SUMMARY
Prader-Willi syndrome (PWS) represents the most common form of genetic obesity. Several studies confirm that obesity is associated with inflammation, oxidative stress and impairment of antioxidant systems; however, no data are available concerning PWS subjects. We compared levels of plasma lipids and C-reactive protein (CRP) in 30 subjects of ‘normal’ weight (18.5–25 kg/m2), 15 PWS obese (>30 kg/m2) subjects and 13 body mass index (BMI)-matched obese subjects not affected by PWS. In all subjects, we evaluated the levels of lipid hydroperoxides and the activity of paraoxonase-1 (PON1), an enzyme involved in the antioxidant and anti-inflammatory properties exerted by high-density lipoproteins (HDLs). Furthermore, using the fluorescent molecule of Laurdan, we investigated the physicochemical properties of HDLs isolated from normal weight and obese individuals. Altogether, our results demonstrated, for the first time, higher levels of lipid hydroperoxides and a lower PON1 activity in plasma of obese individuals with PWS with respect to normal-weight controls. These alterations are related to CRP levels, with a lower PON1:CRP ratio in PWS compared with non-PWS obese subjects. The study of Laurdan fluorescence parameters showed significant modifications of physicochemical properties in HDLs from PWS individuals. Whatever the cause of obesity, the increase of adiposity is associated with inflammation, oxidative stress and alterations in HDL compositional and functional properties.
INTRODUCTION
Environmental and genetic factors are involved in the development of human obesity, a predisposing factor for cardiovascular diseases (CVDs) (Zalesin et al., 2011). Prader-Willi syndrome (PWS), a complex disorder associated with elevated morbidity and mortality both in paediatric and in adult ages (Whittington et al., 2001), is the most common form of genetic obesity. PWS is due to the absent expression of the paternally active genes in the PWS critical region on chromosome 15 (Cassidy and Driscoll, 2009). The syndrome affects multiple body systems; the most consistent major manifestations include muscular hypotonia, hyperphagia, childhood-onset obesity, short stature, hypogonadism and developmental delay (Cassidy and Driscoll, 2009; Faienza et al., 2011). Adults with PWS die prematurely from complications conventionally related to obesity, including type 2 diabetes mellitus (DM2), respiratory insufficiency and CVD (Einfeld et al., 2006; Patel et al., 2007). In this respect, we have previously demonstrated a cardiac cause of death in 58% of adults with PWS (Grugni et al., 2008).
Inflammation, oxidative stress and other metabolic changes related to the increased adiposity could be involved in the development of complications in obese subjects (Higdon and Frei, 2003; Vincent and Taylor, 2006; González-Chávez et al., 2011; Thaler and Schwartz, 2010; Monteiro and Azevedo, 2010; Bondia-Pons et al., 2012). In fact, in addition to serving as a storage depot for lipid energy, adipose tissue is a metabolically active endocrine organ able to secrete a large variety of proteins – including inflammatory cytokines and hormone-like factors, such as leptin, adiponectin and resistin – that can have local effects on adipose tissue physiology but also systemic effects on other organs (Bastard et al., 2006). Furthermore, it has been demonstrated that interleukin-6 (IL-6), tumour necrosis factor-α (TNFα) and leptin secreted by adipose tissue exert a pro-atherogenic effect and modulate signal transduction and gene expression (Vincent and Taylor, 2006; Higdon and Frei, 2003; González-Chávez et al., 2011).
The aim of this study was to investigate, for the first time, whether PWS is associated with oxidative stress and alterations of functional and physicochemical properties (order, polarity) of high-density lipoproteins (HDLs). Therefore, we compared the levels of lipid hydroperoxides and activity of the antioxidant and anti-inflammatory enzyme paraoxonase-1 (PON1) in 30 subjects of ‘normal’ weight (18.5–25 kg/m2), 15 PWS obese (>30 kg/m2) subjects and 13 body mass index (BMI)-matched obese individuals not affected by PWS. The physicochemical properties of HDLs isolated from plasma from normal-weight controls and obese individuals have been investigated by the use of Laurdan [2-dimethylamino-(6-lauroyl)-naphthalene], a fluorescent molecule widely used to investigate the physicochemical properties of model membrane and lipoproteins (Parasassi et al., 1991; Dousset et al., 1994; Ferretti et al., 2004; Ferretti et al., 2010a).
Previous studies have already demonstrated a relationship between obesity, oxidative stress and PON1 in animal models of obesity (Thomas-Moya et al., 2008) and in non-PWS obese subjects (Ferretti et al., 2005; Bajnok et al., 2007; Ferretti et al., 2010b; Koncsos et al., 2010). The interest to further investigate oxidative stress and HDL functions in obesity and in PWS is supported by recent studies that have demonstrated that HDLs exert several physiological effects. HDLs have a role in reverse cholesterol transport, and behave as antioxidant, antithrombotic and anti-inflammatory particles (Barter et al., 2004; Negre-Salvayre et al., 2006; Scanu and Edelstein, 2008; Podrez, 2010). The anti-inflammatory role of HDLs has been previously demonstrated in human studies and in animal models (Säemann et al., 2010; Murphy and Woollard, 2010). Different molecular mechanisms involving either the enzyme PON1 or HDL lipid composition have been hypothesized. The inflammatory process begins with the oxidation of low-density lipoprotein (LDL) in the artery wall (Hansson and Libby, 2006). Endothelial cells activated by oxidized LDLs express several adhesion proteins. Monocytes adhere to stimulated endothelial cells. Once monocytes bind to adhesion proteins on the surface of endothelial cells, they are available for recruitment into the subendothelial space by chemokines such as monocyte chemotactic protein-1 (MCP-1). The ability of HDLs to inhibit the oxidation of LDLs and cell membranes and to promote macrophage cholesterol efflux through the action of associated proteins, particularly PON1, reduces the inflammation related to atherosclerosis (Mackness et al., 2004; Goswami et al., 2009; Mackness and Mackness, 2010). The discovery that human HDLs inhibit endothelial cell adhesion molecules and MCP-1 is thus of potentially great importance because they reduce the recruitment of blood monocytes into the artery wall. Moreover, HDLs inhibit also the expression of endothelial cell adhesion proteins induced by C-reactive protein (CRP), an acute-phase protein synthesized by the liver in response to inflammation (Barter et al., 2004).
