Abstract
Liver fibrosis is the generic response to chronic injury of varying aetiologies. A number of common mechanisms link this response to the pathogenesis of fibrosis in other organs. While long thought to be relentlessly progressive, there is now excellent evidence in both human liver disease and animal models that hepatic fibrosis is potentially reversible. The liver therefore provides an excellent bidirectional model for the study of fibrogenesis and fibrosis resolution. In this article, we will review the evidence for the reversibility of liver fibrosis. We will highlight some of the mechanisms responsible for fibrogenesis and fibrosis regression, focussing on the role of hepatic myofibroblast activation and apoptosis, the importance of matrix metalloproteinases and their tissue inhibitors and the central involvement of hepatic macrophages in orchestrating this process. Finally, we will briefly discuss what renders liver fibrosis irreversible and how this accumulating knowledge base could lead to badly needed anti-fibrotic therapies in the future.
Liver fibrosis is the generic response to chronic injury of varying aetiologies, ultimately leading to cirrhosis, with clinical complications including liver failure, portal hypertension and hepatocellular carcinoma. Currently, no direct anti-fibrotic therapies exist with organ transplantation, the only curative option.
A number of common mechanisms link the pathogenesis of hepatic fibrosis and fibrosis seen in other tissues.1 Tissue fibrogenesis was long thought to be relentlessly progressive. However, emerging data indicates that even in advanced disease, fibrosis is potentially reversible.2–4 This evidence is best developed in human and experimental liver disease, establishing the liver as the paradigm for studying bidirectional fibrosis in a solid organ.5 Given that in clinical practice the majority of patients present with established tissue fibrosis, a greater understanding of the biology of fibrosis resolution is likely to inform novel treatment options. In this short review we will discuss the current knowledge of mechanisms mediating the reversal of hepatic fibrosis and highlight potential avenues for therapeutic translation.
Human liver fibrosis is potentially reversible
Anecdotal reports had suggested that human liver fibrosis was potentially reversible. However, it was only following the development of effective treatments for chronic hepatitis B and hepatitis C infection that this was definitively shown in large cohorts of patients.5 Subsequent studies in chronic liver disease caused by alcohol, autoimmune disease, biliary obstruction, hereditary haemochromatosis and non-alcoholic fatty liver disease (NAFLD) confirmed these findings.5 Additionally, the wide range of aetiologies in which fibrosis resolution occurs suggests that generic rather than disease-specific mechanisms are at play.
Apoptosis of hepatic myofibroblasts accompanies the reversal of fibrosis
To study the mechanisms leading to the reversal of hepatic fibrosis, two tractable rodent models are widely utilized. In bile-duct ligation (BDL) followed by bilio-jejunal anastamosis and chronic carbon tetrachloride (CCl4) administration followed by cessation of dosing, a well-established hepatic fibrosis can resolve to near normal liver architecture within 4–6 weeks.6,7 Analysis of the histological changes during fibrosis resolution in both of these models identifies a rapid loss of activated hepatic myofibroblasts, the principal scar-producing cells in the fibrotic liver,8 by apoptosis.
A number of soluble signals, which may be released by neighbouring inflammatory cells or hepatocytes, have been shown to regulate hepatic myofibroblast apoptosis.9 Interestingly, the physical characteristics of the myofibroblast environment has important effects on cell survival. Specifically, contact between activated myofibroblasts and collagen 1 (the principal fibrillar collagen in hepatic scars) promotes cell survival and fibrogenic activity.10 Furthermore, transgenic mice expressing a non-degradable form of collagen 1, demonstrated a failure to spontaneously remodel hepatic scars and a persistence of activated myofibroblasts following cessation of CCl4 injury.11 These findings suggest an important feedback loop linking the presence of scar tissue, the survival of scar-producing cells and a consequent failure of fibrosis resolution.
TIMP-1 regulates matrix degradation during fibrogenesis and resolution
Clearly the loss of scar-producing myofibroblasts is not sufficient for adequate fibrosis resolution, and degradation of the extracellular matrix (ECM) is a prerequisite. Matrix metalloproteinases (MMPs), a group of endopeptidases, are capable of degrading a range of ECM components. Analysis of human and experimental animal fibrotic liver demonstrates an increase in a number of MMPs with a wide spectrum of activity.5,12,13 Therefore, even in fibrotic liver there remains the capacity for matrix degradation.
