 2010). First, rimonabant and related compounds are not neutral CB1 receptor antagonists, but possess significant inverse agonist activity at these sites (Pertwee, 2006) (see Box 1 for Glossary). Recent preclinical evidence suggests that neutral antagonists might retain the weight loss advantages of rimonabant, but without the adverse effects of this agent (e.g. Cluny et al., 2011). Second, it is known that tolerance develops to the acute anorectic effect but not the weight loss effects of rimonabant-like compounds, and that the weight loss effect might involve CB1 receptors in peripheral tissues. This would support further development of CB1 receptor antagonists that do not cross the blood-brain barrier; indeed, several such agents have recently been reported to produce weight loss in rodent models (e.g. Chen et al., 2010; Randall et al., 2010).
Other realistic possibilities include the development of CB1 receptor partial agonists, allosteric modulators of CB1 receptors and agents that alter the levels of endocannabinoids (Bermudez-Silva et al., 2010). In addition, two other intriguing suggestions merit some attention. The first is based on the low-dose combination of rimonabant with another anorectic agent [e.g. an opioid receptor antagonist (Tallett et al., 2008; Lockie et al., 2011), the 5-HT2C receptor agonist mCPP (Ward et al., 2008) or the gut peptide CCK-8s (Orio et al., 2011)]. These drug combinations have been shown to produce at least additive anorectic effects and, in the case of rimonabant plus the opioid receptor antagonist naloxone, a significant attenuation of rimonabant-induced pruritus (Tallett et al., 2008). The second suggestion derives from recent genomic studies suggesting that variants (polymorphisms) of the CB1 receptor gene, alone or in combination with the gene for the serotonin transporter (SLC6A4), contribute to the development of anxiety and/or depression in response to agents such as rimonabant (Lazary et al., 2011). These observations seem to offer the possibility of personalised medicine based on genetic screening for ‘high-risk’ individuals – i.e. a novel method for the safe use of centrally acting CB1 receptor antagonists. Nevertheless, the CB1 receptor remains stigmatised as a target owing to the massive disappointment surrounding Acomplia®, and even potentially promising alternative approaches understandably continue to face serious scepticism and a challenging regulatory climate.
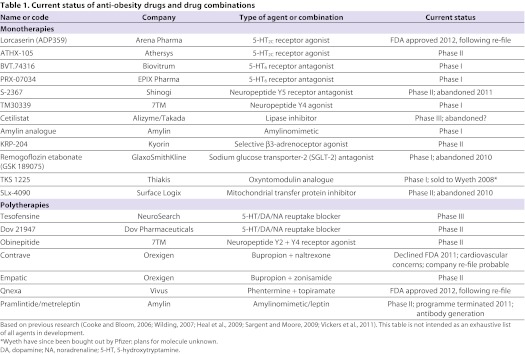
Table 1 lists many of the agents that are either currently or have until very recently been in the anti-obesity drug development pipeline (Cooke and Bloom, 2006; Wilding, 2007; Heal et al., 2009; Sargent and Moore, 2009; Vickers et al., 2011). These treatments are aimed at diverse molecular targets in the CNS and/or periphery and, in some cases, at several targets simultaneously. The large number of potential new therapies should not be surprising given a projected market size of US$3.7 billion for a safe and effective anti-obesity drug (Vickers and Cheetham, 2007). It is beyond the scope of this article to review all the potential therapies listed in Table 1. Instead, we focus on a subset of the most promising and/or exciting lines of enquiry.
Table 1.
Current status of anti-obesity drugs and drug combinations
Table 1 shows that, despite successful progress up to Phase III (Vickers et al., 2011), Contrave® (a polytherapy) was recently turned down by the FDA owing to potential cardiovascular risk. Although the federal authorities still require more evidence that drug-specific concerns are unfounded, it seems probable that Contrave® will be re-filed in the near future. Lorcaserin, a monotherapy, was initially rejected in 2010 owing to concerns about tumour growth in preclinical studies but, following re-file, was approved by the FDA in June 2012: plans are to market the compound under the tradename Belviq®. Similarly, a third agent, Qnexa® (a polytherapy), was initially declined by the FDA in 2010 owing to concerns over possible birth defects but, on re-file, was FDA-approved in July 2012: this compound will apparently be marketed under the tradename Qsymia®. Two other compounds (cetilistat, a monotherapy, and tesofensine, a polytherapy) have reached advanced Phase III testing but have not at the time of writing been formally submitted for approval in the USA or Europe. One area of substantial interest has focused on the weight loss effects of the glucagon-like peptide 1 receptor (GLP-1R) agonists that are already licensed for the treatment of type 2 diabetes. GLP-1 is an endogenous gut peptide that was initially identified as an incretin hormone (Kreymann et al., 1987) and was later found to be part of the endogenous satiety cascade (Turton et al., 2006). Modest weight loss has consistently been observed when individuals are treated for diabetes (Wilding and Hardy, 2011), but there is evidence that higher doses of the GLP-1R agonist liraglutide than are necessary for glucose lowering can produce greater weight loss of up to 10 kg in trials of up to 2 years duration (Astrup et al., 2009; Astrup et al., 2011). Unsurprisingly, an extensive programme is now underway to further test the efficacy and safety of liraglutide. Other treatments that are based largely on preclinical findings and aimed at an impressively wide range of molecular targets remain at relatively early stages of development.