RESULTS
Blood biochemistry data
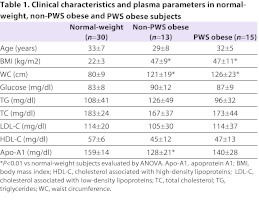
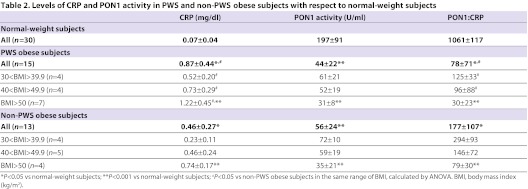
Clinical data, and plasma lipid and apoprotein levels in normal-weight subjects (controls), and obese PWS and non-PWS subjects are shown in Table 1. BMIs of PWS and non-PWS obese subjects were similar. No significant differences between controls, PWS and non-PWS obese subjects were found for glucose, triglycerides (TGs), total cholesterol (TC), LDL or HDL levels (Table 1). The mean levels of apoprotein A1 (Apo-A1) in plasma in both groups of obese individuals were significantly lower with respect to controls (P<0.01). The levels of CRP, a well-recognized marker of inflammation, were in the order PWS obese<non-PWS obese<normal-weight controls, and the differences were statistically significant (Table 2; P<0.05). To investigate whether the levels of CRP are related to BMI, we compared CRP levels in non-PWS and PWS obese subjects in three different subgroups of BMI (kg/m2): 30.1–39.9; 40–49.9; >50. As summarized in Table 2, PWS and non-PWS obese subjects with the highest BMIs showed the highest levels of CRP; moreover, in each BMI range, CRP levels were significantly higher in PWS subjects when compared with non-PWS obese subjects (P<0.05).
Table 1.
Clinical characteristics and plasma parameters in normal-weight, non-PWS obese and PWS obese subjects
Table 2.
Levels of CRP and PON1 activity in PWS and non-PWS obese subjects with respect to normal-weight subjects
Lipid hydroperoxides and PON1 activity
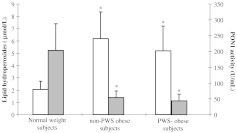
The mean values of the levels of hydroperoxides in plasma of PWS and non-PWS obese subjects were significantly higher (5.2±0.5 μmol/l and 6.2±0.6 μmol/l, respectively) with respect to normal-weight controls (2.1±0.1 μmol/l; P<0.001) (Fig. 1). No significant differences were observed in PWS with respect to non-PWS obese subjects (Fig. 1).
Fig. 1.
Levels of lipid hydroperoxides and PON1 activity in plasma of normal-weight subjects or non-PWS and PWS obese subjects. White bars: lipid hydroperoxides; grey bars: PON1 activity. *P<0.001 vs normal-weight subjects.
The values for PON1 activity in plasma of normal-weight controls ranged from 71 to 369 U/ml of plasma, with a mean value of 197±91 U/ml. In plasma of non-PWS obese subjects, the values of PON1 activity ranged from 10 to 81 U/ml (mean value: 56±24 U/ml) and in PWS subjects ranged from 23 to 80 U/ml (mean value, 44±212 U/ml). As shown in Fig. 1, the mean values of PON1 activity in non-PWS and PWS obese subjects were significantly lower with respect to normal-weight controls (P<0.001). No significant difference has been demonstrated between the two groups of obese subjects (Fig. 1).
To investigate whether the levels of CRP and PON1 activity are related to BMI, we studied the PON1:CRP ratio in the three groups of subjects. The ratio PON1:CRP was in the order PWS obese<non-PWS obese<normal-weight controls (Table 2). The mean values were significantly different (P<0.05). Furthermore, the PON1:CRP ratio was related to BMI; a lower ratio was observed in PWS and non-PWS obese individuals with higher BMI (Table 2). Moreover, in each BMI range, the PON1:CRP ratio was significantly lower in PWS obese subjects when compared with non-PWS obese subjects (Table 2).
HDL physicochemical properties
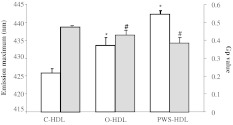
The mean value of emission maximum of Laurdan incorporated in HDLs of normal-weight controls was 426.4±1.1 nm, in agreement with our previous studies (Ferretti et al., 2010a; Ferretti et al., 2004). The emission spectra of Laurdan in HDLs of non-PWS (433.5±7.5 nm) and PWS (442.21±3.28 nm) obese subjects showed a red shifted position of the maximum emission with respect to normal-weight subjects (P<0.001).
In HDLs of controls, the generalized polarization (Gp) value was 0.483±0.003. As summarized in Fig. 2, the mean values of Gp in HDLs of non-PWS (0.437±0.037) and PWS (0.393±0.042) obese subjects were significantly lower with respect to normal-weight subjects (0.483±0.003; P<0.01) (Fig. 2).
Fig. 2.
Fluorescence emission maximum and Gp values of Laurdan incorporated in HDLs isolated from plasma of normal-weight subjects (C-HDL) or non-PWS (O-HDL) and PWS (PWS-HDL) obese subjects. White bars: fluorescence emission maximum; grey bars: Gp value. *P<0.001 vs emission maximum of C-HDL; #P<0.01 vs Gp values of C-HDL.
