SUMMARY
Obesity is associated with an increase in the prevalence and severity of infections. Genetic animal models of obesity (ob/ob and db/db mice) display altered centrally-mediated sickness behaviour in response to acute inflammatory stimuli such as lipopolysaccharide (LPS). However, the effect of diet-induced obesity (DIO) on the anorectic and febrile response to LPS in mice is unknown. This study therefore determined how DIO and ob/ob mice respond to a systemic inflammatory challenge. C57BL/6 DIO and ob/ob mice, and their respective controls, were given an intraperitoneal (i.p.) injection of LPS. Compared with controls, DIO and ob/ob mice exhibited an altered febrile response to LPS (100 μg/kg) over 8 hours. LPS caused a greater and more prolonged anorexic effect in DIO compared with control mice and, in ob/ob mice, LPS induced a reduction in food intake and body weight earlier than it did in controls. These effects of LPS in obese mice were also seen after a fixed dose of LPS (5 μg). LPS (100 μg/kg) induced Fos protein expression in several brain nuclei of control mice, with fewer Fos-positive cells observed in the brains of obese mice. An altered inflammatory response to LPS was also observed in obese mice compared with controls: changes in cytokine expression and release were detected in the plasma, spleen, liver and peritoneal macrophages in obese mice. In summary, DIO and ob/ob mice displayed an altered behavioural response and cytokine release to systemic inflammatory challenge. These findings could help explain why obese humans show increased sensitivity to infections.
INTRODUCTION
Obesity is a very common disease that has reached epidemic status in many developing countries (Bessesen, 2008). It is defined by an excess accumulation of adipose tissue that causes significant health problems, such as cardiovascular disease and type II diabetes. Adipose tissue is a source of numerous inflammatory factors and thus obesity is associated with a change in inflammatory markers, including pro- and anti-inflammatory cytokines (Fantuzzi, 2005; Juge-Aubry et al., 2005). The consequence of this change in inflammatory state is not clear, but might be linked to the increase in susceptibility and morbidity to infections reported in obese individuals (Falagas and Kompoti, 2006). Obesity is associated with poorer wound healing and increased infection following surgical procedures (Vilar-Compte et al., 2000), higher rates of infection and mortality after burns (Gottschlich et al., 1993), and an increased risk of sepsis in the critically ill (Bercault et al., 2004; Yaegashi et al., 2005; Vachharajani, 2008). Furthermore, higher rates of respiratory, periodontal and skin infections occur in the obese population (Al Zahrani et al., 2003; Garcia, 2002; Sabato et al., 2006; Salerno et al., 2004; Thorsteinsdottir et al., 2005; Wood et al., 2003).
An altered immune response to infection has also been observed in several genetic animal models of obesity, including ob/ob and db/db mice, and the Zucker fa/fa rat (Faggioni et al., 1997; Faggioni et al., 1999; Ivanov et al., 2001; Ivanov and Romanovsky, 2002; Lugarini et al., 2005; Mancuso et al., 2002; Rosenthal et al., 1996; Ordway et al., 2008; Hsu et al., 2007; Ikejima et al., 2005; Park et al., 2009; Wehrens et al., 2008; O’Connor et al., 2005; Plotkin et al., 1996). These animals are obese owing to either a deficiency in the adipokine leptin (ob/ob) or a defective leptin receptor (db/db and fa/fa). In addition to its involvement in energy balance, recent evidence suggests that leptin also plays a role in the regulation of immunity (Lam and Lu, 2007). It is possible, therefore, that the effects observed in genetically obese animals are due to a lack of leptin or leptin signalling rather than the effects of obesity per se. Furthermore, although genetically obese rodents have proven to be useful models of obesity, genetic mutations leading to leptin deficiency or a defective leptin receptor have been identified only within a small subset of the human population (Farooqi, 2008). Therefore diet-induced obesity (DIO) in rodents is a more physiologically relevant model of human obesity because the majority of cases of obesity in humans is associated with positive energy balance (increased food intake with or without decreased energy expenditure).
To date, few studies have examined how DIO rodents respond to bacterial or viral infection (Amar et al., 2007; Smith et al., 2007; Strandberg et al., 2009), and only one study in rats has investigated how DIO affects the behavioural and inflammatory response to Gram-negative bacterial endotoxin (Pohl et al., 2009). Endotoxin is the most important microbial trigger in the life-threatening condition sepsis (systemic inflammation due to infection), which kills 4 million people a year worldwide (Cohen, 2002). Most patients with sepsis develop fever; however, in more severe cases, patients develop hypothermia that is often associated with a worse outcome (Arons et al., 1999; Clemmer et al., 1992). In rodents, gram-negative bacterial infections are associated with several host defence responses, such as sickness behaviour, which is an adaptive response to infection characterised by altered sleep patterns, a loss of appetite and body weight, changes in body temperature, and social withdrawal. Lipopolysaccharide (LPS) is the active component of endotoxin from Gram-negative bacteria and causes sickness behaviour in animals, including anorexia and changes in body temperature (fever or hypothermia), effects that are mediated by the central actions of cytokines such as interleukin-1 beta (IL-1β) and IL-6.
The aim of this study was to determine the behavioural consequences of LPS administration in DIO mice by assessing food intake, body weight and core body temperature. In order to identify possible mechanisms underlying the effect of DIO on systemic inflammation, we determined the cytokine profile [IL-1 receptor antagonist (IL-1RA), IL-1β and IL-6] in response to LPS in peripheral tissues, macrophages and blood. Finally, because the actions of LPS on behaviour are mediated by the brain, we assessed the effect of LPS on neuronal activity in DIO mice by quantifying Fos protein expression in key brain regions. We also compared the effects of LPS in DIO mice to ob/ob mice.
RESULTS
Experiment 1: effect of LPS (100 μg/kg) on food intake, body weight and core body temperature in DIO mice
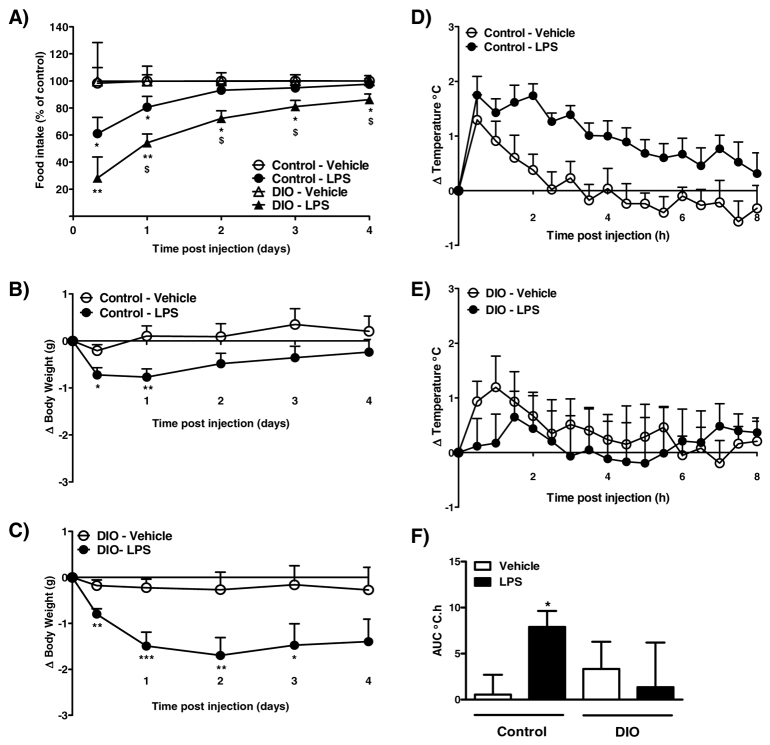
After 20 weeks maintenance on a high-fat diet, DIO mice weighed significantly more than control mice (control, 33.7±1.1 g vs DIO, 52.4±1.1 g; P<0.001). Intraperitoneal (i.p.) administration of 100 μg LPS/kg body weight significantly reduced food intake and body weight in control mice at 8 hours and at 1 day after injection (Fig. 1A,B). The effect of LPS in control mice was transient: there was no difference in food intake or body weight between vehicle- or LPS-treated control mice at 2–4 days. By contrast, LPS had a greater and more prolonged effect in DIO mice. LPS caused a significant reduction in food intake and body weight in DIO mice at 8 hours compared with vehicle-treated DIO mice, and these effects were still observed 3 (body weight) and 4 (food intake) days after injection (Fig. 1A,C). The reduction in food intake and body weight in DIO mice was significantly greater at days 1–4 and 1–3, respectively, compared with control mice (P<0.05).
