Abstract
Graft arteriosclerosis (GA), the major cause of late cardiac allograft failure, Is characterized by a diffuse, concentric arterial intimal hyperplasia composed of infiltrating host T cells, macrophages and predominantly graft-derived smooth muscle–like cells that proliferate and elaborate extracellular matrix, resulting in luminal obstruction and allograft ischemia. IFN-γ, a proinflammatory cytokine produced by effector T cells, is a critical mediator for smooth muscle – like cell proliferation. We have exploited the power of mouse genetics to examine the function of AIP1, a signaling adaptor molecule involved in vascular inflammation, in two newly established IFN-γ-mediated models of GA. Our data suggest that AIP1 inhibits intimal formation in GA by downregulating IFN-γ–activated migratory and proliferative signaling pathways in smooth muscle–like cells.
Introduction
Graft arteriosclerois (GA), also called allograft vasculopathy, is a pathologic lesion that develops over months to years in transplanted organs characterized by diffuse, circumferential stenosis of the entire graft vascular tree (Mitchell, 2009). Early stages may cause eccentric and focal stenoses that are more obvious in arteries, thus more closely resembling stenoses seen in conventional atherosclerosis. The lumen loss of the graft vessels results from intimal expansion due to infiltration of host T cells and macrophages and especially to accumulation of extracellular matrix and smooth muscle-like cells originated from graft, host or both (Minami et al., 2005; Shimizu et al., 2001; Yacoub-Youssef et al., 2005), that is inadequately compensated by outward vessel remodeling. In cardiac allografts, the most clinically significant lesions are those in the epicardial and intramyocardial coronary arteries. Ultimately, GA of the coronary arteries will cause ischemic heart failure. GA is the major cause of late cardiac graft loss. The stenoses of GA stop at the suture lines, strongly implicating the host response to graft alloantigens in its pathogenesis and leading us to classify GA as a form of chronic rejection (Libby and Pober, 2001). However, other forms of arterial injury may increase the risk of GA, either through increasing the net burden of injury or by intensifying and/or modulating the alloimmune response. The endothelial cell (EC) lining of graft arteries is preserved in human GA and the most superficial regions of the intima adjacent to the EC lining is the site most heavily infiltrated by host-derived IFN-γ-producing T cells and macrophages (Salomon et al., 1991); in some patients GA is associated with the development of donor-specific alloantibodies that bind to graft EC (Vassalli et al., 2003) but the vessels show little evidence of the fibrinoid necrosis that is characteristic of acute antibody-mediated rejection (Salomon et al., 1991).
The most critical component of GA pathogenesis is the proliferation of smooth muscle – like cells within the intima; if this process can be arrested, GA is unlikely to progress. Previous work from our group had shown that intimas of human coronary artery segments interposed into the infra-renal aortae of immunodeficient mice expand in response to adoptively transferred human T cells allogeneic to the artery donor and that this process could be inhibited by neutralizing human IFN-γ (Wang et al., 2004). Furthermore exogenous human IFN-γ could cause intimal (and medial) vascular smooth muscle cell (VSMC) proliferation in these arterial grafts in the absence of human T cells (Tellides et al., 2000; Wang et al., 2007). (It’s important to note that human and mouse IFN-γ do not cross species, ruling out indirect effects on the mouse host in this experimental system.) These humanized mouse models have