DISCUSSION
Obesity is associated with oxidative stress, as demonstrated in animal models and in human studies (Higdon and Frei, 2003; Vincent and Taylor, 2006; Bondia-Pons et al., 2012; Ferretti et al., 2005; Ferretti et al., 2010b). In the present study we confirmed a significant increase in lipid hydroperoxides and a decrease in the activity of the antioxidant and anti-inflammatory enzyme PON1 in plasma of non-PWS obese individuals with respect to normal-weight subjects, in good agreement with previous studies carried out by us and by other authors (Higdon and Frei, 2003; Ferretti et al., 2005; Vincent and Taylor, 2006; Ferretti et al., 2010b; Bondia-Pons et al., 2012). For the first time, we demonstrate an increase in the levels of lipid hydroperoxides and a decrease in the activity of the enzyme PON1 in plasma of PWS obese subjects, in the absence of significant changes of plasma lipids [TC, cholesterol associated with LDLs (LDL-C), TG].
The relationship between CRP levels and BMI in both groups of obese subjects confirms that increased adiposity is associated with a low-grade systemic inflammation in these individuals (Visser et al., 1999; Höybye, 2006; Butler et al., 2006; Caixàs et al., 2008; Viardot et al., 2010). Our results demonstrated that CRP levels were significantly higher in PWS obese subjects when compared with non-PWS obese subjects. These results are in agreement with previous studies that demonstrated higher levels of CRP and proinflammatory cytokines, such as IL-18 and IL-6, in obese adults with PWS, in comparison with non-PWS obese subjects (Caixàs et al., 2008; Butler et al., 2006).
Alterations of CRP levels and of serum PON1 activity could reflect modifications of their hepatic synthesis. In fact, inflammatory cytokines, such as IL-6 and TNFα, secreted by human adipose tissue stimulate the hepatic synthesis of CRP and downregulate PON1 hepatic expression (Kumon et al., 2003). Therefore, the low grade of inflammation associated with obesity could explain the lower PON1 activity and increased lipid peroxidation levels in PWS. In agreement with our hypothesis, previous studies have shown a relationship between PON1 activity and CRP levels. Higher plasma levels of CRP are associated with low PON1 activity (Kannampuzha et al., 2010) and a decrease of the PON1:CRP ratio has been observed in individuals with diabetes or end-stage renal disease with respect to healthy subjects (Nowak et al., 2010; Lahrach et al., 2008; Mackness et al., 2006). Both the increase in CRP concentration and the decrease in PON1 activity in serum can disturb the subtle equilibrium between these two parameters. It has been suggested that the PON1:CRP ratio could be a useful indicator of disturbances between the intensity of inflammation processes and anti-inflammatory and antioxidant effects of the HDL fraction, whose functions are closely related to PON1 activity (Mackness et al., 2006). In agreement with these studies, a relationship between serum concentrations of CRP and PON1 activity has been demonstrated; in fact, PWS and non-PWS obese subjects with the highest CRP values showed the lowest PON1 activity. We demonstrated also a lower PON1:CRP ratio in obese PWS compared with obese non-PWS individuals in the same range of BMI. This finding might be related to the different amounts of fat tissue in the two obese groups. In this context, it is known that BMI is not an exact measure of adiposity in PWS, because it underestimates the percentage of body fat. In fact, individuals with PWS harbour a higher fat mass than non-PWS obese subjects, with the same degree of weight excess. In this light, a limitation of this study is the lack of measurement of body composition of our subjects. Dual-energy X-ray photon absorptiometry (DEXA) seems to be the best available technique to evaluate body composition, but its use was impracticable for our study because the weight of the majority of PWS subjects exceeds the instrumental capacity. However, we have evaluated waist circumference (WC) as index of central obesity and no difference was detected between obese individuals with PWS and non-PWS obese subjects.
Other hypotheses could be formulated to explain the decrease of PON1 activity in PWS and non-PWS obese subjects. The significant decrease in the activity of the enzyme PON1 in plasma of obese PWS individuals compared with normal-weight controls could be related to the higher levels of lipid hydroperoxides (Aviram et al., 1998; Ferretti et al., 2005; Ferretti et al., 2010b) and/or to modifications of lipid-apoprotein interactions of HDL in obese individuals with PWS. In fact, PON1 is a lipid-dependent enzyme whose secretion by the liver, stability and stimulation of activity are modulated by HDL composition (James and Deakin, 2004). Apo-A1, the main apoprotein of HDL, seems to be of major importance in defining serum PON1 activity and stability (James and Deakin, 2004). The lower plasma levels of Apo-A1 in PWS and non-PWS obese subjects compared with normal-weight controls suggest alterations in lipid–Apo-A1 interactions at the HDL surface. Modifications of physicochemical properties of HDL in PWS and non-PWS individuals compared with controls have also been demonstrated using the fluorescent molecule Laurdan. Following the currently accepted model for the structure of lipids within the core and surface of lipoproteins, Laurdan is anchored by its lauric tail into the hydrophobic core of the lipoprotein phospholipid layer, with its polar naphthalene fluorescent moiety residing at the level of the glycerol backbone of phospholipids; therefore, it monitors the properties of the outer surface of the particle (Parasassi et al., 1991). The lower value of Gp and red shifted position of the maximum emission of the fluorescent molecule of Laurdan incorporated in HDLs of PWS and non-PWS obese subjects demonstrate a lower molecular order and a higher polarity in the microenvironment of the fluorescent probe with respect to HDL isolated from normal-weight subjects. We could expect that the alterations of compositional and physicochemical properties of HDLs in obese subjects, probably related to modifications of lipid-apoprotein interactions and/or to the increased level of oxidation, could contribute to the modifications of PON1 activity. The modifications of physicochemical properties of HDL of obese subjects have been demonstrated in the absence of significant modifications of plasma lipids routinely evaluated to study plasma lipid metabolism.
