Summary
As exemplified by desmin-related cardiomyopathy and myocardial ischemia/reperfusion injury, proteasome functional insufficiency plays an essential pathogenic role in the progression of cardiac diseases with elevated proteotoxic stress. Upregulation of p62/SQSTM1 and increased selective autophagy in cardiomyocytes may protect against proteotoxic stress in the heart. p62 may serve as a proteotoxic stress sensor, promote segregation and degradation of misfolded proteins by autophagy, and mediate the crosstalk between the ubiquitin-proteasome system and autophagy.
Introduction
Protein homeostasis in the cell is essential to cell function and survival and requires precise control of protein synthesis, processing, and degradation. Like other eukaryotic cells, cardiomyocytes handle misfolded/damaged proteins (i.e., proteotoxic stress) via multi-layered protein quality control (PQC) mechanisms. The degradation of terminally misfolded/damaged proteins is the last line of defense in PQC and is mainly performed by the ubiquitin proteasome system (UPS) and autophagy (Arias et al. 2011, Wang et al. 2011). UPS-mediated proteolysis involves ubiquitination of the target protein molecules and degradation of the ubiquitinated protein by the proteasome (Scruggs et al. 2011). Macroautophagy (commonly known as autophagy) segregates portion of cytoplasm often including organelles such as mitochondria, via a double-membrane bound vacuole known as autophagosome for fusion with and degradation by lysosomes (Codogno et al. 2012). Due to increased production and/or impaired handling of misfolded/damaged proteins, PQC can become inadequate, permitting proteotoxic stress to contribute to aging and the progression of various common human diseases including neurodegenerative disorders and more recently cardiovascular disorders (Wang et al. 2006).
Increased proteotoxic stress in the heart is best exemplified in desmin-related cardiomyopathy (DRC). DRC is the cardiac aspect of desmin-related myopathy which is caused by protein misfolding resulting from mutations in the genes encoding desmin or its partner proteins such as αB-crystallin (CryAB), with intrasarcoplasmic desmin-positive aberrant protein aggregates as its pathological hallmark. Cardiac-specific transgenic (tg) overexpression of DRC-linked misfolded proteins, such as desmin with a 7-amino acid (R172~E178) deletion (D7-des) or CryAB with a missense mutation (CryABR120G), but not their wild-type counterparts, causes formation of desmin-positive protein aggregates and leads to cardiomyopathy in mice, recapitulating the main features of human DRC (McLendon et al. 2011). Studies from these mouse models have demonstrated protein aggregation and proteasome functional insufficiency (PFI) as the proximal pathogenic factors of DRC (Wang et al. 2011). We have recently uncovered that both p62 (a known substrate of autophagy) and autophagy are up-regulated in mouse hearts expressing CryABR120G or D7-des and that p62 mediates protein aggregation and autophagy activation to defend against proteotoxic stress in cardiomyocytes (Zheng et al. 2011a). Here we highlight recent advances in understanding the crosstalk between the UPS and autophagy in cardiac PQC, emphasizing the potential role of p62.
1. PFI in Cardiac Proteinopathy
Although mild proteotoxic stress may initially stimulate the proteasome activities (Aiken et al. 2011), persistent stress eventually suppresses UPS proteolytic function. Taking advantage of a UPS reporter mouse (Kumarapeli et al. 2005), we have demonstrated that cardiomyocyte-restricted tg expression of misfolded proteins, e.g., D7-des and CryABR120G, results in aberrant protein aggregation and proteasome impairment, leading to PFI (Chen et al. 2005, Liu et al. 2006a, Liu et al. 2006b). The PFI is contrasted by increased 20S proteasome peptidase activities, suggesting impairment in the delivery of the ubiquitinated proteins to 20S proteasomes. Indeed, key subunits of 19S proteasomes, which recognize and deubiquitinate the substrates and channel unfold polypeptides into the 20S proteasome, were down-regulated in proteinopathic hearts (Chen et al. 2005).