the benefit of recapitulating human T cell/vascular cell interactions and the intimal lesions are largely composed of human (i.e. graft-derived) cells, as has been observed in clinical specimens, but they do not fully recapitulate the clinical situation because they ignore the role of host macrophages and possibly other cell types involved in clinical transplant lesions. A conventional mouse model of this process could theoretically address this problem, complementing the limitations of the humanized model by involving a complete host immune system and providing the additional advantage of allowing the power of mouse genetic approaches to be applied to GA. The two most widely used mouse models involve heterotopic heart transplantation and orthotopic artery transplantation (George et al., 2005). The lesions that develop in the arteries of heterotopic heart grafts are largely made up of host cells, likely of bone marrow origin, whereas intimal cells of the arteries in human heart grafts are predominantly of graft origin (Minami et al., 2005; Shimizu et al., 2001; Yacoub-Youssef et al., 2005). This is a significant distinction that has led us to develop alternative mouse models. Interposition of a mouse aorta from one strain into another mouse strain recipient is even more limited as a model for chronic rejection in humans because the acute cell-mediated rejection response in this mouse model completely eliminates all donor-derived vascular cells from the graft within two-three weeks (Yacoub-Youssef et al., 2005). Consequently, the subsequent changes seen in the interposed vessel segment are solely a response of host cells that have repopulated the decellularized vessel scaffold, creating a highly artifactual situation of limited relevance as a model for the changes in graft vessels that occur in the clinic. We have recently developed two new mouse models to circumvent these problems (Fig 1). The first model involves interposition of a vessel segment from a male mouse into a female recipient of the same inbred strain (C57Bl/6). Graft rejection in this case is directed only against minor histocompatibility antigens encoded by the Y chromosome (present in the male but not the female) and the rejection response that ensues is sufficiently indolent to preserve donor-derived smooth muscle cells for several weeks (Koulack et al., 1996; Mitchell, 2004; Nagano et al., 1998; Nagano et al., 1997; Raisanen-Sokolowski et al., 1998; Scott et al., 1997; Tellides and Pober, 2007). The second model involves interposing an artery segment from a wild type C57Bl/6 mouse donor into a host mouse of the same strain and gender that lacks the receptor for IFN-γ followed by administration of mouse IFN-γ (delivered via infection of the mouse liver with an adenoviral vector.) There is no rejection in this case as both donor and recipient mice are of the same strain and gender but donor smooth muscle cells proliferate in response to the cytokine while host-derived cells, lacking receptor for this cytokine, are unresponsive (Yu et al., 2011). By backcrossing additional genetic changes into the vessel donor, both models can be used to assess the effect of specific genes on IFN-γ-driven smooth muscle cell proliferation. In this review, we will describe our findings using these models to characterize the role of a key regulatory protein of cytokine signaling, namely ASK-1 interacting protein (AIP1).
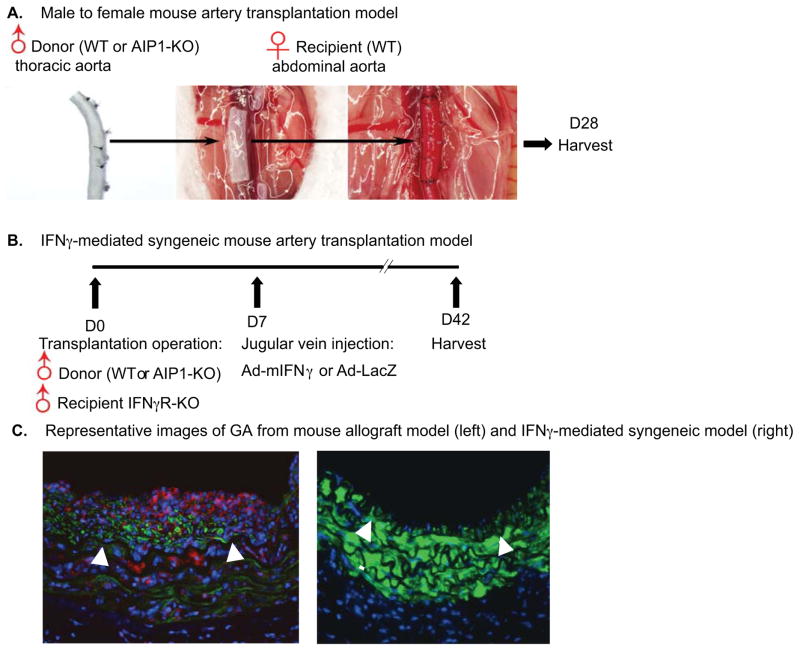
Fig. 1.
A. Illustration of mouse allograft artery transplantation model. A segment of donor male thoracic aorta (WT or AIP1-KO) was surgically interposed into abdominal aorta in a WT female recipient. Aortas were harvested at 4 weeks post-transplantation for morphometric assessment and histological analysis.