Obesity and PWS are associated also with hyperleptinaemia (Haqq et al., 2011; Proto et al., 2007; Thaler and Schwartz, 2010; Oswall and Yeo, 2010). The role of leptin in HDL alterations deserves future study. Previous studies on human obesity by us and other authors have demonstrated that higher levels of leptin are associated with higher levels of lipid hydroperoxides and are negatively correlated with plasma PON1 activity (Ferretti et al., 2005; Koncsos et al., 2010). Moreover, it has been demonstrated that leptin behaves as a hydrophobic peptide that can bind to HDLs and that it could directly inhibit the PON1 enzyme (Holub et al., 1999).
Further studies are also necessary to investigate the role of growth hormone (GH) in PWS. In fact, subjects with PWS are known to be GH deficient (Grugni et al., 2009). GH is reported to be associated with increased systemic inflammation, oxidative stress and endothelial dysfunction (Kokoszko et al., 2008), and GH replacement reduces free radical products in plasma of GH-deficient subjects (Karbownik-Lewinska et al., 2008).
In conclusion, whatever the cause of obesity, the increase of adiposity is associated with inflammation, oxidative stress, and alterations in Apo-A1 levels and HDL structural and functional properties, in agreement with our previous studies (Ferretti et al., 2005; Ferretti et al., 2010b). The alterations of PON1 activity are related to CRP: the PON1:CRP ratio is significantly lower in PWS compared with non-PWS obese subjects; therefore, our results demonstrate metabolic differences between PWS and non-PWS obese subjects.
As far as the physiological relevance of our results is concerned, it has to be stressed that HDLs exert several physiological roles and the multifunctional enzyme PON1 is able to modulate HDL function. PON1 is able to prevent the accumulation of oxidized lipids from lipoproteins (HDLs and LDLs) and membranes, preventing the atherogenic and inflammatory response induced by lipid peroxidation products (Goswami et al., 2009; Mackness et al., 2004; Mackness and Mackness, 2010). Previous studies have demonstrated that systemic inflammation and oxidative stress convert HDLs to a dysfunctional form that loses anti-inflammatory and anti-atherogenic effects (Ferretti et al., 2006; Ragbir and Farmer, 2010). We hypothesize that HDL and PON1 are a common link between oxidative stress and inflammation in human obesity. Oxidized HDLs and the decrease in PON1 activity could contribute to the exacerbated inflammation in subjects with PWS and non-PWS obesity.
METHODS
Chemical substances
Butylated hydroxytoluene (BHT), dihydrogen sulfate (H2SO4), methanol, paraoxon (diethyl p-nitrophenyl phosphate), phosphate-buffer saline (PBS), sodium chloride (NaCl), iron (II) ammonium sulphate, Tris (hydroxymethyl)aminomethane, sodium chloride (NaCl), magnesium chloride (MgCl2), calcium chloride (CaCl2) and xylenol orange were obtained from Sigma Chemical (St Louis, MO). The fluorescent probe 2-dimethylamino-(6-lauroyl)-naphthalene (Laurdan) was purchased from Molecular Probes Inc. (Eugene, OR).
Genetic and clinical characteristics of subjects included in the study
A total of 15 obese subjects with genetically confirmed PWS, nine females and six males, aged 21–40 years were included in the study (Table 1). All patients showed the typical PWS clinical phenotype. Thirteen subjects had interstitial deletion of the proximal long arm of chromosome 15 and two had uniparental maternal disomy for chromosome 15.
Physical examination included determination of height, weight and waist circumference (WC) in fasting conditions and after voiding. Standing height was determined by a Harpenden Stadiometer (Holtain Ltd, Crymych, Dyfed, UK). Body weight was measured to the nearest 0.1 kg, by using standard equipment. BMI was defined as weight in kilograms divided by the square of height in meters (kg/m2). WC was measured in standing position halfway between the inferior margin of the ribs and the superior border of the crista. According to World Health Organization criteria, the BMI cut-off points of 18.5–25 kg/m2 to define normal weight, >25–30 kg/m2 to define overweight and >30 kg/m2 to define obesity were used. Central obesity was defined when WC was >94 cm for men and >80 cm for women (Alberti et al., 2009). All PWS subjects showed central obesity. WC ranged from 113 and 141 cm in males and from 78 and 172 cm in females (Table 1).
PWS individuals were compared with two different groups of subjects. The first group included 13 individuals with obesity due to excessive caloric intake (non-PWS obese subjects) (eight females and five males), matched to PWS subjects for age (range 28–48 years old), BMI (range 30.5–55 kg/m2) and WC [range 108–143 cm (males) and 81–134 cm (females)] (Table 1). The second group included 30 normal-weight healthy control subjects (BMI ranging from 18.5 to 25.2 kg/m2) (controls), 16 females and 14 males, aged 27–52 years (Table 1). All obese patients were chosen from those admitted to the Italian Auxological Institute for evaluation and cure of obesity and its complications. Both PWS and non-PWS obese subjects were diet-free 30 days prior to beginning the study, and none of them was performing standardized physical activity.
Normal-weight controls and obese subjects were not taking lipid-lowering drugs, angiotensin-converting enzyme (ACE) inhibitors or antioxidants, or other medication that can affect lipid metabolism, including GH therapy. At the time of the study, none exhibited evidence of cardiac or renal failure and all were euthyroid with normal liver function tests and normal values for plasma urea, creatinine and electrolytes. Furthermore, subjects having a current or recent illness were excluded from the study, because modifications of PON1 activity were described in humans during the acute phase response (Van Lenten et al., 1995). Informed consent was obtained from each participating subject or by their parents when necessary. The study was approved by the ethics committee of Italian Auxological Institute and was carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Biochemical parameters in plasma of subjects included in the study
Blood samples of all subjects were collected at 8 a.m., after overnight fasting, in heparin-containing vacutainer tubes. Plasma was separated by low-speed centrifugation (1600 g) at 4°C for 20 minutes and thereafter used to evaluate biochemical parameters.