Tissue inhibitors of metalloproteinases (TIMPs), a family of protease inhibitors, are potent inhibitors of MMP activity in vivo. Hepatic myofibroblasts show a marked upregulation of TIMP-1 during activation, preceding collagen expression, and potently inhibiting MMP activity.14,15 In addition, elevated levels of TIMP-1 are seen during progressive fibrosis in humans and experimental models.5,14,16 During fibrosis resolution, there is a rapid decline in TIMP levels, tipping the overall MMP–TIMP balance, resulting in increased matrix degrading activity and net degradation of scar tissue.6,17 The critical role of TIMP-1 in fibrogenesis and resolution was elegantly confirmed using a transgenic system, whereby hepatic TIMP-1 overexpression accelerated fibrogenesis but also caused a failure of scar resolution.18 Additionally, TIMP-1 has anti-apoptotic effects on hepatic myofibroblasts,9 indicating that loss of TIMP-1 during recovery may also contribute to a reduction in the scar-producing cells in the liver (Figure 1). Overall, these data indicate that TIMP production and consequent MMP inhibition is a key regulator in the progression and resolution of hepatic fibrosis.
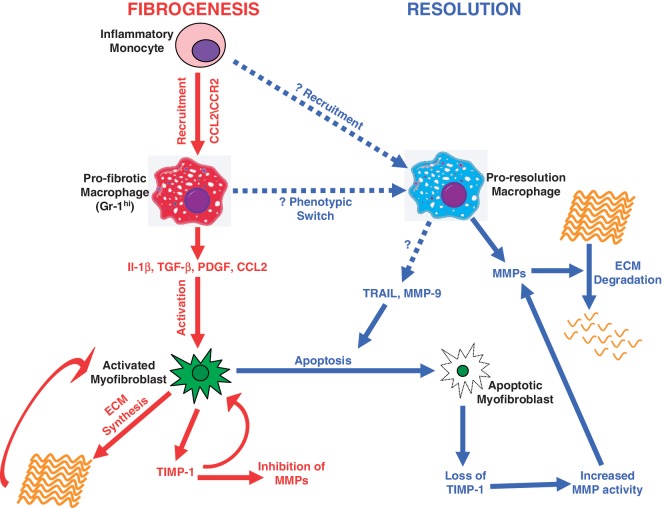
Figure 1.
Macrophages as central orchestrators of hepatic fibrogenesis and fibrosis resolution. During fibrogenesis, inflammatory monocytes are recruited to the inflamed liver via Chemokine (C–C motif) ligand 2 (CCL2) and chemokine (C–C motif) receptor 2 (CCR2) interactions, forming the pro-fibrotic macrophage population. Pro-fibrotic macrophages express mediators such as Il-1β, TGF-β, PDGF and CCL2, which promote activation of hepatic myofibroblasts. Activated myofibroblasts synthesize the ECM and TIMP-1, a potent inhibitor of MMP activity. Both TIMP-1 and ECM interactions promote persistence of the activated myofibroblast phenotype. During fibrosis resolution, there is likely to be a change in macrophage phenotype, either from a phenotypic switch of pro-fibrotic macrophages or a separate recruitment of monocytes. Pro-resolution macrophages express MMPs that promote ECM degradation. Pro-resolution macrophages can also express mediators that induce myofibroblast apoptosis, leading to a reduction in ECM synthesis, loss of TIMP-1 expression and enhanced MMP activity.
Macrophages are critical for fibrogenesis and resolution
Cells of the monocyte/macrophage lineage are central proponents of the innate immune response following tissue damage. A number of experimental studies implicate macrophages in promoting hepatic inflammation and fibrosis.19 Notably, macrophages are located in close proximity to activated hepatic myofibroblasts during fibrogenesis and may produce factors such as TGF-β, IL-1β, PDGF and CCL2 which can enhance the pro-fibrogenic nature of the myofibroblasts by promoting activation, proliferation, chemotaxis and survival.8,19 Functional studies using CCL2 (the principal chemoattractant for the recruitment of inflammatory monocytes) inhibitors or CCL2 knockout animals demonstrated reduced hepatic macrophage infiltration, reduced inflammation and reduced fibrogenesis following chronic injury.13 In seminal work, selective depletion of macrophages during fibrogenesis, using a transgenic CD11B-DTR system, resulted in reduced fibrosis, confirming the importance of macrophages in scar formation.20 Further elegant work identified the specific Gr-1hi subset of hepatic macrophages, derived from recruitment of inflammatory monocytes via CCL2/CCR2 interactions, as responsible for the pro-fibrogenic effects (Figure 1).21
Emerging evidence also implicates macrophages as central mediators of fibrosis resolution. Intriguingly, macrophage depletion during the resolution phase following chronic CCl4 administration caused a failure to degrade the hepatic scar, the opposite effect to that seen with depletion during fibrogenesis.20 Similarly, CCR2 knockout mice subjected to chronic injury with CCl4 had diminished macrophage recruitment and fibrogenesis, but also a delayed ability to resolve fibrosis.22 The mechanisms underpinning macrophage-mediated fibrosis resolution are likely to be multifactorial. Macrophages can produce a range of MMPs and macrophage-derived MMP-13, the major rodent collagenase, has been shown to be important for degrading the hepatic scar.23 Macrophages are also capable of producing molecules such as TRAIL and MMP-9 which can promote myofibroblast apoptosis,9,12,13 although in vivo studies demonstrating this as a relevant mechanism are still lacking (Figure 1).