Although ubiquitination confers target specificity and is perhaps the rate-limiting step of UPS-mediated degradation of native proteins, upregulation of the proteasome activity seems to be more effective in improving the capability of handling proteotoxic stress in the cell. Very few measures are reported to benignly enhance proteasome function in the cell. We have recently shown that expression of proteasome activator (PA) 28α suffices to increase the 11S proteasome and its interaction with the 20S, and enhances the degradation of misfolded but not native proteins by the proteasome in mouse hearts (Li et al. 2011a, Li et al. 2011b). PA28α overexpression in the heart reduces protein aggregation, attenuates cardiac phenotypes, and delays the premature death of CryABR120G-based DRC mice. Moreover, enhancing proteasome function via PA28α overexpression protects against oxidative stress in cultured cardiomyocytes and myocardial ischemia/reperfusion injury in mice (Li et al. 2011a, Li et al. 2011b). These findings provide the first compelling evidence for the necessity of PFI in the genesis of not only proteinopathy but also other cardiac disorders with increased proteotoxic stress.
2. Adaptive Activation of Autophagy in Cardiac Proteinopathy
In addition to its role in energy sustaining during starvation, clearance of superfluous organelles, elimination of pathogens, and tissue renovation, autophagy, especially selective autophagy, may also play a role in intracellular quality control via targeted removal of protein aggregates and defective organelles (Codogno et al. 2012). The role of selective autophagy in defending cardiac proteotoxic stress has just begun to be unveiled. By analyzing the expression of endogenous LC3-II and the distribution of tg GFP-LC3, we found increases in autophagosomes in cultured cardiomyocytes and in the mouse heart that overexpress D7-des or CryABR120G (Zheng et al. 2011a). The increases were caused by enhanced formation, rather than reduced clearance as revealed by autophagic flux assays. Consistently, the lysosomal enzyme (e.g., cathepsin D) activity was concomitantly upregulated in D7-des hearts. Transmission electron microscopy showed rapid increases of autophagic vesicles in D7-des hearts after a brief lysosome inhibition, albeit only rare presence of these structures under baseline. Hence, increased autophagic flux in proteinopathic hearts has been demonstrated by both biochemical and morphological analyses (Zheng et al. 2011a). Moreover, pharmacological activation of autophagy attenuated D7-des-induced accumulation of ubiquitinated proteins in cultured cardiomyocytes while inhibition of autophagy did the opposite (Zheng et al. 2011a). These compelling evidences suggest that the activation of autophagy is compensatory in response to proteotoxic stress in cardiomyocytes. Supporting this notion, Hill group showed that blunting autophagy function by Beclin1 haploinsufficiency exacerbated CryABR120G–induced DRC (Tannous et al. 2008). Robbins laboratory recently reported that enhancement of autophagy by overexpressing Atg7 attenuated CryABR120G-induced cytotoxicity in cultured cardiomyocytes (Pattison et al. 2011).
Degradation of misfolded proteins or defective organelles sequestered in autophagosomes requires fusion of autophagosomes with lysosomes via a process known as autophagosome maturation. The critical role of autophagy induction in cellular homeostasis under both basal and stress conditions has been extensively illustrated but little is known about the regulation of autophagosome maturation and its pathophysiological significance. The COP9 signalosome is responsible for cullin deneddylation which is critical to the functioning of cullin-based ubiquitin E3’s, a large family of ubiquitin ligases (Su et al. 2011b). We have recently uncovered that perinatal knockout of COP9 signalosome subunit 8 (CSN8) in the heart impaired not only UPS-mediated proteolysis but also autophagosome maturation, leading to fatal cardiomyopathy accompanied by massive cardiomyocyte necrosis (Su et al. 2011a, Su et al. 2011b). The defective autophagy in CSN8 deficient hearts is likely attributable to downregulation of Rab7, a key regulator of autophagosome maturation (Hariharan et al. 2010, Su et al. 2011a). These new discoveries signify the importance of autophagosome maturation in cardiac pathogenesis, which is corroborated by a report that the heart of a mouse model of hypertrophic cardiomyopathy displayed increased protein levels of autophagosome markers without alterations in their mRNA levels, indicative of defective autophagosome degradation (Schlossarek et al. 2012). In order to efficiently digest the sequestered content, autophagosome induction should be matched with upregulation of the lysosomal hydrolytic capacity. Therefore, a coordinated programmed upregulation of both autophagosome induction and degradation would more effectively protect against proteotoxic stress.