The future
Traditional pharmacological monotherapies for obesity, although initially successful in achieving weight loss, are often subject to counter-regulation. This is not surprising given the multiplicity and redundancy of mechanisms involved in appetite regulation and energy homeostasis (Adan et al., 2008; Vemuri et al., 2008). It is therefore pertinent to note that two of the three treatments that were most recently submitted for FDA approval (Contrave® and Qnexa®) are effectively ‘polytherapies’ – i.e. combination agents that are designed to simultaneously target more than one biological mechanism and that might ultimately be more effective in producing sustained weight loss and improvements in comorbidities. Advantages of polytherapy, which actually began some 20 years ago with the phentermine and fenfluramine combination, include the use of lower drug doses, possible synergistic but at least additive weight loss, less serious side effects and reduced potential for counter-regulation (Greenway et al., 2009; Padwal, 2009; Roth et al., 2010).
In this context, it would seem reasonable to suggest that man-made CNS-targeted agents would be more likely to engender adverse effects than would naturally occurring biological signals that normally regulate the activity of key CNS circuits. Over the past few decades, basic research (for reviews, see Cooke and Bloom, 2006; Field et al., 2009; Halford et al., 2010; Kennett and Clifton, 2010) has identified a host of signals emanating from the gut (e.g. CCK, ghrelin, GLP-1, glucose-dependent insulinotropic polypeptide, oxyntomodulin, PYY), pancreas (e.g. insulin, amylin, pancreatic polypeptide) and adipose tissue (e.g. leptin, adiponectin). Against this background, basic and applied research has begun to assess the therapeutic potential of combinations of these molecules – either with one another [e.g. amylin with murine leptin (Trevaskis et al., 2008)] or with a centrally acting agent [e.g. amylin or pramlintide with either phentermine or sibutramine (Roth et al., 2008; Arrone et al., 2010)]. As shown in Table 1, one such combination [pramlintide plus metreleptin (Ravussin et al., 2009)] entered Phase II clinical trials but the programme was stopped because of significant problems with antibody generation and skin reactions (http://www.takeda.com/press/article_42791.html).
Another exciting recent strategy involves the development of single-peptide molecules that combine differing modes of action. For example, Day and colleagues recently reported on a peptide with dual agonism at the glucagon receptor and GLP-1R (Day et al., 2009). Glucagon is a pancreatic hormone with well-established thermogenic, anorectic and weight loss effects in animals (Salter, 1960; Woods et al., 2006), whereas GLP-1R agonism (e.g. with exenatide) is known to improve glycaemic control and weight loss in humans with type 2 diabetes (e.g. Hanssen et al., 2009). On the basis of these observations, Day and colleagues reasoned that the antihyperglycaemic property of GLP-1R agonism could minimise any diabetogenic risk of excessive glucagon agonism. They further argued that the lipophilic and thermogenic properties of glucagon, in addition to the satiation-inducing pharmacology of GLP-1, provided a strong scientific basis for the development of a synergistic co-agonist peptide. Consistent with this rationale, the authors report that new peptides with varying ratios of agonism both at glucagon and GLP-1 receptors have potent, sustained satiation-inducing and lipolytic effects in rodents (Day et al., 2009). Detailed analysis revealed that body weight reduction was achieved by a loss of body fat arising from decreased intake and increased expenditure. The authors suggest that it is at least theoretically possible to incorporate factors other than gut hormones in an analogous single-molecule co-agonist. Furthermore, combining more than two endogenous metabolic peptides into a single molecule might lead to receptor occupancy patterns that more closely resemble physiological regulation. The development of new molecules based on this novel strategy is likely to have substantial heuristic value.