Fig. 1.
Effect of 100 μg/kg LPS on food intake, body weight and core body temperature in control and DIO mice. (A–C) LPS or vehicle (5 ml/kg saline) was injected i.p. 2 hours after lights on, and food intake (A; kcal % of control) and change in body weight [B,C; change (Δ)] were measured at 8 hours, and at 1, 2, 3 and 4 days after injections. (D–F) Changes in body temperature were monitored continuously by remote radiotelemetry and data are shown for 0–8 hours. (D,E) Mean change in core body temperature in control (D) and DIO (E) mice. (F) Analysis of the change in core body temperature over 0–8 hours after injection is illustrated as the area under the curve (AUC; °C.h). Data are mean ± s.e.m. for n=8–10 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs respective vehicle-treated animals; $P<0.05 vs LPS-treated control animals.
Injection of 100 μg LPS/kg body weight in control mice caused a significant rise in core body temperature that lasted until approximately 8 hours after injection (Fig. 1D,F). By contrast, LPS did not induce a febrile response in DIO mice (Fig. 1E,F). However, the response in DIO mice was variable, because LPS caused an immediate and long-lasting hypothermia in two (out of eight) DIO mice (data not shown).
Experiment 2: effect of LPS (100 μg/kg) on food intake, body weight and core body temperature in ob/ob mice
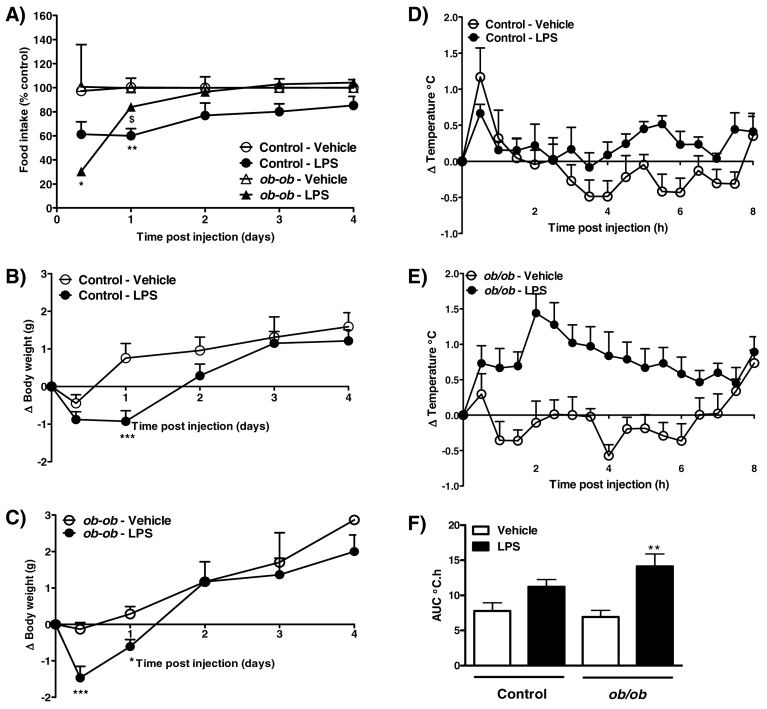
Prior to injection, obese ob/ob mice weighed significantly more than lean controls (control, 26.9±0.3 g vs ob/ob, 41.9±0.6 g; P<0.001). In control mice, i.p. injection of LPS (100 μg/kg) caused a transient reduction in food intake and body weight at 1 day after injection (Fig. 2A,B). However, the effect of LPS in obese ob/ob mice was observed earlier, with LPS inducing a decrease in food intake and body weight at 8 hours after injection. Body weight was also reduced in ob/ob mice at 1 day post-LPS injection, in the absence of a significant effect on food intake (Fig. 2A,C).
Fig. 2.
Effect of 100 μg/kg LPS on food intake, body weight and core body temperature in control and ob/ob mice. (A–C) LPS or vehicle (5 ml/kg saline) was injected i.p. 2 hours after lights on, and food intake (A; kcal % of control) and change in body weight [B,C; change (Δ)] were measured at 8 hours, and at 1, 2, 3 and 4 days after injection. (D–F) Changes in body temperature were monitored continuously by remote radiotelemetry and data are shown for 0–8 h. (D,E) Mean change in core body temperature in control (D) and ob/ob (E) mice. (F) Analysis of the change in core body temperature over 0–8 hours after injection is illustrated as the area under the curve (AUC; °C.h). Data are mean ± s.e.m. for n=6 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs respective vehicle-treated animals; $P<0.05 vs LPS-treated control animals.
Injection of LPS (100 μg/kg) had no significant effect on core body temperature in control mice over the 8-hour monitoring period (Fig. 2D,F). However, a significant increase in core body temperature was observed in ob/ob after LPS injection (Fig. 2E,F).
Experiment 3: effect of LPS (5 μg) on food intake, body weight and core body temperature in DIO mice
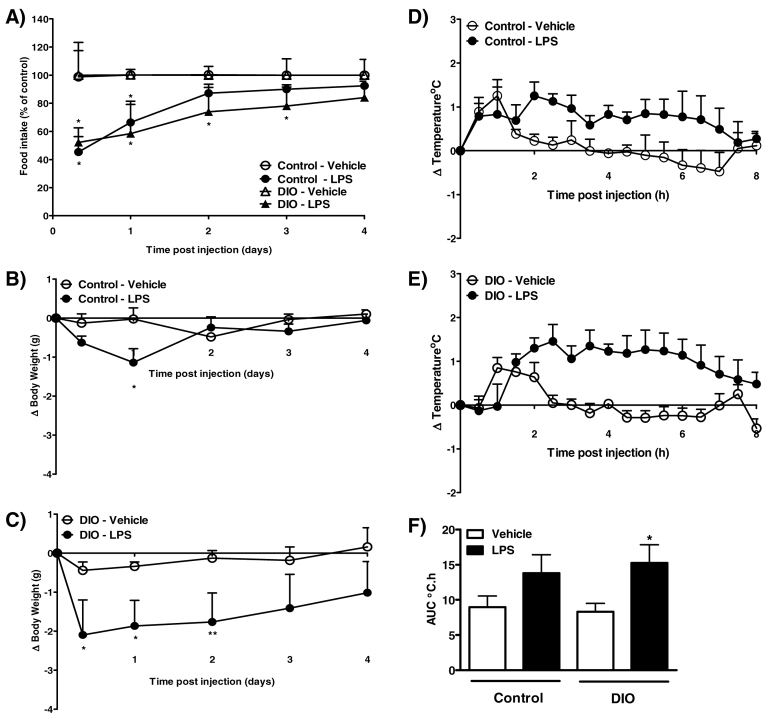
After 20 weeks on a high-fat diet, DIO mice weighed significantly more than mice fed a standard control diet (control, 33.0±0.6 g vs DIO, 47.6±1.3 g; P<0.001). As with 100 μg LPS/kg body weight, 5 μg LPS in control mice produced a transient reduction in food intake at 8 hours and at 1 day after injection, and a decrease in body weight at 1 day after injection (Fig. 3A,B). By contrast, 5 μg LPS had a prolonged effect in DIO mice; in these mice, a decrease in food intake and body weight was observed between 8 hours and 2–3 days after injection (Fig. 3A,C), and the reduction in body weight was greater in LPS-treated DIO mice compared with control mice at 8 hours and at 2 days (P<0.05; Fig. 3B,C).
Fig. 3.