Plasma levels of glucose, lipids and CRP
Enzymatic methods (Roche Molecular Biochemicals, Mannheim, Germany) were used for determination of blood glucose (Frost, 2004), total cholesterol (TC) (Roeschlau et al., 1974), LDL-cholesterol (LDL-C) (Nauck et al., 2000) and triglycerides (TG) (McGowan et al., 1983). A direct HDL-cholesterol assay was used to evaluate HDL-cholesterol (HDL-C) (Warnick and Wood, 1995). Apo-A1 was assessed by immunonephelometry (Lopes-Virella et al., 1980) on IMMAGE analyzer (Beckman) and CRP was measured by immunoturbidimetric assay (Roche, Mannheim, Germany) (Borque et al., 2000).
PON1 activity
PON1 activity was measured in plasma from all subjects using paraoxon (diethyl 4-nitrophenyl phosphate) as substrate, as previously described by Rock et al. (Rock et al., 2008); in fact, the hydrolysis of paraoxon seems most closely related to the inverse relationship with coronary heart disease (Mackness et al., 2003). The assay was conducted at a temperature of 37°C in a microplate reader (Synergy HT). Briefly, an aliquot of plasma (10 μl) was resuspended in a basal assay mixture (5 mM Tris-HCl, pH 7.4 containing 0.15 M NaCl, 4 mM MgCl2, 2 mM CaCl2 and 1.0 mmol/l paraoxon). Paraoxon hydrolysis was kinetically monitored for 8 minutes (every 15 seconds) at 412 nm. Non-enzymatic hydrolysis of paraoxon was subtracted from the total rate of hydrolysis. The enzyme activity was calculated by KC4 software. Previous studies have reported that intra-and inter-assay variation, as well as inter-laboratory variability for PON1 activity, was <10%. These results establish good reproducibility of the PON1 enzymatic assays (Huen et al., 2009).
Levels of lipid hydroperoxides
The extent of lipid peroxidation in plasma was determined using the ferrous oxidation-xylenol orange (FOX) assay (Nourooz-Zadeh, 1999). Briefly, aliquots (200 μl) of plasma were mixed with 1800 μl of FOX-reagent [250 μM ammonium ferrous sulphate, 100 μM xylenol-orange, 25 mM H2SO4 and 4 mM BHT in 90% methanol (v/v) in 100 ml]. After incubation at room temperature for 30 minutes, samples were centrifuged at 2900 g for 10 minutes. The supernatant was carefully decanted into a cuvette and the absorbance was determined at 560 nm. The levels of lipid hydroperoxides were quantified using a stock solution of t-butyl hydroperoxide. The results are shown as μmol/l plasma. Previous studies have reported that the coefficient of variation for individual plasma using the FOX2 assay is <10% (Nourooz-Zadeh, 1999).
HDL physicochemical properties
Preparation of plasma and human HDL
Plasma was used for the preparation of lipoproteins. HDLs [density (d)=1.063–1.210 g/ml] were isolated by single vertical spin density gradient ultracentrifugation for 1.30 hours at 473,200 g and dialyzed at 4°C for 24 hours against 10 mM PBS, pH 7.4 (Chung et al., 1986). A pool of HDL from normal-weight subjects (C-HDL), non-PWS obese subjects (O-HDL) and PWS subjects (PWS-HDL), were used in the following experiments. Protein concentration of HDL was determined by the method of Bradford (Bradford, 1976).
TRANSLATIONAL IMPACT.
Clinical issue
Environmental and genetic factors are involved in the development of human obesity, a predisposing factor for cardiovascular disease (CVD) and other complications. The higher risk of CVD has been attributed to obesity-induced alterations in plasma lipids and lipoproteins [including low- and high-density lipoprotein (LDL and HDL, respectively)], inflammation, oxidative stress, and impaired antioxidant defenses. HDL protects against vascular disease owing in part to its antioxidant properties. The HDL-associated enzyme paraoxonase-1 (PON1) modulates the antioxidant and anti-inflammatory properties of HDLs: oxidatively modified HDLs have reduced PON1 activity and pro-inflammatory properties. Recently, roles for HDL-PON1 activity in lipid metabolism in human adipose tissue, and in the gut-brain axis, have been described.
Results
Here, the authors investigated lipid peroxidation products, the physicochemical properties of HDLs (with Laurdan fluorescent probe) and the activity of the PON1 in normal-weight subjects, obese subjects with Prader-Willi syndrome (PWS; the most common genetic form of obesity) and non-PWS obese subjects with a matched body mass index. They found higher levels of lipid hydroperoxides and a lower level of PON1 activity in the plasma of obese subjects with and without PWS compared with normal-weight subjects. These alterations in PON1 activity were not correlated with significant changes in overall plasma lipid levels. The ratio of PON1 activity to levels of C-reactive protein (CRP; a marker of inflammation) was lower in obese non-PWS subjects compared with normal-weight controls and, notably, the ratio in obese PWS subjects was lowest. In addition, the physicochemical properties of HDLs were significantly modified with respect to polarity and the order of lipoproteins in obese subjects with or without PWS, compared with normal-weight subjects.
Implications and future directions
Our results suggest that obesity, whether induced by genetics (as in PWS) or environmental factors, is associated with inflammation, oxidative stress, and alterations in the composition and functional properties of HDLs. Notably, this is the first report hat has examined such parameters in PWS subjects. The data also suggest that alterations in HDL and PON1 activity in obesity are related to oxidative stress and inflammation, and that further studies examining the role of these factors in obesity-associated pathologies – particularly CVD – are warranted.