Conclusions
Despite an inauspicious history, the pharmacological management of obesity is at an exciting crossroads. New treatments are essentially on the horizon, and novel research strategies have very recently come to the fore. However, it must be emphasised that only limited behavioural data are available on many of these treatments, including those currently undergoing regulatory approval (Halford et al., 2010; Kennett and Clifton, 2010; Rodgers et al., 2010). Much emphasis is being placed on endpoints (reduced food intake and/or body weight) and possibly not enough on process – i.e. how the endpoint has been reached. Without a much deeper understanding of molecular, physiological and behavioural mechanisms, we are likely to witness many more failed ‘magic bullets’ over the next few years. To minimise this prospect, it is essential that detailed behavioural analysis is conducted at an early stage of drug development. Given some of the molecular targets involved (e.g. monoamine transporters, CB1 receptors, 5-HT2C receptors), such analyses should ideally include not only tests of feeding behaviour but also, for example, tests relevant to mood, sexual behaviour, and learning and memory. However, even for those agents that meet preliminary requirements for selectivity of action and potential safety profile, extensive real-world testing is likely to be required by regulators, not only showing efficacy in terms of weight loss but also demonstrating long-term benefits for diabetes prevention and treatment, cardiovascular disease, and psychiatric safety. Finally, successful discovery and development of potent and safe drugs for the prevention and treatment of obesity will probably require polytherapeutic strategies as well as vastly improved tools for the identification and characterisation of specific obese subpopulations that allow for the tailor-made development and appropriate use of personalised medicines.
Footnotes
This article is part of a special issue on obesity: see related articles in Vol. 5, issue 5 of Dis. Model. Mech. at http://dmm.biologists.org/content/5/5.toc.
COMPETING INTERESTS
M.H.T. is a consultant for Roche Pharmaceuticals. J.P.H.W. has received consultancy and speaker fees from Novo Nordisk (manufacturers of liraglutide), and his department has active research grants from Novo Nordisk and Sanofi (who are developing GLP-1 agonists).
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- Adan R. A. H., Vanderschuren L. J. M. J., la Fleur S. E. (2008). Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol. Sci. 29, 208–217 [DOI] [PubMed] [Google Scholar]
- Aronne L. J., Halseth A. E., Burns C. M., Miller S., Shen L. Z. (2010). Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity 18, 1739–1746 [DOI] [PubMed] [Google Scholar]
- Astrup A., Rossner S., Van Gaal L., Rissanen A., Niskanen L., Madsen J., Rasmussen M. F., Lean M. E. J. (2009). Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 [DOI] [PubMed] [Google Scholar]
- Astrup A., Carraro R., Finer N., Harper A., Kunesova M., Lean M. E. J., Niskanen L., Rasmussen M. F., Rissanen A., Rossner S., et al. (2011). Safety, tolerability and sustained weight loss over 2 years with once daily human GLP-1 analog, liraglutide. Int. J. Obesity 36, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Silva F. J., Viveros M. P., McPartland J. M., Rodriguez de Fonseca F. (2010). The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol. Biochem. Behav. 95, 375–382 [DOI] [PubMed] [Google Scholar]
- Borgstrom B. (1988). Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim. Biophys. Acta 962, 308–316 [DOI] [PubMed] [Google Scholar]
- Bray G. A., Greenway F. L. (2007). Pharmacological treatment of the overweight patient. Pharmacol. Rev. 59, 151–184 [DOI] [PubMed] [Google Scholar]
- Broom I., Wilding J., Scott P., Myers N. (2002). Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK multimorbidity study. Int. J. Clin. Pract. 56, 494–499 [PubMed] [Google Scholar]
- Chen W., Tang H., Liu H., Long L., Gong Z., Zheng J., Chi M., Xie Y., Zheng Z., Li S., et al. (2010). Novel selective antagonist of the cannabinoid CB1 receptor, MJ15, with prominent anti-obesity effect in rodent models. Eur. J. Pharmacol. 637, 178–185 [DOI] [PubMed] [Google Scholar]