Effect of 5 μg LPS on food intake, body weight and core body temperature in control and DIO mice. (A–C) LPS or vehicle (5 ml/kg saline) was injected i.p. 2 hours after lights on, and food intake (A; kcal % of control) and change in body weight [B,C; change (Δ)] were measured at 8 hours, and at 1, 2, 3 and 4 days after injection. (D–F) Changes in body temperature were monitored continuously by remote radiotelemetry and data are shown for 0–8 hours. (D,E) Mean change in core body temperature in control (D) and DIO (E) mice. (F) Analysis of the change in core body temperature over 0–8 hours after injection is illustrated as the area under the curve (AUC; °C.h). Data are mean ± s.e.m. for n=5 mice per group. *P<0.05, **P<0.01 vs respective vehicle-treated animals.
I.p. injection of 5 μg LPS in control mice had no significant effect on core body temperature during the first 8 hours after injection (Fig. 3D,F). However, LPS induced an increase in core body temperature during this time in DIO mice (Fig. 3E,F).
Experiment 4: effect of LPS (5 μg) on food intake, body weight and core body temperature in ob/ob mice
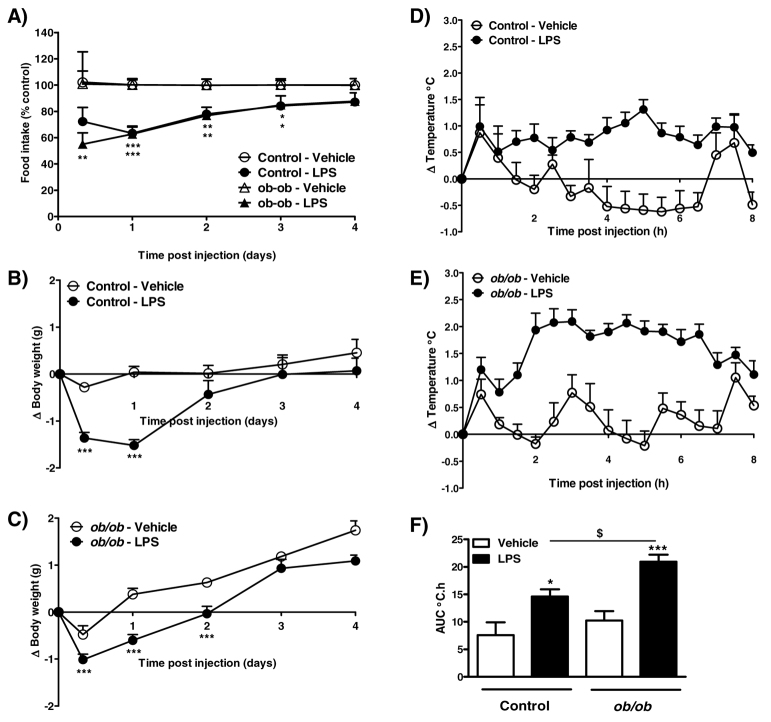
Prior to injection, obese ob/ob mice weighed significantly more than lean controls (control, 31.6±0.3 g vs ob/ob, 49.1±0.8 g; P<0.001). In control mice, LPS (5 μg, i.p.) caused a significant decrease in food intake between 1 and 3 days after injection (Fig. 4A). Compared with control mice, an anorexic effect of LPS was observed earlier in ob/ob mice: a reduction in food intake was noted at 8 hours and lasted until 3 days after injection (Fig. 4A). A reduction in body weight in response to LPS was observed in control mice at 8 hours and at 1 day after injection (Fig. 4B), an effect that lasted until 2 days in obese ob/ob mice (Fig. 4C).
Fig. 4.
Effect of 5 μg LPS on food intake, body weight and core body temperature in control and ob/ob mice. (A–C) LPS or vehicle (5 ml/kg saline) was injected i.p. 2 hours after lights on, and food intake (A; kcal % of control) and change in body weight [B,C; change (Δ)] were measured at 8 hours, and at 1, 2, 3 and 4 days after injection. (D–F) Changes in body temperature were monitored continuously by remote radiotelemetry and data are shown for 0–8 hours. (D,E) Mean change in core body temperature in control (D) and ob/ob (E) mice. (F) Analysis of the change in core body temperature over 0–8 hours after injection is illustrated as the area under the curve (AUC; °C.h). Data are mean ± s.e.m. for n=6 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs respective vehicle-treated animals; $P<0.05 vs LPS-treated control animals.
LPS (5 μg, i.p.) caused a significant increase in core body temperature over 8 hours in both control and ob/ob mice (Fig. 4D–F). However, the effect of LPS on temperature in ob/ob mice was greater, with the rise in body temperature in these mice being significantly higher compared with LPS-treated controls (Fig. 4F).
Experiment 5: effect of LPS on Fos expression in the brain and on peripheral cytokine release in DIO mice
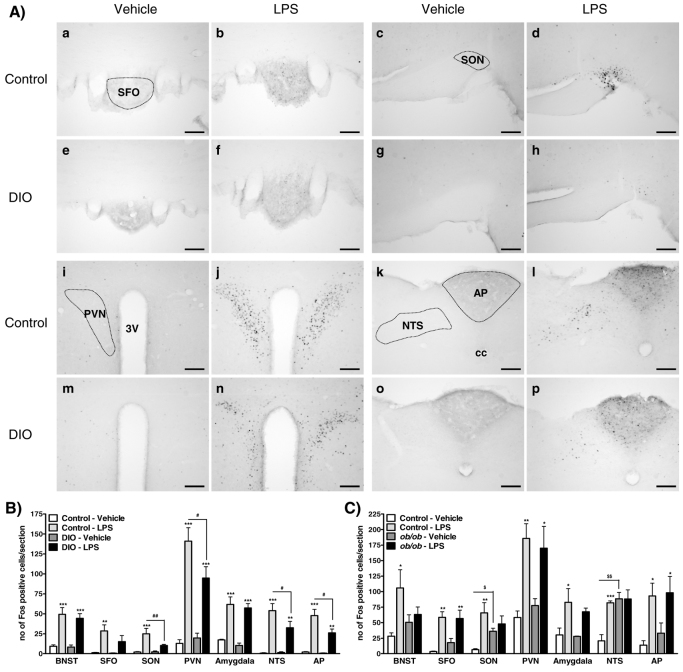
After 20 weeks maintenance on their respective diets, DIO mice were significantly heavier than control mice (body weight: control, 35.3±1.4 g vs DIO, 45.0±1.0 g; P<0.001). At 2 hours after i.p. administration of LPS (100 μg/kg) to control mice, a significant increase in Fos expression was observed in several forebrain regions, including the bed nucleus of stria terminalis (BNST), subfornical organ (SFO), supraoptic nucleus of the hypothalamus (SON), paraventricular nucleus of the hypothalamus (PVN), amygdala, and the nucleus of the tractus solarius (NTS) and area postrema (AP) of the brainstem when compared with vehicle-treated control mice (Fig. 5A,B). In LPS-treated DIO mice, a significant increase in the number of Fos-positive cells was observed in the same brain regions reported for control mice, apart from the SFO and SON, where no significant change in Fos expression was detected compared with vehicle-treated DIO mice. However, the number of cells positive for Fos protein in the PVN, NTS and AP was significantly lower in LPS-treated DIO mice compared with LPS-treated controls.
Fig. 5.
Effect of i.p. injection of LPS on Fos protein expression in the brains of control, DIO and ob/ob mice. (A) Representative photomicrographs illustrating Fos expression in the brain of control (a–d and i–l) or DIO (e–h and m–p) mice after i.p. injection of vehicle (5 ml saline/kg body weight) or LPS (100 μg/kg body weight). After LPS injection, significant increases in Fos expression were observed in the SFO (a,b,e,f) and SON (c,d,g,h) in control mice only, and in the PVN (i,j,m,n), NTS (k,l,o,p) and AP (k,l,o,p) of control and DIO mice, although the number of Fos-positive cells in response to LPS was lower in DIO compared with control mice. Scale bars: 100 μm. 3V, third ventricle; cc, central canal. (B) Quantification of the number of Fos-positive nuclei per section in DIO mice. (C) Quantification of the number of Fos-positive nuclei per section in ob/ob mice. No photomicrographs are shown for ob/ob mice because findings were similar to DIO mice. Data are mean ± s.e.m. for n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs respective vehicle-treated group; #P<0.05, ##P<0.01 versus LPS-treated control; $P<0.05, $$P<0.01 versus vehicle-treated control.