B. A scheme of the IFN-γ-induced mouse syngeneic model. Male donor thoracic artery from WT or AIP1-KO was dissected and transplanted into abdominal aorta in male recipient IFN-γR KO mice. One week post surgery, 1X109 pfu Ad-mIFN-γ or Ad-LacZ was injected into recipient mice via jugular vein. Grafts were harvested 6 weeks for morphometric assessment and histological analysis.
C. A representative image of GA from a mouse allograft transplantation model (left), characterized by infiltration of leukocytes (red) and neointima formation with accumulation of VSMC (green). A representative image of GA from an IFN-γ-induced mouse syngeneic model (right). IFN-γ is sufficient to induce neointimal formation with no obvious infiltration of leukocytes. Arrowheads in B and C and F mark the internal elastic lamina to delineate the intima from media.
AIP1 is a signaling adaptor molecule involved in vascular inflammation
AIP1, was initially identified as a binding partner for Apoptosis Signaling Kinase (ASK)-1; it is also known as DAB2-interacting protein (DAB2IP). AIP1 contains multiple domains including an N-terminal plekstrin homology (PH) domain for membrane targeting, the C2 domain for interactions with ASK1, a Ras-GTPase activating protein (GAP) domain for inhibition of Ras signaling (so that AIP1 may be considered as a novel member of RAS-GAP family of proteins), the C-terminal period-like domain for inhibition of transcription factor NF-κB, and a proline-rich region that can inhibit the PI3K-Akt survival pathway (Fig. 2A: Summary of AIP1 domains and function) (Min et al., 2008; Pober et al., 2009; Xie et al., 2010; Xie et al., 2009; Zhang et al., 2008; Zhang et al., 2007; Zhang et al., 2004; Zhang et al., 2003). We previously have investigated the biological function of AIP1 in vascular EC. We initially found that AIP1 functions as an adaptor molecule in TNF signaling where it specifically allows the TNFR1/TRADD/RIPK1/TRAF2 signaling complex to activate a pro-apoptotic ASK1/JNK signaling pathway while inhibiting the same complex from activating a prosurvival IκB kinase (IKK)/NF-kB signaling pathway. In this system AIP1 activates ASK1 by recruiting phosphatase PP2A to dephosphorylate the 14-3-3 binding site on ASK1, leading to dissociation of this inhibitory protein from the enzyme, thereby allowing activation by the TNFR1 signaling complex (Min et al., 2008; Zhang et al., 2003). The mechanism by which AIP1 inhibits IKK/NF-κB signaling is not exactly known. AIP1 appears to directly bind to TRAF2 and RIPK1 and thus may block the recruitment and activation of the IKK complex. AIP1 in vascular EC also functions as an inhibitor of VEGFR2 signaling (Zhang et al., 2008), a cell surface receptor tyrosine kinase critical for angiogenesis and lymphangiogenesis. In this case, AIP1 is recruited to VEGFR2-PI3K complex, binding to both VEGFR2 and PI3K p85 and inhibiting signaling through this pathway. Consistent with these in vitro observations, AIP1-deficient mice (AIP1-KO) exhibit dramatically enhanced NF-κB and VEGFR2 activities resulting in enhanced angiogenesis in several different mouse models.
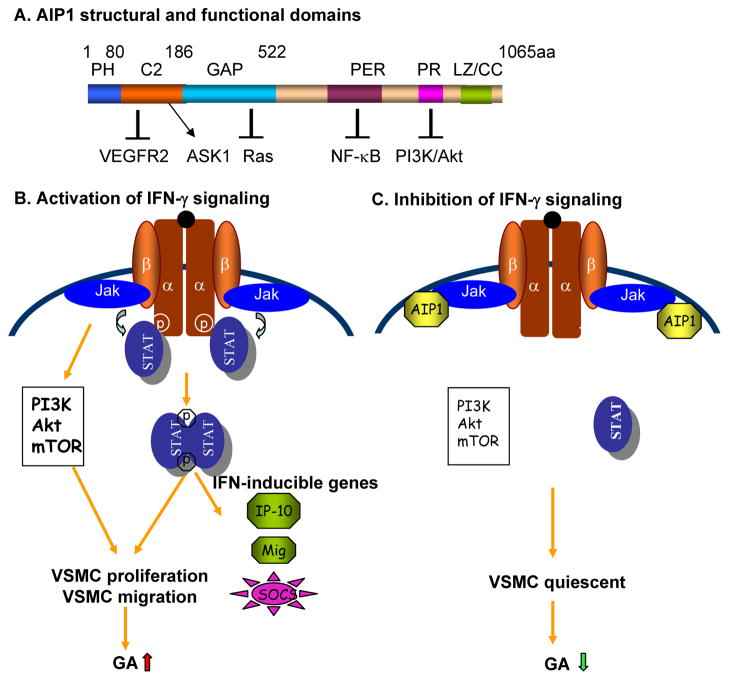
Fig. 2.