- Cluny N. L., Chambers A. P., Vemuri V. K., Wood J. T., Eller L. K., Freni C., Reimer R. A., Makriyannis A., Sharkey K. A. (2011). The neutral cannabinoid CB1 receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol. Biochem. Behav. 97, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman E. (2005). Anorectics on trial: a half-century of federal regulation of prescription appetite suppressants. Ann. Int. Med. 143, 380–385 [DOI] [PubMed] [Google Scholar]
- Connolly H. M., Crary J. L., McGoon M. D., Hensrud D. D., Edwards B. S., Edwards W. D., Schaff H. V. (1997). Valvular heart disease associated with fenfluramine-phentermine. N. Eng. J. Med. 337, 581–588 [DOI] [PubMed] [Google Scholar]
- Cooke D., Bloom S. (2006). The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat. Rev. Drug Discov. 5, 919–931 [DOI] [PubMed] [Google Scholar]
- Daniels J. (2006a). Obesity: America’s epidemic. Am. J. Nurs. 106, 40–49 [DOI] [PubMed] [Google Scholar]
- Daniels S. R. (2006b). The consequences of childhood overweight and obesity. Future Child 16, 47–67 [DOI] [PubMed] [Google Scholar]
- Dansinger M. L., Gleason J. A., Griffith J. L., Selker H. P., Schaefer E. J. (2005). Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease reduction: a randomised trial. JAMA 293, 43–53 [DOI] [PubMed] [Google Scholar]
- Day J. W., Ottaway N., Patterson J. T., Gelfanov V., Smiley D., Gidda J., Findeisen H., Bruemmer D., Drucker D. J., Chaudhary N., et al. (2009). A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 [DOI] [PubMed] [Google Scholar]
- Despres J. P., Golay A., Sjostrom L. (2005). Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Eng. J. Med. 353, 2121–2134 [DOI] [PubMed] [Google Scholar]
- Field B. C. T., Chaudhri O. B., Bloom S. R. (2009). Obesity treatment: novel peripheral targets. Br. J. Clin. Pharmacol. 68, 830–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Graubard B. I., Williamson D. F., Gail M. H. (2007). Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298, 2028–2037 [DOI] [PubMed] [Google Scholar]
- Fontaine K. R., Redden D. T., Wang C., Westfall A. J., Allison D. B. (2003). Years of life lost due to obesity. JAMA 298, 187–193 [DOI] [PubMed] [Google Scholar]
- Freedman D. S., Khan L. K., Dietz W. H., Srinivasan S. R., Berenson G. S. (2001). Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 108, 712–718 [DOI] [PubMed] [Google Scholar]
- Greenway F. L., Whitehouse M. J., Guttadauria M., Anderson J. W., Atkinson R. L., Fujioka K., Gadde K. M., Gupta A. K., O’Neil P., Schumacher D., et al. (2009). Rational design of a combination medication for the treatment of obesity. Obesity 17, 30–39 [DOI] [PubMed] [Google Scholar]
- Halford J. C. G., Boyland E. J., Blundell J. E., Kirkham T. C., Harrold J. A. (2010). Pharmacological management of appetite expression in obesity. Nat. Rev. Endocrinol. 6, 255–269 [DOI] [PubMed] [Google Scholar]
- Hanssen K. B., Knop F. K., Holst J. J., Vilsboll T. (2009). Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int. J. Clin. Pract. 63, 1154–1160 [DOI] [PubMed] [Google Scholar]
- Haslam D. W., James W. P. T. (2005). Obesity. Lancet 366, 1197–1209 [DOI] [PubMed] [Google Scholar]
- Heal D. J., Gosden J., Smith S. L. (2009). Regulatory challenges for new drugs to treat obesity and comorbid metabolic disorders. Br. J. Clin. Pharmacol. 68, 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. P. T., Caterson I. D., Couthino W., Finer N., Van Gaal L. F., Maggioni A. P., Torp-Pederson C., Sharma A. M., Shepherd G. M., Rode R. A., et al. (2010). Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Eng. J. Med. 363, 905–917 [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Clifton P. G. (2010). New approaches to the pharmacological treatment of obesity: Can they break through the efficacy barrier? Pharmacol. Biochem. Behav. 97, 63–83 [DOI] [PubMed] [Google Scholar]
- Kirkham T. C. (2009). Cannabinoids and appetite: food craving and food pleasure. Int. Rev. Psychiat. 21, 163–171 [DOI] [PubMed] [Google Scholar]
- Kopelman P. G. (2000). Obesity as a medical problem. Nature 404, 635–643 [DOI] [PubMed] [Google Scholar]