Fluorescence studies
Laurdan is an amphipathic fluorescent probe that localizes at the hydrophilic-hydrophobic interface of the lipoprotein surface with its lauric acid tail anchored in the phospholipid acyl chains region. Laurdan spectroscopic properties reflect the probe sensitivity to the polarity and to the dynamics of its environment (Parasassi et al., 1991). Laurdan was incorporated into HDLs of all subjects as previously described (Dousset et al., 1994; Ferretti et al., 2004; Ferretti et al., 2010a). Laurdan probe was dissolved in a 100% methanol solution (concentration 1 mM) and stored at −20°C. Briefly, an aliquot of Laurdan was incorporated with HDLs (100 μg/ml of protein) for 30 minutes at 37°C, using a final probe concentration of 1 μM. The fluorescence emission spectra of Laurdan were obtained using an excitation wavelength of 340 nm. The value of Gp of Laurdan was calculated using the formula: Gp=(I435–I490)/(I435+I490), where I435 and I490 are the emission intensities at 435 and 490 nm, respectively.
Previous studies demonstrated that the position of the maximum emission and the Gp value of Laurdan are sensitive to modifications of physicochemical properties in the microenvironment surrounding the probe. An increase of Gp indicates a decrease of polarity and an increase of molecular order, and vice versa (Parasassi et al., 1991).
Data analysis
Statistical analysis was performed with SPSS 15.0 statistical software version. All experiments were performed in triplicate and the results were shown as mean ± s.d. The differences in all parameters between non-PWS obese, PWS obese subjects and normal-weight individuals were evaluated by one-way ANOVA. Post-hoc analysis was performed using the LSD test. Values were considered to be significant at P<0.05.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the intellectual development of this work and approved the final manuscript. G.F. was responsible for experimental design, coordination of research and preparation of the manuscript; T.B. and S.M. carried out evaluation of plasma PON1 activity and of levels of markers of oxidative stress, lipoprotein isolation, fluorescence studies, statistical analysis, and participated in preparation of the manuscript; G.G. and V.B. were involved in the recruitment of normal weight, obese and PWS subjects and provided critical corrections to the manuscript.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- Alberti K. G. M. M., Eckel R. H., Grundy S. M., Zimmet P. Z., Cleeman J. I., Donato K. A., Fruchart J. C., James W. P. T., Loria C. M., Smith S. C., Jr (2009). Harmonizing the metabolic syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 [DOI] [PubMed] [Google Scholar]
- Aviram M., Rosenblat M., Bisgaier C. L., Newton R. S., Primo-Parmo S. L., La Du B. N. (1998). Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Invest. 101, 1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajnok L., Seres I., Varga Z., Jeges S., Peti A., Karanyi Z., Juhasz A., Csongradi E., Mezosi E., Nagy E. V., et al. (2007). Relationship of endogenous hyperleptinemia to serum paraoxonase 1, cholesteryl ester transfer protein, and lecithin cholesterol acyltransferase in obese individuals. Metabolism 56, 1542–1549 [DOI] [PubMed] [Google Scholar]
- Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. (2004). Antiinflammatory properties of HDL. Circ. Res. 95, 764–772 [DOI] [PubMed] [Google Scholar]
- Bastard J. P., Maachi M., Lagathu C., Kim M. J., Caron M., Vidal H., Capeau J., Feve B. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17, 4–12 [PubMed] [Google Scholar]
- Bondia-Pons I., Ryan L., Martinez J. A. (2012). Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. In Press. [DOI] [PubMed] [Google Scholar]
- Borque L., Bellod L., Rus A., Seco M. L., Galisteo-González F. (2000). Development and validation of an automated and ultrasensitive immunoturbidimetric assay for C-reactive protein. Clin. Chem. 46, 1839–1842 [PubMed] [Google Scholar]
- Bradford M. A. (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Butler M. G., Bittel D. C., Kibiryeva N., Garg U. (2006). C-reactive protein levels in subjects with Prader-Willi syndrome and obesity. Genet. Med. 8, 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixàs A., Giménez-Palop O., Broch M., Vilardell C., Megía A., Simón I., Giménez-Pérez G., Mauricio D., Vendrell J., Richart C., et al. (2008). Adult subjects with Prader-Willi syndrome show more low-grade systemic inflammation than matched obese subjects. J. Endocrinol. Invest. 31, 169–175 [DOI] [PubMed] [Google Scholar]
- Cassidy S. B., Driscoll D. J. (2009). Prader-Willi syndrome. Eur. J. Hum. Genet. 17, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P., Ray M. J., Brunzell J. D., Hokanson J. E., Krauss R. M., Beaudrie K., Cone J. T. (1986). Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 128, 181–209 [DOI] [PubMed] [Google Scholar]
- Dousset N., Ferretti G., Taus M., Valdiguiè P., Curatola G. (1994). Fluorescence analysis of lipoprotein peroxidation. Methods Enzymol. 233, 459–469 [DOI] [PubMed] [Google Scholar]
- Einfeld S. L., Kavanagh S. J., Smith A., Evans E. J., Tonge B. J., Taffe J. (2006). Mortality in Prader-Willi syndrome. Am. J. Ment. Retard. 111, 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faienza M. F., Ventura A., Lauciello R., Crinò A., Ragusa L., Cavallo L., Spera S., Grugni G. (2011). Analysis of endothelial protein C receptor gene and metabolic profile in Prader-Willi syndrome and obese subjects. Obesity. doi: 10.1038/oby.2011.349 [DOI] [PubMed] [Google Scholar]