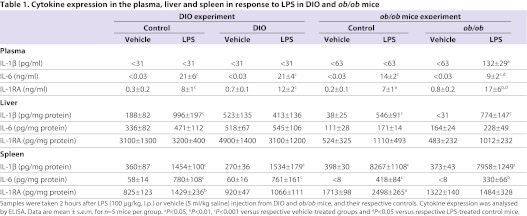
At 2 hours after i.p. administration of 100 μg LPS/kg body weight, there was a significant increase in IL-6 and IL-1RA levels in the plasma of control and DIO mice compared with their respective vehicle-treated groups (P<0.001; Table 1). There was no significant difference between plasma levels of IL-6 or IL-1RA after LPS treatment in control versus DIO mice. The amount of IL-1β detected in the plasma 2 hours after LPS in control or DIO mice was below the level of detection.
Table 1.
Cytokine expression in the plasma, liver and spleen in response to LPS in DIO and ob/ob mice
LPS caused a significant increase in IL-1β protein in the liver of control mice (P<0.001) but failed to stimulate expression in DIO mice. LPS had no effect on the production of IL-6 or IL-1RA in the liver 2 hours after injection in DIO or control mice.
Peripheral administration of LPS stimulated the expression of IL-1β and IL-6 to the same extent in the spleen of control compared with DIO mice. LPS also significantly increased IL-1RA expression in the spleen of control mice, but had no effect on IL-1RA expression in the spleen of DIO mice.
Experiment 6: effect of LPS on Fos expression in the brain and on peripheral cytokine release in ob/ob mice
At the time of injection, ob/ob mice were significantly heavier compared with control mice (body weight: control, 24.1±0.3 g vs ob/ob, 44.5±1.0 g; P<0.001). At 2 hours after i.p. administration of LPS (100 μg/kg) to control mice, a significant increase in Fos expression was observed in several forebrain regions, including the BNST, SFO, SON, PVN, amygdala, and the NTS and AP of the brainstem when compared with vehicle-treated control mice (Fig. 5C). In ob/ob mice, LPS caused a significant increase in the number of Fos-positive cells only in the SFO, PVN and AP; no significant change was detected in the BNST, SON, amygdala and NTS when compared with vehicle-treated ob/ob mice. In addition, the number of cells positive for Fos protein in the SON and NTS was significantly higher in vehicle-treated ob/ob compared with control mice.
Peripheral injection of LPS (100 μg/kg) had no effect on the plasma levels of IL-1β in control mice but significantly increased IL-1β in ob/ob mice 2 hours after injection (Table 1). LPS significantly increased the levels of IL-6 and IL-1RA in the plasma of both control and ob/ob mice when compared with vehicle-treated mice. However, there was less IL-6 but more IL-1RA in the plasma of LPS-treated ob/ob mice compared with LPS-treated controls.
LPS significantly increased IL-1β to the same extent in the liver of both control and ob/ob mice, but had no effect on the levels of IL-6 or IL-1RA in either group of mice. At 2 hours after injection, LPS increased IL-1β and IL-6 protein in the spleen of both control and ob/ob mice. LPS also stimulated the expression of IL-1RA in the spleen of control mice, but had no effect in ob/ob mice.
Experiments 7 and 8: effect of LPS on the release of cytokines from peritoneal macrophages in DIO and ob/ob mice
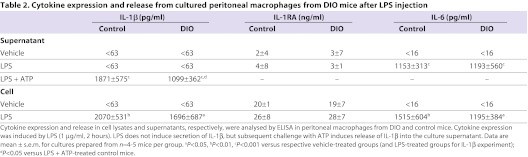
LPS significantly increased peritoneal macrophage IL-6 content and release to the same degree in cells from control or DIO mice (Table 2). LPS also caused a significant increase in IL-1β expression, to an equivalent level, in macrophages isolated from either control or DIO mice (P<0.01 and P<0.05, respectively). ATP-induced release of IL-1β was significantly increased in the supernatant of cells from LPS-treated control and DIO mice compared with their respective vehicle-treated groups. However, there was significantly less IL-1β released in response to LPS and ATP in macrophages from DIO compared with control mice. Consistent with this, reduced IL-1β release was also observed in the supernatants of LPS-treated macrophages from ob/ob mice compared with macrophages isolated from control mice (data not shown). LPS had no effect on the amount of IL-1RA detected in the supernatant or cell lysate of peritoneal macrophages from either control or DIO mice.
Table 2.
Cytokine expression and release from cultured peritoneal macrophages from DIO mice after LPS injection
DISCUSSION
This study demonstrates, for the first time, that DIO mice exhibit an altered anorexic, temperature and inflammatory response to bacterial endotoxin (LPS) compared with control mice, and suggests that obesity might impair the ability of the immune system to appropriately respond to bacterial infection. Furthermore, we also report for the first time that LPS causes a greater febrile response in obese ob/ob mice than in controls.
The anorexic response to peripheral administration of LPS in DIO mice was enhanced and prolonged compared with control mice: DIO animals showed a greater reduction in food intake and body weight, and took longer to recover after LPS treatment. Although previous studies have demonstrated that DIO rats display a greater febrile response to LPS (Pohl et al., 2009), and genetically obese leptin-deficient ob/ob mice are more sensitive to the anorexic actions of LPS (Faggioni et al., 1997), the present study is the first to report on the anorexic and febrile effects of LPS in DIO and ob/ob mice. Because only a small number of obese humans are deficient in leptin (Farooqi, 2008), DIO is arguably a more relevant model of human obesity and, therefore, our data in DIO mice could have relevance to the increase in susceptibility and morbidity to infection reported in obese individuals (Falagas and Kompoti, 2006).
In the present study, the initial dose of LPS administered was based on body weight (i.e. 100 μg LPS/kg body weight). Therefore, it is likely that, owing to their increased body weight, obese mice would have elevated levels of peritoneal LPS that could be responsible for the heightened anorexic response observed. In order to address this issue, we assessed the anorexic (and febrile) effects of a fixed dose of LPS (i.e. 5 μg) regardless of body weight, and observed that DIO and ob/ob mice again displayed an altered response to LPS compared with controls. These data suggest, therefore, that obese mice are highly sensitive to the anorexic actions of LPS.
The present study also demonstrates that DIO and ob/ob mice exhibit an altered temperature response to LPS. In some experiments, LPS did not induce a rise in core body temperature (fever) in control mice. In our hands it is difficult to induce a consistent febrile response in mice at the doses of LPS used here because these effects can also be influenced by slight fluctuations in ambient temperature. However, both DIO and ob/ob mice showed a greater rise in core body temperature to a dose of 5 μg LPS, compared with controls, and only ob/ob mice displayed an enhanced febrile response to 100 μg/kg LPS: this latter dose of LPS did not induce a fever in DIO mice. However, interpretation of these data is complicated because, in some DIO mice, LPS induced hypothermia (data not shown). The thermoregulatory response to LPS in rodents is complex and depends on the ambient temperature and dose administered (Rudaya et al., 2005; Wang et al., 1997; Romanovsky et al., 2005). The ambient temperature range in the present study (19–24°C) was sub-neutral and, at these temperatures, LPS induces a fever at low doses, mild hypothermia followed by fever at intermediary doses, and prolonged hypothermia at high doses that induce septic shock (Rudaya et al., 2005). Because LPS (at 100 μg/kg) induced hypothermia in some DIO mice in the present study, it is possible that these mice are more sensitive to the thermoregulatory effects of LPS.