- Kral J. G., Naslund E. (2007). Surgical treatment of obesity. Nature Clin Pract: Endocrinol. Metab. 3, 574–583 [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. (1987). Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2, 1300–1304 [DOI] [PubMed] [Google Scholar]
- Kunos G., Osei-Hyiaman D., Batkai S., Sharkey K. A., Makriyannis A. (2008). Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 30, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J., Juhasz G., Hunyady L., Bagdy G. (2011). Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol. Sci. 32, 270–280 [DOI] [PubMed] [Google Scholar]
- LeBlanc E. S., O’Connor E., Wtitlock P. D., Patnode C. D., Kapka T. (2011). Effectiveness of primary care -relevant treatments for obesity in adults: a systematic evidence review for the U. S. Preventive Services Task Force. Ann. Int. Med. 155, 434–447 [DOI] [PubMed] [Google Scholar]
- Li Z., Maglione M., Tu W., Mojica D., Arterburn D., Shugaman L. R., Hilton L., Suttorp M., Solomon V., Shekelle P. G., et al. (2005). Meta-analysis: pharmacologic treatment of obesity. Ann. Intern. Med. 142, 532–546 [DOI] [PubMed] [Google Scholar]
- Lockie S. H., Czyzyk T. A., Chaudhary N., Perez-Tilve D., Woods S. C., Oldfield B. J., Statnick M. A., Tschöp M. H. (2011). CNS opioid signaling separates cannabinoid receptor 1-mediated effects on body weight and mood-related behavior in mice. Endocrinol. 152, 3661–3667 [DOI] [PubMed] [Google Scholar]
- Luque C. A., Rey J. A. (2002). The discovery and status of sibutramine as an anti-obesity drug. Eur. J. Pharmacol. 440, 119–128 [DOI] [PubMed] [Google Scholar]
- McNeely W., Goa K. L. (1998). Sibutramine. A review of its contribution to the management of obesity. Drugs 56, 1093–1124 [DOI] [PubMed] [Google Scholar]
- McPherson K., Marsh T., Brown M. (2007). Tackling obesities: future choices. Modelling future trends in obesity and their impact on health. 2nd Edition Government Office for Science: London [Google Scholar]
- Melnikova I., Wages D. (2006). Anti-obesity therapies. Nat. Rev. Drug Discov. 5, 369–370 [DOI] [PubMed] [Google Scholar]
- National Audit Office (2001). Tackling obesity in England. Report by the Comptroller and Auditor General. The Stationery Office: London [Google Scholar]
- Orio L., Crespo I., Lopez-Moreno J. A., Reyes-Cabello C., Rodriguez de Fonseca F., Gomez de Heras R. (2011). Additive effects of cannabinoid CB1 receptors blockade and cholecystokinin on feeding inhibition. Pharmacol. Biochem. Behav. 98, 220–226 [DOI] [PubMed] [Google Scholar]
- Padwal R. (2009). Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. Curr. Opin. Invest. Drugs 10, 1117–1125 [PubMed] [Google Scholar]
- Pertwee R. G. (2006). The pharmacology of cannabinoid receptors and their ligands: an overview. Int. J. Obesity 30, S13–S18 [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer F., Aronne L. J., Heshmati H. M., Devin J., Rosenstock J. (2006). Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk in overweight or obese patients - RIO-North America: a randomised controlled trial. JAMA 295, 761–775 [DOI] [PubMed] [Google Scholar]
- Plieth J. (2008). Obesity: what next after the CB1 antagonists’ failure. Scrip 2008, 44–47 [Google Scholar]
- Randall P. A., Vemuri V. K., Segovia K. N., Torres E. F., Hosmer S., Nunes E. J., Santerre J. L., Makriyannis A., Salamone J. D. (2010). The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol. Biochem. Behav. 97, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E., Smith S. R., Mitchell J. A., Shringarpure R., Shan K., Maier H., Koda J. E., Weyer C. (2009). Enhanced weight loss with pramlintide/metreleptin: an integrated neurohumoral approach to obesity pharmacotherapy. Obesity 17, 1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers R. J., Holch P., Tallett A. J. (2010). Behavioural satiety sequence (BSS): Separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol. Biochem. Behav. 97, 3–14 [DOI] [PubMed] [Google Scholar]
- Roth J. D., Trevaskis J. L., Wilson J., Lei C., Athanacio J., Mack C., Kesty N. C., Coffey T., Weyer C., Parkes D. G. (2008). Antiobesity effects of the beta-cell hormone amylin in combination with phenteramine or sibutramine in diety-induced obese rats. Int. J. Obesity 32, 1201–1210 [DOI] [PubMed] [Google Scholar]