- Ferretti G., Bacchetti T., Menanno F., Curatola G. (2004). Effect of genistein against copper-induced lipid peroxidation of human high density lipoproteins (HDL). Atherosclerosis 172, 55–61 [DOI] [PubMed] [Google Scholar]
- Ferretti G., Bacchetti T., Moroni C., Savino S., Liuzzi A., Balzola F., Bicchiega V. (2005). Paraoxonase activity in high-density lipoproteins: a comparison between healthy and obese females. J. Clin. Endocrinol. Metab. 90, 1728–1733 [DOI] [PubMed] [Google Scholar]
- Ferretti G., Bacchetti T., Nègre-Salvayre A., Salvayre R., Dousset N., Curatola G. (2006). Structural modifications of HDL and functional consequences. Atherosclerosis 184, 1–7 [DOI] [PubMed] [Google Scholar]
- Ferretti G., Bacchetti T., Masciangelo S., Bicchiega V. (2010a). Effect of homocysteinylation on high density lipoprotein physico-chemical properties. Chem. Phys. Lipids 163, 228–235 [DOI] [PubMed] [Google Scholar]
- Ferretti G., Bacchetti T., Masciangelo S., Bicchiega V. (2010b). HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity 18, 1079–1084 [DOI] [PubMed] [Google Scholar]
- Frost L. D. (2004). Glucose assays revisited: experimental determination of the glucose concentration in honey. Chem. Educator 9, 239–241 [Google Scholar]
- González-Chávez A., Elizondo-Argueta S., Gutiérrez-Reye G., León-Pedroza J. I. (2011). Pathophysiological implications between chronic inflammation and the development of diabetes and obesity. Cir. Cir. 79, 209–216 [PubMed] [Google Scholar]
- Goswami B., Tayal D., Gupta N., Mallika V. (2009). Paraoxonase: a multifaceted biomolecule. Clin. Chim. Acta 410, 1–12 [DOI] [PubMed] [Google Scholar]
- Grugni G., Crinò A., Bosio L., Corrias A., Cuttini M., De Toni T., Di Battista E., Franzese A., Gargantini L., Greggio N., et al. (2008). The Italian National Survey for Prader-Willi syndrome: An epidemiologic study. Am. J. Med. Genet. A 146, 861–872 [DOI] [PubMed] [Google Scholar]
- Grugni G., Crinò A., Bertocco P., Marzullo P. (2009). Body fat excess and stimulated growth hormone levels in adult patients with Prader-Willi syndrome. Am. J. Med. Genet. A 149, 726–731 [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Libby P. (2006). The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 [DOI] [PubMed] [Google Scholar]
- Haqq A. M., Muehlbauer M. J., Newgard C. B., Grambow S., Freemark M. (2011). The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J. Clin. Endocrinol. Metab. 96, E225–E232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon J. V., Frei B. (2003). Obesity and oxidative stress: a direct link to CVD? Arterioscler. Thromb. Vasc. Biol. 23, 365–367 [DOI] [PubMed] [Google Scholar]
- Holub M., Zwiauer K., Winkler C., Dillinger-Paller B., Schuller E., Schober E., Stöckler-Ipsiroglou S., Patsch W., Strobl W. (1999). Relation of plasma leptin to lipoproteins in overweight children undergoing weight reduction. Int. J. Obes. Relat. Metab. Disord. 23, 60–66 [DOI] [PubMed] [Google Scholar]
- Höybye C. (2006). Inflammatory markers in adults with Prader-Willi syndrome before and during 12 months growth hormone treatment. Horm. Res. 66, 27–32 [DOI] [PubMed] [Google Scholar]
- Huen K., Richter R., Furlong C., Eskenazi B., Holland N. (2009). Validation of PON1 enzyme activity assays for longitudinal studies. Clin. Chim. Acta 402, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. W., Deakin S. P. (2004). The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic. Biol. Med. 37, 1986–1994 [DOI] [PubMed] [Google Scholar]
- Kannampuzha J., Darling P. B., Maguire G. F., Donnelly S., McFarlane P., Chan C. T., Connelly P. W. (2010). Paraoxonase 1 arylesterase activity and mass are reduced and inversely related to C-reactive protein in patients on either standard or home nocturnal hemodialysis. Clin. Nephrol. 73, 131–138 [PubMed] [Google Scholar]
- Karbownik-Lewinska M., Kokoszko A., Lewandowski K. C., Shalet S. M., Lewinski A. (2008). GH replacement reduces increased lipid peroxidation in GH-deficient adults. Clin. Endocrinol. 68, 957–964 [DOI] [PubMed] [Google Scholar]
- Kokoszko A., Lewinski A., Karbownik-Lewinska M. (2008). The role of growth hormone and insulin-like growth factor I in oxidative processes. Endokrynol. Pol. 59, 496–501 [PubMed] [Google Scholar]
- Koncsos P., Seres I., Harangi M., Illyés I., Józsa L., Gönczi F., Bajnok L., Paragh G. (2010). Human paraoxonase-1 activity in childhood obesity and its relation to leptin and adiponectin levels. Pediatr. Res. 67, 309–313 [DOI] [PubMed] [Google Scholar]
- Kumon Y., Suehiro T., Ikeda Y., Hashimoto K. (2003). Human paraoxonase-1 gene expression by HepG2 cells is downregulated by interleukin-1beta and tumor necrosis factor-alpha, but is upregulated by interleukin-6. Life Sci. 73, 2807–2815 [DOI] [PubMed] [Google Scholar]
- Lahrach H., Ghalim N., Taki H., Kettani A., Er-Rachdi L., Ramdani B., Saïle R. (2008). Serum paraoxonase activity, high-sensitivity C-reactive protein, and lipoprotein disturbances in end-stage renal disease patients on long-term hemodialysis. J. Clin. Lipidol. 2, 43–50 [DOI] [PubMed] [Google Scholar]
- Lopes-Virella M. F., Virella G., Evans G., Malenkos S. B., Colwell J. A. (1980). Immunonephelometric assay of human apolipoprotein AI. Clin. Chem. 26, 1205–1208 [PubMed] [Google Scholar]
- Mackness B., Mackness M. (2010). Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv. Exp. Med. Biol. 660, 143–151 [DOI] [PubMed] [Google Scholar]