3. Is p62 a Sensor of Proteotoxic stress in Cardiomyocytes?
It is largely accepted that the UPS and autophagy collaborate in defending against proteotoxic stress but the identity of the stress sensor, if any, and the mechanism by which these catabolic pathways are coordinated remain unclear. p62/SQSTM1 is a multifunctional protein containing a number of protein-protein interaction motifs that is involved in the regulation of cellular signaling and protein aggregation and degradation (Moscat et al. 2009). Multiple lines of evidence indicate that p62 is a sensor of proteotoxic stress. First, p62 transcripts and proteins are up-regulated upon expression of aggregation-prone proteins (Nagaoka et al. 2004, Zheng et al. 2011a), the latter impairs the proteasome (Wang et al. 2006). Second, p62 is selectively degraded by autophagy but not the UPS (Bjorkoy et al. 2005), and accumulates when autophagy function is inhibited (Nakai et al. 2007, Su et al. 2011a). Third, pharmacological inhibition of the proteasome also increased p62 expression (Kuusisto et al. 2001, Nakaso et al. 2004). Lastly, p62 is increased in mouse hearts with defects in UPS- and/or autophagy-mediated proteolysis (Schlossarek et al. 2012, Su et al. 2011a).
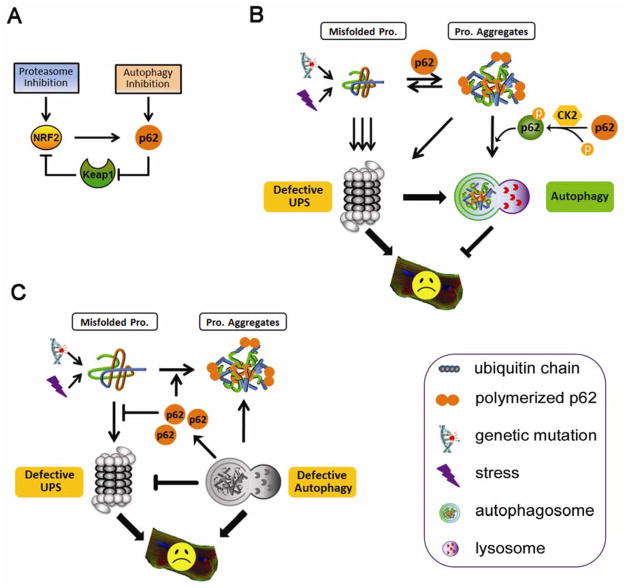
As illustrated in Figure 1, the expression of p62 can be transcriptionally regulated by NRF2 (NF-E2-related factor 2), a transcription factor that controls the expression of an array of anti-oxidant genes. NRF2 protein is constitutively degraded by the proteasome under the control of cullin3-Keap1 ubiquitin ligase in non-stressed condition. In response to oxidative stress, the interaction between Keap1 and NRF2 is disrupted, resulting in the stabilization and activation of NRF2 (Cullinan et al. 2004, Kobayashi et al. 2004), which in turn binds to the antioxidant responsive element (ARE) in the promoter of p62 and induces its expression (Jain et al. 2010). Interestingly, p62 was recently shown to interact with Keap1 at the NRF2 binding site, and compete for NRF2 against Keap1 when upregulated. Therefore, p62 can control its own expression by regulating NRF2 activation (Jain et al. 2010).
Figure 1. p62 and protein quality control (PQC) in cardiomyocytes.
(A) Upregulation of p62 in response to proteotoxic stress sequesters NRF2 from its interaction with Keap1, leading to the stablization and activation of NRF2, which in turn induces p62 expression. (B) Proteasome functional insufficiency leads to the accumulation of protein aggregates and compensatory activation of selective autophagy in a p62-dependent manner. Casein kinase 2 (CK2)-mediated p62 phosphorylation regulates selective degradation of protein aggregates by autophagy. Autophagy activation protects cardiomyocytes from defective UPS-induced proteotoxic stress. (C) Defective autophagy accumulates p62, which binds ubiquitinated proteins and promotes their agregation, hindering proteasomal proteolysis. Both defective autophagy and impaired UPS function are detrimental to cardiomyocyte function and survival.