The mechanism underlying the altered thermoregulatory response to LPS in DIO and ob/ob mice in the present study is yet to be determined. Previous studies have demonstrated that the obese Koletsky rat (which lacks the leptin receptor) exhibits a prolonged hypothermia in response to high doses of LPS (Steiner et al., 2004), which, among other things, could be due to impaired brown adipose tissue (BAT) thermogenesis (Steiner and Romanovsky, 2007). However, although it remains to be determined whether DIO and ob/ob mice have a reduced capacity to increase BAT thermogenesis after infection, our data do suggest that DIO mice are more likely than control mice to develop septic shock in response to systemic inflammation, because the incidence of LPS-induced hypothermia was greater in DIO mice compared with controls. Hypothermia in mice correlates with mortality after bacterial infection (Vlach et al., 2000) and, in support, mice fed a high-fat/cholesterol diet show enhanced mortality to a high dose (2 mg/kg, i.p.) of LPS (Huang et al., 2007). Some studies in humans also suggest that sepsis might be linked to increased mortality in critically ill obese patients (Vachharajani, 2008). The mechanism underlying this effect of obesity on outcome after sepsis in humans might be due to an increased susceptibility to hypothermia, because a drop in core body temperature is associated with increased mortality in normal-weight subjects with sepsis (Arons et al., 1999; Clemmer et al., 1992).
To further understand why DIO and ob/ob mice exhibited an enhanced behavioural response to an immune stimulus, the present study determined the levels of pro- and anti-inflammatory cytokines in plasma and peripheral tissues in response to LPS. Because the actions of LPS in rodents (e.g. fever) are mediated by cytokines (Cartmell et al., 1999; Cartmell et al., 2000; Dantzer, 2004; Roth and De Souza, 2001), we measured the peripheral expression of IL-1β, IL-6 and IL-1RA. Obese ob/ob mice displayed an altered cytokine response to LPS in the plasma. Furthermore, in contrast to control mice, LPS failed to stimulate the expression of IL-1RA in the spleen of DIO or ob/ob mice, and of IL-1β in the liver of DIO mice. In addition, peritoneal macrophages from either DIO or ob/ob mice released less IL-1β in response to LPS compared with control mice. These latter findings suggest a detrimental effect of macrophage cytokine release, not production, in obese individuals, because LPS stimulated IL-1β expression to the same degree in obese and control macrophages. Overall, this attenuated cytokine release in DIO and ob/ob mice might seem unexpected because these mice show greater behavioural responses to LPS. However, a blunted, or delayed, peripheral cytokine response is also associated with an increase in mortality and morbidity in DIO mice after bacterial Porphyromonas gingivalis or influenza virus infection (Amar et al., 2007; Smith et al., 2007), or in ob/ob mice in response to Listeria monocytogenes or LPS (Ikejima et al., 2005; Faggioni et al., 1999). Furthermore, DIO mice showed a delayed recovery in response to LPS and, thus, the impairment in the release of IL-1 and IL-1RA suggests that DIO mice might have a defect in mounting an appropriate immune response, resulting in inadequate elimination of bacterial infection.
In agreement with the present study, a reduction in IL-1RA expression in response to LPS is also observed in obese db/db (O’Connor et al., 2005) and ob/ob (Faggioni et al., 1999) mice in the spleen and plasma, respectively. As demonstrated here and by others, these genetically obese mice also display increased sensitivity to the actions of LPS (O’Connor et al., 2005; Faggioni et al., 1997; Faggioni et al., 1999). Mice deficient in IL-1RA show an increase in endotoxin-induced lethality (Hirsch et al., 1996), and endogenous IL-1RA limits LPS-induced fever (Cartmell et al., 2001); thus, it is possible that the lack of IL-1RA release from the spleen in response to LPS is responsible for the enhanced sickness behaviour in DIO and ob/ob mice reported here. However, because the present study only assessed cytokine expression at 2 hours after LPS, a more detailed time course is required in order to assess the temporal profile of the inflammatory response to infection in DIO and ob/ob mice.
The mechanism underlying the altered systemic immune response in DIO and ob/ob mice reported here and by others (Amar et al., 2007; Smith et al., 2007; Faggioni et al., 1999; Ikejima et al., 2005) is unclear. In the present study, peritoneal macrophages and immune cells within the spleen and liver of DIO and ob/ob mice showed a reduced capacity to release the pro- and anti-inflammatory mediators IL-1β and IL-1RA. Several functional and phenotypic abnormalities, such as a reduction in cytotoxic or phagocytic activity, are seen in macrophages from obese ob/ob (Cousin et al., 2001; Kjerrulf et al., 2006; Lee et al., 1999; Loffreda et al., 1998; Mancuso et al., 2002) and db/db (Loffreda et al., 1998) mice, the obese fa/fa rat (Plotkin et al., 1996), and mice fed a high-fat diet (Morrow et al., 1985; Stewart-Phillips et al., 1991). Furthermore, in agreement with the results shown here, macrophages from mice fed a high-fat diet show altered responses compared with controls, including a reduction in the release of inflammatory mediators such as IL-1β in response to infection (Amar et al., 2007; Stewart-Phillips et al., 1991; Wallace et al., 2000). Obesity and a diet high in fat can also affect the liver and spleen, leading to structural changes (Altunkaynak et al., 2007; Kiki et al., 2007; Chandra, 1980) and a reduction in natural killer cell number and activity (Jeffery et al., 1997; Li et al., 2005; Miyazaki et al., 2008; Smith et al., 2007; Yaqoob et al., 1994). Furthermore, altered cytokine production is also seen in the spleen of DIO mice (Lamas et al., 2004) and in cultured splenic lymphocytes from DIO mice in response to LPS (Mito et al., 2000). The reason for these effects of obesity and a high-fat diet on macrophage and on liver and spleen immune cell function are unknown, but they might be responsible for the reduced release of inflammatory mediators in obese mice in the present study.
The release of peripheral cytokines mediates the behavioural responses to LPS by directly or indirectly activating several key brain regions (Dantzer, 2004; Dantzer and Kelley, 2007). Because, overall, a blunted peripheral cytokine release was observed in DIO and ob/ob mice in the present study, we assessed the effect of obesity on the expression of Fos, a marker for cell activation, in response to LPS. In control mice, LPS induced Fos expression in several brain nuclei (the BNST, SFO, SON, PVN, amygdala, NTS and AP) that have previously been reported to be responsive to LPS (Sagar et al., 1995). However, the brains of obese mice were less responsive to LPS, with several brains regions either expressing fewer Fos-positive cells or showing no significant activation. This study is, therefore, the first to report a difference in the activity of the brain of obese compared with non-obese mice in response to an immune challenge. Because DIO and ob/ob mice showed enhanced responses to LPS, an increase in Fos expression might have been expected. However, the attenuation of LPS-induced Fos expression in obese mice could be due to the reduction in cytokine expression, such as the decreased macrophage IL-1β release seen here, because peripheral administration of IL-1β stimulates Fos protein in the same brain regions as LPS (Day and Akil, 1996).
In summary, this study demonstrates that obesity caused by a high-fat diet (DIO) or leptin deficiency (ob/ob) leads to an impaired innate immune response to LPS and an increased or prolonged behavioural response. The reasons for these effects of obesity on the immune function are likely to be complex. However, the changes that occur in response to a high-fat diet and excess adiposity seem to negatively impact on the function of the immune system. These results might have relevance in understanding why obese individuals show an increase in susceptibility and morbidity to infections (Falagas and Kompoti, 2006), including the life-threatening condition sepsis.
METHODS
Animals and diets
C57BL/6 male mice (8 weeks old; Harlan UK Limited, UK) were randomly assigned to either a high-fat diet (60% fat by energy; Test Diets, supplied by IPS Product Supplies, UK) or a standard diet (12% fat by energy; Test Diets, supplied by IPS Product Supplies) for 20 weeks. Obese ob/ob mice (10–12 weeks of age; Harlan UK Limited, UK) and normal weight controls (10–12 weeks of age; Harlan UK Limited) were maintained on a standard laboratory chow diet (10% fat by energy; Beekay International, UK). Mice on their respective diets were group housed and body weight and food intake of mice were measured weekly. All mice were given ad libitum access to food and water, and were housed at a constant ambient temperature of 21±2°C on a 12-hour light, 12-hour dark cycle (lights on at 08:00 h). All procedures conformed to the requirements of the UK Animals (Scientific Procedures) Act, 1986. Animals fed a high-fat diet are referred to as DIO mice, and mice fed a control diet as controls.