How can one cell type have such divergent functional effects? While this question remains incompletely answered, it is probable that heterogeneity in macrophage populations will be critical. It is well recognized that macrophages can adopt distinct functional characteristics depending on the stimuli to which they are exposed.24 It is likely that a specific macrophage phenotype will predominate during fibrosis resolution, and this will be distinct from the phenotype which promotes fibrogenesis.21 Studies aimed at characterizing the macrophage population responsible for fibrosis reversal will yield novel mechanistic insights. Specifically, determining if the same macrophage population switches from a pro-fibrotic to pro-resolution phenotype in situ and identifying the factors mediating this switch may enable the development of novel therapies designed to promote this change in vivo and thus induce fibrosis resolution.
What renders a fibrosis irreversible?
Having discussed the factors mediating the resolution of hepatic fibrosis, it is also important to consider what might render fibrosis irreversible. In a rat CCl4 model, an increasing duration of injury induced hepatic fibrosis which was resistant to full resolution, even with protracted recovery times of up to 1 year.17 Careful analysis of the non-degraded scar tissue indicated that matrix cross-linking was a key feature, making the fibrotic bands more resistant to proteolytic degradation. Additionally, the presence of elastin fibres in the more mature scars suggests a change in the biochemical composition of the fibrotic bands may be relevant.17 Another striking feature of the persistent scars was the relative hypocellularity.17 This raises the intriguing possibility that a degree of ongoing inflammation and thus the influx of macrophages around the fibrotic bands is essential for adequate scar remodelling.
Potential for therapy
Clearly, using the liver as a model organ, our knowledge regarding the mechanisms orchestrating scar resolution has improved greatly. But are we any closer to the ‘holy grail’, a therapy which can reverse established fibrosis and improve organ function? A number of strategies have been employed in experimental fibrosis, variously targeting myofibroblast activation, myofibroblast apoptosis and the critical MMP–TIMP balance.12,13 While no large-scale clinical studies have yet been conducted, promising results are seen in the experimental models. Interestingly, drugs such as sulphasalazine, angiotensin receptor antagonists and PPAR-γ agonists, already widely used in clinical practice, show some early promise as anti-fibrotic agents and may speed up the route to translation.12,13
An alternative strategy is the use of cell therapy to treat hepatic fibrosis, which has shown some beneficial effects.12,13 Specifically, the use of macrophages as a therapy has the potential to harness the pro-resolution capabilities of this cell type and can be anti-fibrotic in vivo.25 However, given the plasticity of macrophages, caution should be used with this approach, as macrophages delivered at an inappropriate time in the disease course have the potential to exacerbate inflammation and fibrosis. Thus, treatments aimed at inducing the pro-resolution macrophage phenotype in vivo may represent a useful methodology in the future.
Conclusions
In using the liver as a paradigm for studying fibrosis resolution, we have made significant advances in our understanding of the key cellular and molecular mechanisms at play. However, further work is needed to determine the specific phenotype of the cells involved, which of these mechanisms are relevant in other fibrotic organs and to develop methods to enhance this process with a therapeutic benefit.
Funding
The Wellcome Trust (to P.R.); the Medical Research Council (to J.P.I.).
Conflict of interest: None declared.
References
- 1.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddy AA. Can renal fibrosis be reversed? Pediatr Nephrol. 2005;20:1369–75. doi: 10.1007/s00467-005-1995-5. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–2. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 4.Tyralla K, Adamczak M, Benz K, Campean V, Gross ML, Hilgers KF, et al. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS One. 2011;6:e15287. doi: 10.1371/journal.pone.0015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–48. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–57. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–39. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 11.Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–9. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 12.Iredale J. Defining therapeutic targets for liver fibrosis: exploiting the biology of inflammation and repair. Pharmacol Res. 2008;58:129–36. doi: 10.1016/j.phrs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8:283–91. [PubMed] [Google Scholar]
- 14.Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, et al. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–84. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 15.Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJ. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest. 1992;90:282–7. doi: 10.1172/JCI115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–31. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 17.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, et al. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36(4 Pt 1):850–60. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 19.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766–75. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–95. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 24.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 53:2003–15. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]