4. p62 Promotes Protein Aggregation and Selective Autophagy
The significance of p62 in defending proteotoxic stress is largely reflected by its critical role in mediating the formation and selective degradation of protein aggregates. One plausible model (Figure 1B) is that p62 acts to: 1) recognize the non-functional ubiquitinated proteins via its ubiquitin-associating domain (UBA); 2) promote protein aggregation via its ability to multimerize through PB1 domain, thus reducing the toxicity of soluble misfolded proteins; and 3) deliver the aggregates for autophagic degradation through both LC3-interacting region (LIR) and PB1 domains (Johansen et al. 2011). Obviously this model remains to be fully established but it is well supported by our recent findings that expression of CryABR120G or D7-des in cardiomyocytes upregulates p62 in the heart and that depletion of p62 reduces the formation of protein aggregates and ubiquitinated proteins and aggravates stress-induced cell injury (Zheng et al. 2011a).
The activity of p62 in selective autophagy appears to be controlled by its phosphorylation status. Phosphorylation at Ser403 of the p62 UBA domain, as mediated by casein kinase 2 (CK2), enhances the affinity of the UBA domain to polyubiquitin chains, promoting inclusion body formation and efficient autophagic degradation of the polyubiquitinated proteins (Matsumoto et al. 2011).
5. Does p62 mediate the Crosstalk between the UPS and Autophagy in the Heart?
A crosstalk between the UPS and autophagy in PQC and a mediating role of p62 in the crosstalk are suggested by several lines of evidence. First, proteasome inhibition is sufficient to activate autophagy in the heart (Zheng et al. 2011b) and autophagy is activated in mouse hearts with PFI (Zheng et al. 2011a). Second, chronic inhibition of autophagy has also been shown to hinder the proteasome from degrading ubiquitinated substrates (Korolchuk et al. 2009) or to lead to a delayed decrease in proteasome peptidase activities (Qiao et al. 2008) but both remain to be confirmed in cardiomyocytes. Third, p62 is increased during both PFI and autophagy inhibition (Komatsu et al. 2007, Zheng et al. 2011a).
The mechanism by which p62 mediates the induction of autophagy during PFI or proteasome inhibition appears to be straightforward and is discussed in Section 4. Interestingly, p62 accumulation during autophagy inhibition appears to be responsible for the defective UPS function, as p62 silencing attenuated the accumulation of proteasome substrates caused by autophagy inhibition (Korolchuk et al. 2009). One explanation for this observation is that accumulated p62 catches the ubiquitinated proteins before they reach the proteasome and promote the formation of protein aggregates, which thus become inaccessible to the proteasome. Indeed, overexpression of p62 is also sufficient to accumulate proteasome substrates under basal condition (Korolchuk et al. 2009). Loss of p62 in mice was shown to attenuate the accumulation of ubiquitinated proteins in Atg5-deficient cells (Komatsu et al. 2007). Ablation of NRF2, which transcriptionally activates p62 expression as aforementioned, also attenuated the accumulation of polyubiquitinated proteins in Atg7 knockout cells (Riley et al. 2010). These data suggest impaired autophagy may, via accumulating p62, impair proteasome function, thereby severely compromising the removal of misfolded proteins in the cell.
6. Concluding remarks
Recent research on the UPS, autophagy and p62 in the heart has yielded exciting insights into the molecular pathogenesis of proteotoxic stress. Proteotoxicity from increased expression of misfolded/damaged proteins causes cardiac PFI, upregulates p62, and triggers compensatory autophagy. Notably, the increased prevalence of pre-amyloid oligomers and ubiquitinated proteins have been observed in most human failing hearts (Sanbe et al. 2005, Weekes et al. 2003), implicating that increased proteotoxic stress is likely a common pathogenic factor for the progression of a large subset of heart disease to congestive heart failure. Therefore, improving PQC may become a novel therapeutic strategy to treat many forms of heart disease with excessive proteotoxic stress. Indeed, we showed that genetic enhancement of proteasome function in cardiomyocytes protects against not only a bona fide proteinopathy but also myocardial ischemia/reperfusion injury in mice (Li et al. 2011a). Given that a small molecule inhibitor (IU-1) of ubiquitin-specific protease 14 (USP14, a 19S proteasome associated deubiquitinating enzyme) was recently shown to enhance proteasome function (Lee et al. 2010), it is in the horizon to pharmacologically enhance proteasome-mediated degradation of misfolded proteins to treat disease with elevated proteotoxic stress. To this end, it will be important to test whether enhancing p62 phosphorylation and increasing lysosomal biogenesis can protect against diseases with increased proteotoxic stress.