- Mackness B., Durrington P., McElduff P., Yarnell J., Azam N., Watt M., Mackness M. (2003). Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 107, 2775–2279 [DOI] [PubMed] [Google Scholar]
- Mackness B., Hine D., Liu Y., Mastorikou M., Mackness M. (2004). Paraoxonase-1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem. Biophys. Res. Commun. 318, 680–683 [DOI] [PubMed] [Google Scholar]
- Mackness B., Hine D., McElduff P., Mackness M. (2006). High C-reactive protein and low paraoxonase1 in diabetes as risk factors for coronary heart disease. Atherosclerosis 186, 396–401 [DOI] [PubMed] [Google Scholar]
- McGowan M. W., Artiss J. D., Stranberg D. R., Zak B. A. (1983). A peroxidase coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 29, 538–542 [PubMed] [Google Scholar]
- Monteiro R., Azevedo I. (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. J., Woollard K. J. (2010). High-density lipoprotein: a potent inhibitor of inflammation. Clin. Exp. Pharmacol. Physiol. 37, 710–718 [DOI] [PubMed] [Google Scholar]
- Nauck M., Graziani M. S., Bruton D., Cobbaert C., Cole T. G., Lefevre F. (2000). Analytical and clinical performance of a detergent-based homogeneous LDL-cholesterol assay: a multicenter evaluation. Clin. Chem. 46, 506–514 [PubMed] [Google Scholar]
- Negre-Salvayre A., Dousset N., Ferretti G., Bacchetti T., Curatola G., Salvayre R. (2006). Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic. Biol. Med. 41, 1031–1040 [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J. (1999). Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol. 300, 58–62 [DOI] [PubMed] [Google Scholar]
- Nowak M., Wielkoszynski T., Marek B., Kos-Kudla B., Swietochowska E., Sieminska L., Karpe J., Kajdaniuk D., Glogowska-Szelag J., Nowak K. (2010). Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clin. Exp. Med. 10, 185–192 [DOI] [PubMed] [Google Scholar]
- Oswall A., Yeo G. (2010). Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity 18, 221–229 [DOI] [PubMed] [Google Scholar]
- Parasassi T., DeStasio G., Ravagnan G., Rusch R. M., Gratton E. (1991). Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Harmer J. A., Loughnan G., Skilton M. R., Steinbeck K., Celermajer D. S. (2007). Characteristics of cardiac and vascular structure and function in Prader-Willi syndrome. Clin. Endocrinol. 66, 771–777 [DOI] [PubMed] [Google Scholar]
- Podrez E. A. (2010). Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin. Exp. Pharmacol. Physiol. 7, 719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto C., Romualdi D., Cento R. M., Romano C., Campagna G., Lanzone A. (2007). Free and total leptin serum levels and soluble leptin receptors levels in two models of genetic obesity: the Prader-Willi and the Down syndromes. Metabolism 56, 1076–1080 [DOI] [PubMed] [Google Scholar]
- Ragbir S., Farmer J. A. (2010). Dysfunctional high-density lipoprotein and atherosclerosis. Curr. Atheroscler. Rep. 12, 343–348 [DOI] [PubMed] [Google Scholar]
- Rock W., Rosenblat M., Miller-Lotan R., Levy A. P., Elias M., Aviram M. (2008). Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 56, 8704–8713 [DOI] [PubMed] [Google Scholar]
- Roeschlau P., Bernt E., Gruber W. A. (1974). Enzymatische bestimmung des gesamtcholesterins im serum. Clin. Chem. Clin. Biochem. 12, 226. [PubMed] [Google Scholar]
- Säemann M. D., Poglitsch M., Kopecky C., Haidinger M., Hörl W. H., Weichhart T. (2010). The versatility of HDL: a crucial anti-inflammatory regulator. Eur. J. Clin. Invest. 40, 1131–1143 [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. (2008). HDL: bridging past and present with a look at the future. FASEB J. 22, 4044–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. P., Schwartz M. V. (2010). Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Moya E., Gomez-Perez Y., Fiol M., Gianotti M., Llado I., Proenza A. M. (2008). Gender related differences in paraoxonase 1 response to high-fat diet-induced oxidative stress. Obesity 16, 2232–2238 [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Hama S. Y., de Beer F. C., Stafforini D. M., McIntyre T. M., Prescott S. M., La Du B. N., Fogelman A. M., Navab M. (1995). Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest. 96, 2758–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viardot A., Sze L., Purtell L., Sainsbury A., Loughnan G., Smith E., Herzog H., Steinbeck K., Campbell L. V. (2010). Prader-Willi syndrome is associated with activation of the innate immune system independently of central adiposity and insulin resistance. J. Clin. Endocrinol. Metab. 95, 3392–3399 [DOI] [PubMed] [Google Scholar]
- Vincent H. K., Taylor A. G. (2006). Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 30, 400–418 [DOI] [PubMed] [Google Scholar]
- Visser M., Bouter L. M., McQuillan G. M., Wener M. H. (1999). Elevated C-reactive protein levels in overweight and obese adults. JAMA 282, 2131–2135 [DOI] [PubMed] [Google Scholar]
- Warnick G. R., Wood P. D. (1995). National Cholesterol Education Program recommendations for measurement of high-density lipoprotein cholesterol: executive summary. Clin. Chem. 41, 1427–1433 [PubMed] [Google Scholar]
- Whittington J. E., Holland A. J., Webb T., Butler J., Clarke D., Boer H. (2001). Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J. Med. Genet. 38, 792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesin K. C., Franklin B. A., Miller W. M., Peterson E. D., McCullough P. A. (2011). Impact of obesity on cardiovascular disease. Med. Clin. North Am. 95, 919–937 [DOI] [PubMed] [Google Scholar]