Experiments 1–4: the effect of LPS on food intake, body weight and core body temperature in DIO and ob/ob mice
DIO and control mice after 20 weeks of maintenance on their respective diets, or ob/ob and control mice at 10–11 weeks of age, were anaesthetised with isoflurane (1.5–2.5% in O2) and radiotransmitters (TA10TA-F20; Data Sciences, MN) were implanted abdominally into the peritoneum to allow for measurement of core body temperature by remote radiotelemetry. After surgery, mice were allowed to recover for 7–9 days before being housed individually 24 hours before experimental injections. Mice (n=5–10 per group) were then injected intraperitoneally (i.p., at 10:00 h) with either LPS (0127:B8 from Escherichia coli; Sigma-Aldrich, UK) or vehicle (sterile saline at 5 ml/kg body weight). After injections, animals were given a pre-weighed amount of their respective diets, and core body temperature was measured continuously in undisturbed animals by radiotelemetry.
In the first experiment, LPS was administered at a dose of 100 μg/kg (based on body weight). However, because DIO and ob/ob mice received a larger total amount of LPS, owing to their greater body weight compared with control mice, separate experiments were performed using a fixed dose of LPS (5 μg). Food intake and body weight were monitored daily for 4 days and core body temperature was monitored for 8 hours after injection. Experiments involving DIO or ob/ob mice were performed as separate studies.
Experiments 5 and 6: effect of LPS on Fos expression in the brain and peripheral release of cytokines in DIO and ob/ob mice
Separate groups of DIO (high-fat fed for 20 weeks) or ob/ob mice (10 weeks of age) and their respective controls (n=5 per group) were handled daily for 7 days (to reduce stress), after which they were given an i.p. injection of either LPS (100 μg/kg) or vehicle (5 ml/kg sterile saline). At 2 hours post-injection, animals were anaesthetised with sodium pentobarbitone (i.p.; Sagatal, Rhône-Mérieux, UK), blood was sampled by cardiac puncture and plasma obtained after centrifugation (13,000 g, 10 minutes). Mice were then perfused transcardially with 0.9% heparinised saline, and samples of liver and spleen taken. Plasma, liver and spleen samples were frozen and stored at −80°C until analysis by ELISA. Following perfusion with saline, animals were then perfuse-fixed with 4% paraformaldehyde [in 0.1 M phosphate buffer (PB)]. Brains were removed, post-fixed overnight in the same fixative, and cryopreserved in 30% sucrose. Coronal 30 μm brain sections were cut throughout the level of the hypothalamus and brainstem on a freezing sledge microtome. Immunohistochemistry for Fos protein was then performed on free-floating sections. Endogenous peroxidase was removed before treatment in blocking solution (2% normal goat serum in PB/0.3% Triton X-100). Sections were then incubated at 4°C overnight with a rabbit polyclonal anti-Fos antibody (1:5000, Ab5; Merck Chemicals, UK), washed in PB/0.3% Triton X-100 and treated for 2 hours in a peroxidase-labelled goat anti-rabbit IgG antibody (1:500; Vector Laboratories, UK). Following washes (in 0.1 M PB), nuclear Fos was detected by incubation in a nickel-diaminobenzidine solution (Sigma-Aldrich) that produced a blue-black precipitate.
The brain regions analysed for Fos expression were those previously reported to express Fos after i.p. injection of LPS (Sagar et al., 1995). The number of immunopositive cells expressing Fos protein per section (2–11 sections depending on the region analysed) was counted bilaterally, using a light microscope, in nuclei defined by Franklin and Paxinos (Franklin and Paxinos, 1997): BNST, 0.38 to 0.14 mm; SFO, 0.02 to −0.82 mm; SON, −0.58 to −0.94 mm; PVN, −0.58 to −1.22 mm; central nucleus of the amygdala, −0.70 to −1.82 mm; NTS, −6.34 to −7.48 mm; AP, −7.20 to −7.48 mm. The average number of cells per section was calculated and the group mean determined for each brain region.
Experiments 7 and 8: effect of LPS on cytokine release from peritoneal macrophages in DIO and ob/ob mice
Macrophages were prepared as described previously (Le Feuvre et al., 2002) from DIO or control mice after 20 weeks on their respective diets (n=4–5 per group) or from ob/ob or control mice (n=5–6 per group) at 12 weeks of age. Briefly, following sacrifice, the peritoneal cavity was lavaged with 8 ml RPMI 1640 containing 5% fetal bovine serum, 100 mg/ml streptomycin and 100 IU penicillin (all from Invitrogen, UK). The medium was recovered and cells were collected by centrifugation (80 g, 5 minutes) and seeded onto a 24-well plate at a density of 1×106 cells/ml; the macrophages were then incubated overnight (37°C, 5% CO2). To induce the synthesis of pro-IL-1β, IL-6 and IL-1RA, the macrophages were incubated with 1 mg/ml LPS (026:B6, E. coli; Sigma-Aldrich) for 2 hours. A further stimulation of the macrophages with ATP (5 mM, 10 minutes) to activate the P2X7 receptor and induce the release of IL-1β into the culture supernatant (Le Feuvre et al., 2002) was performed to investigate the effects of obesity on the capability of the macrophages to secrete IL-1β. Cell supernatant and lysate were then collected and stored at −20°C until analysis by ELISA.
TRANSLATIONAL IMPACT.
Clinical issue
Obesity has reached epidemic status in the Western world and is associated with many other serious illnesses, as well as with impaired wound healing and increased rates of infection following surgical procedures; higher rates of infection and mortality after burns; higher rates of respiratory, periodontal and skin infection; and an increased risk of sepsis in patients who are critically ill. Obesity induces chronic inflammation, which might increase susceptibility to and morbidity caused by infections in obese individuals. Thus, understanding how inflammatory responses are affected by obesity is crucial. This issue has been addressed in genetic models of obesity, but not yet in a diet-induced obesity (DIO) model, which might be of greater relevance to humans.
Results
In this study, the authors determined the consequences of intraperitoneal administration of lipopolysaccharide (LPS) (bacterial endotoxin) in mouse models of obesity by assessing food intake, body weight and core body temperature. LPS injection caused altered febrile responses in both DIO and genetic (ob/ob) mouse models of obesity compared with control animals. In addition, LPS caused a greater and more prolonged anorexic effect in DIO mice than control mice. Notably, the anorexic effect and drop in body weight induced by LPS in ob/ob mice occurred at an earlier time point than in controls. Compared with controls, liver expression of the pro-inflammatory cytokine IL-1β was lower in DIO mice (but not in ob/ob mice) and neither DIO nor ob/ob mice upregulated expression of the receptor antagonist of IL-1 (IL-1RA) in the spleen in response to LPS. Furthermore, macrophages isolated from DIO and ob/ob mice secreted less IL-1β in response to stimulation with LPS and ATP than macrophages from control mice. Finally, induced expression of Fos protein (a marker of cellular activation) in several brain regions was lower in the brains of obese compared with control mice following LPS injection.
Implications and future directions
In summary, this study demonstrates that obesity caused by a high-fat diet (i.e. DIO mice) or leptin deficiency (ob/ob mice) results in impaired innate immune responses to LPS and an increased or prolonged behavioural response. There were notable differences between the two models with respect to the effect of LPS, which might be important in understanding human obesity. These data might help to explain the greater susceptibility to and morbidity caused by infections in obese individuals, and suggest that more successful treatment of infection or inflammatory episodes in such individuals could be achieved by pharmacological intervention that enhances immune responses.
Cytokine measurement: ELISA
Saline-perfused liver and spleen samples (from experiments 5 and 6) were homogenised in buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2 and 0.02% NaN3) containing 1% Triton X-100 and a protease inhibitor cocktail (Set I; Calbiochem, Merck Chemicals). All homogenates were centrifuged at 10,000 g for 30 minutes at 4°C. The supernatant from the liver samples was further ultracentrifuged at 100,000 g for 1 hour at 4°C. Supernatant for both spleen and liver were then stored at −20°C until analysis. Mouse IL-1β, IL-6 or IL-1RA concentrations in plasma, homogenates from liver and spleen, and supernatant and cell lysates from macrophages (from experiments 7 and 8) were analysed by ELISA (Duosets; R&D Systems, UK) according to the manufacturer’s instructions. Cytokine concentrations were determined by reference to the relevant standard curves. For liver and spleen supernatant, protein concentration was assessed by a BCA protein assay (Pierce Biotechnology, Rockford, IL) and results expressed as pg/mg protein.