The crosstalk between the UPS and autophagy remains incompletely understood. Since autophagy deficiency is shown to compromise the degradation of UPS substrates, it will be important to elucidate if autophagy inhibition, by blocking either the initiation or the degradation of autophagosomes, compromises UPS function in the heart. Given the implication of p62 accumulation and the impairment of UPS function in proteinopathic hearts, further investigation on the impact of p62 in UPS proteolysis in proteinopathic hearts may provide another underlying mechanism for the pathogenesis of DRC.
Acknowledgments
We are in debt to the former and current members of our laboratory for their contributions to the work summarized here. Dr. X. Wang is a recipient of the Established Investigator Award of the American Heart Association. This work is in part supported by NIH grants R01HL085629, R01HL072166, and R01HL068936 and American Heart Association grants 0740025N (to X. W.) and 11SDG6960011 (to H.S.).
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken CT, Kaake RM, Wang X, Huang L. Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. 2011;10:R110 006924. doi: 10.1074/mcp.M110.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Current opinion in cell biology. 2011;23:184–9. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liu JB, Horak KM, et al. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–26. doi: 10.1161/01.RES.0000189262.92896.0b. [DOI] [PubMed] [Google Scholar]
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy. variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase. oxidative stress sensing by a Cul3-eap1 ligase. Mol Cell Biol. 2004;24:8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–82. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–96. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–27. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarapeli AR, Horak KM, Glasford JW, et al. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J. 2005;19:2051–3. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Suuronen T, Salminen A. Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. Biochem Biophys Res Commun. 2001;280:223–8. doi: 10.1006/bbrc.2000.4107. [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee MJ, Park S, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–84. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Horak KM, Su H, et al. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011a;121:3689–700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. FASEB J. 2011b;25:883–93. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen Q, Huang W, et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006a;20:362–4. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- Liu J, Tang M, Mestril R, Wang X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J Mol Cell Cardiol. 2006b;40:451–4. doi: 10.1016/j.yjmcc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell. 2011;44:279–89. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- McLendon PM, Robbins J. Desmin-related cardiomyopathy. an unfolding story. Am J Physiol Heart Circ Physiol. 2011;301:H1220–8. doi: 10.1152/ajpheart.00601.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–4. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka U, Kim K, Jana NR, et al. Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J Neurochem. 2004;91:57–68. doi: 10.1111/j.1471-4159.2004.02692.x. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yoshimoto Y, Nakano T, et al. Transcriptional activation of p62/A170/ZIP during the formation of the aggregates. possible mechanisms and the role in Lewy body formation in Parkinson’s disease. Brain Res. 2004;1012:42–51. doi: 10.1016/j.brainres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–60. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hamamichi S, Caldwell KA, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BE, Kaiser SE, Shaler TA, et al. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway. a potential role for protein aggregation in autophagic substrate selection. J Cell Biol. 2010;191:537–52. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbe A, Osinska H, Villa C, et al. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2005;102:13592–7. doi: 10.1073/pnas.0503324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossarek S, Englmann DR, Sultan KR, et al. Defective proteolytic systems in Mybpc3-targeted mice with cardiac hypertrophy. Basic Res Cardiol. 2012;107:1–13. doi: 10.1007/s00395-011-0235-3. [DOI] [PubMed] [Google Scholar]
- Scruggs SB, Ping P, Zong C. Heterogeneous cardiac proteasomes. mandated by diverse substrates? Physiology (Bethesda) 2011;26:106–14. doi: 10.1152/physiol.00039.2010. [DOI] [PubMed] [Google Scholar]
- Su H, Li F, Ranek MJ, Wei N, Wang X. COP9 signalosome regulates autophagosome maturation. Circulation. 2011a;124:2117–28. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Li J, Menon S, et al. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res. 2011b;108:40–50. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous P, Zhu H, Johnstone JL, et al. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–50. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H2207–19. doi: 10.1152/ajpheart.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–28. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- Weekes J, Morrison K, Mullen A, et al. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics. 2003;3:208–16. doi: 10.1002/pmic.200390029. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Su H, Ranek MJ, Wang X. Autophagy and p62 in cardiac proteinopathy. Circ Res. 2011a;109:296–308. doi: 10.1161/CIRCRESAHA.111.244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Su H, Tian Z, Wang X. Proteasome malfunction activates macroautophagy in the heart. Am J Cardiovasc Dis. 2011b;1:214–26. [PMC free article] [PubMed] [Google Scholar]