A. Summary of AIP1 structure and functional domains. Schematic diagram of AIP1 domains with amino acid residues denoted above. Domains responsible for AIP1 inhibition on VEGFR2, Ras, NF-κB and PI3K/Akt as well as activation on ASK1 are indicated.
B–C. A model for the role of AIP1 in IFN-γ signaling, VSMC function and GA. AIP1 directly binds to JAK2 and inhibits its kinase activity, limiting JAK2-dependent STAT1/3 and PI3K/Akt activation, VSMC proliferation and GA (C). In AIP1-KO VSMC (B), increased active JAK2 activity leads to enhanced STAT1/3 and PI3K/Akt/mTOR-dependent VSMC proliferation/migration, promoting GA progression. Enhancing AIP1 expression or activity could be used as a therapeutic strategy for the treatment of GA (C).
A critical role of AIP1 in IFN-γ-induced neointimal formation
AIP1 is highly expressed in vascular smooth muscle cells (VSMC) as well as in EC (Gretarsdottir et al.; Zhang et al., 2008; Zhang et al., 2003). This prompted us to examine the function of AIP1 in the two mouse GA models described in the Introduction, both of which are characterized by VSMC proliferation and intimal expansion. In the basic allograft model, WT male to WT female transplantation induces a GA-like lesion, characterized by infiltration of leukocytes and neointima formation that involves accumulation of donor-derived VSMC. In a variant version of the model, grafts from IFN-γR-KO donors show less leukocyte infiltration and VSMC accumulation in the neointima, consistent with previous findings from the humanized mouse xenograft transplantation model in which IFN-γ signaling in the graft was critical for GA progression (Tellides et al., 2000; Wang et al., 2007; Wang et al., 2004). In the second, syngeneic graft model, in which a male aorta is transplanted into a male IFN-γR-KO recipient animal and mouse IFN-γ is then systemically expressed by adenoviral gene transfer, expression of IFN-γ alone induces intimal expansion of the graft in the absence of infiltrating leukocytes (Yu et al., 2011), once again consistent with the previous observations from the human artery-SCID mouse transplantation model in which vascular IFN-γ signaling alone was sufficient to induce neointima formation in the absence of leukocytes (Tellides et al., 2000; Wang et al., 2007). We have now used these new mouse models to test the role of AIP1 in donor graft cells on the progression of GA.
Transplantation of an AIP1-KO male donor graft to WT female recipients generates significantly more neointima containing more smooth muscle α-actin (SMA) expressing cells compared to grafts from WT male donors. We observe no significant differences between AIP1-KO and WT grafts re the number of infiltrating leukocytes or CD4+ T cell subsets (Th1 and Treg). However, IFN-γ-induced genes such as IP-10 and Mig are significantly higher in AIP1-KO, indicating that AIP1 deletion augments IFN-γ responses of VSMC. Consistent with this interpretation the, augmentation of GA in grafts from AIP1-KO donors is not seen in grafts from AIP1/IFN-γR double KO donors. These results identify AIP1 as an endogenous inhibitor of IFN-γ-dependent VSMC proliferation in this mouse allograft model of GA. We further tested this idea using the syngeneic graft model. As to be expected, AIP1 deletion in the graft has no effect on systemic expression of mouse IFN-γ in the serum induced by an adenoviral vector. There is little change in either type of syngeneic graft in the absence of exogenous IFN-γ. However, AIP1-KO donor grafts develop significantly more neointima containing more SMA-expressing cells compared to grafts from WT donors when IFN-γ is introduced by the adenoviral vector. Moreover, AIP1-KO donor grafts show augmented IFN-γ-activated phospho-JAK2, p-STAT1 and p-STAT3, as well as increased IFN-γ-induced VSMC proliferation as measured by SMA+ BrdU+ double staining of cells in the neointima. We conclude that AIP1 deletion directly augments IFN-γ effects on VSMC in these two new mouse GA models (Yu et al., 2011).