- Roth J. D., Trevaskis J. L., Turek V. F., Parkes D. G. (2010). ‘Weighing in’ on synergy: preclinical research on neurohumoral anti-obesity combinations. Brain Res. 1350, 86–94 [DOI] [PubMed] [Google Scholar]
- Salter J. M. (1960). Metabolic effects of glucagon in the Wistar rat. Am. J. Clin. Nutr. 8, 535–539 [Google Scholar]
- Sargent B. J., Moore N. A. (2009). New central targets for the treatment of obesity. Br. J. Clin. Pharmacol. 68, 852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen A. J., Finer N., Hollander P., Jensen M. D., Van Gaal L. F. (2006). Efficacy and tolerability of rimonabant in oiverweight or obese patients with Type 2 diabetes: a randomised controlled study. Lancet 368, 1660–1672 [DOI] [PubMed] [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C. D., Karason K., Larsson B., Wedel H., Lystig T., Sullivan M., Bouchard C., Carlsson B., et al. (2007). Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Eng. J. Med. 357, 741–752 [DOI] [PubMed] [Google Scholar]
- Tallett A. J., Blundell J. E., Rodgers R. J. (2008). Endogenous opioids and cannabinoids: system interactions in the regulation of appetite, grooming and scratching. Physiol. Behav. 94, 422–431 [DOI] [PubMed] [Google Scholar]
- Torgerson J. S., Hauptman J., Boldrin M. N., Sjostrom L. (2004). Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomised study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27, 155–161 [DOI] [PubMed] [Google Scholar]
- Trevaskis J. L., Coffey T., Cole R., Lei C., Wittmer C., Walsh B., Weyer C., Koda J., Baron A. D., Parkes D. G., et al. (2008). Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 149, 5679–5687 [DOI] [PubMed] [Google Scholar]
- Turton M. D., O’Shea D., Gunn I., Edwards C. M. B., Meeran K., Heath M., Lambert P. D., Wilding J. P. H., Smith D. M., Ghatei M. A., et al. (2006). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 [DOI] [PubMed] [Google Scholar]
- Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rossner S. (2005). Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from RIO-Europe study. Lancet 365, 1389–1397 [DOI] [PubMed] [Google Scholar]
- Vemuri V. K., Janero D. R., Makriyannis A. (2008). Therapeutic targeting of the endocannabinoid signaling system: drugs for obesity and the metabolic syndrome. Physiol. Behav. 93, 671–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers S. P., Cheetham S. C. (2007). Preclinical developments in antiobesity drugs. In Appetite and Body Weight (ed. Kirkham T. C., Cooper S. J.), pp. 323–336 London: Academic Press [Google Scholar]
- Vickers S. P., Jackson H. C., Cheetham S. C. (2011). The utility of animal models to evaluate novel anti-obesity agents. Br. J. Pharmacol. 164, 1248–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. J., Lefever T. W., Jackson C., Tallarida R. J., Walker E. A. (2008). Effects of a cannabinoid1 receptor antagonist and serotonin2C receptor agonist alone and in combination on motivation for palatable food: a dose addition analysis study in mice. J. Pharmacol. Exp. Ther. 325, 567–576 [DOI] [PubMed] [Google Scholar]
- Weintraub M., Sundaresan P. R., Madan M., Schuster B., Balder A., Lasagna L., Cox C. (1992). Long-term weight control study, 1 (weeks 0–34) – the enhancement of behavior-modification, caloric restriction, and exercise by fenfluramine plus phentermine. Clin. Pharmacol. Ther. 51, 586–594 [DOI] [PubMed] [Google Scholar]
- Wilding J. (2007). Clinical investigations of antiobesity drugs. In Appetite and Body Weight (ed. Kirkham T. C., Cooper S. J.), pp. 337–355 London: Academic Press [Google Scholar]
- Wilding J. P. H., Hardy K. (2011). New drugs for diabetes: glucagon-like peptide 1 analogues. BMJ 342, 343–346 [DOI] [PubMed] [Google Scholar]
- Wing R. R., Tate D. F., Gorin A. A., Raynor H. A., Fava J. L. (2006). A self-regulation program for maintenance of weight loss. N. Eng. J. Med. 355, 1563–1571 [DOI] [PubMed] [Google Scholar]
- Woods S. C., Lutz T. A., Geary N., Langhans W. (2006). Pancreatic signals controlling food intake: insulin, glucagon and amylin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1219–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]