Data analyses and statistics
All data are presented as mean ± s.e.m. Cumulative food intake is calculated as kcal and expressed as % of control, because the control and high-fat diets have different metabolisable energy contents based on weight (g). Body weight and body temperatures are plotted as the mean change from the point of injection (time zero). For body temperature, the integrated temperature response between 0 and 8 hours [area under the curve (AUC), °C.h] was calculated for each animal by the trapezoidal method. To take into account the animals that exhibited a hypothermic response, the baseline was set at 0 and peaks both above and below the baseline were considered. The AUC for the positive and negative peaks was determined and the net AUC calculated. Average net AUC values were then determined for each treatment group.
Statistical comparisons for food intake and body weight were performed using a two-way ANOVA with repeated measures, followed by a Tukey’s multiple comparisons test. All other data were analysed by a two-way ANOVA followed by a Tukey’s multiple comparisons test. For clarity, body weight and body temperature data for control and obese groups are plotted in separate figures. Statistical significance was taken when P<0.05.
Acknowledgments
We thank Sarah Gumusgoz and Sally J. Shelmerdine for their technical assistance.
Footnotes
FUNDING
This work was supported by the Research Council UK (RCUK).
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
C.B.L. conceived and designed the experiments, analysed the data and wrote the paper. C.B.L. performed the majority of the in vivo experiments and ex vivo analyses, with the help of E.M.K., and D.B. performed the experiments involving peritoneal macrophages.
REFERENCES
- Al Zahrani M. S., Bissada N. F., Borawskit E. A. (2003). Obesity and periodontal disease in young, middle-aged, and older adults. J. Periodontol. 74, 610–615 [DOI] [PubMed] [Google Scholar]
- Altunkaynak B. Z., Ozbek E., Altunkaynak M. E. (2007). A stereological and histological analysis of spleen on obese female rats, fed with high fat diet. Saudi Med. J. 28, 353–357 [PubMed] [Google Scholar]
- Amar S., Zhou Q., Shaik-Dasthagirisaheb Y., Leeman S. (2007). Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc. Natl. Acad. Sci. USA 104, 20466–20471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons M. M., Wheeler A. P., Bernard G. R., Christman B. W., Russell J. A., Schein R., Summer W. R., Steinberg K. P., Fulkerson W., Wright P., Dupont W. D., Swindell B. B. (1999). Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit. Care Med. 27, 699–707 [DOI] [PubMed] [Google Scholar]
- Bercault N., Boulain T., Kuteifan K., Wolf M., Runge I., Fleury J. C. (2004). Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit. Care Med. 32, 998–1003 [DOI] [PubMed] [Google Scholar]
- Bessesen D. H. (2008). Update on obesity. J. Clin. Endocrinol. Metab. 93, 2027–2034 [DOI] [PubMed] [Google Scholar]
- Cartmell T., Luheshi G. N., Rothwell N. J. (1999). Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J. Physiol. 518, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T., Poole S., Turnbull A. V., Rothwell N. J., Luheshi G. N. (2000). Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J. Physiol. 526, 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T., Luheshi G. N., Hopkins S. J., Rothwell N. J., Poole S. (2001). Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J. Physiol. 531, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R. K. (1980). Cell-mediated immunity in genetically obese C57BL/6J ob/ob) mice. Am. J. Clin. Nutr. 33, 13–16 [DOI] [PubMed] [Google Scholar]
- Clemmer T. P., Fisher C. J., Jr, Bone R. C., Slotman G. J., Metz C. A., Thomas F. O. (1992). Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit. Care Med. 20, 1395–1401 [DOI] [PubMed] [Google Scholar]
- Cohen J. (2002). The immunopathogenesis of sepsis. Nature 420, 885–891 [DOI] [PubMed] [Google Scholar]
- Cousin B., Andre M., Casteilla L., Penicaud L. (2001). Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J. Cell Physiol. 186, 380–386 [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2004). Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 500, 399–411 [DOI] [PubMed] [Google Scholar]
- Dantzer R., Kelley K. W. (2007). Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day H. E., Akil H. (1996). Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology 63, 207–218 [DOI] [PubMed] [Google Scholar]
- Faggioni R., Fuller J., Moser A., Feingold K. R., Grunfeld C. (1997). LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. Am. J. Physiol. 273, R181–R186 [DOI] [PubMed] [Google Scholar]
- Faggioni R., Fantuzzi G., Gabay C., Moser A., Dinarello C. A., Feingold K. R., Grunfeld C. (1999). Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 276, R136–R142 [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Kompoti M. (2006). Obesity and infection. Lancet. Infect. Dis. 6, 438–446 [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. (2005). Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S. (2008). Monogenic human obesity. Front. Horm. Res. 36, 1–11 [DOI] [PubMed] [Google Scholar]
- Franklin K., Paxinos G. (1997). The Mouse Brain in Stereotaxic Coordinates. New York, USA: Elsevier [Google Scholar]
- Garcia H. L. (2002). Dermatological complications of obesity. Am. J. Clin. Dermatol. 3, 497–506 [DOI] [PubMed] [Google Scholar]
- Gottschlich M. M., Mayes T., Khoury J. C., Warden G. D. (1993). Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. J. Am. Diet. Assoc. 93, 1261–1268 [DOI] [PubMed] [Google Scholar]
- Hirsch E., Irikura V. M., Paul S. M., Hirsh D. (1996). Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc. Natl. Acad. Sci. USA 93, 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A., Aronoff D. M., Phipps J., Goel D., Mancuso P. (2007). Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin. Exp. Immunol. 150, 332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Liu T., Rose J. L., Stevens R. L., Hoyt D. G. (2007). Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J. Inflamm. (Lond.) 4, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima S., Sasaki S., Sashinami H., Mori F., Ogawa Y., Nakamura T., Abe Y., Wakabayashi K., Suda T., Nakane A. (2005). Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes 54, 182–189 [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., Romanovsky A. A. (2002). Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R311–R316 [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., Kulchitsky V. A., Romanovsky A. A. (2001). Does obesity affect febrile responsiveness? Int. J. Obes. Relat. Metab. Disord. 25, 586–589 [DOI] [PubMed] [Google Scholar]
- Jeffery N. M., Sanderson P., Newsholme E. A., Calder P. C. (1997). Effects of varying the type of saturated fatty acid in the rat diet upon serum lipid levels and spleen lymphocyte functions. Biochim. Biophys. Acta 1345, 223–236 [DOI] [PubMed] [Google Scholar]
- Juge-Aubry C. E., Henrichot E., Meier C. A. (2005). Adipose tissue: a regulator of inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 19, 547–566 [DOI] [PubMed] [Google Scholar]
- Kiki I., Altunkaynak B. Z., Altunkaynak M. E., Vuraler O., Unal D., Kaplan S. (2007). Effect of high fat diet on the volume of liver and quantitative feature of Kupffer cells in the female rat: a stereological and ultrastructural study. Obes. Surg. 17, 1381–1388 [DOI] [PubMed] [Google Scholar]
- Kjerrulf M., Berke Z., Aspegren A., Umaerus M., Nilsson T., Svensson L., Hurt-Camejo E. (2006). Reduced cholesterol accumulation by leptin deficient (ob/ob) mouse macrophages. Inflamm. Res. 55, 300–309 [DOI] [PubMed] [Google Scholar]