Mechanisms of AIP1 regulation of IFN-γ signaling
Human VSMC show variable responses to IFN-γ in cell culture, but we have found it to consistently stimulate VSMC proliferation in cultured aortae or cells that have been quiesced by serum withdrawal. Under this condition, effect of IFN-γ on VSMC support our in vivo observations that IFN-γ causes proliferation of arterial wall human and mouse VSMCs. Therefore, we use these in vitro models to determine effects of AIP1 on IFN-γ responses in organ cultures of aortae as well as in cultured aortic VSMC isolated from WT and AIP1-KO mice. AIP1 deletion in both whole aorta and isolated VSMC caused enhanced IFN-γ-induced activation of JAK2 and STAT1/3. Similarly, knockdown of AIP1 by siRNA in human VSMC also augmented IFN-γ signaling as well as IFN-γ-induced VSMC proliferation and migration (Yu et al., 2011). The molecular mechanism by which IFN-γ induces VSMC proliferation depends, at least in part, upon a PI3K/Akt/mTOR pathway whereas the mechanism underlying migration is less well understood. The canonical IFN-γ signaling pathways are initiated by ligand –induced receptor dimerization, trans-activation of receptor-associated JAK1 and JAK2 cytosolic tyrosine kinases, and subsequent phosphorylation of specific tyrosine residues within the cytosolic tails of the IFN-γR. Phosphorylation of IFN-γR results in recruitment, JAK-mediated tyrosine phosphorylation, dimerization and nuclear translocation of STAT1 and (in VSMC but not EC) STAT3, which in turn induces expression of many (but not all) IFN-γ-responsive genes (Darnell Jr et al., 1994; Stark et al., 1998). Complete activation of STAT proteins also requires serine phosphorylation mediated through the less well understood IFN-γ-activated PI3K/Akt pathway (Nguyen et al., 2001). While the STAT1 pathway is generally thought to inhibit proliferation and may contribute to cell death, the STAT3 pathway is more classically associated with proliferation and cell survival. (Peilot et al., 2000; Qing and Stark, 2004; Ramana et al., 2005; Shen et al., 2001). However, it may also induce the expression of pro-apoptotic genes in VSMC, sensitizing these cells to the effects of death-inducing signals such as TRAIL (Bai et al., 2008). Our recent analysis of IFN-γ signaling in both mouse and human cultured VSMC, using gene knock out or siRNA knock down approaches, respectively, has revealed that AIP1 functions as a novel negative regulator of IFN-γ/JAK1,2/STAT1/3 signaling in this cell type (Yu et al., 2011). Specifically, we have found AIP1 associates with JAK2 in response to IFN-γ and inhibits JAK2 autokinase activity, thereby preventing JAK2-dependent STAT1/3 tyrosine phosphorylation. AIP1 deletion in mouse aortic VSMC or siRNA knockdown in human VSMC resulted in enhanced IFN-γ-induced phosphorylation of Akt and mTOR. A JAK2 inhibitor, and to a lesser extent an Akt inhbitor, could reverse the effects of diminished AIP1. Interestingly, the JAK2 inhibitor could also reverse AIP1 deletion-enhanced Akt activity, suggesting that JAK2 may be an upstream activator of PI3K-Akt in IFN-γ signaling. (Fig. 2B–C: A model for AIP1 regulation of the IFN-γ signaling in VSMC). Our data suggest that AIP1 functions as a brake in IFN-γdependent VSMC proliferation and GA progression. It has previously been shown that JAK-STAT signaling can be negatively regulated through three main mechanisms: the dephosphorylation of JAKs or STATs by various protein tyrosine phosphatases such as Src homology region 2 domain-containing phosphatases 1 and 2; the inactivation of JAKs by the suppressor of cytokine signaling (SOCS) family of proteins; and the inhibition of the transcriptional activity of STATs by protein inhibitor of STAT (PIAS) proteins (Krebs and Hilton, 2000; Shuai and Liu, 2005). Since AIP1 directly binds to JAK2 and inhibits the JAK2 kinase activity, AIP1 acts in a similar manner as the SOCS family (SOCS1). It will be interesting to determine if AIP1 and SOCS1 function synergistically or independently to inhibit IFN-γ signaling and GA.
Prospective
Expression of AIP1 is strong in EC and VSMC, but much weaker in T cells and macrophages. AIP1 in vascular EC functions as an endogenous inhibitor of inflammatory responses in mouse models (Zhang et al., 2008). In transplanted organs, graft vascular EC may selectively recruit and then activate graft vessel-infiltrating IFN-γ-secreting T cells to graft vessels, and IFN-γ in turn contributes to pathogenesis of GA by modulating functions of VSMC. Although the studies using mouse models reviewed here support a critical role of regulation by VSMC-expressed AIP1 as a check upon intimal expansion, a key step in the development of GA, the role of EC-expressed AIP1 as a regulator of the immune and inflammatory functions of this cell type is still largely unexplored. Moreover, clinical studies and evidence from other experimental systems have suggested that non-immune factors, especially peri-operative stress-induced alterations in the graft, are also important contributors to GA pathogenesis. We have proposed that such changes operate by altering the initial T cell response to graft vascular cells, examples being increased expression of IL-1α and IL-6. It will be important to examine if AIP1 regulates the coupling peri-operative stresses, such ischemia and reperfusion, to the production and release of such mediators by EC and/or VSMC. Furthermore, a recent human genome-wide association study (GWAS) has identified AIP1 (DAB2IP) as a susceptibility gene for abdominal aortic aneurysm, peripheral vascular disease, early onset of myocardial infarction and pulmonary embolism (Gretarsdottir et al., 2010). It is not known if similar genetic polymorphisms contribute to the risk of transplant vasculopathies. Finally, AIP1 expression is primarily regulated by epigenetic modification in its promoter region in tumor cells (Chen et al., 2003; Min et al., 2010). A future analysis of the regulation of AIP1 expression during GA progression could help to define if induction of AIP1 could be a therapeutic approach for the prevention of GA and other vascular diseases.
Acknowledgments
This work was supported by NIH grants R01 HL109420 to WM and R01 HL109455 to JSP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai Y, Ahmad U, Wang Y, Li JH, Choy JC, Kim RW, Kirkiles-Smith N, Maher SE, Karras JG, Bennett CF, et al. Interferon-gamma induces X-linked inhibitor of apoptosis-associated factor-1 and Noxa expression and potentiates human vascular smooth muscle cell apoptosis by STAT3 activation. J Biol Chem. 2008;283:6832–6842. doi: 10.1074/jbc.M706021200. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Toyooka S, Gazdar AF, Hsieh JT. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J Biol Chem. 2003;278:3121–3130. doi: 10.1074/jbc.M208230200. [DOI] [PubMed] [Google Scholar]
- 3.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 4.George JF, Pinderski LJ, Litovsky S, Kirklin JK. Of mice and men: mouse models and the molecular mechanisms of post-transplant coronary artery disease. J Heart Lung Transplant. 2005;24:2003–2014. doi: 10.1016/j.healun.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Gretarsdottir S, Baas AF, Thorleifsson G, Holm H, den Heijer M, de Vries JP, Kranendonk SE, Zeebregts CJ, van Sterkenburg SM, Geelkerken RH, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD. Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin Immunol Immunopathol. 1996;80:273–277. doi: 10.1006/clin.1996.0123. [DOI] [PubMed] [Google Scholar]
- 7.Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 9.Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE, Beroukhim R, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, Luhn T, Fu H, Chen H. AIP1 recruits phosphatase PP2A to ASK1 in tumor necrosis factor-induced ASK1-JNK activation. Circ Res. 2008;102:840–848. doi: 10.1161/CIRCRESAHA.107.168153. [DOI] [PubMed] [Google Scholar]
- 11.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell RN. Allograft arteriopathy: pathogenesis update. Cardiovasc Pathol. 2004;13:33–40. doi: 10.1016/S1054-8807(03)00108-X. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- 14.Nagano H, Libby P, Taylor MK, Hasegawa S, Stinn JL, Becker G, Tilney NL, Mitchell RN. Coronary arteriosclerosis after T-cell-mediated injury in transplanted mouse hearts: role of interferon-gamma. Am J Pathol. 1998;152:1187–1197. [PMC free article] [PubMed] [Google Scholar]