- Lam Q. L., Lu L. (2007). Role of leptin in immunity. Cell Mol. Immunol. 4, 1–13 [PubMed] [Google Scholar]
- Lamas O., Martinez J. A., Marti A. (2004). Decreased splenic mRNA expression levels of TNF-alpha and IL-6 in diet-induced obese animals. J. Physiol. Biochem. 60, 279–283 [DOI] [PubMed] [Google Scholar]
- Le Feuvre R. A., Brough D., Iwakura Y., Takeda K., Rothwell N. J. (2002). Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 277, 3210–3218 [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Li Y., Yang E. K., Yang S. Q., Lin H. Z., Trush M. A., Dannenberg A. J., Diehl A. M. (1999). Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am. J. Physiol. 276, C386–C394 [DOI] [PubMed] [Google Scholar]
- Li Z., Soloski M. J., Diehl A. M. (2005). Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology 42, 880–885 [DOI] [PubMed] [Google Scholar]
- Loffreda S., Yang S. Q., Lin H. Z., Karp C. L., Brengman M. L., Wang D. J., Klein A. S., Bulkley G. B., Bao C., Noble P. W., Lane M. D., Diehl A. M. (1998). Leptin regulates proinflammatory immune responses. FASEB J. 12, 57–65 [PubMed] [Google Scholar]
- Lugarini F., Hrupka B. J., Schwartz G. J., Plata-Salaman C. R., Langhans W. (2005). Acute and chronic administration of immunomodulators induces anorexia in Zucker rats. Physiol. Behav. 84, 165–173 [DOI] [PubMed] [Google Scholar]
- Mancuso P., Gottschalk A., Phare S. M., Peters-Golden M., Lukacs N. W., Huffnagle G. B. (2002). Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J. Immunol. 168, 4018–4024 [DOI] [PubMed] [Google Scholar]
- Mito N., Hosoda T., Kato C., Sato K. (2000). Change of cytokine balance in diet-induced obese mice. Metabolism 49, 1295–1300 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Iwabuchi K., Iwata D., Miyazaki A., Kon Y., Niino M., Kikuchi S., Yanagawa Y., Kaer L. V., Sasaki H., Onoe K. (2008). Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand. J. Immunol. 67, 230–237 [DOI] [PubMed] [Google Scholar]
- Morrow W. J., Ohashi Y., Hall J., Pribnow J., Hirose S., Shirai T., Levy J. A. (1985). Dietary fat and immune function. I. Antibody responses, lymphocyte and accessory cell function in (NZB x NZW)F1 mice. J. Immunol. 135, 3857–3863 [PubMed] [Google Scholar]
- O’Connor J. C., Satpathy A., Hartman M. E., Horvath E. M., Kelley K. W., Dantzer R., Johnson R. W., Freund G. G. (2005). IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J. Immunol. 174, 4991–4997 [DOI] [PubMed] [Google Scholar]
- Ordway D., Henao-Tamayo M., Smith E., Shanley C., Harton M., Troudt J., Bai X., Basaraba R. J., Orme I. M., Chan E. D. (2008). Animal model of Mycobacterium abscessus lung infection. J. Leukoc. Biol. 83, 1502–1511 [DOI] [PubMed] [Google Scholar]
- Park S., Rich J., Hanses F., Lee J. C. (2009). Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect. Immun. 77, 1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin B. J., Paulson D., Chelich A., Jurak D., Cole J., Kasimos J., Burdick J. R., Casteel N. (1996). Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): susceptibility to Candida albicans infection and leucocyte function. J. Med. Microbiol. 44, 277–283 [DOI] [PubMed] [Google Scholar]
- Pohl J., Woodside B., Luheshi G. N. (2009). Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150, 4901–4910 [DOI] [PubMed] [Google Scholar]
- Romanovsky A. A., Almeida M. C., Aronoff D. M., Ivanov A. I., Konsman J. P., Steiner A. A., Turek V. F. (2005). Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front. Biosci. 10, 2193–2216 [DOI] [PubMed] [Google Scholar]
- Rosenthal M., Roth J., Storr B., Zeisberger E. (1996). Fever response in lean (Fa/-) and obese (fa/fa) Zucker rats and its lack to repeated injections of LPS. Physiol. Behav. 59, 787–793 [DOI] [PubMed] [Google Scholar]
- Roth J., De Souza G. E. (2001). Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz. J. Med. Biol. Res. 34, 301–314 [DOI] [PubMed] [Google Scholar]
- Rudaya A. Y., Steiner A. A., Robbins J. R., Dragic A. S., Romanovsky A. A. (2005). Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1244–R1252 [DOI] [PubMed] [Google Scholar]
- Sabato R., Guido P., Salerno F. G., Resta O., Spanevello A., Barbaro M. P. (2006). Airway inflammation in patients affected by obstructive sleep apnea. Monaldi. Arch. Chest Dis. 65, 102–105 [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Price K. J., Kasting N. W., Sharp F. R. (1995). Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res. Bull. 36, 381–392 [DOI] [PubMed] [Google Scholar]
- Salerno F. G., Carpagnano E., Guido P., Bonsignore M. R., Roberti A., Aliani M., Vignola A. M., Spanevello A. (2004). Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir. Med. 98, 25–28 [DOI] [PubMed] [Google Scholar]
- Smith A. G., Sheridan P. A., Harp J. B., Beck M. A. (2007). Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J. Nutr. 137, 1236–1243 [DOI] [PubMed] [Google Scholar]
- Steiner A. A., Romanovsky A. A. (2007). Leptin: at the crossroads of energy balance and systemic inflammation. Prog. Lipid Res. 46, 89–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. A., Dogan M. D., Ivanov A. I., Patel S., Rudaya A. Y., Jennings D. H., Orchinik M., Pace T. W., O’connor K. A., Watkins L. R., Romanovsky A. A. (2004). A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 18, 1949–1951 [DOI] [PubMed] [Google Scholar]
- Stewart-Phillips J. L., Lough J., Phillips N. C. (1991). The effect of a high-fat diet on murine macrophage activity. Int. J. Immunopharmacol. 13, 325–332 [DOI] [PubMed] [Google Scholar]
- Strandberg L., Verdrengh M., Enge M., Andersson N., Amu S., Onnheim K., Benrick A., Brisslert M., Bylund J., Bokarewa M., Nilsson S., Jansson J. O. (2009). Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS ONE 4, e7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir B., Tleyjeh I. M., Baddour L. M. (2005). Abdominal wall cellulitis in the morbidly obese. Scand. J. Infect. Dis. 37, 605–608 [DOI] [PubMed] [Google Scholar]
- Vachharajani V. (2008). Influence of obesity on sepsis. Pathophysiology. 15, 123–134 [DOI] [PubMed] [Google Scholar]
- Vilar-Compte D., Mohar A., Sandoval S., de la Rosa M., Gordillo P., Volkow P. (2000). Surgical site infections at the National Cancer Institute in Mexico: a case-control study. Am. J. Infect. Control 28, 14–20 [DOI] [PubMed] [Google Scholar]
- Vlach K. D., Boles J. W., Stiles B. G. (2000). Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp. Med. 50, 160–166 [PubMed] [Google Scholar]
- Wallace F. A., Neely S. J., Miles E. A., Calder P. C. (2000). Dietary fats affect macrophage-mediated cytotoxicity towards tumour cells. Immunol. Cell Biol. 78, 40–48 [DOI] [PubMed] [Google Scholar]
- Wang J., Ando T., Dunn A. J. (1997). Effect of homologous interleukin-1, interleukin-6 and tumor necrosis factor-alpha on the core body temperature of mice. Neuroimmunomodulation. 4, 230–236 [DOI] [PubMed] [Google Scholar]
- Wehrens A., Aebischer T., Meyer T. F., Walduck A. K. (2008). Leptin receptor signaling is required for vaccine-induced protection against Helicobacter pylori. Helicobacter 13, 94–102 [DOI] [PubMed] [Google Scholar]
- Wood N., Johnson R. B., Streckfus C. F. (2003). Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Periodontol. 30, 321–327 [DOI] [PubMed] [Google Scholar]
- Yaegashi M., Jean R., Zuriqat M., Noack S., Homel P. (2005). Outcome of morbid obesity in the intensive care unit. J. Intensive Care Med. 20, 147–154 [DOI] [PubMed] [Google Scholar]
- Yaqoob P., Newsholme E. A., Calder P. C. (1994). Inhibition of natural killer cell activity by dietary lipids. Immunol. Lett. 41, 241–247 [DOI] [PubMed] [Google Scholar]