- 15.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100:550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–33368. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 17.Peilot H, Rosengren B, Bondjers G, Hurt-Camejo E. Interferon-gamma induces secretory group IIA phospholipase A2 in human arterial smooth muscle cells. Involvement of cell differentiation, STAT-3 activation, and modulation by other cytokines. J Biol Chem. 2000;275:22895–22904. doi: 10.1074/jbc.M002783200. [DOI] [PubMed] [Google Scholar]
- 18.Pober JS, Min W, Bradley JR. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 19.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 20.Raisanen-Sokolowski A, Glysing-Jensen T, Koglin J, Russell ME. Reduced transplant arteriosclerosis in murine cardiac allografts placed in interferon-gamma knockout recipients. Am J Pathol. 1998;152:359–365. [PMC free article] [PubMed] [Google Scholar]
- 21.Ramana CV, Kumar A, Enelow R. Stat1-independent induction of SOCS-3 by interferon-gamma is mediated by sustained activation of Stat3 in mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2005;327:727–733. doi: 10.1016/j.bbrc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 22.Salomon RN, Hughes CCW, Schoen FJ, Payne DD, Pober JS, Libby P. Human Coronary Transplantation-Associated Arteriosclerosis - Evidence for a Chronic Immune Reaction to Activated Graft Endothelial Cells. Am J Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- 23.Scott DM, Ehrmann IE, Ellis PS, Chandler PR, Simpson E. Why do some females reject males? The molecular basis for male-specific graft rejection. J Mol Med. 1997;75:103–114. doi: 10.1007/s001090050095. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Devgan G, Darnell JE, Jr, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A. 2001;98:1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 26.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol. 2005;5:593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- 27.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 28.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 29.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 30.Vassalli G, Gallino A, Weis M, von Scheidt W, Kappenberger L, von Segesser LK, Goy JJ. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. Eur Heart J. 2003;24:1180–1188. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, Sessa WC, Lorber MI, Pober JS, Tellides G. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. Faseb J. 2004;18:606–608. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- 33.Xie D, Gore C, Liu J, Pong RC, Mason R, Hao G, Long M, Kabbani W, Yu L, Zhang H, et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2010;107:2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie D, Gore C, Zhou J, Pong RC, Zhang H, Yu L, Vessella RL, Min W, Hsieh JT. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci U S A. 2009;106:19878–19883. doi: 10.1073/pnas.0908458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yacoub-Youssef H, Marcheix B, Calise D, Thiers JC, Benoist H, Blaes N, Segui B, Dambrin C, Thomsen M. Chronic vascular rejection: histologic comparison between two murine experimental models. Transplant Proc. 2005;37:2886–2887. doi: 10.1016/j.transproceed.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, Qin L, Zhang H, He Y, Chen H, Pober J, Tellides G, Min W. AIP1 prevents graft arteriosclerosis by inhibiting IFN-γ-dependent smooth muscle cell proliferation and intimal expansion. Cir Res. 2011;109:418–427. doi: 10.1161/CIRCRESAHA.111.248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, et al. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W. RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem. 2007;282:14788–14796. doi: 10.1074/jbc.M701148200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Zhang R, Luo Y, D’Alessio A, Pober JS, Min W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J Biol Chem. 2004;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. AIP1 mediates TNF-alpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]